Abstract
Relative expressions of structural genes and a number of transcription factors of the anthocyanin pathway relevant in Vaccinium species, and related key enzyme activities were compared with the composition and content of metabolites in skins of ripe fruits of wild albino and blue bilberry (Vaccinium myrtillus) found in Slovenia. Compared to the common blue type, the albino variant had a 151-fold lower total anthocyanin and a 7-fold lower total phenolic content in their berry skin, which correlated with lower gene expression of flavonoid 3-O-glycosyltransferase (FGT; 33-fold), flavanone 3-hydroxylase (FHT; 18-fold), anthocyanidin synthase (ANS; 11-fold), chalcone synthase (CHS, 7.6-fold) and MYBPA1 transcription factor (22-fold). The expression of chalcone isomerase (CHI), dihydroflavonol 4-reductase (DFR), leucoanthocyanidin reductase (LAR), anthocyanidin reductase (ANR) and MYBC2 transcription factor was reduced only by a factor of 1.5–2 in the albino berry skins, while MYBR3 and flavonoid 3’,5’-hydroxylase (F3’5’H) were increased to a similar extent. Expression of the SQUAMOSA class transcription factor TDR4, in contrast, was independent of the color type and does therefore not seem to be correlated with anthocyanin formation in this variant. At the level of enzymes, significantly lower FHT and DFR activities, but not of phenylalanine ammonia-lyase (PAL) and CHS/CHI, were observed in the fruit skins of albino bilberries. A strong increase in relative hydroxycinnamic acid derivative concentrations indicates the presence of an additional bottleneck in the general phenylpropanoid pathway at a so far unknown step between PAL and CHS.
Introduction
Bilberry (Vaccinium myrtillus L.) is a well-known deciduous dwarf shrub growing mostly in cool temperate regions and mountain areas of Europe and Asia. The berries are a rich source of various phenolic compounds, including large amounts of anthocyanins [1–3]. This flavonoid subclass provides the main red, violet and blue pigments in flowers and fruits, in which they act as insect and animal attractants, possess protective roles against various biotic and abiotic stresses and also provide benefits for human health [4]. In fruits, anthocyanins are predominantly found in the vacuoles of skin, although anthocyanins can also be found in the pulp in some berries [5, 6].
Differences in the composition and content of anthocyanins and other polyphenols in fruits are the consequence of complex metabolic networks, regulated by genetic, developmental and environmental factors [7–11]. Flavonoids are synthesized via the phenylpropanoid/flavonoid pathway, the main steps of which are well known [4, 12, 13]. The regulation of this pathway occurs by the interaction of various transcription factors; R2R3 MYB, basic helix–loop–helix (bHLH), WD40-like proteins and MADS-box genes [7, 14, 15]. R2R3-MYB transcription factors are the key switches for secondary metabolite gene regulation and are therefore important regulators of anthocyanin, proanthocyanidin and flavonol biosynthesis in plants [14]. They are known to regulate the expression of chalcone synthase (CHS), flavanone 3-hydroxylase (FHT), dihydroflavonol 4-reductase (DFR), anthocyanidin synthase (ANS) and other flavonoid pathway genes in various plant parts (leaves, flowers and fruits) of different horticultural plants such as anthurium [16], apple [17], bog bilberry [18] etc. One of the factors that determines whether flower and fruit color is also the competition of flavonoid 3’-hydroxylase (F3’H) and flavonoid 3’, 5’-hydroxylase (F3’5’H) with DFR for substrates, as well as substrate specificity of DFR itself [19, 20]. This results in different compositions of pelargonidin (orange), cyanidin (red) and delphinidin (blue) derivatives. Mutations of structural and regulatory genes can also result in different yellow or white anthocyanin-free phenotypes [19, 21]. Such color mutants have always been valuable study objects to obtain insights into the regulation of anthocyanins in nature [22]. Previous studies of rare berry colors of other Vaccinium species have thus provided a detailed insight into the gene expression of structural and regulatory genes of the anthocyanin pathway [18, 23, 24].
A downregulation of the structural genes of the anthocyanin pathway in Vaccinium seems to be correlated with strongly decreased expression of the transcription factors VuMYBPA1 and VuMYBR3 and a moderately but significantly lowered gene expression of VuTDR4 and VuMYBC2 [18]. VuMYBPA1 is a R2R3 MYB transcription factor with a presumed role in anthocyanin formation in Vaccinium species [18], although a closely related transcription factor in Vitis vinifera (VvMYBPA1) rather controls proanthocyanidin formation in grapevine [25]. PhMYB27 and VvMYBC2-L1 are negative regulators of the pathway to anthocyanidins and proanthocyanidins in petunia and grapevine, respectively [26, 27]. TDR4 has recently been suggested to play an important role in the accumulation of anthocyanins in Vaccinium species [24] but final evidence is not yet available.
We recently discovered a rare Slovenian wild-growing albino bilberry (V. myrtillus) differing from blue wildtypes in terms of fruit quality parameters such as fruit weight, color and primary and secondary metabolite composition [28]. Common blue ripe bilberries, typically accumulate high amounts of blue colored delphinidin- and red colored cyanidin-derived pigments and additionally contain significant levels of hydroxycinnamic acid derivatives, flavanols and flavonol glycosides. Our albino variant generally showed strongly reduced polyphenol content, which was in contrast to a recently reported albino variant of the closely related wild-type bog bilberries (Vaccinium uliginosum L.) which had reduced amounts of anthocyanins but unchanged flavonol and flavanol levels in comparison common blue berries and thus possesses a bottleneck in the late anthocyanin pathway [18]. Our almost anthocyanin-free albino fruits contained predominantly hydroxycinnamic acid derivatives and only moderate amounts of flavanols and flavonol glycosides.
