Abstract
Campylobacter jejuni and C. coli are important food-borne pathogens that are widespread in animal husbandry. To combat Campylobacter along the food chain, the application of lytic phages has been shown to be a promising tool. Campylobacter phages are currently classified into three groups, of which group II and group III phages are the most common. Members of each group are closely related, whereas the two groups share only little DNA similarity. Moreover, while group III phages are specific for C. jejuni, group II phages additionally infect C. coli. Phage cocktails intended to be used for applications should be composed of various phages that differ in their host range and growth kinetics. The isolation of phages is generally performed by plaque assays. This approach has the limitation that phages are merely identified by their lytic activity on certain indicator strains and that relatively high numbers of phages must be present in a tested sample. Therefore, a more sensitive molecular detection system would be beneficial, which allows a pre-screening of samples and the quick detection and discrimination of group II and group III phages. New phages can then be isolated by use of indicator strains that may be different from those typically applied. On the basis of available Campylobacter phage genome sequences, we developed a multiplex PCR system for group II and group III phages selecting the tail tube gene and the gene for the base plate wedge, respectively, as target. Phages of both groups could be identified with primers deduced from the putative tail fiber gene. Efficient release of phage DNA from capsids was achieved by an extended heat treatment or digestion of phage particles with proteinase K/SDS yielding a detection limit of 1 pfu/ml. Individual detection of group II phages, group III phages and of both groups was studied with artificially contaminated chicken skin. To recover phages that had strongly adhered to the skin, stomaching was the most efficient technique. The developed PCR protocol was employed to detect Campylobacter phages in food and environmental samples. In 50 out of 110 samples group II and/or group III phages were identified.
Introduction
Campylobacteriosis is a worldwide zoonosis and the most frequent bacterial enteritis in the European Union (EU) [1]. Typical symptoms are diarrhea, cramping, abdominal pain, and fever. The disease is mainly caused by the thermophilic species Campylobacter jejuni (C. jejuni) and its close relative C. coli. Human infections generally occur by the consumption of undercooked meats, especially poultry [2]. Campylobacter is a common commensal of the gastrointestinal tract of various mammals and birds [3] and frequently found in chicken farms where the bacteria may spread rapidly [4]. To reduce the number of this pathogen in chicken and on chicken products, phages that have also been used for typing of Campylobacter strains [5–7] might be an appropriate means. Indeed phage administration in the laboratory reduced C. jejuni colonization of the broiler gut and the contamination on chicken skin by several orders of magnitude [1, 3, 4, 8–19]. In addition, a field trial with Campylobacter phages has been successfully carried out in commercial broiler flocks [20, 21].
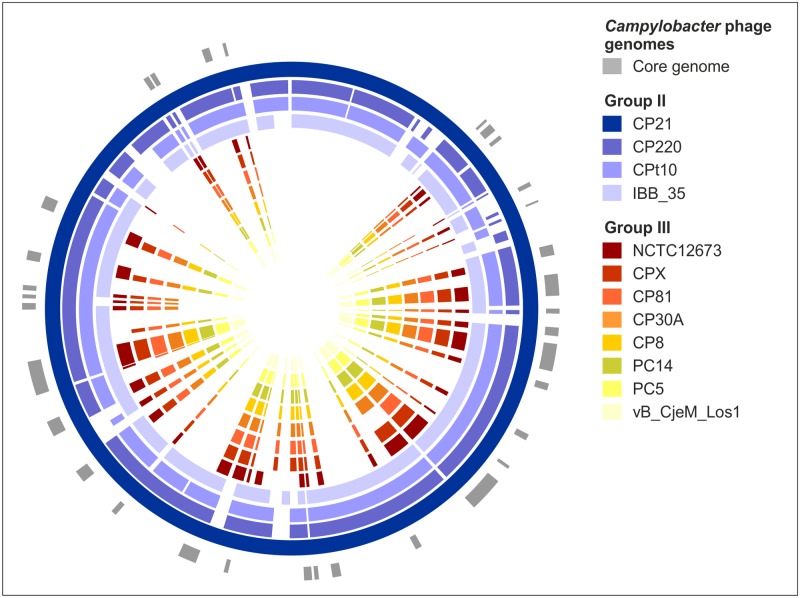
On the basis of their genome size and morphology, the currently known lytic Campylobacter phages are divided into three groups [22]. While group I phages (~320 kb) have been rarely isolated and have not been used for applications yet, members of group II (~175 to 183 kb) and group III (~131 to 135 kb) are very common [22]. Group II phages have generally a broader host range than phages of group III since they frequently infect both C. jejuni and C. coli strains. Though, the successive application of a group III and a group II phage reduced the numbers of C. jejuni in chickens most efficiently [15]. What is common to group II and group III phages are a similar myoviridal morphology, a low burst size, a very low GC content of 26 to 27% and a resistance