Abstract
Background and Objectives
The present study investigated whether MSCs derived microvesicles (MVs) or (Exosomes) can exert therapeutic effects on an experimental model of cutaneous injury and explored the underlying involving mechanisms.
Methods and Results
Three bilateral full thickness circular wounds were created on the back of two groups of dogs using 2-cm dermal punch. The wounds were at least 2.5 cm apart. Saline was subcutaneously injected in 4 places around each wound area in group-I (control), whereas an equal volume of exosomal solution of MSCs derived MVs was similarly injected in group-II. The findings demonstrated that MSCs derived MVs had significantly promoted cutaneous wound healing, collagen synthesis, and vascularization at wound sites. The application of the exosomal solution had not only promoted the generation of newly formed vessels, but also have accelerated their development and maturation leading to a faster healing process.
Conclusions
MSC-Exosomes appeared to be a superior candidate for treating cutaneous wounds than their originator cells, and may represent a promising opportunity to develop a novel cell-free therapy approach that might overcome the obstacles and risks associated with the use of native or engineered stem cells transplantation therapy.
Keywords: Histological, Critical size defect, Mesenchymal stem cells, Microvesicles, Healing
Introduction
The repair of wounds is one of the most complex biological processes that occur during human life (1). It requires a coordinated interplay among cells, growth factors, and extracellular matrix proteins (2). Microvesicles (MVs) or Exosomes are membrane vesicles that are released by many types of cells from the endosomal compartment, or as shedding vesicles from the cell surface (3). These exosomes are considered important mediators of cell-to-cell communication (3, 4). As vehicles of information, these exosomes may act as paracrine or endocrine mediators that interact with neighboring cells, modulate immune responses, promote self-repair from cells that survive injury and play important role in communication between stem and injured tissue cells (3, 4).
Other than being mediators of cell-to-cell communication, MSC-derived exosomes have functions similar to those of MSCs, such as repairing tissue damage and suppressing inflammatory responses (4). Compared with the cells from where they originate, exosomes produced by MSCs are expected to have several advantages in the wound environment. For example, exosomes are more stable due to their lipid bilayer shell which could avert proteolytic degradation and hence can effectively transfer signals to target cells (e.g., fibroblasts and endothelial cells). Additionally, exosomes contain many potential regulatory components including miRNAs, mRNAs, and proteins, which can be transferred as a type of “physiological lipofection” to recipient cells to modify their characteristics (5).
Moreover, exosomes are more re-servable, have no risk of aneuploidy, a lower possibility of immune rejection following in vivo allogeneic administration, and therefore may provide an alternative therapy for various diseases (4, 5).
In the present study, we histologically studied the therapeutic effects of MSC derived exosomes in cutaneous wound healing. Healing was assessed using histological parameters such as: re-epithelialization, cellularity, angiogenesis, and dermal structural regeneration. Confirmatory immuno-histochemistry tests were conducted.
Materials and Methods
Experimental animals
Six apparently healthy dogs weighing (20~25 kg), age (2~3 years) were used in this study. The study was approved by Institutional Animal Care and Use Committee. Dogs were housed at separate kennels, fed on standard dog’s food with free access to drinking water.
Animal grouping
Dogs were randomly and equally allocated into 2 groups.
Group-I (control)
treated with saline.
Group-II (treated)
treated with MSCs-derived exosomes.
Three full-thickness skin wounds were induced bilaterally on the back of each dog. Wounds were evaluated daily for appearance and size. Histological samples were taken at the 3rd, 7th and the 14th day post treatment. At the end of the experiment, all dogs received medical treatment to the remaining of their skin wounds (Mepore sterile dermal patches, Molnlycke® health care) and Bivatracin topical antibiotic spray (Neomycin and bacitracin, ACDIMA® International) until complete healing.
Induction of skin wound
Experimental dogs were anesthetized using general anesthetic regimen after overnight fasting. Three bilateral circular wounds of 2 cm in diameter were created on the back of each dog by using a dermal punch. The wounds were at least 2.5 cm apart. The day on which the wounds were created was designed as day 0.
Isolation and expansion of canine MSCs-derived MVs
One month before commence of the experiment, fresh Bone Marrow (BM) was aspirated from the iliac crest of healthy dogs and MSCs were isolated, propagated, and identified. Microvesicles where then isolated for usage.
Bone marrow MSCs isolation
Proper sterile technique was used and all solutions and equipment coming into contact with cells were sterile.