We here present a detailed study of this albino variant of V. myrtillus and show how the two color types of wild bilberry (albino and blue) vary in their relative expressions of selected structural and regulatory genes of anthocyanin biosynthesis. In addition, we measured the specific activities of selected enzymes and concentrations of primary and secondary metabolites. We particularly focused on the polyphenol metabolism in berry skins, since previous studies have indicated that some key genes that are related to anthocyanin accumulation are expressed mainly in the skin [29, 30].
Materials and methods
Plant material
Wild blue and albino bilberry fruits were collected at the fully ripe stage on 11 June 2015 from native population in a forest near Žiri, 40 km west of Ljubljana, Slovenia. Blue and albino type fruits were randomly collected (approx. 300 and 150 g of fruit, respectively) and only undamaged fruits were selected for the analysis. Immediately after harvest, berry skin was separated from the pulp, shock-frozen in liquid nitrogen, and stored at -80°C until analyses of enzyme activities, relative expression and secondary metabolite concentrations. For analysis of primary metabolites, whole berries were stored at -20°C.
Experimental design
For extraction of sugars, organic acids and phenolic compounds for each bilberry type (blue and albino), five biological replications were carried out (n = 5), while for enzyme and gene expression analysis three biological replications were carried out (n = 3). Each replication included at least 15 berries. For enzyme assays, each replication was analyzed with 2 technical replications each. RT-qPCR was carried out in biological triplicates with three technical replications each. Primary metabolites were analyzed in whole bilberry fruit, while secondary metabolites in addition to gene expression and enzyme activity were analyzed only in berry skin.
Chemicals
For the determination of primary and secondary metabolites, same reference compounds were used as previously reported in our study [28]. Methanol, ethanol, and gallic acid were obtained from Sigma-Aldrich Chemie (Steinheim, Germany) and sodium carbonate from Merck (Darmstadt, Germany). The Folin-Ciocalteu phenol reagent and the solvents for the mobile phases, HPLC-MS grade acetonitrile and formic acid, was purchased from Fluka Chemie (Buchs, Switzerland). Water for the mobile phase was double distilled and purified with the Milli-Q system (Millipore, Bedford, MA, USA). L-(U-14C) Phenylalanine and (2-14C)-malonyl-coenzyme A were obtained from Amersham International (Freiburg, Germany). (14C)-Labeled flavonoids naringenin, dihydrokaempferol (DHK), dihydromyricetin (DHM), and dihydroquercetin (DHQ) were prepared as described previously [20, 31].
Extraction and determination of sugars and organic acids
Berries (2 g) were ground to a fine paste in a mortar, homogenized with 8 mL of double distilled water and left for 30 min at room temperature. After the extraction, the homogenate was centrifuged and the supernatant was filtered and transferred into a vial. The further analysis was made as described by our previous study [28]. The results were expressed in mg g-1 FW.
Extraction and determination of individual phenolic compounds
Berry skins were ground to a fine paste in a mortar chilled with liquid nitrogen, and 0.5 g were extracted with 6 (albino) or 8 mL (blue bilberries) methanol containing 3% (v/v) formic acid in a cooled ultrasonic bath for 1 h. Skin extracts were centrifuged and each supernatant was filtered and transferred to a vial prior to injection into the HPLC system. The further analysis was made as described by our previous study [28]. The results were expressed in mg kg-1 FW.
Determination of total phenolic content
The extraction of skin berry samples for the determination of total polyphenols was carried out according to the same protocol as for individual polyphenols. Total polyphenol concentrations of extracts were estimated by the Folin-Ciocalteu phenol reagent method [32] and expressed as gallic acid equivalents in mg kg−1 FW. Absorption was measured in five replications.
Extraction and enzyme assays
Shock-frozen bilberry skin was ground to powder with liquid nitrogen. A total of 0.20 g fine skin powder was homogenized with 0.20 g quartz sand, 0.20 g Polyclar AT, and 3 mL extraction buffer (prepared as described by Thill et al. [33]. The homogenate was centrifuged for 10 min at 4°C and 13.000 x g. To remove low molecular compounds, 400 μL of supernatant were passed through a gel chromatography column (Sephadex G25 medium). The protein solution eluted in the excluded volume of the column (crude extract) was used for enzyme assays.
Enzyme assays were performed as described previously [34] using the assay conditions optimized for bilberry skin (S1 Table). The assays were incubated for 15 min at 30°C. To determine the specific enzymatic activity, a modified Lowry method for protein determination [35] with BSA as a standard was used. Specific activities of PAL (phenylalanine ammonia-lyase), CHS/CHI, FHT and DFR were calculated and expressed as kat kg-1 protein.
Gene expression studies
Total RNA was prepared according to Chang et al. [36] and subsequently used for the isolation of mRNA via the μMACS mRNA isolation kit (Miltenyi Biotec, Auburn, CA, USA). cDNA was prepared using the RevertAid H Minus MuLV reverse transcriptase (Fermentas Life Science, St. Leon-Rot, Germany) with the oligo(-dT) anchor Primer GACCACGCGTATCGATGTCGAC(T)16V.