against cleavage by many restriction endonucleases, probably caused by modification of the bases cytosine or guanine [7, 23, 24]. These properties hampered the quick identification of group II and group III phages and the molecular characterization of these phages for many years. Up to now three group II (CP21, CP220 and CPt10) and eight group III (CP81, CP30A, NCTC12673, PC14, PC5, vB_CjeM_Los1, CPX and CP8) phages have been completely sequenced. The genomic sequence of group II phage vB_CcoM-IBB_35 (IBB_35) has been deposited in the form of five contigs [25]. Phages within each group revealed strong DNA homologies whereas only weak similarities exist between group II and group III phages for which two new genera, the "Cp220likevirus" and "Cp8unalikevirus", respectively, have been proposed [22, 26]. Both groups are distantly related to T4-like phages [22, 23, 27, 28]. They possess a core genome comprising genes for virion assembly and for proteins involved in replication and DNA packaging. A striking feature of group II phages is that their genomes are composed of large modules separated by long DNA repeat regions, which could lead to rearrangements [26–29]. Thus far two subgroups of group II phages exhibiting a different modular genome organization and host range have been identified [27]. Campylobacter phages are generally isolated by plaque assays using indicator strains (e.g. C. jejuni NCTC12662), which are susceptible to a broad range of phages [8, 9, 30, 31]. However, the choice of indicator strains may influence the finding of new phages possibly suitable for applications [32]. Although phages within each group are genetically very similar, they may differ in terms of their host range and lytic activity. Thus, a molecular approach could help to detect even low numbers of group II and group III phages quickly, which can then be isolated by use of common or uncommon indicator strains.
In this study, a multiplex real-time PCR (qPCR) system has been developed for the sensitive detection of group II- and group III-related phages. To achieve this the genomes of the hitherto described Campylobacter phages were searched for the presence of possible target sequences. After examination of all targets by conventional PCR, the best suited sites were selected to design primers and probes for qPCR. Individual detection of group II phages, group III phages and of both groups together was studied with spiked chicken meat. Following this, an optimized protocol was established and used to analyse food and environmental samples for the presence of Campylobacter phages.
Material and methods
Bacterial strains and phages used in this study
All strains were provided by the National Reference Laboratory for Campylobacter of the German Federal Institute for Risk Assessment. Cultivation of Campylobacter spp. was performed on blood agar Base II (Oxoid, Wesel, Germany) supplemented with 5% calf blood [15]. Phages used to design and verify the PCR are listed in Tables 1 and 2.
Table 1. Phage genomes analysed for the presence of possible target sequences.
| Campylobacter phage | Accession number | Description (genome size, origin) | Group | Reference |
|---|---|---|---|---|
| CP8unalikevirus | ||||
| CP81 | FR823450.1 | 132,454 bp, Germany | III | [23] |
| CP30A | JX569801.1 | 133,572 bp, UK | III | [33] |
| NCTC12673 | GU296433.1 | 135,041 bp, UK | III | [34] |
| PC14 | KX236333.1 | 134,927 bp, Slovenia | III | [35] |
| PC5 | KX229736.1 | 131,095 bp, Slovenia | III | [35] |
| vB_CjeM_Los1 | KX879627.1 | 134,073 bp, Ireland | III | [36] |
| CPX | NC_016562.1 | 132,662 bp, UK | III | Unpublished |
| CP8 | KF148616.1 | 132,667 bp, UK | III | [33] |
| CP220likevirus | ||||
| CP21 | HE815464.1 | 182,833 bp, Germany | II | [27, 29] |
| CP220 | FN667788.1 | 177,534 bp, UK | II | [28] |
| CPt10 | FN667789.1 | 175,720 bp, UK | II | [28] |
| vB_CcoM-IBB_351 | HM246720.1 to HM246724.11 | 172,065 bp1, Portugal | II | [25] |
1 The genome of vB_CcoM-IBB_35 (IBB_35) is available in five sequence contigs.
Table 2. Phages used to verify the developed PCR.