Bone marrow MSCs propagation
Bone marrow samples were over-layered on 20 ml Ficoll, and then centrifuged for 15 min. at 400×g. Bone marrow mononuclear cell fractions (BM-MNCs) were isolated. The layer of BM-MNC layer was carefully aspirated and resuspended in PBS and centrifuged for 10 minutes at 200×g. Then the cell pellet was resuspended in PBS for washing and centrifuged for 10 minutes at 200×g. And the pellet was re-suspended in a final volume of 2 ml of RBCs lysis buffer and incubated for 10 minutes at 4°C. PBs was added for a final volume of 10 ml and centrifuged again to remove the lysis buffer. Cell pellet was suspended in complete culture medium containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). Cells were incubated at 37°C in 5% CO2 for 24 hours. Media was removed to remove non-specific cells leaving only adherent cells. Fresh complete medium was added and incubated at 37°C in 5% CO2 and media was changed every 2~3 days. When cells reached 70~80% confluence, cultures were washed twice with PBS and cells were trypsinized with 0.25% Trypsin-EDTA (GIBCO) for 3 minutes at 37°C, complete medium was added to stop the action of trypsin. Centrifuged for 10 minutes at 200×g. Cell pellets were resuspended in complete medium. The resulting cultures were referred to as first-passage cultures. The last 3 fore mentioned steps were repeated until the fourth passage was attained.
Identification of BM-derived MSCs
Cells were identified as MSCs by their morphology and adherence on tissue culture flask. Then Flow cytometric analysis of the cultured MSCs surface markers was done using CD105, CD90 and CD73.
Isolation and FACS Analysis of MVs
MVs obtained from the supernatants of the third passage of MSCs (5×106 cells/ml) were cultured in RPMI deprived of FBS and supplemented with 0.5% of bovine serum albumin (BSA, Sigma). After centrifugation at 2000 g for 20 min to remove debris, cell-free supernatants were centrifuged at 100,000 g (Beckman Coulter Optima L 90K ultracentrifuge) for 1 h at 4°C, washed in serum-free medium 199 containing HEPES 25 mM (Sigma) and submitted to a second ultracentrifugation under the same conditions. The protein content was quantified by the Bradford method (BioRad, Hercules, CA). Electron microscopy analyses of MVs were performed. Purified MVs cultured overnight in the medium used for collection of MVs. Images were obtained by secondary electron at a working distance of 15 to 25 mm and an accelerating voltage of 20 and 30 kV. Digital acquisition and analysis were performed using the Jeol T300 system. Cytofluorimetric analysis was performed using the following FITC- or PE-conjugated antibodies: CD63, CD44 (MiltenyiBiotec) and CD73 (Becton Dickinson). FITC or PE mouse nonimmune isotypic IgG (DakoCytomation) was used as a control.
Treatment
Twenty-four hours after wound creation, equal amounts (1 ml) of saline and exosomal solution were subcutaneously injected in 4 cardinal places around each wound. The amount of exosomes injected equals the amount produced by 2×106 MSCs/1 ml/wound.
Monitoring of the general condition and wound healing of the experimental dogs was performed daily, and the wounds were photographed.
Evaluation
Physical evaluation of the wounds
Wound-size reduction was calculated using the equation: wound-size reduction=( Ai–Af)/Ai×100, (where Ai is the initial wound area, and Af is the wound area at Day 3, 7 or 14 post-wounding) (6).
Histological analyses
On days 3, 7 and 14, each experimental dog was sedated and tissue samples were harvested with 0.5 cm of the peripheral unwounded skin using 2.5 cm dermal biopsy. Samples were pinned on a plastic plate (to keep the tissue flat) and fixed in 10% buffered Formalin, dehydrated with a graded Alcohol series, embedded in Paraffin, and sectioned into 4-μm-thick sections (6).
Hematoxylin and Eosin (H&E) staining was used for histological observations
For comparison of the rate of re-epithelialization in different groups, and the epithelial gap was evaluated. Masson’s trichrome staining was used to determine the degree of collagen synthesis.
Immunohistochemistry
CD31 and alpha smooth muscle actin (α-SMA) markers were detected by immunoperoxidase technique and evaluated by light microscope to study exosome-induced angiogenesis during the wound healing process. For immunoperoxidase staining, excised skin from the wound sites was fixed in 10% formalin, dehydrated with different grades of alcohol series, embedded in paraffin, and sectioned into 4-μm-thick sections on positive slides.
