Abstract
Background
Extreme BMI (either very high or very low) has been associated with increased risk of adverse perioperative outcome in adults undergoing cardiac surgery. The effect of body-mass index (BMI) on perioperative outcomes in congenital heart disease patients has not been evaluated.
Methods
A multicenter retrospective cohort study was performed studying patients 10–35 years undergoing a congenital heart disease operation in the Society of Thoracic Surgeons Congenital Heart Surgery Database between 1/1/2010–12/31/2015. The primary outcomes were operative mortality and a composite outcome (one or more of operative mortality, major adverse event, prolonged hospital length of stay, and wound infection/dehiscence). The associations between age and sex-adjusted BMI percentiles and these outcomes were assessed, adjusting for patient level risk factors, using multivariate logistic regression.
Results
Of 18,337 patients (118 centers), 16% were obese, 15% overweight, 53% normal weight, 7% underweight and 9% were severely underweight. Observed risks of operative mortality (p=0.04) and composite outcome (p<0.0001) were higher in severely underweight and obese subjects. Severely underweight BMI was associated with increased unplanned cardiac operation and reoperation for bleeding. Obesity was associated with increased risk of wound infection. In multivariable analysis, the association between BMI and operative mortality was no longer significant. Obese (OR: 1.28 p=0.008), severely underweight (OR: 1.29 p<0.0001) and underweight subjects (OR: 1.39 p=0.002) subjects were associated with increased risk of composite outcome.
Conclusion
Obesity and underweight BMI were associated with increased risk of composite adverse outcome independent of other risk factors. Further research is necessary to determine whether BMI represents a modifiable risk factor for perioperative outcome.
Keywords: Outcomes research, pediatric cardiology, congenital heart surgery, mortality, obesity
Introduction
The effect of body mass on outcomes following operations for congenital heart disease (CHD) is not well understood. Obesity and associated co-morbid conditions are associated with increased lifetime risks of morbidity and mortality1,2, especially due to cardiovascular disease3. For patients undergoing cardiothoracic surgery, obesity might specifically increase the risk of adverse outcomes in the perioperative period. It is frequently coincident with diabetes mellitus, hypertension, peripheral vascular disease, and pulmonary disease, which are indicators of medical frailty. Moreover, these comorbid conditions may also interfere with wound healing and recovery from surgery. Finally, the body habitus of obese patients can pose a technical challenge for the surgical approach, an obstacle to closure of the chest, and an impediment to wound healing.
In older adults undergoing coronary artery bypass graft and valve replacement operations, subjects with extreme BMI (underweight and extreme or morbid obesity) had higher risk of mortality and major adverse outcome4–7. It is not clear that these results can be extrapolated for younger patients with CHD, and data on the subject is limited. A single previous matched cohort study attempted to measure the effect of obesity on perioperative outcomes8 with equivocal results, but may have been limited by its dependence on billing data to differentiate obese and non-obese subjects.
Low BMI also is also a potential risk factor for adverse perioperative outcome. In previous studies of adult patients undergoing surgery, overweight and obese BMI have been associated with reduced risk of adverse outcomes, especially in comparison to underweight subjects4,9,10. A proposed mechanisms underlying this “obesity paradox” is that chronic illness, especially heart failure, results in a malnourished state, manifest as unintended weight loss and accompanied by deficits in immune function, tissue maintenance, and other processes that are potentially relevant to recovery from an operation. In elderly cardiac surgery patients, underweight status is associated with increased risk of mortality and adverse events4–7. In longitudinal studies of elderly heart failure patients, unintended weight loss is independently associated with increased risk of adverse events11–13. In CHD patients, residual anatomic disease, myocardial dysfunction, and non-cardiac disease all could result in an analogous heart failure syndrome. Though markers of malnutrition have been shown to be associated with risk of adverse outcome following cardiac surgery in young children14,15, to our knowledge no studies have been performed in older CHD patients.
We utilized data from the Society for Thoracic Surgeons Congenital Heart Surgery Database (STSCHSD) to perform a multicenter retrospective cohort study to determine if body mass was associated with perioperative risk in children, adolescents, and young adults. The STSCHSD provides a large and representative study sample with a well-validated risk stratification model16. We hypothesized that obesity and underweight BMI would be associated with increased risk of adverse perioperative outcome, specifically operative mortality and major morbidity.
Methods
Data Source
Data from the STSCHSD was used for this study. As of June 30, 2016, the database contains de-identified data on 414,174 operations performed since 2000 at 122 centers in North America, representing 96% of all centers performing CHD surgery in the United States and >96% of all operations17–19. Data collected is comprised of preoperative condition, details of the operation, and early postoperative outcomes on all pediatric and/or CHD patients at participating centers. Operations are stratified on the basis of mortality risk using the Society of Thoracic Surgeons- European Association for Cardio-Thoracic Surgery Congenital Heart Surgery Mortality Categories (STAT mortality categories)16. Coding for this database is accomplished by clinicians and ancillary staff using the International Pediatric and Congenital Cardiac Code17,18. The Duke Clinical Research Institute serves as the data warehouse and analysis center for all of the Society for Thoracic Surgeons (STS) national databases, including STSCHSD. Evaluation of data quality includes intrinsic verification and formal in-person site visits and data audits conducted by a panel of independent data quality personnel and pediatric cardiac surgeons at 10% of sites per year17–19. The study was approved by STSCHSD Access and Publications Committee. It was reviewed by Duke University Institutional Review Board, which determined that the project was exempt from review in accordance with the Common Rule (45 CFR 46.102(f)). As an analysis of aggregated de-identified data, contacting and obtaining informed consent from subjects was not possible and was not pursued.
Study population
Children, adolescents, and young adults between 10 and 35 years of age undergoing a cardiothoracic operation with or without cardiopulmonary bypass recorded in STSCHD from 1/1/2010 to 12/31/2015 were studied. The timeframe was chosen to maintain a consistent version of the database. Operations were excluded if the STAT Mortality Category, sex, BMI percentile, operative mortality, or major morbidity were missing. Operations for organ procurement were also excluded. If more than one operation was performed during a single admission, only the index (first operation of the hospital admission) operation was included. For multi-component operations, a primary procedure (component procedure with the highest STAT Score) was identified, and analysis was based on the primary procedure of the index operation16,19. Centers were excluded if they reported >15% missing data for operative mortality, major morbidity, or key pre-operative factors.
Data collection
Data was extracted directly from the database and included demographics, baseline pre-operative characteristics, operative variables including STAT Mortality Category, and outcomes.
Exposure and outcomes
The primary exposure was BMI. Through childhood and adolescence, the population distribution of BMI varies. These changes are also different for boys and girls. To provide a comparable statistic, we converted BMI to BMI percentile for age and sex. For descriptive purposes, BMI percentile is divided into strata defined by the United States Center for Disease Control and Prevention BMI categories: obese (BMI≥95%), overweight (BMI ≥85 and <95%), normal weight (>15 and <85%), underweight (5–15%), and severely underweight (≤5%) (http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html). For adult subjects (20–35 years), BMI-percentiles for 20 year olds were used. Though there are alternative methods for calculating BMI percentiles20, the CDC categories were chosen because they were derived from a population sample that is most similar to our study population. The CDC also publishes electronic calculators to convert from BMI to BMI percentile using their methodology.
Two primary outcomes were identified prior to analysis: 1) operative mortality (all deaths, regardless of cause, occurring during the hospitalization in which the operation was performed even if after 30 days (including patients transferred to other acute or chronic care facilities); and all deaths, regardless of cause, occurring after discharge from the hospital but before the end of the 30th post-operative day) and 2) a composite outcome of any one or more of the following: operative mortality, major adverse event, wound infection, and prolonged length of stay (postoperative length of stay >14 days). Major adverse outcome was defined as previously described16 and was comprised of temporary or permanent renal failure at discharge, neurological deficit at discharge, atrioventricular block or arrhythmia requiring permanent pacemaker, mechanical circulatory support, phrenic nerve injury, unplanned re-operation or unplanned catheterization during the post-operative period. Wound infection was defined as any of the following: wound dehiscence, wound infection including deep wound infection, mediastinitis, and superficial wound infection. The risks for each of the individual adverse outcomes were also recorded. A composite outcome was chosen as one of the two primary outcomes, because perioperative mortality was known to have a relatively low event rate. The higher event rate of the composite outcome was expected to improve the statistical power in a study with a fixed population, and we also felt that BMI potentially influenced all of the components of the composite outcome.