| Campylobacter phage | Accession number | Description | Group | Reference |
|---|---|---|---|---|
| CP8unalikevirus | ||||
| CP81 | FR823450.1 | 132,454 bp, Germany | III | [23] |
| CP1 | n.a. | ~135 kb*, Germany | III | [15] |
| CP14 | n.a. | ~135 kb*, Germany | III | [15] |
| F14 | n.a. | ~135 kb*, Denmark | III | [15, 30] |
| CP32 | n.a. | ~135 kb*, UK | III | [15] |
| CP220likevirus | ||||
| CP21 | HE815464.1 | 182,833 bp, Germany | II | [27, 29] |
| CP75 | n.a. | ~185 kb*, UK | II | [27] |
| CP7 | n.a. | ~185 kb*, Germany | II | [27] |
| CP68 | n.a. | ~185 kb*, Germany | II | [27] |
| CP84 | n.a. | ~185 kb*, UK | II | [27] |
| CP83 | n.a. | ~185 kb*, UK | II | [27] |
n.a.: not available;
*: genome sizes were estimated by PFGE analysis of phage DNA.
Isolation, propagation and purification of Campylobacter phages
To detect phage-induced lysis, plaque and spot tests were conducted using the softagar double-layer technique [37]. For the preparation of overlay agar plates, 500 μl of bacterial cultures (OD588 nm 0.9 to 1.4) were added to NZCYM soft agar (Sigma Aldrich, Taufkirchen, Germany) [27]. For phage propagation and activity tests, the following indicator strains were used: C. coli NCTC12668 (CP21, CP68, CP83, CP84), C. jejuni NCTC12662 (CP7, CP75, IBB_35, F325), C. jejuni NCTC11168 (CP1, CP14, CP32, CP81, F14) and C. jejuni RM1221 (F376-F389).
High-titre lysates of phages were obtained as previously described [27] Bacterial DNA and RNA was removed by three consecutive DNaseI (20 μg/ml) and RNaseA (20 μg/ml) treatment steps for at least 4 h at 37°C. The lysates were concentrated by ultracentrifugation and purified by CsCl step gradients [27].
Preparation and quantification of phage DNA for PCR analyses
Phage DNA was prepared as previously described [27]. Determination of the concentration and purity of all phage DNAs was carried out by Qubit analysis according to the manufacturer´s recommendations. Results obtained with the Qubit 2.0 spectrophotometer (Life Technologies, Darmstadt, Germany) were compared with band intensities of phage DNA analysed in ethidium bromide stained 0.8% agarose gels.
Examination of possible target sequences
Conventional PCR analyses were performed in an Eppendorf Mastercycler ep Gradient (Eppendorf, Hamburg, Germany). PCR reactions were prepared in a final volume of 25 μl using the DreamTaq DNA polymerase amplification components (Fisher Scientific, Schwerte, Germany). The mastermix of each reaction comprised 13.35 μl of RNase-free water, 2.5 μl of 10 x DreamTaq Buffer, 2.5 μl of dNTP solution (2 mM), 0.65 μl of DreamTaq Enzyme, 2.5 μl of each primer and 1.0 μl of template DNA (10–20 ng/μl). The following protocol was used: initial PCR activation and template denaturation at 94°C for 120 s followed by 35 cycles comprising a denaturation step at 94°C for 15 s, annealing at 47°C for 15 s and elongation for 60 s at 72°C. In addition, the protocol contained a final elongation step at 72°C for 1 min before the PCR reactions were stored at 4°C until further processing. If necessary, PCR products were purified using the MSB Spin PCRapace kit (Stratec, Birkenfeld, Germany). Commercial Sanger sequencing was conducted by Eurofins Genomics (Ebersberg, Germany).
Real-time PCR detection assay
In general, real-time PCR (qPCR) runs were performed in duplicate using an ABI 7500 Fast real-time PCR System (Applied Biosystems, Darmstadt, Germany). qPCRs were performed using the Qiagen Pathogen Detection Kit including a synthetic internal amplification control (Qiagen, Hilden; Germany). Validation of the qPCR assay was performed by use of both the ABI 7500 Fast real-time PCR System and the CTX96 cycler (Bio-Rad Laboratories GmbH, Munich, Germany). Each individual reaction had a final volume of 20 μl containing 0.2 mM of forward and reverse primers, 0.3 nM of the probe (Table 3), 10 μl of Pathogen Detection Master Mix (Qiagen, Hilden, Germany) and 1 μl of purified phage DNA. The amplification protocol included an initial activation step at 95°C for 20 s followed by 40 cycles of denaturation at 95°C for 15 s and annealing at 60°C for 30 s. Threshold values were automatically generated by the 7500 Fast real-time PCR software and the Bio-Rad-CFX-Manager (v 3.1, Bio-Rad Laboratories Inc.). In each experiment, a non-template control (NTC), negative control (NC) and DNA of group II (CP21) and group III (CP81) Campylobacter phages (positive controls) were included. To determine the sensitivity of the detection assay, a standard curve of CP81 and CP21 phage DNA was created in triplicate for different DNA concentrations. Standard curves were generated by amplifying 1:10 DNA dilution series of 5 x 108 phages. The specificity of the detection assay was determined by examination of all available Campylobacter phages and phages of other host bacteria (E. coli, Vibrio, Klebsiella, Salmonella, Yersinia, MRSA, etc.). For further analyses, phage lysates were used as template for real-time PCR.