Results
Physical evaluation of the wounds
Wound reduction size
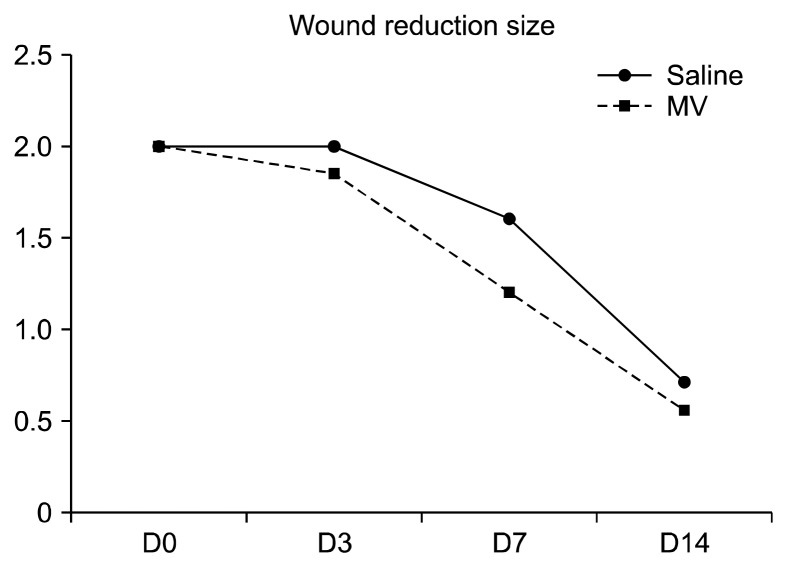
Induced wounds treated with exosomal solution showed quicker healing than their counterparts treated with saline (Fig. 1). All results were significant during the three observation periods, (p=0.001) at 3 and 7 days and (p=0.003) at the 14th day. The reduction percentages for the two experimental groups are shown in (Table 1) and drawn (Fig. 2).
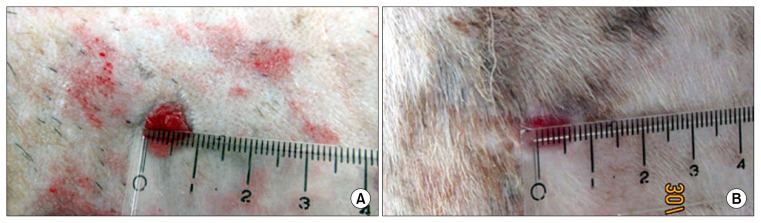
Fig. 1.
Photographs showing the wound area in the two groups at 14th day of treatment: (A) wound area from Group-I treated with saline compared to (B) wound area from Group-II treated with exosomes.
Table 1.
Percentage of wound reduction size (compared to initial wound size) and the contraction difference between saline and exosomes groups
| Saline | Exosomes | Exosomes vs Saline | |
|---|---|---|---|
| Day 3 | 0% | 7.5% | 7.5% |
| Day 7 | 20% | 40% | 20% |
| Day 14 | 65% | 72.5% | 7.5% |
Fig. 2.
Graph showing increased wound-size reduction % in wounds treated with saline and exosomes at 3, 7 and 14 days.
Histological evaluation of wound healing
Histopathological examination
Histopathological evaluation of induced wounds of the control group at the 3rd day post-wounding disclosed evident wound edges and complete removal of epidermis in the surface of the wound area. The dermis was populated with inflammatory exudates mainly neutrophils, fibrin deposition admixed with necrotic tissue debris, in addition to newly formed capillaries (angiogenesis) that populated the area with proliferation of fibroblasts (Fig. 3A).
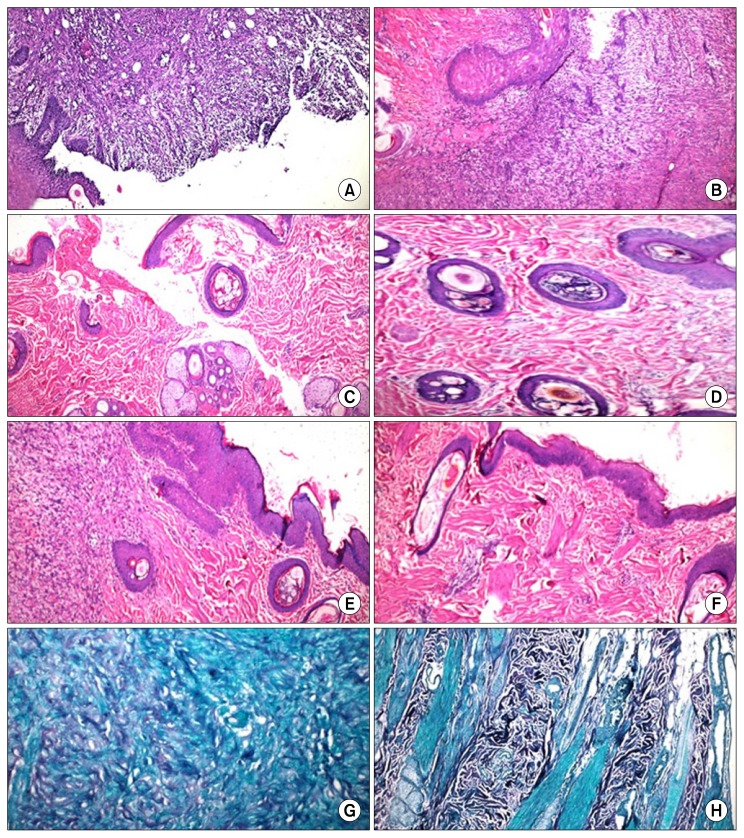
Fig. 3.