Relative gene expressions of ANR (anthocyanidin reductase), ANS, CHS, CHI, DFR, F3’5’H, FHT, FGT (flavonoid 3-O-glycosyltransferase), LAR (leucoanthocyanidin reductase), MYBC2, MYBPA1, MYBR3 and TDR4 in comparison to the glycerine aldehyde 3-phosphate dehydrogenase (GAPDH) control gene were analyzed by qPCR using a StepOnePlus system and the SYBR Green PCR Master Mix (Applied Biosystems, Darmstadt, Germany) according to the supplier’s instruction. Primers for RT-qPCR were used as published elsewhere for V. uliginosum [18]. Specificity was confirmed by melting curve analysis. All primers showed an efficiency between the limits of 90 and 110%. Primer suitability of V. uliginosum for the orthologous genes of V. myrtillus was confirmed by sequencing of the amplification products. Additionally, we designed primers for V. myrtillus FHT, by using a public available sequence (NCBI AY123766). A summary of the primers used in this study are provided in S2 Table.
Differences between the cycle threshold (Ct) of the target gene and the GAPDH gene were used to obtain relative transcript levels of the target gene, and calculated as 2 exp-(Cttarget – CtGAPDH). The efficiency of the RT-qPCR-reaction was determined on the basis of standard curves which were obtained by applying different DNA concentrations. Results were calculated in relation to the control gene.
Statistical analysis
Results were evaluated with the Statgraphics Centurion XV.II program (Statpoint Technologies Inc., Warrenton, VA, USA). The significance of the type on the content of primary and secondary metabolites, relative expressions of flavonoid genes, transcription factors and enzyme activities were tested using One-Way ANOVA. Differences between forms were tested with the LSD test at a significance level of 0.05.
Results
Relative expression of structural and regulatory genes of the anthocyanin pathway
The relative expression of the structural genes CHS, CHI, FHT, F3’5’H, DFR, LAR, ANR, ANS and FGT of the flavonoid pathway and of four transcription factors previously described as influencing anthocyanin accumulation in Vaccinium was determined in the blue and albino bilberry skins (Figs 1 and 2). The latter included transcription factors of the R2R3 and R3 MYB family (MYBC2, MYBPA1 and MYBR3) and the SQUAMOSA class transcription factor TDR4 (Fig 2). After evaluation of nine candidates of housekeeping genes with respect to expression stability and quality of signals obtained with published primer sequences for Vaccinium sp. [37], GAPDH, tubulin ß and clathrin adaptor complexes subunit family protein remained as suitable housekeeping genes. Considering the fact that only fruits from the same location and grown under identical conditions were analyzed, we used GAPDH as single housekeeping gene for the studies.
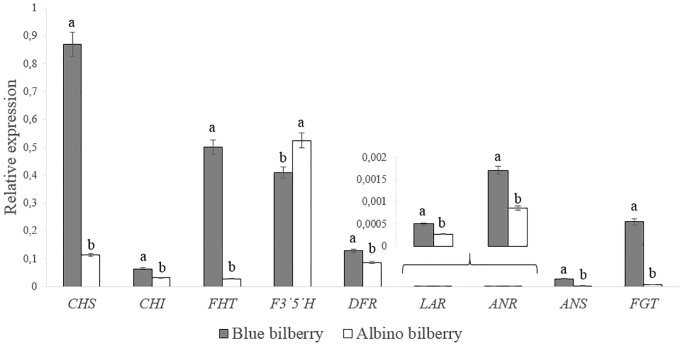
Fig 1. Relative expression of genes from the anthocyanin pathway (CHS, CHI, FHT, F3’5’H, DFR, LAR, ANR, ANS and FGT) of blue and albino bilberry normalized to GAPDH.
Different letters (a, b) above the columns denote significant differences among bilberries (LSD test P < 0.05).
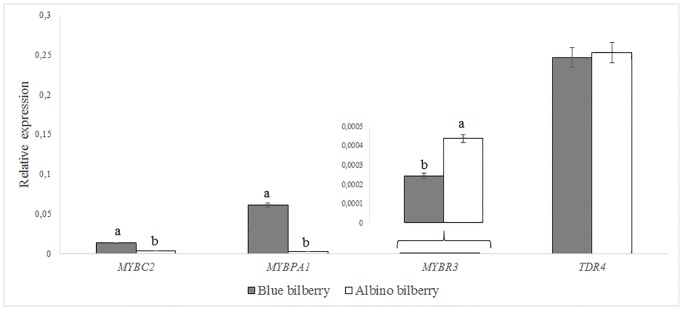
Fig 2. Relative expression of transcription factors (MYBC2, MYBPA1, MYBR3 and TDR4) of blue and albino bilberry normalized to GAPDH.
Different letters (a, b) above the columns denote significant differences among bilberries (LSD test P < 0.05).
Most of the primers were derived from studies on V. uliginosum but could be used for our studies as well. The successful application of RT-qPCR primers of V. uliginosum for genes of the closely related V. myrtillus showed that the interspecific usage of primers can be successfully applied if sequence information is lacking.
In comparison to common blue berry skins of V. myrtillus, the albino type showed strongly decreased gene expression of all tested structural genes, with the exception of F3’5’H expression, which was only 1.3-fold higher in albino bilberry and thus almost unchanged (Fig 1). The most affected genes were FGT (33-fold lower), FHT (18-fold lower gene expression), ANS (11-fold lower) and CHS (7.6-fold lower), while the other structural genes (CHI, DFR, LAR and ANR) showed only a 1.5–2.1-fold lower gene expression in albino bilberry (Fig 1).