Covariates
Covariates of interest were identified before analysis and based on the components of the STSCHSD risk adjustment model16, specifically patient age, sex, race, STAT mortality score, previous cardiothoracic surgery, non-cardiac anatomic abnormalities, genetic syndrome/chromosomal abnormalities, and preoperative factors (mechanical circulatory support, shock, mechanical ventilation, renal failure, neurological deficit, and other coded pre-operative risk factors). Genetic variations or syndromes that have frequent or prominent cardiac manifestations (some of which may also affect BMI) are likely to have a higher prevalence in a database of cardiac surgical outcomes, whereas syndromes that affect BMI without other symptoms are unlikely to be tested for, and therefore less likely to be recorded.. Diabetes mellitus (both type I and II) was added to the model, because of possible association with both obesity and perioperative outcome.
Analysis
Descriptive statistics were calculated to characterize the study population. The preoperative characteristics, operations, and outcomes are summarized for the cohort stratified by modified Centers for Disease Control BMI category to assess whether other preoperative risk factors were distributed uniformly across BMI classes. A similar comparison was done by STAT mortality category. Patients’ baseline characteristics were compared across BMI or STAT mortality categories using chi-square tests for categorical variables and Kruskal-Wallis tests for continuous variables.
Mixed effects multivariable logistic regression models were used to assess the relationship between our primary exposure and outcomes. These models included fixed effects for patient level covariates and a random intercept for center to account for clustering within centers. Modeling was performed in two steps. First, BMI percentile was treated as a continuous variable. To evaluate a possible non-linear association between BMI and the study outcomes restricted linear splines were used, and if possible models with cut-points based on the splines were constructed21. Second, to confirm these results using categories that are immediately recognizable to clinicians, models were constructed using the modified BMI categories as the primary exposure. For all models, pre-identified covariates were included in the light of prior knowledge of the disease and based on clinical expert opinion. No secondary analyses or sensitivity analyses were performed.
As noted above, missing data for critical fields were eliminated by case restriction. Missing data in non-critical fields was uncommon (less than 2%). Therefore, simple imputation for missing covariate data was used in multivariable models where the mode was imputed for categorical covariate and the median for continuous covariates. No special adjustments were made for multiple comparisons. The study population was fixed, and so no power calculations were performed. The threshold for significance was set at p<0.05. All analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, NC).
Results
Study population
The initial data query generated a cohort of 27,965 procedures from 122 hospitals (Figure 1). Applying exclusion criteria generated an analytic cohort of 18,337 operations from 118 hospitals. This cohort was 43% female and 64% white with median age of 15.9 years (IQR: 13.1–20.0). Dividing the study population by modified CDC categories, 9% of the cohort was severely underweight, 7% underweight, 53% normal weight, 15% overweight, and 16% obese. In terms of the operations performed, 39% were STAT 1, 38% were STAT 2, 9% STAT 3, and 14% STAT 4/5.
Figure 1.
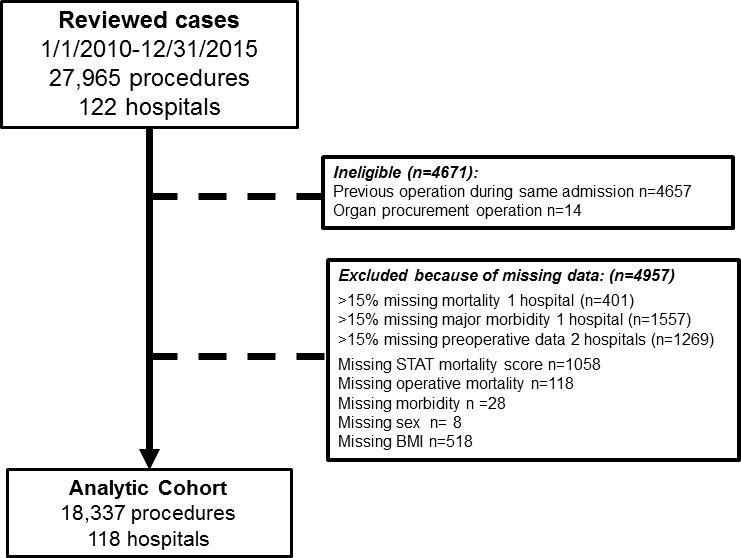
Study population
Stratifying the population by modified CDC BMI classification revealed several significant differences in baseline characteristics (Table 1). These included a number of factors identified as risk factors for operative mortality in the STS mortality risk adjustment model. A higher proportion of black and Hispanic subjects were obese, while a higher portion of Asian patients were underweight or severely underweight (p<0.001). Non-cardiac anomalies and genetic syndromes were more prevalent in underweight and severely underweight subjects than in normal weight subjects (both p<0.0001). Mechanical circulatory support (p=0.04), mechanical ventilation (p=0.007), shock (p=0.03), neurologic deficits (p=0.0004), hypocoagulable state independent of medication (p=0.04), and “other” preoperative risk factors were all more prevalent in severely underweight and underweight patients. Type II diabetes mellitus was more prevalent in obese patients (p<0.0001). Additionally, underweight and severely underweight subjects underwent higher mortality risk (STAT 3 and 4/5) operations more frequently than other subjects (Figure 2, p<0.0001). Additionally, when subjects were grouped by the STAT mortality score of their operation (Table 2), a larger proportion of patients undergoing STAT 3 and 4/5 operations had genetic syndromes, non-cardiac anatomic abnormalities, and preoperative risk factors (p<0.001).
Table 1.
Study Population stratified by CDC BMI category
| Severely Underweight (<5%) |
Underweight (5–15%) |
Normal (5–85%) |
Overweight (85–95%) |
Obese (>95%) |
p | |
|---|---|---|---|---|---|---|
| n | 1694 | 1352 | 9637 | 2789 | 2865 | |
| Age (years) | 15.4 (12.5, 19.1) | 15.1 (12.5, 18.3) | 15.9 (13.1, 19.9) | 16.3 (13.4, 21.6) | 16.2 (13.4, 20.7) | <0.0001 |
| Female sex | 36% (608) | 40% (536) | 45% (4305) | 47% (1301) | 37% (1069) | <0.0001 |
| Race | ||||||
| White | 63% (1060) | 65% (881) | 65% (6241) | 65% (1801) | 61% (1751) | <0.0001 |
| Black | 14% (244) | 13% (177) | 13% (1253) | 12% (344) | 16% (458) | |
| Hispanic | 10% (161) | 11% (153) | 11% (1070) | 13% (361) | 14% (411) | |
| Asian | 5% (88) | 4% (56) | 4% (347) | 3% (73) | 2% (49) | |
| Other | 6% (108) | 5% (64) | 5% (525) | 6% (159) | 6% (160) | |
| Weight (kg) | 38 (29, 48) | 43 (33, 52) | 55 (44, 65) | 69 (58, 80) | 87 (72, 102) | <0.0001 |
| Height (cm) | 157 (144, 170) | 158 (144, 169) | 162 (152, 171) | 162 (153, 171) | 164 (153, 173) | <0.0001 |
| Body mass index (kg/m2) | 15.4 (14.0, 16.9) | 17.0 (15.6, 18.5) | 20.6 (18.7, 22.7) | 26.3 (23.9, 28.1) | 32.0 (28.9, 35.6) | |
| Chromosomal abnormalities | 5% (84) | 5% (66) | 5% (512) | 8% (215) | 11% (301) | <0.0001 |
| Non-cardiac anatomic abnormalities | 12% (205) | 8% (106) | 5% (470) | 4% (111) | 4% (125) | <0.0001 |
| Genetic syndrome | 19% (322) | 14% (195) | 11% (1102) | 13% (358) | 15% (415) | <0.0001 |
| Number of prior cardiac operation(s) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | <0.001 |
| STAT mortality category | ||||||
| 1 | 33% (557) | 39% (530) | 40% (3875) | 38% (1061) | 38% (1100) | <0.0001 |
| 2 | 38% (641) | 35% (474) | 38% (3621) | 40% (1110) | 39% (1110) | |
| 3 | 11% (190) | 11% (151) | 9% (815) | 8% (214) | 8% (239) | |
| 4/5 | 18% (306) | 15% (197) | 14% (1326) | 15% (404) | 15% (416) | |
| Preoperative condition | ||||||
| Hypercoagulable state | 1% (12) | 1% (12) | 1% (56) | 1% (15) | 1% (23) | 0.47 |
| Hypocoagulable state not secondary to medication | 1% (14) | 2% (20) | 1% (67) | 1% (27) | 1% (21) | 0.04 |
| Hypocoagulable state secondary to medication | 3% (55) | 3% (35) | 2% (207) | 2% (63) | 2% (66) | 0.09 |
| Diabetes mellitus | ||||||
| Diabetes mellitus – Type I | 1% (14) | 0.4% (6) | 1% (58) | 1% (21) | 1% (17) | 0.62 |
| Diabetes mellitus – Type II | 0.1% (1) | 0% (0) | 0.1% (13) | 0.2% (6) | 0.6% (18) | <0.0001 |
| Renal failure | 2% (29) | 2% (22) | 1% (106) | 1% 932) | 1% (37) | 0.16 |
| Mechanical ventilation | 2% (34) | 1% (19) | 1% (111) | 1% (23) | 2% (42) | 0.007 |
| Mechanical circulatory support | 1% (23) | 1% (16) | 1% (78) | 1% (24) | 1% (38) | 0.04 |
| Persistent shock | 0.4% (7) | 1% (9) | 0.4% (34) | 1% (15) | 1% (23) | 0.03 |
| Stroke or intracranial hemorrhage within 48 hours preceding operation | 5% (77) | 4% (57) | 3% (273) | 3% (79) | 3% (85) | 0.0004 |
Data are presented as % (n) or median (interquartile range).