Histopathological skin sections of wounds treated with saline and exosomes: (A) Group-I at 3 days of treatment disclosing the wound edge, complete removal of epidermis in wound area. The dermis has inflammatory exudates, fibrin deposition admixed with necrotic tissue debris and new blood capillaries. H&E×100; (B) Group-II at 3 days revealing wound edges populated with inflammatory exudates, fibrin deposition, necrotic debris and newly formed blood capillaries with proliferation of fibroblasts and few disorganized collagen. H&E×400; (C) Group-I at the 7th day showing reduced wound edges and proliferation of kerationcytes. The re-epithelialized wound is higher than the surrounding surface. Most of the cells from the previous stage have migrated from the wound site, few inflammatory exudates and clotted blood are still present. The dermal extracellular matrix exhibits disorganized collagen under the re-epithelialized surface. H&E×100; (D) Group-II at the 7th day: wound area has contracted. Collagen has been laid down under the re-epithelized wound surface associated with newly formed blood capillaries and few fibroblasts with intact skin appendages. H&E×400; (E) Group-I at the 14th day of treatment showing complete re-epithelialization of wound surface, large amount of granulation tissue formation under the re-epithelialized epidermis with few disorganized immature collagen. H&E×100; (F) Group-II at the 14th day showing complete re-epithelialization of wound surface with complete wound closure, tissue remodeling took place below the epidermal surface and mature collagen laid down in the dermis, the skin appendages and the blood vessels are intact. H&E×200; (G) Masson’s trichrome stain section, Group-I, day 14, showing few disorganized collagen and fibroblastic proliferation×400; (H) Masson’s trichrome section, Group-II, day 14, showing large amount of collagen deposition×200.
At the 7th day, the wound edges were reduced with proliferation of kerationcytes. The re-epithelialized wound was higher than the surrounding surface. Most of the cells from the previous stage of repair appeared to have migrated from the wound site, with the exception of few inflammatory exudates and clotted blood that were still present. The extracellular matrix in the dermis revealed formation of disorganized collagen under the re-epithelialized surface. Outside the wound area, the epidermis, the skin appendages and the collagen were intact (Fig. 3C).
At the 14th day post-wounding, the wound surface showed complete re-epithelialization with large amount of granulation tissue that has been formed under the re-epithelialized epidermis with few disorganized immature collagen (extracellular matrix) laid down. Outside the wound area, intact epidermis, mature collagen fibers and skin appendages were observed (Fig. 3E). Disorganized few collagen produced by proliferative fibroblast that have migrated into the wound were observed and stained positively with Masson’s trichrome (Fig. 3G).
Histopathological evaluation of induced wounds injected with MSC-derived exosomes at the 3rd day after wounds induction, disclosed apparent wound edges that were populated with inflammatory exudates mainly neutrophils and macrophages with fibrin deposition and necrotic tissue debris. Also newly formed blood capillaries (angiogenesis) were populate at the area with proliferation of large number of fibroblasts and few disorganized collagen (the extracellular matrix). Outside the wound area, the epidermis and the skin appendages were intact with mature organized collagen in the dermis (extracellular matrix) (Fig. 3B).
While at the 7th day the wound area had contracted with narrowing of its edges, collagen has been laid down under the re-epithelialized wound surface and was associated with the newly formed blood capillaries and few fibroblasts with intact skin appendages (Fig. 3D).
At the 14th day, wound sites showed enhanced cellularity, complete re-epithelization and closure of wound. Tissue remodeling took place below the epidermal surface and mature collagen (extracellular matrix) was laid down in the dermis. The skin appendages and the blood vessels were intact (Fig. 3F). Enhanced dermal fibroblasts and large amount of mature organized collagen deposition were stained positive with Masson’s trichrome stain (Fig. 3H).