Among transcription factors, the highest difference between V. myrtillus color types, with almost 22-fold lower relative expression level for albino bilberry, was measured for MYBPA1 (Fig 2). In addition, albino bilberry had 3.7-fold lower relative expression of MYBC2 but a 1.8-fold higher relative expression of MYBR3 (Fig 2). For TDR4 gene expression, no significant differences were observed between blue and albino V. myrtillus berries (Fig 2).
Specific activities of selected flavonoid enzymes
In addition to the gene expression studies, we also measured for the first time the activities of the enzymes of the main pathway to anthocyanins, PAL, CHS/CHI, FHT and DFR (Fig 3). ANS could unfortunately not be included, since its activity can so far only be measured with recombinant enzymes [13].
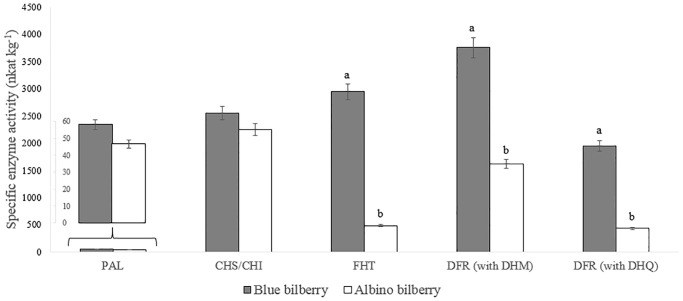
Fig 3. Specific enzyme activities (PAL, CHS/CHI, FHT and DFR (with DHQ and DHM as substrates) (nkat kg-1 protein) of blue and albino bilberry.
Different letters (a, b) above the columns denote significant differences among bilberries (LSD test P < 0.05).
Significant differences between the two V. myrtillus color types were observed for FHT and DFR activities, but not for PAL and CHS/CHI (Fig 3). A 6.1-fold lower specific activity of FHT was measured in the albino type (Fig 3). DFR enzyme activities were tested with DHK, DHQ and DHM as substrates (S1 Table). Enzyme preparations from V. myrtillus did not convert DHK; moreover DFR preferred DHM over DHQ (1.9-fold and 3.7-fold higher conversation rates for blue and albino bilberry, respectively). Higher differences in DFR enzyme activities among the tested bilberries were measured with DHQ, with a 4.5-fold lower activity in the albino bilberry (Fig 3). DFR with DHM as a substrate had only a 2.3-fold lower activity in the albino compared to the blue bilberry (Fig 3).
Phenolic profile of bilberry fruit skins
To provide a more detailed insight into the complex flavonoid pathway in the two V. myrtillus types, the composition and content of primary and secondary metabolites were additionally analyzed (S3–S5 Tables). Since detailed phenolic characterization of both bilberry fruits was reported in our previously published work [28], in this study we analyzed only fruit skin phenolics, which are presented in S4 and S5 Tables. Fig 4 provides an overview on the relative levels of the main polyphenol classes of both bilberry skins.
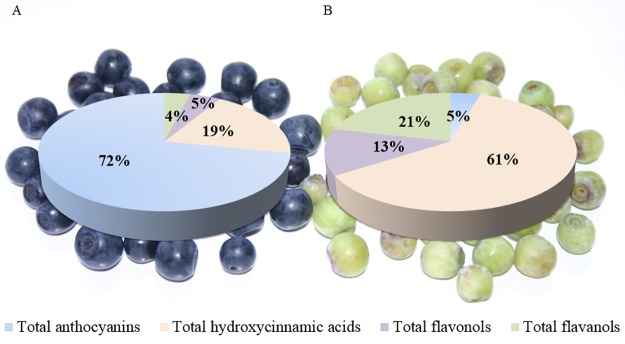
Fig 4. Relative levels of the main polyphenol classes of bilberry fruit skins.
(A) Blue bilberry. (B) Albino bilberry.
In the albino type, the majority of polyphenols were found to be hydroxycinnamic acid derivatives (61%), followed by flavanols (21%) and flavonol glycosides (13%), whereas total anthocyanins contributed only 4.5% (Fig 4 and S4 Table). The polyphenol spectrum of blue V. myrtillus berry skins, in contrast, consisted of 72% anthocyanins, 19% hydroxycinnamic acid derivatives and 4–5% of flavanols and flavonol glycosides (Fig 4 and S4 Table). Due to the very low polyphenol concentrations (7-fold lower total phenolic content in albino compared to blue bilberry), the absolute amounts of each polyphenol class were higher in blue than albino type berry skins (S5 Table). Although there were differences in the individual composition of polyphenol classes (S4 Table), no striking qualitative differences could be found between the two color types. Even in the anthocyanin spectrum, albino V. myrtillus berry skins accumulated the same 15 anthocyanins, albeit in drastically lower amounts (151-fold lower) (S5 Table).
An overview of the flavonoid pathway in V. myrtillus according to our results obtained in this study is summarized in Fig 5.