Figure 2. Distribution of subject BMI category and STAT mortality category.
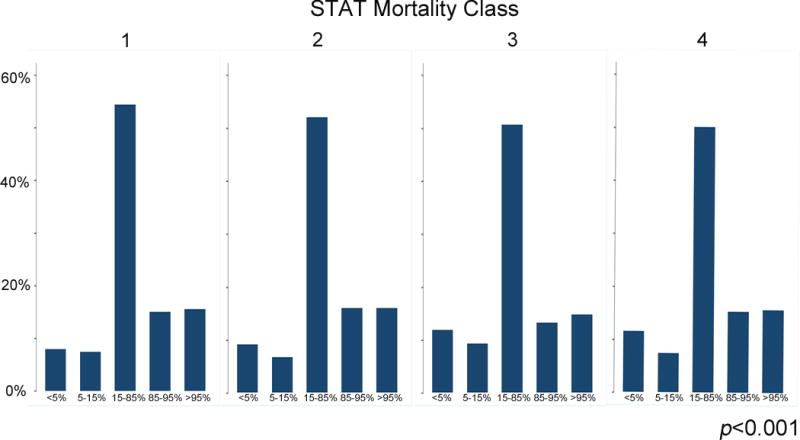
This histogram depicts the distribution (percent) of subject BMI category (x-axis) within each STAT Mortality Class (y-axis).
Table 2.
Study Population stratified by STAT category
| STAT Mortality Category | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4/5 | p | |
| n | 7123 | 6956 | 1609 | 2649 | |
| Age (years) | 16.0 (13.0, 20.4) | 16.1 (13.3, 20.1) | 15.2 (12.6, 18.9) | 15.7 (13.1, 19.2) | <0.0001 |
| Female sex | 46% (3245) | 39% (2710) | 43% (698) | 44% (1166) | <0.0001 |
| Race | <0.0001 | ||||
| White | 64% (4571) | 65% (4493) | 67% (1073) | 60% (1597) | |
| Black | 12% (864) | 14% (981) | 14% (217) | 16% (414) | |
| Hispanic | 13% (890) | 11% (772) | 11% (181) | 12% (313) | |
| Asian | 4% (275) | 3% (203) | 3% (44) | 3% (91) | |
| Other | 6% (390) | 6% (379) | 4% (67) | 7% (180) | |
| Weight (kg) | 57 (45, 71) | 59 (46, 74) | 53 (39, 68) | 56 (42, 70) | <0.0001 |
| Height (cm) | 162 (151, 170) | 163 (152, 173) | 158 (147, 168) | 160 (150, 170) | <0.0001 |
| Body mass index (kg/m2) | 21.5 (18.5, 25.7) | 21.7 (18.5, 26.0) | 20.6 (17.6, 24.8) | 21.4 (18.0, 25.6) | <0.0001 |
| Chromosomal abnormality | 6% (408) | 7% (463) | 11% (172) | 5% (135) | <0.0001 |
| Non-cardiac anatomic abnormality | 4% (307) | 6% (417) | 9% (136) | 6% (157) | <0.0001 |
| Genetic syndrome | 10% (711) | 15% (1035) | 17% (269) | 13% (336) | <0.0001 |
| Number of prior cardiac operation(s) | 0 (0, 2) | 1 (0, 2) | 1 (1, 2) | 1 (0, 2) | <0.0001 |
| CDC BMI category | |||||
| Severely underweight | 8% (557) | 9% (641) | 12% (190) | 12% (306) | <0.0001 |
| Underweight | 7% (530) | 7% (474) | 9% (151) | 7% (197) | |
| Normal | 54% (3875) | 52% (3621) | 51% (815) | 50% (1326) | |
| Overweight | 15% (1061) | 16% (1110) | 13% (214) | 15% (404) | |
| Obese | 15% (1100) | 16% (1110) | 15% (239) | 16% (416) | |
| Preoperative condition | |||||
| Hypercoagulable state | 0.4% (32) | 1% (36) | 1% (10) | 2% (40) | <0.0001 |
| Hypocoagulable state not secondary to medication | 0.4% (32) | 1% (61) | 1% (12) | 2% (43) | <0.0001 |
| Hypocoagulable state secondary to medication | 1% (70) | 2% (121) | 1% (16) | 8% (219) | <0.0001 |
| Diabetes mellitus | 0.6% (43) | 0.6% (42) | 2.5% (40) | 1.1% (29) | <0.0001 |
| Diabetes mellitus – Type I | 0.4% (32) | 0.4% (26) | 2.2% (35) | 0.9% (23) | <0.0001 |
| Diabetes mellitus – Type II | 0.2% (11) | 0.2% (16) | 0.3% (5) | 0.2% (6) | 0.57 |
| Renal failure | 0.5% (39) | 1.0% (69) | 1.4% (23) | 3.6% (95) | <0.0001 |
| Mechanical ventilation | 1% (44) | 1% (64) | 2% (29) | 4% (92) | <0.0001 |
| Mechanical circulatory support | 0.2% (15) | 0.1% (8) | 1% (19) | 5% (137) | <0.0001 |
| Persistent shock | 0.2% (12) | 0.3% (23) | 0.4% (6) | 2% (47) | <0.0001 |
| Stroke or intracranial hemorrhage within 48 hours preceding operation | 3% (199) | 3% (216) | 4% (64) | 4% (92) | 0.06 |
Data are presented as % (n) or median (interquartile range).
Operative outcomes
The observed operative mortality was higher in severely underweight (1.6%) and obese subjects (1.2%) than in underweight, normal weight, and overweight subjects (0.9% in each, p=0.04, Table 3). Risk of composite outcome (mortality, major adverse events, prolonged length of stay, and wound infection) and major adverse events were both higher in obese, underweight, and severely underweight subjects than in normal weight or overweight subjects (both p<0.0001). The rates of reoperation for bleeding, unplanned cardiac operation, and non-cardiac procedures were all significantly higher in severely underweight subjects. The risk of wound infection or dehiscence was significantly higher in overweight and obese patients (p<0.0001). Hospital length of stay was higher in these groups as well (p<0.0001), as was the risk of prolonged hospital length of stay (>14 days) (p<0.0001). As expected the risk of all perioperative adverse events increased with STAT mortality score (Supplemental Table 1).
Table 3.