Immunohistochemistry
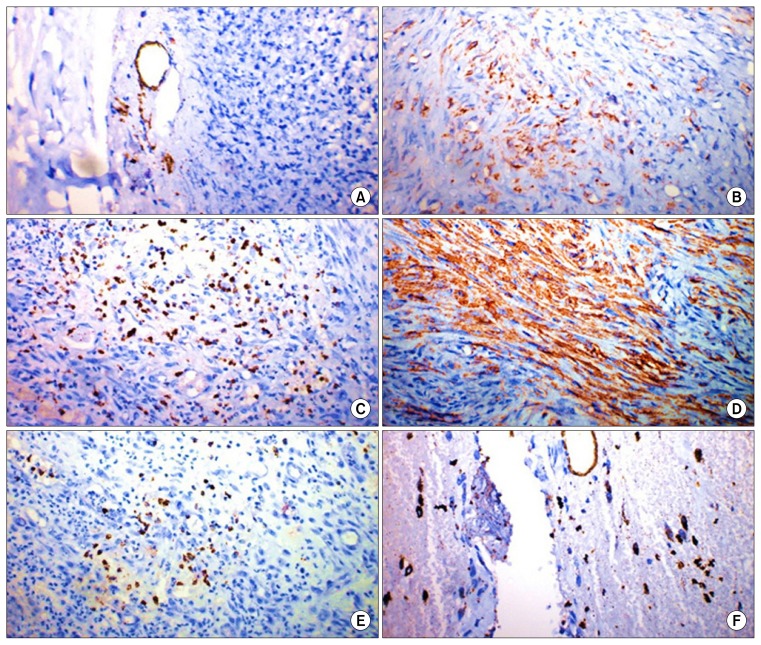
Immunohistochemical staining of skin wound sections of control group at 3 days revealed mild expression of both angiogenesis markers CD31 and α-SMA. While at 7th and 14th days, moderate positive expression of both markers was noticed with the existence of large number of macrophages and lymphocytes (Figs. 4, 5).
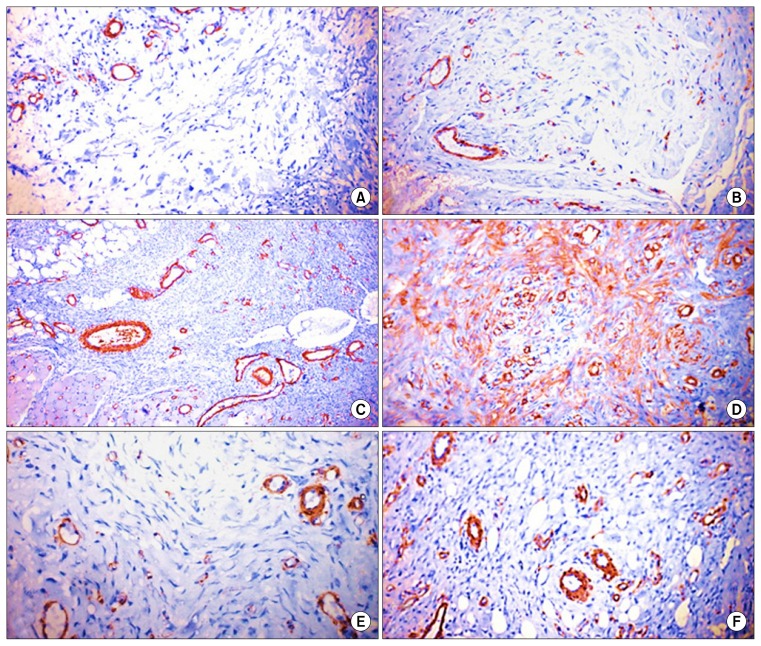
Fig. 4.
α-SMA-Immunohistochemistry of skin wound sections: (A) Group-I, 3 days of treatment disclosing mild expression of α-SMA. Immunoperoxidase×200; (B) Group-II, 3 days of treatment showing mild immunoreactivity. Immunoperoxidase×400; (C) Group-I, 7 days of treatment revealing moderate positive expression of α-SMA. Immunoperoxidase×200; (D) Group-II, 7 days of treatment disclosing, marked positive expression of α-SMA. Immunoperoxidase×200; (E) Group-I at 14 days of treatment showing moderate immunoreactivity. Immunoperoxidase×400; (F) Group-II, 14 days of treatment revealing moderate positive expression of α-SMA. Immunoperoxidase×200.
Fig. 5.
CD31-Immunohistochemistry of skin wound sections: (A) Group-I, 3 days of treatment revealing scarce immunoreactivity. Immunoperoxidase×400; (B) Group-II, 3 days of treatment revealing limited immunoreactivity. Immunoperoxidase×200; (C) Group-I, 7 days of treatment revealing moderate positive expression of CD31 with large number of macrophages and lymphocytes. Immunoperoxidase×100; (D) Group-II, 7 days of treatment disclosing marked positive expression of CD31. Immunoperoxidase×400; (E) Group-I, 14 days of treatment disclosing few capillaries formation with infiltration of lymphocytes and macrophages. Immunoperoxidase×400; (F) Group-II, 14 days of treatment showing moderate immunoreactivity and moderate angiogenesis. Immunoperoxidase×400.