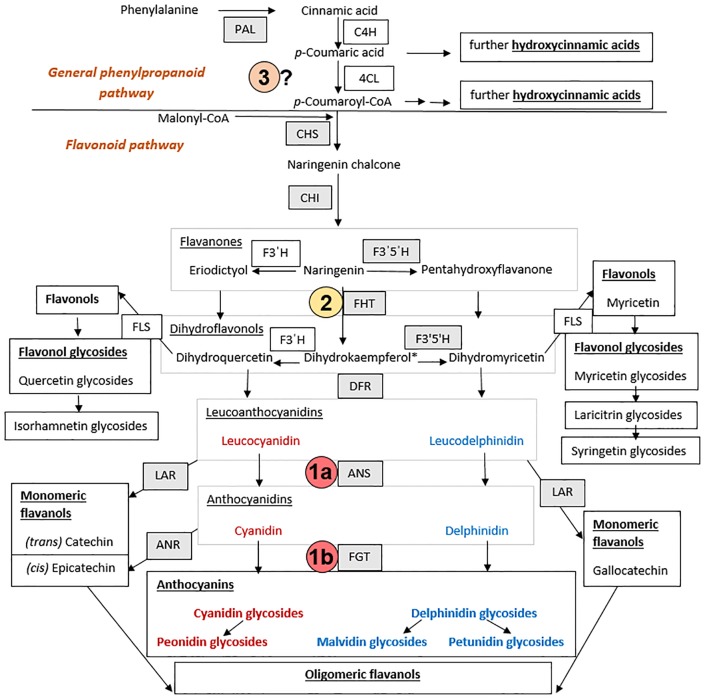
Fig 5. Simplified flavonoid biosynthesis pathway (combined studies of gene expression, enzyme activity and metabolite analysis) leading to anthocyanin accumulation in Vaccinium myrtillus L.
Abbreviations: ANR, anthocyanidin reductase; ANS, anthocyanidin synthase; C4H, cinnamate 4-hydroxylase; CHI, chalcone isomerase; CHS, chalcone synthase; 4CL, hydroxycinnamate: CoA ligase; DFR, dihydroflavonol reductase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′, 5′-hydroxylase; FGT, flavonoid-3-O-glucosyltransferase; FHT, flavanone 3-hydroxylase; FLS, flavonol synthase; LAR, leucoanthocyanidin reductase; PAL, phenylalanine ammonia lyase. *leading to small amounts of kaempferol-3-O-glucuronide. The three bottlenecks identified in the anthocyanin pathway of the albino type berry skins are numbered with 1 (a, b: ANS, FGT), 2 (FHT) and 3 (a so far unknown step located in the general phenylpropanoid pathway between PAL and CHS). Grey shaded boxes, enzymes/genes analyzed in our work.
Discussion
In this study we characterized for the first time the flavonoid pathway of an albino type of V. myrtillus found in Slovenia [28] by measuring the expression of a range of structural and regulatory genes and selected enzyme activities correlated with the polyphenols accumulated in the berry skins. Whereas the flavonoid pathway is well established and structural genes can be found in the databases, if not always for V. myrtillus then at least for another Vaccinium species, the knowledge on the regulatory genes influencing the formation of polyphenols, especially flavonoids is fragmented. We included, however, a number of transcription factors, that have been previously assumed to play a role in the anthocyanin pathway in any of the Vaccinium species, to shed first light on the question if our V. myrtillus albino variant can provide novel insights into the pathway or if it is similar to other Vaccinium color types previously reported.
Our study revealed significant differences in the expression levels of key structural genes and specific enzyme activities of the anthocyanin pathway between albino and blue colored V. myrtillus fruit skins. Key structural genes showed considerably lower expression levels in albino bilberry skins, compared to common blue type. Among them, the most affected genes were FGT, FHT, ANS and CHS, followed by CHI, DFR, LAR and ANR, while F3’5’H gene expression was slightly higher in albino V. myrtillus (Fig 1). This is in line with reports on albino V. uliginosum and V. myrtillus, in which the whole pathway with the exception of F3’5’H was strongly downregulated in ripe fruits [18, 23]. It also confirms that F3’5’H is regulated separately as suggested from earlier studies of V. uliginosum [18].
Our albino V. myrtillus, however, showed interesting differences in the expression of transcription factors compared to other albino fruits of V. uliginosum and V. myrtillus. We observed significantly lower relative expression levels of MYBPA1 and MYBC2, but higher relative expression of MYBR3 in albino compared to blue bilberry, while for TDR4 gene expression, no differences were observed (Fig 2). A high correlation of MYBPA1 expression with the anthocyanin pathway gene expression has largely been reported earlier [15, 16, 18, 24, 38]. Other albino Vaccinium fruits, however, showed a strong correlation between the expression of TDR4 and various MYB factors, suggesting that TDR4 plays an important role in the control of anthocyanin biosynthesis in Vaccinium berries [18, 24]. This was supported by virus-induced gene silencing of TDR4, leading to a strong reduction of anthocyanin concentrations in the berries. It seems, however, that in this experiment the flesh was much more affected than skin, since the ripe fruits had a clearly faded flesh color, despite an intense skin coloration [24]. In agreement with this, our data also suggest that TDR4 is not necessarily correlated with anthocyanin based coloration in V. myrtillus, at least not in the skins. The moderate increase of MYBR3 gene expression in the albino V. myrtillus type is in contrast to the observed downregulation in albino V. uliginosum [18] and to PhMYBx (R3-MYB) from Petunia, which was upregulated when plants begin to accumulate anthocyanins [39]. Although anthocyanin biosynthesis in plants usually involve R2R3 and R3 MYB activators, there are also multiple types of repressors, which are less understood [26, 39]. In Petunia hybrida, a putative R2R3-MYB repressor of anthocyanin synthesis PhMYB27 was identified and in strawberry structurally similar FaMYB1, though there are also some other known ones [39, 40].