Perioperative outcomes stratified by BMI categories
| Severely underweight | Underweight | Normal weight | Overweight | Obese | p | |
|---|---|---|---|---|---|---|
| Operative mortality | 1.6% (27) | 0.9% (12) | 0.9% (82) | 0.9% (25) | 1.2% (34) | 0.04 |
| Cardiopulmonary bypass time (minutes) | 105 (IQR: 66–170) | 100 (IQR: 63–158) | 96 (IQR: 62–147) | 101 (IQR: 67–159) | 104 (IQR: 69–164) | <0.0001 |
| Operating room time (hours) | 5.9 (IQR: 4.2–7.7) | 5.6 (IQR: 4.2–7.4) | 5.4 (IQR: 4.1–7.1) | 5.7 (IQR: 4.3–7.4) | 6.0 (IQR: 4.5–7.9) | <0.0001 |
| Length of stay (days) | 5.0 (IQR: 3.0–8.0) | 5.0 (IQR: 3.0–7.0) | 4.0 (IQR: 3.0–6.0) | 4.0 (IQR: 3.0–6.0) | 5.0 (IQR: 4.0–7.0) | <0.0001 |
| Major complication | 9.4% (159) | 7.1% (96) | 5.9% (564) | 5.9% (165) | 6.4% (182) | <0.0001 |
| Arrhythmia requiring pacemaker | 2.1% (32) | 1.5% (18) | 1.6% (136) | 1.4% (35) | 1.7% (44) | 0.51 |
| New mechanical circulatory support | 1.4% (21) | 0.7% (8) | 0.7% (64) | 0.8% (21) | 1.1% (29) | 0.07 |
| Renal failure requiring permanent dialysis | 0.4% (6) | 0.1% (1) | 0.3% (22) | 0.2% (5) | 0.3% (8) | 0.53 |
| Renal failure requiring temporary dialysis | 0.2% (5) | 0.3% (4) | 0.4% (30) | 0.3% (8) | 0.5% (12) | 0.91 |
| Neurological deficit at discharge | 1.1% (17) | 0.8% (10) | 0.5% (44) | 0.5% (12) | 0.7% (17) | 0.05 |
| Phrenic nerve injury or diaphragm hemiparesis | 0.1% (1) | 0.3% (4) | 0.4% (30) | 0.3% (8) | 0.5% (12) | 0.91 |
| Reoperation for bleeding | 3.4% (52) | 2.6% (31) | 2.1% (179) | 2.0% (50) | 1.6% (40) | 0.002 |
| Unplanned cardiac operation | 2.9% (49 | 1.6% (22) | 1.4% (132) | 1.5% (43) | 1.9% (54) | 0.0002 |
| Unplanned interventional catheterization procedure | 0.7% (12) | 0.9% (12) | 0.7% (63) | 0.5% (13) | 0.7% (19) | 0.60 |
| Unplanned non-cardiovascular procedure | 1.5% (25) | 1.3% (17) | 0.8% (79) | 0.9% (24) | 0.6% (17) | 0.02 |
| Composite Outcome | 17.4% (294) | 14.1% (190) | 10.6% (1021) | 10.9% (305) | 13.1% (375) | <0.0001 |
| Wound infection or dehiscence | 0.9% (16) | 1.0% (14) | 0.9% (85) | 1.3% (36) | 2.1% (61) | <0.0001 |
| Prolonged length of stay | 12.0% (203) | 9.4% (127) | 6.4% (614) | 6.2% (174) | 8.0% (229) | <0.0001 |
Abbreviations: IQR interquartile range
Multivariate models
Multivariable models were constructed for both primary outcomes: operative mortality and composite outcome. Using the restricted cubic splines for BMI percentiles, there was not a significant non-linear association between BMI percentile and mortality (Figure 3). When BMI percentile was treated as a continuous variable, there was no significant association between it and the risk of operative mortality (p=1.00, Supplemental Table 2). When BMI was treated as a categorical variable, dividing by modified CDC categories, the unadjusted risk of operative mortality was higher for severely underweight (OR 1.9, 95% CI: 1.2–2.9, p=0.005) subjects (Table 4). In the same model, the point estimate for unadjusted odds ratio for obesity was 1.4 but the association was not statistically significant (95% CI: 0.9–2.1, p=0.10). When adjustment was performed with multivariate analysis, no significant association was seen between any of the BMI categories and the risk of operative mortality. In this model, risk factors for operative mortality were previous cardiothoracic operation, increasing STAT score, preoperative mechanical ventilation, preoperative renal failure, preoperative neurologic deficit, and other preoperative factor. Preoperative diabetes mellitus was not a risk factor for operative mortality (p=0.43).
Figure 3. Linear spline model for operative mortality by BMI percentile.
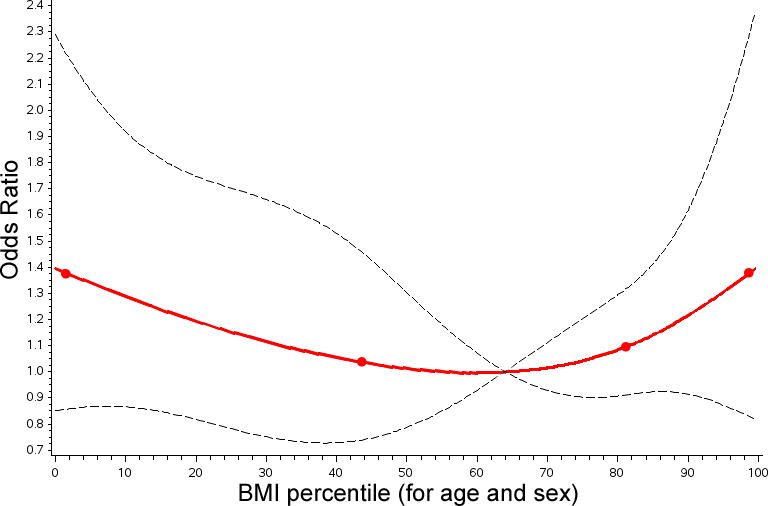
The results of a multivariate model utilizing restricted cubic splines for risk of operative mortality are depicted. The line of best fit (red line), knots (red circles), and 95% confidence intervals (black hashed line) are shown. For operative mortality the nonlinear association depicted is not statistically significant.
Table 4.
Multivariate analysis of operative mortality BMI as a categorical variable
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Risk Factor | OR (95% CI) | p | OR (95% CI) | p |
| Severely underweight vs. Normal weight | 1.87 (1.21,2.90) | 0.005 | 1.42 (0.90,2.24) | 0.13 |
| Underweight vs. Normal weight | 1.04 (0.57,1.92) | 0.90 | 0.91 (0.48,1.71) | 0.77 |
| Overweight vs. Normal weight | 1.06 (0.68,1.67) | 0.79 | 1.08 (0.68,1.71) | 0.76 |
| Obese vs. Normal weight | 1.41 (0.94,2.11) | 0.10 | 1.30 (0.86,1.98) | 0.21 |
| Age (per 5 years) | 1.13 (0.99,1.28) | 0.07 | ||
| Female sex | 0.95 (0.69,1.29) | 0.72 | ||
| Race | ||||
| White | 1 | n/a | ||
| Black | 1.38 (0.92,2.07) | 0.12 | ||
| Other | 0.92 (0.61,1.40) | 0.71 | ||
| Previous cardiothoracic operations | 1.50 (1.06,2.13) | 0.02 | ||
| Presence of non-cardiac anatomic abnormalities | 1.53 (0.63,3.71) | 0.34 | ||
| Presence of genetic syndrome or chromosomal abnormality | 1.39 (0.94,2.06) | 0.09 | ||
| STAT mortality category | ||||
| STAT 1 | 1 | n/a | ||
| STAT 2 | 2.58 (1.49,4.46) | 0.0007 | ||
| STAT 3 | 4.99 (2.67,9.36) | <0.0001 | ||
| STAT 4/5 | 9.88 (5.85,16.70) | <0.0001 | ||
| Preoperative mechanical circulatory support | 1.24 (0.62,2.48) | 0.54 | ||
| Preoperative persistent shock | 1.90 (0.80,4.51) | 0.15 | ||
| Preoperative mechanical ventilation | 5.17 (2.91,9.20) | <0.0001 | ||
| Preoperative renal failure or need for dialysis | 2.05 (1.11,3.76) | 0.02 | ||
| Preoperative neurological deficit/stroke/intracranial hemorrhage | 2.16 (1.26,3.70) | 0.005 | ||
| Preoperative diabetes mellitus | 1.59 (0.58,4.37) | 0.37 | ||
| Any other preoperative factor | 1.93 (1.39,2.69) | 0.0001 | ||
Again, using restricted cubic splines for BMI percentile, a significant non-linear association between BMI percentile and composite outcome was demonstrated (Figure 4). Therefore, linear splines of BMI percentiles was included in the final model (Table 5). For subjects with BMI percentile greater than 57%, increases in BMI percentile were associated with increased risk of composite outcome in both univariate and multivariate analysis (OR: 1.1 per 10% increase, 95% CI: 1.02–1.09, p=0.003). At the same time, in subjects with BMI percent less than 57%, increasing BMI was associated with reduced risk in univariate and multivariate analyses (OR: 0.9 per 10% increase, 95% CI: 0.89–0.91). To restate this for clarity, the OR for a hypothetical severely underweight patient (BMI of 5%) is 1.53. For an underweight patient (BMI in the 15th percentile) the OR is 1.33. An obese patient (BMI in the 95th percentile) would have an OR of 1.21. This is similar to the OR demonstrated in a multivariable model that divides the study population by modified CDC BMI categories (Table 6). In both models, increasing STAT mortality score, presence of a syndrome or genetic abnormality, mechanical circulatory support, shock, preoperative renal failure, neurologic injury, and other preoperative factors were associated with increased risk of the outcome. Preoperative diabetes mellitus was independently associated with increased risk of composite outcome (OR: 2.7, 95% CI: 1.83–3.99, p<0.0001).