Wound sections treated with MSC-derived exosomes, revealed variable degrees of CD31 and α-SMA markers expression. At the 3rd day of treatment, mild expression of the two markers was observed. High expression of the markers was evident at the 7th day of treatment followed by moderate expression of both markers at the 14th day of treatment (Figs. 4, 5).
Discussion
It was originally thought that MSCs homed to injured tissue differentiate into various cell types such as chondrocytes, adipocytes, osteoblasts or endothelial cells and replace damaged tissue (7, 8). However, subsequent studies have shown that MSC engraftment and differentiation to injured sites are low and transient (4).
Conversely, it has now emerged that MSCs may predominantly act in a paracrine fashion and secretory factors being the mediators of tissue repair and wound healing (9–11). Exosomes are one of the many secretory factors released by the MSCs (7, 8) and have been described as the most important effective ingredients that play an important role in cell-to-cell communication (12, 13). Thus, it was hypothesized that if the benefits of stem cell therapy are mediated by its exosomal secretions, then direct treatment with these exosomes may overcome the limitations and risks associated with stem cell therapy (6, 7).
Faster epithelialization, following BM-derived stem cell transplantation for cutaneous wound healing, has been published in previous studies on rabbits, mice, rats and pigs (14–17) but not on dogs.
In the present study three bilateral full thickness circular wounds were created on the back of six dogs using 2 cm dermal punch similar to (6, 18, 19). The wounds were 2 cm in diameter as estimated to be the Critical Size Defect (CSD) for dogs (20, 21). And they were at least 2.5 cm apart (18).
One month before the commence of the experimental wounds, fresh BM was aspirated from the iliac crest of healthy dogs under general anesthesia then MSCs were isolated and cultured similar to (18, 22, 23). Other possibilities for obtaining BM included the femur (24) and the humerus (25).
Studies have reported a strong direct-correlation between the number of cells applied per cm2 of the wound surface and the subsequent decrease in wound size. It was suggested that at least 1×106 cells/cm2 of wound is needed for a significant therapeutic effect (17). In this study, the amount of exosomes used is the amount of exosomes obtained from 2×106 MSCs/wound similar to (19, 26, 27). The route of administration of the therapeutic material also varied. Cell-treatment could be achieved through intra-dermal injection (18, 26), local and systemic injection (27, 28), or spray (17). Topical methods were statistically more effective than other routes of administration. In this study the exosomal solution was subcutaneously injected in 4 places around each wound similar to (6, 29).
The present work demonstrated better and faster healing of the exosomes treated wound compared with the control group. Dogs treated with MSC-Exosomes showed faster wound closure than observed in the control group at Days 3, 7, and 14 post-wounding. Reduced scar widths and increased collagen maturity were parameters used to assess the degree of wound healing. The gross evaluation of exosomes-treated wounds revealed early and better wound contraction and closure of wounds, early crustation and deposition of healthy granulation tissue consequently resulting in complete healing and covering of wound with regenerated skin by the end of the second week. On the other hand, delayed crustation, deposition of unhealthy granulation tissue and delayed closure of the wound in the control group. The MSC-Exosomal treatment significantly enhanced wound reduction compared to that observed in the control group. The narrowest scar widths and largest collagen deposition areas were observed in the MSC-Exosomes group at days 14 post-wounding, compared to the control group. The macroscopic findings revealed that, the wound size reduction percentage for the exosomes treated group was 7.5% at the 3rd day, 40% at the 7th day and 72.5% the 14th day after treatment. While, the wound size reduction percentage for the control group was 0% at the 3rd day, 20% at the 7th day and 65% at the 14th after treatment. The peak of wound reduction was during the second week post-treatment in both groups.
Exosomes are proven to contribute to an important mechanism for cutaneous wound healing including proangiogenic effects (increase angiogenesis and enhance tubular formation), activating wound-healing pathways as well as promoting growth factor secretion and collagen synthesis (6, 30).
The histological samples were taken at the 3rd, 7th and the 14th day post-treatment similar to (6). In previous studies, histological sampling varied from twelve days (31), five weeks (18) and six weeks (32).
Evidences of rapid re-epithelialization, increased collagen deposition and angiogenesis following topical injection of exosomes were noticed. By the end of the second week, complete re-epithelialization of wound surface and collagen deposition and intact hair follicles were evident at day 14th post-wounding at the exosomes group while, the re-epithelized wound was slightly higher than the surrounding surface at the saline group (33).