Table 3. Primers and probes for the qPCR detection of Campylobacter phages.
| Oligonucleotide | Sequence (5’-3’) | Specificity (target) |
| CPGII-F | TATTTTTGTCACGCTACAAGTTTT | Group II phages (CP21 ORF186) |
| CPGII-R | ACATTTGTTGGAAATACATTCATC | |
| CPGII-probe | FAM-CCGGGATTGACTGTAGAAACA-BHQ-1 | |
| CPGIII-F | TTCAGGGATAAATGAAAACCAAA | Group III phages, (CP81 ORF008) |
| CPGIII-R | AGTTGGCACTGATGAAGAAACC | |
| CPGIII-probe | Cy5-TGTAACTGCCCTGTTTGCTG-BBQ-650 | |
| CPGII/GIII-F1 | TGTAGATCTTTCTAGTGGDAGTAAYG | Group II & III phages (CP21 ORF096 and CP81 ORF002) |
| CPGII/GIII-R1 | ACTATTATTTYCAGAGCTKCCTTTA | |
| CPGII/GIII-probe | YY-TTTGGAACTAGTGCTACAAATCC-BHQ-1 |
Abbreviations: F: forward primer; R: reverse primer; probe: fluorescently labeled primer; FAM: 6-Carboxyfluorescein, BHQ-1: Black Hole Quencher Dye 1, Cy5: Cyanine Dye 5, YY: Yakima Yellow, BBQ-650: BlackBerry Quencher 650, Y: C or T, K: G or T, D: G, A or T.
Testing of the developed qPCR detection system with spiked meat samples
To evaluate the suitability of the detection assay for routine testing, natural contamination of food samples was simulated by spiking of chicken meat purchased at local supermarkets (Berlin, Germany) in January 2015. Before spiking the samples were investigated by qPCR and plaque assays for the presence of Campylobacter phages. Spiking was performed by adding SM-buffer containing the Campylobacter phages CP81 and CP21 (103 to 109 pfu) to the surface of the meat samples (25 cm2). To allow adherence of the phages to the surface of the meat, the samples were incubated for 1 h at 4°C. Meat samples without phages were used as negative controls (NCs). To ascertain by which technique the highest yield of phages added to chicken skin can be recovered, three different sample preparations were compared, (A) swab sampling, (B) homogenization of the skin and (C) flushing of chicken skin pieces (25 cm2). To open the pores of the chicken skin, meat samples were incubated for 30 min at 42°C after adding phage. For swab sampling, swabs were moisturized with 2 ml buffered peptone water. Three consecutive sampling steps were conducted. After each step the swabs were resuspended in 1 ml buffered peptone water. Homogenization was carried out by diluting (1:10) meat samples in buffered peptone water in sterile stomacher bags (BagPage 400 ml; Interscience, St. Nom la Bretèche, France). Stomaching was performed at the highest intensity in a BagMixer 400SW (Interscience) for 5 min. Flushing was conducted by three steps using 10 ml buffered peptone water. Aliquots of suspensions from each procedure were tested in triplicate by qPCR and plaque testing.
Bioinformatic analyses
Sequence alignments and primer design were carried out using the Accelrys DS Gene software package (Accelrys Inc., USA) and primer 3web (v 4.0.0) [38]. Similarity and identity values were calculated using different BLAST algorithms (http://www.ncbi.nlm.nih.gov/BLAST/) at the NCBI homepage. In addition, primers already described in another study [28] have been tested.