The albino type V. myrtillus berry skins showed comparable results as the previously reported whole bilberry fruits [28]. As expected, a pronounced shift in the composition of polyphenol classes was observed, which was also characterized by a drastically lower accumulation of total polyphenols, which was as low as 15% of the concentrations found in the blue berries (S5 Table). The low content of total polyphenols indicates the presence of a bottleneck early in the pathway, located between PAL and CHS, which was not encompassed by the enzyme and gene expression studies as the sequences from Vaccinium are not yet available (Fig 3). The discrepancies between CHS/CHI enzyme activity and CHS gene expression is most probably the result of the presence of isoenzymes as frequently observed for flavonoid enzymes [41]. In Dahlia x variabilis two phylogenetically different chalcone synthases were described sharing only 69% nucleotide sequence identity, of which one is generally present, whereas the second is specifically upregulated together with DFR and ANS during anthocyanin formation [42]. Indeed the presence of several isoforms of the genes from the anthocyanin pathway was reported for Vaccinium species [23]. This underpins the importance of measuring enzyme activities in addition to gene expression to obtain a better picture of the pathway.
The shift in the other polyphenol classes correlated nicely with the observed enzyme activities and gene expression levels. In general, a lower FHT and DFR activity in albino bilberry was found and correlated with the higher relative FHT and DFR expression in blue bilberry (Figs 1 and 2). Additionally, the bottleneck created by low ANS and FGT expression resulted in drastically lower anthocyanin accumulation in albino skins (S5 Table), as described for other plants [18, 19, 43]. In a previous study [23], a reduction in levels of DFR and ANS in pink and white colored bilberries was also demonstrated but their transcript abundance was not measured by RT-qPCR. The increase in the relative epicatechin concentrations in our albino V. myrtillus berry skins (S4 Table) may reflect the redirection of anthocyanidin formation to epicatechin formation, since ANR and ANS expression were reduced to a minor extent compared to FGT. The slightly increased F3’5’H expression in albino V. myrtillus berry skins, however, was not reflected in elevated amounts of 3’, 4’, 5-hydroxylated anthocyanins (delphinidin type) or flavonols (myricetin type). In our albino type V. myrtillus, FHT and DFR seem to form a two-step bottleneck, with a lower flux at the first FHT step. The low amounts of dihydroflavonols formed are common substrates for DFR and flavonol synthase (FLS). Substrate competition between DFR (anthocyanin pathway) and FLS (side branch to flavonols) has been reported previously for many plants [19, 33, 44]. In detail, enzyme preparations from V. myrtillus did not convert DHK, which is in line with the absence of pelargonidin derived anthocyanins. A changed flavonoid flux due to the DHM preference of DFR may provide an explanation of the relatively lower myricetin concentrations in the albino type compared to the blue (S4 Table). Although DFR preferred DHM over DHQ, higher differences in DFR enzyme activities among the tested bilberries were measured with DHQ (Fig 2). The 4.5-fold higher activity in blue bilberry with DHQ compared with a 2.3-fold higher activity with DHM confirms the presence of DFR isoenzymes in bilberry that show different substrate specificities, as recently shown for Fragaria species [20]. If only one isoform were to be present, a similar reduction in the conversion of DHM and DHQ would be expected. Actually at least two different types of DFRs (Accession numbers AY780883, AF483836) have been identified in the closely related V. macrocarpon [45, 46].
Conclusions
This study provides an in-depth characterization of gene expression and enzyme activities and metabolites of the polyphenol pathway of an albino type of wild V. myrtillus recently found in Slovenia. We identified three bottlenecks in the anthocyanin pathway in the berry skins of the albino type at the level of (1) ANS/FGT, (2) FHT and (3) a so far unknown step located in the general phenylpropanoid pathway between PAL and CHS. The latter is clearly reflected by drastically lower total polyphenol concentrations in the albino V. myrtillus berry skins and a shift in the polyphenol profile towards a prevalent presence of hydroxycinnamic acids and increased relative (but not absolute) concentrations of flavanols and flavonols. Thus our albino type adds another model type for studying the anthocyanin pathway and its regulation in Vaccinium fruits, which particularly offers the possibility to focus on the general phenylpropanoid pathway upstream of flavonoid formation. Further work will focus on the identification of the bottleneck located in the general phenylpropanoid pathway, the identification of isoenzymes of the flavonoid pathway and putative differences in their substrate specificity as well as transcriptome studies to identify so far unknown further regulatory genes involved in the formation of anthocyanins in Vaccinium species.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We are grateful to Mr. Matjaž Maležič for providing the plant material.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the Slovenian Research Agency (ARRS); Horticulture No. P4-0013-0481 (https://www.arrs.gov.si/sl/) and the Austrian Science Fund (AT) grant no. P 28134-B25 to MHC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Može S, Polak T, Gašperlin L, Koron D, Vanzo A, Poklar Ulrih N, et al. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J Agric Food Chem. 2011;59: 6998–7004. doi: 10.1021/jf200765n [DOI] [PubMed] [Google Scholar]