Figure 4. Linear spline model for composite outcome by BMI percentile.
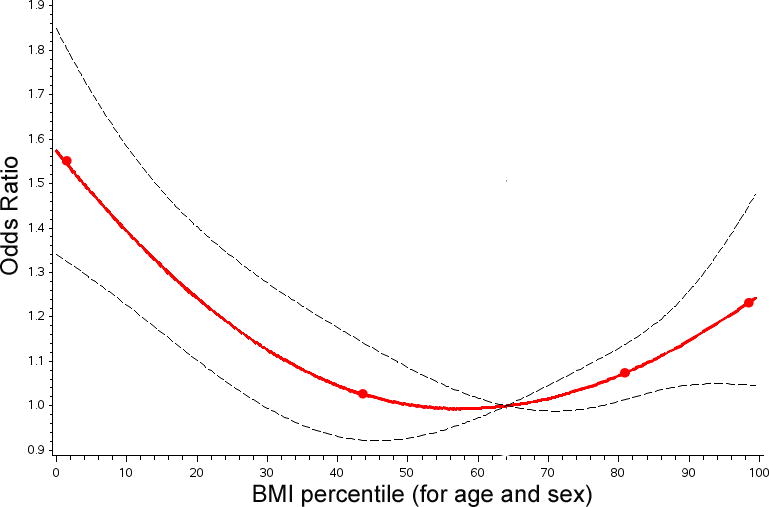
The results of a multivariate model utilizing restricted cubic splines to measure the risk for a composite outcome are depicted. The line of best fit (red line), knots (red circles), and 95% confidence intervals (black hashed line) are shown. For composite outcome the nonlinear association depicted is statistically significant. The odds of a composite outcome are increased at either extreme of BMI. Above the inflection point (a BMI percentile of 57%), increasing BMI increases risk of composite outcome. Below that same inflection point decreasing BMI is also associated with increased risk of composite outcome. Compared to a patient with normal weight (BMI percentile of 64%), the odds of composite outcome are 1.53:1 for a severely underweight patient (BMI percentile of 5%) and 1.21:1 for an obese patient (BMI percentile of 95%).
Table 5.
Multivariate analysis of composite outcome with BMI modeled using spline methodology
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Risk Factor | OR (95% CI) | p | OR (95% CI) | p |
| Severely underweight vs. Normal weight | 1.79 (1.55,2.06) | <0.0001 | 1.53 (1.31,1.79) | <0.0001 |
| Underweight vs. Normal weight | 1.39 (1.18,1.65) | 0.0001 | 1.33 (1.11,1.60) | 0.002 |
| Overweight vs. Normal weight | 1.04 (0.91,1.20) | 0.54 | 1.03 (0.89,1.19) | 0.72 |
| Obese vs. Normal weight | 1.28 (1.12,1.45) | 0.0002 | 1.21 (1.05,1.39) | 0.008 |
| Age (per 5 years) | 1.02 (0.98,1.06) | 0.40 | ||
| Female sex | 0.96 (0.87,1.07) | 0.47 | ||
| Race | ||||
| White | 1 | n/a | ||
| Black | 1.32 (1.14,1.52) | 0.0001 | ||
| Other | 1.26 (1.11,1.43) | 0.0005 | ||
| Previous cardiothoracic operations | 1.24 (1.12,1.38) | <.0001 | ||
| Presence of non-cardiac anatomic abnormalities | 1.03 (0.70,1.52) | 0.86 | ||
| Presence of genetic syndrome or chromosomal abnormality | 1.20 (1.04,1.37) | 0.01 | ||
| STAT mortality category | ||||
| STAT 1 | 1 | n/a | ||
| STAT 2 | 1.79 (1.56,2.05) | <0.0001 | ||
| STAT 3 | 2.61 (2.17,3.14) | <0.0001 | ||
| STAT 4/5 | 7.49 (6.52,8.60) | <0.0001 | ||
| Preoperative mechanical circulatory support | 2.59 (1.81,3.69) | <0.0001 | ||
| Preoperative persistent shock | 2.75 (1.63,4.63) | 0.0001 | ||
| Preoperative mechanical ventilation | 5.89 (4.21,8.25) | <0.0001 | ||
| Preoperative renal failure or need for dialysis | 2.68 (1.95,3.68) | <0.0001 | ||
| Preoperative neurological deficit/stroke/intracranial hemorrhage | 1.51 (1.19,1.90) | 0.0006 | ||
| Preoperative diabetes mellitus | 2.70 (1.83,3.99) | <0.0001 | ||
| Any other preoperative factor | 1.90 (1.70,2.12) | <0.0001 | ||
Table 6.
Multivariate model of composite outcome with BMI divided by modified CDC categories
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Risk Factor | OR (95% CI) | p | OR (95% CI) | P |
| CDC BMI Percentile when > 57, % per 10 unit increase | 1.10 (1.06,1.14) | <0.0001 | 1.07 (1.03,1.11) | 0.0012 |
| CDC BMI Percentile when <= 57, % per 10 unit increase | 0.89 (0.86,0.91) | <0.0001 | 0.92 (0.89,0.95) | <.0001 |
| Age (per 5 years) | 1.02 (0.98,1.06) | 0.37 | ||
| Female sex | 0.96 (0.87,1.06) | 0.41 | ||
| Race | ||||
| White | 1 | n/a | ||
| Black | 1.32 (1.15,1.52) | 0.0001 | ||
| Other | 1.26 (1.10,1.43) | 0.0005 | ||
| Previous cardiothoracic operations | 1.24 (1.12,1.38) | <0.0001 | ||
| Presence of non-cardiac anatomic abnormalities | 1.04 (0.70,1.53) | 0.86 | ||
| Presence of genetic syndrome or chromosomal abnormality | 1.20 (1.05,1.38) | 0.008 | ||
| STAT mortality category | ||||
| STAT 1 | 1 | n/a | ||
| STAT 2 | 1.79 (1.56,2.04) | <0.0001 | ||
| STAT 3 | 2.61 (2.17,3.14) | <0.0001 | ||
| STAT 4/5 | 7.48 (6.51,8.60) | <0.0001 | ||
| Preoperative mechanical circulatory support | 2.61 (1.83,3.73) | <0.0001 | ||
| Preoperative persistent shock | 2.75 (1.63,4.64) | 0.0001 | ||
| Preoperative mechanical ventilation | 5.89 (4.21,8.24) | <0.0001 | ||
| Preoperative renal failure or need for dialysis | 2.65 (1.93,3.64) | <0.0001 | ||
| Preoperative neurological deficit/stroke/intracranial hemorrhage | 1.51 (1.19,1.90) | 0.0007 | ||
| Preoperative diabetes mellitus | 2.70 (1.83,3.98) | <0.0001 | ||
| Any other preoperative factor | 1.90 (1.70,2.13) | <0.0001 | ||
Discussion
In this retrospective multicenter cohort study, obese and underweight BMI were associated with increased risk of perioperative adverse outcome. This is a potentially significant issue for the health of patients with CHD. As the long-term survival of CHD patients has improved, the distribution of patients with CHD has skewed progressively older22,23, forcing physicians to contend with aspects of patient health beyond their CHD. Concurrently, obesity has become increasingly prevalent. In the National Health and Nutrition Examination Survey, 31% of children between 6–19 years are overweight or obese24, and smaller series have demonstrated a similar prevalence in children and adolescents with CHD25–27. The prevalence of subjects with obese (16%) and overweight (15%) BMI in the current series closely matched these previous reports. To our knowledge, the prevalence of underweight BMI in the young patients with CHD, but in the current series it was 17%. Therefore, over a third of patients have BMI associated with increased risk of adverse perioperative outcome, underscoring the potential impact of interventions that address the nutritional status of CHD patients. Further research is necessary to determine whether interventions can affect BMI and more importantly if these interventions modify the increased risks associated with extreme BMI.