Results and discussion
Identification of suitable PCR target sequences on the phage genomes
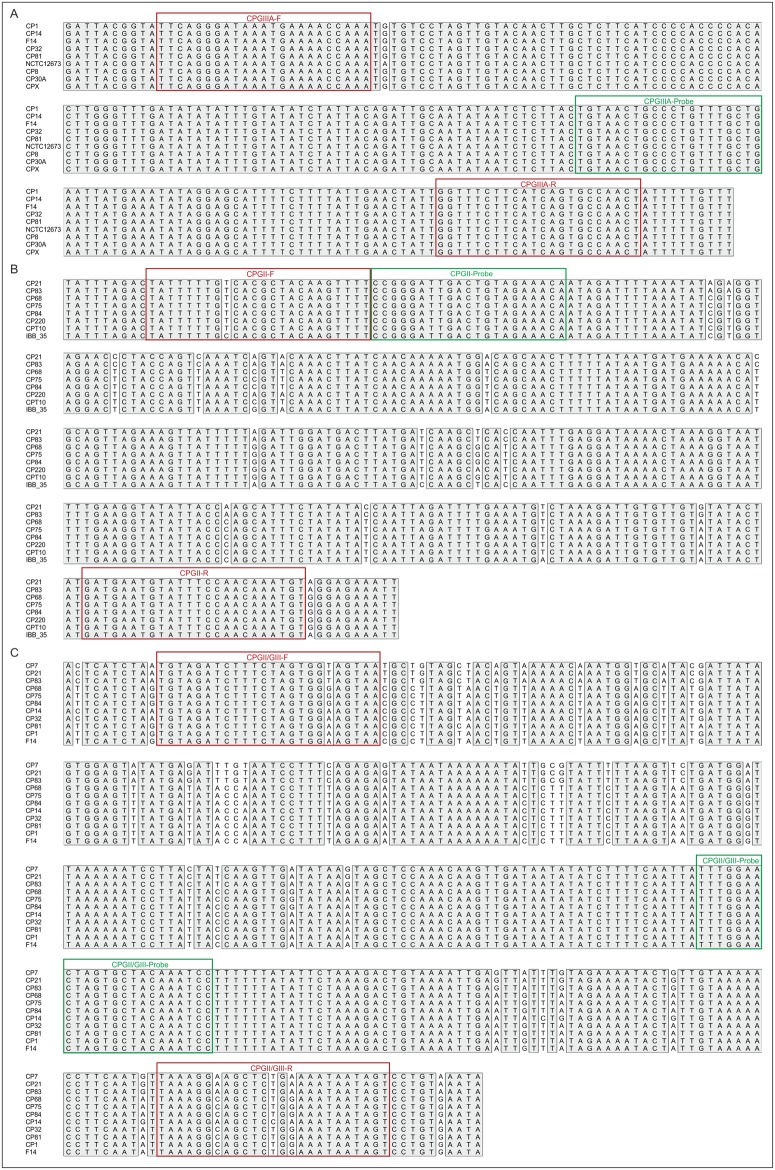
To identify nucleotide sequences that are specific for group II or group III Campylobacter phages as well as sequences existing in both groups, the genomes of four group II and eight group III phages (Table 1) were compared. Group II and group III phages possess a core genome (Fig 1) comprising genes for virion assembly and proteins, which are important for replication and regulation [26, 27]. Focussing on this core genome, conserved DNA sequences were analysed in detail. For the detection of group II phages, twenty-two possible loci were identified, whereas fourteen potential target candidates were found on the genomes of group III phages (S1 Table). Group II phages generally show only weak DNA similarities to phages of group III but one DNA region within the putative tail fiber gene is conserved in these phages. Primers were deduced from all potential target sequences and examined by conventional PCR (amplicon lengths 800 to 1,500 bp) with our whole collection of Campylobacter phages (Table 2). Gel electrophoretic analyses of the amplicons revealed that only few loci were suited to establish a specific PCR detection system for the differentiation of group II and group III phages and for the identification of both groups. Some phages did not show the expected amplicons or the obtained products did not have the predicted sizes. In other cases, several amplicons were produced probably caused by the very low GC content of the phages [23, 25]. Finally, some primers were not specific enough to detect group II or group III phages individually. Nevertheless, for each group, several possible targets were identified. The respective amplicons of all phages were analysed by Sanger sequencing. Unique regions of 150 to 300 bp with an appropriate GC content that were identical or showed only single polymorphisms were used to design primers and probes for real-time PCR. For the detection of group II and group III phages, the tail tube gene (ORF186 of CP21) [29] and the gene for the base plate wedge (ORF008 of CP81) [23], respectively, were selected. Primers (termed “general primers” throughout this publication) derived from the tail fiber gene (ORF096 of CP21 and ORF002 of CP81) were used for the identification of both groups (Figs 1 and 2). The specificity of the primers and probes was confirmed by BLAST searches at the NCBI homepage. All primers and probes were identical or similar to Campylobacter phage sequences, whereas they did not match to any other phage DNA, even if up to three mismatches were tolerated. Similarly, none of the primers and probes matched perfectly to any eukaryotic DNA. Initial experiments performed by conventional PCR with DNA of all Campylobacter phages demonstrated that the designed primers were suited to obtain the predicted amplicons. The same results were achieved with phage lysates and phages recovered from single plaques. It has been reported that Campylobacter phage DNA was refractory to PCR [25]. In this study, DNA of all investigated phages could be amplified using DreamTaq polymerase. We did not test other DNA polymerases, but it might be recommendable to choose the DreamTaq enzyme for the amplification of Campylobacter phage DNA.