Dermal fibroblasts provide critical functions during wound healing, including wound contraction, extracellular matrix deposition, and tissue remodeling (30), providing a scaffold for the assembly of neighboring cells at wound margins and consequently contributing to wound closure. On the instance of skin wound, fibroblasts present in the dermis proliferate rapidly and migrate to wound site where they can secrete type I and III collagens and elastin, which are the central components of the extracellular matrix (34, 35). Studies have demonstrated that a paracrine mechanism of MSCs (conducted through exosomes) is involved in promoting the proliferation, migration, and collagen secretion of fibroblasts (36).
The finding showed new capillaries formation with fibroblasts and few collagen deposition at 7th day post-wounding at exosomes group, while, inflammatory cells, few new capillaries and few fibroblast cells were present at the 7th day post-wounding at the control group.
Masson’s trichrome staining was used to determine the degree of collagen synthesis (6, 18). Evidences suggested that MSC-Exosomes have stimulated the proliferation and migration of dermal fibroblasts and large amount of collagen deposition in the wound at 14th day after treatment with MSC-Exosomes. On the other hand, Masson’s trichrome stain showing disorganized few collagen and proliferation of fibroblasts in the wound at 14th day post wounding in control group.
Vascularization is an essential step in the wound-healing process, angiogenesis and maturation of the new blood vessels is necessary to maintain a healthy granulation tissue (14, 34). To study exosome-induced angiogenesis during the wound healing process, CD31 and alpha smooth muscle actin (α-SMA) were detected. The newly formed vessels were detected by CD31 positive staining, whereas, mature vessels were characterized as CD31 and α-SMA double-positive vascular structures (6, 18, 37, 38).
Results showed that the number of newly formed vessels and mature vessels both have increased alongside the healing process. The MSC derived exosomes group showed the highest vessel densities and numbers of mature vessels, compared with the control group. The exosomes-treated group had more CD31 positive cells in the wound area than control group at both 1st and 2nd weeks after treatment. Results also revealed that the granulation tissue angiogenesis was more active in wounds treated with MSC-derived exosomes than those in the control group at both 1st and 2nd weeks. These findings indicated that exosomes have improved the angiogenesis, in agreement with similar results (6).
The highest increase expression of the two markers was at 7th day of treatment indicating significant angiogenesis and high rate of cellular regenerative activities. Moderate positive expression of CD31 and α-SMA were noticed at 14th day of treatment indicating moderate angiogenesis, which agree with (18).
Exosomes treated group exhibited reduced permeability of the capillary membrane, less fibrosis, decreased inflammation than the control group, all of which had contributed to a better tissue repair. The superior and faster wound healing following the injection of exosomes can be explained by the release of cytokines which may have balanced the wound environment. Exosomes may also have induced better healing changes through the activation of growth factor that enhanced the proliferation and migration of wound fibroblasts and promoted angiogenesis (30).
These findings suggested that MSC-Exosomes have similar protective and reparative properties as their cellular counter parts in tissue repair. Moreover, MSC-Exosomes appeared to be a superior candidate for treating cutaneous wounds, and represented a promising opportunity to develop a novel cell-free therapy approach that might overcome the obstacles and risks associated with the use of native or engineered stem cells transplantation therapy (3, 39).
Conclusion
The findings demonstrated that MSCs derived exosomes significantly promote cutaneous wound healing, collagen synthesis, and vascularization at wound sites in a dog full-thickness skin defect model and showed more rapid wound closure. It was also concluded that the application of exosomes not only promoted the generation of newly formed vessels, but also have accelerated their maturation at wound sites.