Obesity is associated with increased risk of conditions such as diabetes mellitus, coronary artery disease, and peripheral vascular disease. In elderly patients these conditions are suggestive of medical frailty. At the same time, increased adiposity and obese body habitus are potential technical obstacles particularly for the surgical approach and chest closure. Peripheral vascular disease, adiposity, and impaired glucose metabolism can also further impair wound healing. In elderly adults undergoing CT surgery, data regarding the effect of obesity on risk have been contradictory. Small increases in operative mortality have been observed in patients with extreme obesity5,6 along with a more pronounced increase in the risk of wound infections and prolonged intubation5–7. In other studies of elderly patients, overweight and obese BMI were associated with reduced risk of perioperative mortality and other adverse outcome10. Whether this “obesity paradox” is the result of a biologically protective effect of obesity or the result of a limitation of these early studies was not clear. Proposed mechanisms have included selection bias (reduced propensity to operate on obese patients, especially those with other co-morbidities), residual confounding or interaction, and collider bias (where the low risk in obesity is actually a reflection that underweight subjects are at higher risk due to medical frailty and other comorbidities).
Recently, Mariscalco and colleagues performed a retrospective cohort study utilizing a large study cohort of cardiothoracic surgeries performed in Ireland and the United Kingdon over a 12-year period and bolstered with data from a concurrent meta-analysis, demonstrating that overweight and obese subjects had significantly reduced risk of perioperative mortality relative to both underweight and normal weight subjects10. This association was seen in an analysis of the entire study population as well as subgroup analyses stratified by co-morbid conditions, leading the authors to suggest that confounding and interaction were not responsible for the observed associations, while acknowledging that residual confounding is impossible to fully overcome. The authors addressed collider bias by performing two other sensitivity analyses 1) restricting underweight subjects and 2) restricting underweight subjects and those with chronic medical conditions. In both analyses, elevated BMI continued to be associated with reduced risk of perioperative mortality. In terms of selection bias, the large study population included a large number of obese subjects with co-morbid conditions. This study, the largest of its kind to date, appears to confirm that in elderly patients undergoing cardiothoracic surgery, obesity is associated with reduced risk of perioperative mortality.
The current study used data from a prospective national registry of cardiothoracic operations, to address these same issues. CHD is less prevalent than acquired heart disease, which is reflected in a smaller study population. This limited our ability to perform sensitivity analyses described above. However, there was a sufficient population to apply a well-validated risk adjustment model and address selection bias and confounding based on preoperative risk factors. In contrast to the work by Mariscalco and colleagues, the operations included in the study cohort included the full range of congenital heart operations performed in this age group and enabling us to better address the risk of selection bias28. Though there were differences in the proportion of subjects of different BMI by STAT category, there was sufficient overlap to address concerns for selection bias in our analysis. In this analysis, the observed risk of perioperative mortality was increased in obese patients, but after adjustment the association was no longer significant. This may be due to the combination of a low event rate and limited statistical power. At the same time, the high BMI was consistently associated with risk of composite outcome and prominent increases in the observed risk of wound infection/dehiscence and prolonged hospitalization. It is important to note that the increased risk of composite outcome was independent of the additional risk posed by concomitant diabetes mellitus. Obesity appears to have affected the risk of perioperative outcome differently in children, adolescents, and young adults with CHD differently than in older adults with acquired heart disease. This is a surprising observation. The younger patients in our study were exposed to obesity and the resultant comorbidities for a shorter duration than the predominantly elderly patients in the study by Mariscalco et al. Further research is necessary to explore how elevated BMI increases risk of perioperative adverse events in this population and whether interventions to prevent and/or treat obesity can improved outcomes in CHD patients.
To our knowledge, only one study has previously attempted to study the risk associated with obesity following congenital cardiac surgery. Shamszad and colleagues used data from the Pediatric Health Information Systems Database to perform a matched cohort study of obese and control subjects undergoing congenital heart surgery. This study failed to demonstrate a significant difference in mortality or other perioperative adverse outcomes8, but was notably limited by a small study population and no patient- or procedure-level risk stratification. In addition, classification of subject’s as obese or otherwise was dependent on billing codes, since height and weight data are not available in the data source. This is relevant because billing codes are generally accurate when coded, but are potentially insensitive. Failing to correctly classify portion of the potential obese patients would contaminate the study sample and biases it towards a null finding. The study also did not differentiate normal weight and underweight or severely underweight subjects, leading to an inflated risk in normal-weight patients. Given these differences, it is not surprising that the findings were dramatically different.
We acknowledge that the observed association between very high BMI and increased risk of adverse outcomes does not imply that reducing BMI would result in improved outcomes. In an observational study design, this inference would be erroneous. However, if perioperative risk in obese patients arises from the technical challenge of large body habitus and corporeal adiposity and wound healing, weight reduction could plausibly have real benefits. In elderly heart failure patients, weight loss has been shown to improve outcomes during long-term follow-up. Specifically, weight loss after bariatric surgery is associated with reduced risk of heart failure exacerbations29, and weight loss from calorie restriction and behavior modification has been associated with improved performance during exercise testing and quality of life in obese, elderly heart failure patients 30. To our knowledge, no studies have assessed whether weight loss in obese patients prior to a cardiac operation in adult cardiac patients improves perioperative outcomes. Numerous behavioral31, pharmacologic32, and surgical strategies33 for weight loss have been tested in obese but otherwise healthy children. To our knowledge, none of these strategies has been tested in children and young adults with CHD. The presence of CHD potentially complicates management of obesity in several ways. First, exercise intolerance is a common symptom of heart failure and can make the implementation of an exercise program challenging. Second, exercise restriction is indicated in some forms of CHD34. Finally, pharmacologic and surgical interventions may have higher risk in children with CHD than in other patient populations. For example, bariatric surgery is effective in helping obese children lose weight, recent studies have demonstrated that general surgical procedures have increased risk of mortality and morbidity in congenital heart patients35. Longitudinal studies are necessary to assess the risks and benefits of weight loss in obese subjects prior to cardiothoracic surgery. An important distinction exists between obesity treatment (e.g. pharmacological and surgical treatments described above) and obesity prevention. The latter is part of lifelong anticipatory guidance for healthy cardiac health and could incorporate patient and family education about healthy eating, tailoring portion sizes to the patient’s individual metabolic requirements, and participation where possible in healthful activity. The results of the current study should add to the expanding literature demonstrating benefit of habitual exercise and directed exercise regimens on the exercise performance in survivors of congenital heart surgery36–39. Obesity prevention is potentially complementary avenue for future research in improving the health of children with CHD.
The risks associated with underweight patients likely have a different mechanism from those associated with obesity. As noted earlier chronic disease, especially heart failure, is associated with decreased body mass and may be associate with additional deficits in immune function, tissue function, and other functions that might impact post-operative recovery. Low body mass is potentially both a marker of severe heart disease and also a marker of other downstream biological processes that impact adverse outcome. Therefore, it is important to try to differentiate between the contributions to risk associated with severity of heart disease and BMI. In the current study, underweight patients underwent more complicated operations, suggesting more severe heart disease or at the very least an intrinsically higher risk of undesirable outcome. They were also more likely to have increased risk of genetic syndromes and anatomic abnormalities, which have also been shown in other studies to be associated independently with worse perioperative outcome16,40,41. Pre-operative symptom scores (e.g. New York Heart Association Scores) were not widely available in the current database, nor were surrogate markers of nutritional status (e.g. albumin or pre-albumin). We used multivariable modeling using the previously validated STS risk adjustment model to differentiate between the contributions of BMI and pre-operative patient level risk, including severity of heart disease. Even after adjustment, there was an association between underweight BMI and the risk of adverse outcomes. Again, because of the observational design of this study, it is not possible to infer that interventions aimed at normalizing the BMI in underweight patients would reduce the risk of adverse events.
To our knowledge no interventions to improve nutrition in cachectic children and young adults with CHD have been studied. Growth failure is a common problem in infants with CHD and has received significant attention. Attempts to support weight gain in infants undergoing staged palliation for single ventricle heart disease are potentially instructive. These patients frequently experience growth failure between their initial Norwood operation and second-stage operation. While various feeding strategies and pharmacologic therapies have not resulted in improved growth in these patients in large observational studies42–44, there is evidence that patients enrolled in longitudinal follow-up programs experience significantly better growth than those without this support45. This simultaneously highlights the challenge of overcoming cachexia due to heart disease and reinforces that longitudinal support from the cardiac care team is vital regardless of the chosen intervention. Further research is necessary to determine if interventions can effectively ameliorate low BMI in this population and if these interventions reduce the observed risk of adverse perioperative outcome.