Fig 1. Synteny plot based on the amino acid similarity of Campylobacter group II and group III phages.
Fig 2. Alignments of target sequences used for the detection of Campylobacter phages.
Primers and probes for the specific detection of group III phages (A), group II phages (B) and of both groups (C) are boxed by red and green lines, respectively.
Validation of the qPCR and determination of the sensitivity and specificity of the assay
The qPCR assay was validated using lysates of the Campylobacter phages CP21 (group II) and CP81 (group III) of the BfR phage collection. Both phages contain the group-specific target region (Tables 1 and 2). Various non-Campylobacter phages (NCP, e.g. T1 and T4, phages of other genera) were used as negative controls while two non template controls (NTC) did not contain any DNA. For validation, the assay was performed in triplicate using two different real-time cyclers. The obtained Ct-values (cut-off: Ct ≤ 37.0) indicate that in the presence of Campylobacter phages, each of the three primer/probe combinations showed a specific amplification of the respective target sequence with a detection limit of 10 pfu/ml, whereas no amplification was observed with any NCP and the NTC sample. The two real-time cyclers gave similar results (S2 Table). Thus, a reliable detection of Campylobacter phages using this assay was independent from the amplification platform. All group II and group III Campylobacter phages of the BfR phage collection were detectable with this assay.
Testing and optimization of the PCR protocol
By testing a 1:10 dilution series of a group II (CP21) and group III (CP81) lysate containing 5 x 108 pfu/ml a detection limit of ~10 pfu/ml was determined (S2 Table). By introduction of an initial heating step (95°C) for 20 min, we were able to decrease the detection limit further by one order of magnitude suggesting that efficient disintegration of the capsids by heat required an extended incubation time. Similar results were obtained by digestion of phage particles with proteinase K/SDS prior to PCR. Table 4 gives an overview about detection limits of conventional and real-time PCR using the designed primers and probes. It can be seen that real-time PCR was two to three orders of magnitude more sensitive than conventional PCR and that the group II- and group III-specific primers allowed a more sensitive detection than the primer pair designed for both groups. The reason for the latter finding might be wobble bases present in the primers targeting the putative tail fiber gene (Fig 2).
Table 4. Detection limits of Campylobacter phages by conventional PCR and qPCR.
| CPGII detection (Group II-phages) |
CPGIII detection (Group III-phages) |
CPGII/GIII detection (Both phage groups) |
||||
|---|---|---|---|---|---|---|
| PCR | qPCR | PCR | qPCR | PCR | qPCR | |
| CP21 lysate | ||||||
| Untreated control | 104 | 101 | n.d. | n.d. | 104−103 | 102 |
| Heat treatment (20 min, 96°C) | 103 | 100 | n.d. | n.d. | 103 | 101 |
| Proteinase K/SDS treatment | 103 | 100 | n.d. | n.d. | 103 | 101−100 |
| CP81 lysate | ||||||
| Untreated control | n.d. | n.d. | 104−103 | 101 | 105 | 103 |
| Heat treatment (20 min, 96°C) | n.d. | n.d. | 103 | 100 | 104 | 102 |
| Proteinase K/SDS treatment | n.d. | n.d. | 103−102 | 100 | 104 | 102 |
Results are given in pfu/ml.
To test the practicability of the protocol, chicken skin samples were spiked with diluted lysates of group II phage CP21 and group III phage CP81 (see Materials and methods). Upon rinsing of the skin, lytic activity of the phages was quantified by plaque assays. Compared to the phage numbers added to the skin, approximately two orders of magnitude lower titers were determined for recovered phages. Similar results were obtained by PCR indicating that most phages had obviously not been removed from the chicken skin, probably due to a strong adherence to certain surface structures. Since swabbing did not yield more phages, it may be recommendable to stomach chicken skin samples instead of rinsing and swabbing, because this may increase the recovery of phages [8]. In our experiments twice as many phages could be recovered by use of a stomacher. To enhance the sensitivity of the method more significantly, phage particles washed down from skin were concentrated tenfold by centrifugal filter units. By this additional step, the sensitivity could be increased by one log10 unit.