- 2.Mikulic-Petkovsek M, Schmitzer V, Slatnar A, Stampar F, Veberic R. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J Sci Food Agric. 2014;95: 776–785. doi: 10.1002/jsfa.6897 [DOI] [PubMed] [Google Scholar]
- 3.Veberic R, Slatnar A, Bizjak J, Stampar F, Mikulic-Petkovsek M. Anthocyanin composition of different wild and cultivated berry species. LWT-Food Sci Technol. 2015;60: 509–517. [Google Scholar]
- 4.Falcone Ferreyra ML, Rius SP, Casati P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci. 2012;3 doi: 10.3389/fpls.2012.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riihinen K, Jaakola L, Karenlampi S, Hohtola A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ‘northblue’ blueberry (Vaccinium corymbosum x V. angustifolium). Food Chem. 2008;110(1): 156–60. doi: 10.1016/j.foodchem.2008.01.057 [DOI] [PubMed] [Google Scholar]
- 6.He JJ, Liu YX, Pan QH, Cui XY, Duan CQ. Different anthocyanin profiles of the skin and the pulp of Yan73 (Muscat Hamburg × Alicante Bouschet) grape berries. Molecules. 2010;15: 1141–1153. doi: 10.3390/molecules15031141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaakola L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013;18: 477–483. doi: 10.1016/j.tplants.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 8.Luengo-Escobar A, Alberdi M, Acevedo P, Machado M, Nunes-Nesi A, Inostroza-Blancheteau C, et al. Distinct physiological and metabolic reprogramming undergoing long term UV-B radiation revealed in highbush blueberry cultivars (Vaccinium corymbosum L.). Physiol Plant. 2017;160: 46–64. doi: 10.1111/ppl.12536 [DOI] [PubMed] [Google Scholar]
- 9.Serrano A, Espinoza C, Armijo G, Inostroza-Blancheteau C, Poblete E, Meyer-Regueiro C, et al. Omics approaches for understanding grapevine berry development: Regulatory networks associated with endogenous processes and environmental responses. Front Plant Sci. 2017;8 doi: 10.3389/fpls.2017.01486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoratti L, Sarala M, Carvalho E, Karppinen K, Martens S, Giongo L, et al. Monochromatic light increases anthocyanin content during fruit development in bilberry. BMC Plant Biol. 2014;14:377 doi: 10.1186/s12870-014-0377-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zorenc Z, Veberic R, Koron D, Miosic S, Hutabarat OS, Halbwirth H, et al. Polyphenol metabolism in differently colored cultivars of red currant (Ribes rubrum L.) through fruit ripening. Planta. 2017;246(2): 217–226. doi: 10.1007/s00425-017-2670-3 [DOI] [PubMed] [Google Scholar]
- 12.Halbwirth H, Puhl I, Haas U, Jezik K, Treutter D, Stich K. Two-phase flavonoid formation in developing strawberry (Fragaria x ananassa) fruit. J Agric Food Chem. 2006;54: 1479–1485. doi: 10.1021/jf0524170 [DOI] [PubMed] [Google Scholar]
- 13.Halbwirth H, Muster G, Stich K. Unraveling the biochemical base of dahlia flower coloration. Nat Prod Commun. 2008;3(8): 1259–1266. [Google Scholar]
- 14.Liu J, Osbourn A, Ma P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol Plant. 2015;8(5): 689–708. doi: 10.1016/j.molp.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 15.Zifkin M, Jin A, Ozga JA, Zaharia LI, Schernthaner JP, Gesell A, et al. Gene expression and metabolite profiling of developing highbush blueberry fruit indicates transcriptional regulation of flavonoid metabolism and activation of abscisic acid metabolism. Plant Physiol. 2012;158: 200–224. doi: 10.1104/pp.111.180950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Qiu J, Yang G, Huang S, Yin J. Isolation and characterization of a R2R3-MYB transcription factor gene related to anthocyanin biosynthesis in the spathes of Anthurium andraeanum (Hort.). Plant Cell Rep. 2016;35(10): 2151–2165. doi: 10.1007/s00299-016-2025-8 [DOI] [PubMed] [Google Scholar]
- 17.Vimolmangkang S, Han Y, Wei G, Korban SS. An apple MYB transcription factor, MdMYB3, is involved in regulation of anthocyanin biosynthesis and flower development. BMC Plant Biol. 2013;13: 176 doi: 10.1186/1471-2229-13-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Primetta AK, Karppinen K, Riihinen KR, Jaakola L. Metabolic and molecular analyses of white mutant Vaccinium berries show down-regulation of MYBPA1-type R2R3 MYB regulatory factor. Planta. 2015;242: 631–643. doi: 10.1007/s00425-015-2363-8 [DOI] [PubMed] [Google Scholar]
- 19.Jin X, Huang H, Wang L, Sun Y, Dai S. Transcriptomics and metabolite analysis reveals the molecular mechanism of anthocyanin biosynthesis branch pathway in different Senecio cruentus cultivars. Front Plant Sci. 2016. doi: 10.3389/fpls.2016.01307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miosic S, Thill J, Milosevic M, Gosch C, Pober S, Molitor C, et al. Dihydroflavonol 4-reductase genes encode enzymes with contrasting substrate specificity and show divergent gene expression profiles in Fragaria species. PLoS ONE. 2014. doi: 10.1371/journal.pone.0112707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafique MZ, Carvalho E, Stracke R, Palmieri L, Herrera L, Feller A, et al. Nonsense mutation inside anthocyanidin synthase gene controls pigmentation in yellow raspberry (Rubus idaeus L.). Front Plant Sci. 2016;7 doi: 10.3389/fpls.2016.01892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forkmann G. Flavonoids as flower pigments: the formation of the natural spectrum and its extension by genetic engineering. Plant Breeding. 1991;106: 1–26. [Google Scholar]