The possibility of confounding by indication must also be addressed. For instance if, clinicians believe (rightly or wrongly) that low and high BMI patients are at higher risk and do not refer these patients for an operation unless they have severe disease, the measured risk of adverse events might be falsely elevated relative to the “true” risk. The study sample in this case had a similar proportion of overweight and obese patients as previous unselected series25–27, suggesting that the study sample was representative of the existent population at risk. As noted, previous series have not identified the prevalence of underweight and severely underweight BMI in this population, and we must acknowledge that the prevalence of several pre-operative risk factors was higher in the severely underweight stratum. We felt there was sufficient overlap to allow us to have used statistical adjustment (with multivariate modeling) to isolate the effect of BMI on our outcomes. It is possible; however, that some of the observed effect was the result of unmeasured confounding.
We also acknowledge that in a retrospective analysis the study population is fixed. Even with a relatively large study sample, there may be insufficient statistical power to detect small differences in event rates. Despite following conventions regarding limiting the ratio of events to covariates, it is possible that we were underpowered to detect differences in operative mortality.
BMI is a static assessment of body mass corrected for height. Recent changes in their body mass are another measure of both severity of illness and current nutritional status. In longitudinal studies of elderly heart failure patients, unintended weight loss was associated with increased risk of cardiovascular adverse events11–13. Patient height and weight in STSCHSSD are limited to measurements at the time of their operation, and thus it was not possible to identify recent changes in BMI. Serum albumin, a surrogate for nutritional status46,47, is also not available in the database. The current study was restricted to studying the association pre-operative BMI and outcome. Future studies should measure both BMI at operation and recent changes in BMI to determine the degree to which each of these affect risk of perioperative adverse outcomes.
BMI is also an imperfect measurement of adiposity and body composition. Other measures may more accurately measure adiposity and more accurately predict the associated risks but are not possible in a retrospective study. BMI remains a straightforward statistic that can be calculated with readily available data without special tools or training and remains widely used in both clinical practice and epidemiologic studies. Secondly, there are differences in the distribution of BMI between children of different races. Hispanic and Black boys and girls are more likely to have higher BMI than age-matched non-Hispanic white children48,49, while South Asian children have been noted to have lower BMI than white children of similar age49. These trends were born out in data from the current study, where the proportion of obese black subjects was higher than in whites, along with a higher proportion of underweight Asian subjects than in whites. These patterns may reflect differences in the “healthy” or “normal” BMI between children of different racial and ethnic groups or differences in the prevalence of obesity between racial and ethnic groups. Differentiating between these possibilities is beyond the scope of this study. We addressed the contribution of race as a confounder by including it in all multivariable models.
The current study demonstrated that obesity and underweight BMI were both associated with increased risk of postoperative adverse events. These conditions are prevalent in children with CHD. Therefore, there is a pressing need to determine whether interventions aimed at achieving a more normal BMI in both groups can improve perioperative outcomes.
Supplementary Material
Clinical Perspective.
What Is New?
This analysis studied the association of body mass index and perioperative outcome in 18,377 children, adolescents, and young adults undergoing congenital cardiac surgery at 118 hospitals in the United States over a 6-year span.
After adjusting for patient- and procedure-level risk factors as well as accounting for clustering of patients by hospital, the authors found that obese, underweight, and severely underweight subjects were associated with increased risk of composite adverse outcome (death, major adverse event, and/or wound infection).
What Are the Clinical Implications?
There are significant associations between body composition and perioperative outcome after congenital heart surgery, which appear not to be otherwise explained by patient and procedure factors.
Additional efforts to understand the mechanisms underlying these associations are needed.
Ultimately, interventions aimed at improving nutrition in underweight patients and weight loss in obese subjects need to be studied both for their efficacy in affecting BMI and effect on perioperative outcome.
Acknowledgments
Funding Sources
Dr. O’Byrne receives research support from the National Institute of Health/National Heart Lung and Blood Institute (K23 HL130420-01). The analysis in this manuscript was funded by the Society for Thoracic Surgeons. The proposed project and manuscript were reviewed by Society For Thoracic Surgeons Access and Publications Committee. The funding agencies had no role in the drafting of the manuscript or influencing its content. This manuscript represents the opinion of the authors alone.
Footnotes
Disclosures:
None.
References
- 1.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee I-M, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 4.Valentijn TM, Galal W, Tjeertes EKM, Hoeks SE, Verhagen HJ, Stolker RJ. The obesity paradox in the surgical population. The Surgeon. 2013;11:169–176. doi: 10.1016/j.surge.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer NJ, Charlesworth DC, Hernandez F, Leavitt BJ, Marrin CA, Morton JR, Olmstead EM, O’Connor GT. Obesity and risk of adverse outcomes associated with coronary artery bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 1998;97:1689–1694. doi: 10.1161/01.cir.97.17.1689. [DOI] [PubMed] [Google Scholar]
- 6.Prabhakar G, Haan CK, Peterson ED, Coombs LP, Cruzzavala JL, Murray GF. The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: a study from the Society of Thoracic Surgeons’ database. Ann Thoracic Surg. 2002;74:1125–30. doi: 10.1016/s0003-4975(02)03899-7. discussion 1130–1. [DOI] [PubMed] [Google Scholar]
- 7.Reeves BC, Ascione R, Chamberlain MH, Angelini GD. Effect of body mass index on early outcomes in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol. 2003;42:668–676. doi: 10.1016/s0735-1097(03)00777-0. [DOI] [PubMed] [Google Scholar]
- 8.Shamszad P, Rossano JW, Marino BS, Lowry AW, Knudson JD. Obesity and Diabetes Mellitus Adversely Affect Outcomes after Cardiac Surgery in Children’s Hospitals. Cong Heart Dis. 2016:1–6. doi: 10.1111/chd.12325. epublish ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Stamou SC, Nussbaum M, Stiegel RM, Reames MK, Skipper ER, Robicsek F, Lobdell KW. Effect of Body Mass Index on Outcomes After Cardiac Surgery: Is There an Obesity Paradox? Ann Thoracic Surg. 2011;91:42–47. doi: 10.1016/j.athoracsur.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 10.Mariscalco G, Wozniak MJ, Dawson AG, Serraino GF, Porter R, Nath M, Klersy C, Kumar T, Murphy GJ. Body Mass Index and Mortality Among Adults Undergoing Cardiac Surgery: A Nationwide Study With a Systematic Review and Meta-Analysis. Circulation. 2017;135:850–863. doi: 10.1161/CIRCULATIONAHA.116.022840. [DOI] [PubMed] [Google Scholar]
- 11.Pocock SJ, McMurray JJV, Dobson J, Yusuf S, Granger CB, Michelson EL, Ostergren J, Pfeffer MA, Solomon SD, Anker SD, Swedberg KB, on behalf of the CHARM Investigators Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:2641–2650. doi: 10.1093/eurheartj/ehn420. [DOI] [PubMed] [Google Scholar]
- 12.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 13.Rossignol P, Masson S, Barlera S, Girerd N, Castelnovo A, Zannad F, Clemenza F, Tognoni G, Anand IS, Cohn JN, Anker SD, Tavazzi L, Latini R, on the behalf of GISSI-HF and Val-HeFT Investigators Loss in body weight is an independent prognostic factor for mortality in chronic heart failure: insights from the GISSI-HF and Val-HeFT trials. Eur J of Heart Failure. 2015;17:424–433. doi: 10.1002/ejhf.240. [DOI] [PubMed] [Google Scholar]