Footnotes
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;41:283–287. doi: 10.1042/BST20120192. [DOI] [PubMed] [Google Scholar]
- 4.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleissner F, Goerzig Y, Haverich A, Thum T. Microvesicles as novel biomarkers and therapeutic targets in transplantation medicine. Am J Transplant. 2012;12:289–297. doi: 10.1111/j.1600-6143.2011.03790.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi: 10.1186/s12967-015-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 9.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296:H1888–H1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shabbir A, Zisa D, Leiker M, Johnston C, Lin H, Lee T. Muscular dystrophy therapy by nonautologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation. 2009;87:1275–1282. doi: 10.1097/TP.0b013e3181a1719b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 14.Borena BM, Pawde AM, Amarpal, Aithal HP, Kinjavdekar P, Singh R, Kumar D. Evaluation of autologous bone marrow-derived nucleated cells for healing of full-thickness skin wounds in rabbits. Int Wound J. 2010;7:249–260. doi: 10.1111/j.1742-481X.2010.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo G, Cheng W, He W, Wang X, Tan J, Fitzgerald M, Li X, Wu J. Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair Regen. 2010;18:506–513. doi: 10.1111/j.1524-475X.2010.00616.x. [DOI] [PubMed] [Google Scholar]
- 16.Badiavas EV, Ford D, Liu P, Kouttab N, Morgan J, Richards A, Maizel A. Long-term bone marrow culture and its clinical potential in chronic wound healing. Wound Repair Regen. 2007;15:856–865. doi: 10.1111/j.1524-475X.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 17.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 18.Kim JW, Lee JH, Lyoo YS, Jung DI, Park HM. The effects of topical mesenchymal stem cell transplantation in canine experimental cutaneous wounds. Vet Dermatol. 2013;24:242–e53. doi: 10.1111/vde.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu X, Fang L, Li X, Cheng B, Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006;14:325–335. doi: 10.1111/j.1743-6109.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 20.Karayannopoulou M, Tsioli V, Loukopoulos P, Anagnostou TL, Giannakas N, Savvas I, Papazoglou LG, Kaldrymidou E. Evaluation of the effectiveness of an ointment based on Alkannins/Shikonins on second intention wound healing in the dog. Can J Vet Res. 2011;75:42–48. [PMC free article] [PubMed] [Google Scholar]
- 21.Adam JA. A simplified model of wound healing (with particular reference to the critical size defect) Mathematical and Computer Modelling. 1999;30:23–32. doi: 10.1016/S0895-7177(99)00145-4. [DOI] [Google Scholar]
- 22.Ansari MM, Sreekumar TR, Chandra V, Dubey PK, Kumar GS, Amarpal, Sharma GT. Therapeutic potential of canine bone marrow derived mesenchymal stem cells and its conditioned media in diabetic rat wound healing. J Stem Cell Res Ther. 2013;3:141. doi: 10.4172/2157-7633.1000141. [DOI] [Google Scholar]
- 23.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, Tamura F, Sakai D. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26:589–600. doi: 10.1002/jor.20584. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Lin HK, Frimberger D, Epstein RB, Kropp BP. Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int. 2005;96:1120–1125. doi: 10.1111/j.1464-410X.2005.05741.x. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya V, McSweeney PA, Shi Q, Bruno B, Ishida A, Nash R, Storb RF, Sauvage LR, Hammond WP, Wu MH. Enhanced endothelialization and microvessel formation in polyester grafts seeded with CD34(+) bone marrow cells. Blood. 2000;95:581–585. [PubMed] [Google Scholar]
- 26.Abd-Allah SH, El-Shal AS, Shalaby SM, Abd-Elbary E, Mazen NF, Abdel Kader RR. The role of placenta-derived mesenchymal stem cells in healing of induced full-thickness skin wound in a mouse model. IUBMB Life. 2015;67:701–709. doi: 10.1002/iub.1427. [DOI] [PubMed] [Google Scholar]
- 27.Basiouny HS, Salama NM, Maadawi ZM, Farag EA. Effect of bone marrow derived mesenchymal stem cells on healing of induced full-thickness skin wounds in albino rat. Int J Stem Cells. 2013;6:12–25. doi: 10.15283/ijsc.2013.6.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tark KC, Hong JW, Kim YS, Hahn SB, Lee WJ, Lew DH. Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg. 2010;65:565–572. doi: 10.1097/SAP.0b013e3181d9aae2. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24:1635–1647. doi: 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azari O, Babaei H, Derakhshanfar A, Nematollahi-Mahani SN, Poursahebi R, Moshrefi M. Effects of transplanted mesenchymal stem cells isolated from Wharton’s jelly of caprine umbilical cord on cutaneous wound healing; histopathological evaluation. Vet Res Commun. 2011;35:211–222. doi: 10.1007/s11259-011-9464-z. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa H, Akita S, Fukui M, Fujii T, Akino K. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol. 2005;153:29–36. doi: 10.1111/j.1365-2133.2005.06554.x. [DOI] [PubMed] [Google Scholar]
- 33.Shi W, Liu L, Li J, Qu L, Pang X, Yu H, Zhang Y, Wang T. Bioactive flavonoids from Flos Sophorae. J Nat Med. 2017;71:513–522. doi: 10.1007/s11418-017-1084-7. [DOI] [PubMed] [Google Scholar]
- 34.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 35.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Xu Y, Zhao J, Zhang Z, Yang R, Xie J, Liu X, Qi S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9:e96161. doi: 10.1371/journal.pone.0096161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hattori N, Mochizuki S, Kishi K, Nakajima T, Takaishi H, D’Armiento J, Okada Y. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol. 2009;175:533–546. doi: 10.2353/ajpath.2009.081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]