Detection of Campylobacter phages in food and environmental samples
The developed PCR system was applied to detect Campylobacter phages in various meat products and samples (water, dust, faeces) collected from chicken and pig farms (Table 5). Altogether 110 samples were analysed of which 65 samples had previously been tested positive for the presence of Campylobacter. In 50 samples (45.5%) group II and/or group III phages were identified. The highest incidence rates (57%) were found in poultry and in fecal samples from poultry and swine. Group III phages were twice as frequently found as phages belonging to group II. Overall there was a good agreement between results obtained with the group-specific primers and the general primer pair designed to detect both groups. One exception were two chicken samples which were positively tested with the group II primers but not with the general primers. It is conceivable that these samples contained a novel subgroup of group II phages which may differ from the known subgroups in its tail fiber gene. We recently analysed a set of group II phages isolated in Denmark by PCR. Some phages generated products only with the group II-specific primers. Moreover, restriction patterns of these phages diverged from those of the known subgroups suggesting that they may possess a different modular genome organization [27]. Hence, the combination of the group-specific and general primers allows the detection not only of common, but also of uncommon Campylobacter phages which might possess unusual properties and could be suitable candidates for applications.
Table 5. Detection of Campylobacter phages in environmental and food samples by qPCR.
| Sample | No. of samples | CPGII-detection | CPGIII-detection | CPGII/GIII-detection | Negatives |
|---|---|---|---|---|---|
| Meat | 74 (100%) | 14 (19%) | 30 (41%) | 40 (54%) | 32 (43%) |
| Turkey | 19 (100%) | 4 (21%) | 6 (32%) | 10 (53%) | 9 (47%) |
| Chicken | 51 (100%) | 9 (18%) | 22 (43%) | 28 (55%) | 21 (41%) |
| Duck | 4 (100%) | 1 (25%) | 2 (50%) | 2 (50%) | 2 (50%) |
| Entrails | 6 (100%) | n.d. | n.d. | n.d. | n.d |
| Lamb | 2 (100%) | n.d. | n.d. | n.d. | n.d. |
| Pork | 4 (100%) | n.d. | n.d. | n.d. | n.d. |
| Milk | 4 (100%) | 1 (25%) | 2 (50%) | 2 (50%) | 2 (50%) |
| Feces | 7 (100%) | 1 (14%) | 3 (43%) | 4 (57%) | 2 (29%) |
| Pig | 3 (100%) | 1 (33%) | 1 (33%) | 2 (66%) | 1 (33%) |
| Fowl | 3 (100%) | n.d. | 2 (66%) | 2 (66%) | 1 (33%) |
| Wild birds | 1 (100%) | n.d. | n.d. | n.d. | n.d. |
| Environment | 19 (100%) | 2 (11%) | 2 (11%) | 4 (21%) | 15 (79%) |
| Dust & surface | 6 (100%) | n.d. | n.d. | n.d. | 6 (100%) |
| Water | 13 (100%) | 2 (15%) | 2 (15%) | 4 (31%) | 9 (69%) |
| Total | 110 (100%) | 18 (16%) | 37 (34%) | 50 (45%) | 51 (46%) |
n.d., not detected
Two samples (one chicken leg and one fecal sample of swine) gave products with the general primer pair but not with the group II- and group III-specific primers. The reason for this result is yet not clear but it cannot be excluded that the samples contained Campylobacter phages which do not belong to group II or III. The fact that phages exhibiting lytic activity on the used indicator strains could be isolated from only one out of 110 samples suggests that some samples may have contained Campylobacter phages with an uncommon host range or that the number of phages was very low In addition, it has to be taken into account that most samples were stored frozen, which may have had a negative effect on the lytic activity of the phages [8].
Our study showed that the majority (52%) of the 65 Campylobacter-positive samples contained group II or group III phages whereas only 38% of the remaining 45 samples whose Campylobacter content was unknown, were phage-positive. In another study Campylobacter phages were isolated from 34/300 (11%) chilled retail chicken portions by plaque assays. Phages were exclusively recovered from samples that also harbored Campylobacter [8]. These findings demonstrate that on the one hand Campylobacter-specific phages can be readily found at locations where their hosts occur, but that on the other hand the presence of those phages is a good indicator for Campylobacter. The data also show that Campylobacter phages can be detected with much greater sensitivity by PCR than by plaque assays and that the molecular approach can help to identify phages that might possess novel properties, e. g. an uncommon host range. However, it should be emphasized that although the use of two primer/probe sets allows a more reliable detection of Campylobacter phages than a single set, it cannot be ruled out that other yet unknown phages may cause false-positive results. Since both active and inactivated phages are detected by PCR, a combination of this technique with plaque assays is the most promising strategy to identify and isolate new Campylobacter phages.