- 23.Jaakola L, Määttä K, Pirttila AM, Törrönen R, Kärenlampi S, Hohtola A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 2002;130: 729–739. doi: 10.1104/pp.006957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaakola L, Poole M, Jones MO, Kämäräinen-Karppinen T, Koskimäki JJ, Hohtola A, et al. A SQUAMOSA MADS box gene involved in the regulation of anthocyanin accumulation in bilberry fruits. Plant Physiol. 2010;153: 1619–1629. doi: 10.1104/pp.110.158279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogs J, Jaffe FW, Takos AM, Walker AR, Robinson AS. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol. 2007;143: 1347–1361. doi: 10.1104/pp.106.093203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albert NW, Davies KM, Lewis DH, Zhang H, Montefiori M, Brendolise C, et al. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell. 2014;26: 962–980. doi: 10.1105/tpc.113.122069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang YF, Vialet S, Guiraud JL, Torregrosa L, Bertrand Y, Cheynier V, et al. A negative MYB regulator of proanthocyanidin accumulation, identified through expression quantitative locus mapping in the grape berry. New Phytol. 2014;201: 795–809. doi: 10.1111/nph.12557 [DOI] [PubMed] [Google Scholar]
- 28.Zorenc Z, Veberic R, Stampar F, Koron D, Mikulic-Petkovsek M. White versus blue: Does the wild ‘albino’ bilberry (Vaccinium myrtillus L.) differ in fruit quality compared to the blue one? Food Chem. 2016;211: 876–882. doi: 10.1016/j.foodchem.2016.05.142 [DOI] [PubMed] [Google Scholar]
- 29.Grimplet J, Deluc LG, Tillett RL, Wheatley MD, Schlauch KA, Cramer GR, et al. Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genom. 2007;187: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mu L, He JJ, Pan QH, He F, Duan CQ. Tissue-specific accumulation of flavonoids in grape berries is related to transcriptional expression of VvF3’H and VvF3’5’H. S Afr J Enol Vitic. 2014;35(1): 68–81. [Google Scholar]
- 31.Fischer TC, Halbwirth H, Meisel B, Stich K, Forkmann G. Molecular cloning, substrate specificity of the functionally expressed dihydroflavanol 4-reductases from Malus domestica and Pyrus comunis cultivars and the consequences for flavonoid metabolism. Arch Biochem Biophys. 2003;412: 223–230. [DOI] [PubMed] [Google Scholar]
- 32.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol.1999;299: 152–178. [Google Scholar]
- 33.Thill J, Regos I, Farag MA, Ahmad AF, Kusek J, Castro A, et al. Polyphenol metabolism provides a screening tool for beneficial effects of Onobrychis viciifolia (sainfoin). Phytochemistry. 2012;82: 67–80. doi: 10.1016/j.phytochem.2012.05.030 [DOI] [PubMed] [Google Scholar]
- 34.Slatnar A, Mikulic-Petkovsek M, Halbwirth H, Stampar F, Stich K, Veberic R. Polyphenol metabolism of developing apple skin of a scab resistant and a susceptible apple cultivar. Trees. 2012;26: 109–119. [Google Scholar]
- 35.Sandermann H, Strominger JL. Purification and properties of C55-isoprenoid alcohol phosphokinase from Staphylococcus aureus. J Biol Chem. 1972;247: 5123–5131. [PubMed] [Google Scholar]
- 36.Chang S, Pur Year J, Carney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11: 113–116. [Google Scholar]
- 37.Vashisth T, Johnson LK, Malladi A. An efficient RNA isolation procedure and identification of reference genes for normalization of gene expression in blueberry. Plant Cell Rep. 2011;30(12): 2167–76. doi: 10.1007/s00299-011-1121-z [DOI] [PubMed] [Google Scholar]
- 38.Inostroza-Blancheteau C, Reyes-Díaz M, Arellano A, Latsague M, Acevedo P, Loyola R, et al. Effects of UV-B radiation on anatomical characteristics, phenolic compounds and gene expression of the phenylpropanoid pathway in highbush blueberry leaves. Plant Physiol Biochem. 2014;85: 85–95. doi: 10.1016/j.plaphy.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 39.Albert NW, Lewis DH, Zhang H, Schwinn KE, Jameson PE, Davies KM. Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant J. 2011;65: 771–784. doi: 10.1111/j.1365-313X.2010.04465.x [DOI] [PubMed] [Google Scholar]
- 40.Aharoni A, De Vos CH, Wein M, Sun Z, Greco R, Kroon A, et al. The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J. 2001;28(3): 319–32. [DOI] [PubMed] [Google Scholar]
- 41.Holton TA, Cornish EC. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 1995;7(7): 1071–1083. doi: 10.1105/tpc.7.7.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohno S, Hosokawa M, Kojima M, Kitamura Y, Hoshino A, Tatsuzawa F, et al. Simultaneous post-transcriptional gene silencing of two different chalcone synthase genes resulting in pure white flowers in the octoploid dahlia. Planta. 2011;234(5): 945–958. doi: 10.1007/s00425-011-1456-2 [DOI] [PubMed] [Google Scholar]
- 43.Wang YR, Lu YF, Hao SX, Zhang ML, Zhang J, Tian J, et al. Different coloration patterns between the red- and white-fleshed fruits of malus crabapples. Sci Hort. 2015;194: 26–33. [Google Scholar]
- 44.Davies KM, Schwinn KE, Deroles SC, Manson DG, Lewis DH, Bloor SJ, et al. Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase. Euphytica. 2003;131: 259–268. [Google Scholar]
- 45.Gosch C, Puhl I, Halbwirth H, Schlangen K, Roemmelt S, Andreotti C, et al. Effect of Prohexadione-Ca on various fruit crops: flavonoid composition and substrate specificity of their dihydroflavonol 4-reductases. Eur J Hortic Sci. 2003;68(3): 144–151. [Google Scholar]
- 46.Polashock JJ, Griesbach RJ, Sullivan RF, Vorsa N. Cloning of a cDNA encoding the cranberry dihydroflavonol-4-reductase (DFR) and expression in transgenic tobacco. Plant Sci. 2002;163(2): 241–251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.