- 14.Radman M, Mack R, Barnoya J, Castañeda A, Rosales M, Azakie A, Mehta N, Keller R, Datar S, Oishi P, Fineman J. The effect of preoperative nutritional status on postoperative outcomes in children undergoing surgery for congenital heart defects in San Francisco (UCSF) and Guatemala City (UNICAR) J Thorac Cardiovasc Surg. 2014;147:442–450. doi: 10.1016/j.jtcvs.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossano JW, Grenier MA, Dreyer WJ, Kim JJ, Price JF, Jefferies JL, Smith EO, Clunie SK, Moulik M, Decker JA, Breinholt JP, Morales DLS, McKenzie ED, Towbin JA, Denfield SW. Effect of body mass index on outcome in pediatric heart transplant patients. J Heart Lung Transplant. 2007;26:718–723. doi: 10.1016/j.healun.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien SM, Clarke DR, Jacobs JP, Jacobs ML, Lacour-Gayet FG, Pizarro C, Welke KF, Maruszewski B, Tobota Z, Miller WJ, Hamilton L, Peterson ED, Mavroudis C, Edwards FH. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 17.Clarke DR, Breen LS, Jacobs ML, Franklin RCG, Tobota Z, Maruszewski B, Jacobs JP. Verification of data in congenital cardiac surgery. Cardiology in the Young. 2008;18(Suppl 2):177–187. doi: 10.1017/S1047951108002862. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs ML, Daniel M, Mavroudis C, Morales DLS, Jacobs JP, Fraser CD, Turek JW, Mayer JE, Tchervenkov C, Conte J. Report of the 2010 society of thoracic surgeons congenital heart surgery practice and manpower survey. Ann Thoracic Surg. 2011;92:762–8. doi: 10.1016/j.athoracsur.2011.03.133. discussion 768–9. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs ML, O’Brien SM, Jacobs JP, Mavroudis C, Lacour-Gayet F, Pasquali SK, Welke K, Pizarro C, Tsai F, Clarke DR. An empirically based tool for analyzing morbidity associated with operations for congenital heart disease. J Thorac Cardiovasc Surg. 2013;145:1046–1057.e1. doi: 10.1016/j.jtcvs.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole TJ, T L. Extended International (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obesity. 2012;7:284–294. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 21.Harrell F. Regression Modeling Strategies With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York: Springer; 2015. General Aspects of Fitting Regression Models; pp. 13–42. [Google Scholar]
- 22.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 23.Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime Prevalence of Congenital Heart Disease in the General Population From 2000 to 2010. Circulation. 2014;130:749–756. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 24.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of Overweight and Obesity Among US Children, Adolescents,and Adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 25.Pinto NM, Marino BS, Wernovsky G, de Ferranti SD, Walsh AZ, Laronde M, Hyland K, Dunn SO, Cohen MS. Obesity Is a Common Comorbidity in Children With Congenital and Acquired Heart Disease. Pediatrics. 2015;120:1–1. doi: 10.1542/peds.2007-0306. [DOI] [PubMed] [Google Scholar]
- 26.Shustak RJ, McGuire SB, October TW, Phoon CKL, Chun AJL. Prevalence of Obesity Among Patients With Congenital and Acquired Heart Disease. Pediatr Cardiol. 2011;33:8–14. doi: 10.1007/s00246-011-0049-y. [DOI] [PubMed] [Google Scholar]
- 27.Pasquali SK, Marino BS, Pudusseri A, Wernovsky G, Paridon SM, Walker SA, Cohen MS. Risk factors and comorbidities associated with obesity in children and adolescents after the arterial switch operation and Ross procedure. Am Heart J. 2009;158:473–479. doi: 10.1016/j.ahj.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Carnethon MR, Khan SS. An Apparent Obesity Paradox in Cardiac Surgery. Circulation. 2017;135:864–866. doi: 10.1161/CIRCULATIONAHA.117.026856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimada YJ, Tsugawa Y, Brown DFM, Hasegawa K. Bariatric Surgery and Emergency Department Visits and Hospitalizations for Heart Failure Exacerbation: Population-Based, Self-Controlled Series. J Am Coll Cardiol. 2016;67:895–903. doi: 10.1016/j.jacc.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010;125:e396–418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 32.McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, Erwin PJ, Montori VM. Clinical review: treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. J Clinic Endocrin Metabol. 2008;93:4600–4605. doi: 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- 33.Treadwell JR, Sun F, Schoelles K. Systematic review and meta-analysis of bariatric surgery for pediatric obesity. Ann Surg. 2008;248:763–776. doi: 10.1097/SLA.0b013e31818702f4. [DOI] [PubMed] [Google Scholar]
- 34.Maron BJ, Zipes DP, Kovacs RJ. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Preamble, Principles, and General Considerations: A Scientific Statement From the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66:2343–2349. doi: 10.1016/j.jacc.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 35.Faraoni D, Zurakowski D, Vo D, Goobie SM, Yuki K, Brown ML, DiNardo JA. Post-Operative Outcomes in Children With and Without Congenital Heart Disease Undergoing Noncardiac Surgery. J Am Coll Cardiol. 2016;67:793–801. doi: 10.1016/j.jacc.2015.11.057. [DOI] [PubMed] [Google Scholar]
- 36.O’Byrne ML, Mercer-Rosa L, Ingall E, McBride MG, Paridon S, Goldmuntz E. Habitual exercise correlates with exercise performance in patients with conotruncal abnormalities. Pediatr Cardiol. 2013;34:853–860. doi: 10.1007/s00246-012-0556-5. [DOI] [PubMed] [Google Scholar]
- 37.Duppen N, Etnel JR, Spaans L, Takken T, van den Berg-Emons RJ, Boersma E, Schokking M, Dulfer K, Utens EM, Helbing W, Hopman MT. Does exercise training improve cardiopulmonary fitness and daily physical activity in children and young adults with corrected tetralogy of Fallot or Fontan circulation? A randomized controlled trial. Am Heart J. 2015;170:606–614. doi: 10.1016/j.ahj.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 38.Cordina RL, O’Meagher S, Karmali A, Rae CL, Liess C, Kemp GJ, Puranik R, Singh N, Celermajer DS. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol. 2013;168:780–788. doi: 10.1016/j.ijcard.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Rhodes J, Curran TJ, Camil L, Rabideau N, Fulton DR, Gauthier NS, Gauvreau K, Jenkins KJ. Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics. 2005;116:1339–1345. doi: 10.1542/peds.2004-2697. [DOI] [PubMed] [Google Scholar]
- 40.O’Byrne ML, Yang W, Mercer-Rosa L, Parnell AS, Oster ME, Levenbrown Y, Tanel RE, Goldmuntz E. 22q11.2 Deletion syndrome is associated with increased perioperative events and more complicated postoperative course in infants undergoing infant operative correction of truncus arteriosus communis or interrupted aortic arch. J Thorac Cardiovasc Surg. 2014;148:1597–1605. doi: 10.1016/j.jtcvs.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercer-Rosa L, Pinto N, Yang W, Tanel R, Goldmuntz E. 22q11.2 Deletion syndrome is associated with perioperative outcome in tetralogy of Fallot. J Thorac Cardiovasc Surg. 2013;146:868–873. doi: 10.1016/j.jtcvs.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams RV, Zak V, Ravishankar C, Altmann K, Anderson J, Atz AM, Dunbar-Masterson C, Ghanayem N, Lambert L, Lurito K, Medoff-Cooper B, Margossian R, Pemberton VL, Russell J, Stylianou M, Hsu D, Pediatric Heart Network Investigators Factors affecting growth in infants with single ventricle physiology: a report from the Pediatric Heart Network Infant Single Ventricle Trial. J Pediatr. 2011;159:1017–22.e2. doi: 10.1016/j.jpeds.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medoff-Cooper B, Ravishankar C. Nutrition and growth in congenital heart disease: a challenge in children. Curr Opin Cardiol. 2013;28:122–129. doi: 10.1097/HCO.0b013e32835dd005. [DOI] [PubMed] [Google Scholar]
- 44.Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, Barker PC, Ravishankar C, McCrindle BW, Williams RV, Altmann K, Ghanayem NS, Margossian R, Chung WK, Border WL, Pearson GD, Stylianou MP, MITAL S, the Pediatric Heart Network Investigators Enalapril in Infants With Single Ventricle: Results of a Multicenter Randomized Trial. Circulation. 2010;122:333–340. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson JB, Iyer SB, Schidlow DN, Williams R, Varadarajan K, Horsley M, Slicker J, Pratt J, King E, Lannon C, National Pediatric Cardiology Quality Improvement Collaborative Variation in growth of infants with a single ventricle. J Pediatr. 2012;161:16–21.e1. doi: 10.1016/j.jpeds.2012.01.009. quiz 21.e2–3. [DOI] [PubMed] [Google Scholar]
- 46.Engelman DT, Adams DH, Byrne JG, Aranki SF, Collins JJ, Couper GS, Allred EN, Cohn LH, Rizzo RJ. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J Thorac Cardiovasc Surg. 1999;118:866–873. doi: 10.1016/s0022-5223(99)70056-5. [DOI] [PubMed] [Google Scholar]
- 47.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 48.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 49.Nightingale CM, Rudnicka AR, Owen CG, Cook DG, Whincup PH. Patterns of body size and adiposity among UK children of South Asian, black African-Caribbean and white European origin: Child Heart And health Study in England (CHASE Study) Int J Epidemiol. 2011;40:33–44. doi: 10.1093/ije/dyq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