Conclusions
Phages are a promising tool to reduce Campylobacter along the food chain. Though, some phenotypic and genotypic properties of Campylobacter phages make it rather difficult to deal with them. The multiplex real-time PCR developed in this study may help to overcome some of the problems. First and foremost, the PCR system allows a quick and sensitive detection and discrimination of group II and group III phages. It can be used to pre-screen even large numbers of samples possibly containing Campylobacter phages. Due to the high sensitivity of the PCR system, negative samples are not very likely to harbor phages belonging to one of these groups. On the other hand, PCR-positive samples, which did not show lytic activity on common indicator strains could be tested with other strains that may be suitable hosts for group II or group III phages. The PCR system can also be used in host phage interaction studies, where even low numbers of active and inactive phages can be determined very quickly. Finally, it can be included in metagenomic analyses of phage communities, as it facilitates the detection of Campylobacter phages containing modified DNA.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors thank Barbara Freytag and Andrea Barac for excellent technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was supported by a grant of the Bundesanstalt für Landwirtschaft und Ernährung (BLE project: CAMPYQUANT - Einsatz von Bakteriophagen zur quantitativen Senkung der Campylobacter-Belastung von Masthähnchen; No. 2816100107); http://www.ble.de/EN/00_Home/homepage_node.html). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.ECDC. Annual epidemiological report 2012: Annual epidemiological report reporting on 2010 surveillance data and 2011 epidemic intelligence data. 2013.
- 2.Moore JE, Corcoran D, Dooley JS, Fanning S, Lucey B, Matsuda M, et al. Campylobacter. Vet Res. 2005;36(3):351–82. doi: 10.1051/vetres:2005012 [DOI] [PubMed] [Google Scholar]
- 3.Lee MD, Newell DG. Campylobacter in poultry: filling an ecological niche. Avian Dis. 2006;50(1):1–9. doi: 10.1637/7474-111605R.1 [DOI] [PubMed] [Google Scholar]
- 4.Humphrey TJ, Jorgensen F, Mattick KL. Fit to eat? Food scares and safe food production. Microbiol Today. 2000;27:10–2. [Google Scholar]
- 5.Frost JA, Kramer JM, Gillanders SA. Phage typing of Campylobacter jejuni and Campylobacter coli and its use as an adjunct to serotyping. Epidemiol Infect. 1999;123(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grajewski BA, Kusek JW, Gelfand HM. Development of a bacteriophage typing system for Campylobacter jejuni and Campylobacter coli. J Clin Microbiol. 1985;22(1):13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sails AD, Wareing DR, Bolton FJ, Fox AJ, Curry A. Characterisation of 16 Campylobacter jejuni and C. coli typing bacteriophages. J Med Microbiol. 1998;47(2):123–8. doi: 10.1099/00222615-47-2-123 [DOI] [PubMed] [Google Scholar]
- 8.Atterbury RJ, Connerton PL, Dodd CE, Rees CE, Connerton IF. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl Environ Microbiol. 2003;69(10):6302–6. doi: 10.1128/AEM.69.10.6302-6306.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho CM, Gannon BW, Halfhide DE, Santos SB, Hayes CM, Roe JM, et al. The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC Microbiol. 2010;10:232 doi: 10.1186/1471-2180-10-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connerton PL, Timms AR, Connerton IF. Campylobacter bacteriophages and bacteriophage therapy. J Appl Microbiol. 2011;111(2):255–65. doi: 10.1111/j.1365-2672.2011.05012.x [DOI] [PubMed] [Google Scholar]
- 11.El-Shibiny A, Scott A, Timms A, Metawea Y, Connerton P, Connerton I. Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. J Food Prot. 2009;72(4):733–40. [DOI] [PubMed] [Google Scholar]
- 12.Ghareeb K, Awad WA, Mohnl M, Porta R, Biarnes M, Bohm J, et al. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poult Sci. 2012;91(8):1825–32. doi: 10.3382/ps.2012-02168 [DOI] [PubMed] [Google Scholar]
- 13.Gibbens JC, Pascoe SJ, Evans SJ, Davies RH, Sayers AR. A trial of biosecurity as a means to control Campylobacter infection of broiler chickens. Prev Vet Med. 2001;48(2):85–99. [DOI] [PubMed] [Google Scholar]
- 14.Goode D, Allen VM, Barrow PA. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl Environ Microbiol. 2003;69(8):5032–6. doi: 10.1128/AEM.69.8.5032-5036.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammerl JA, Jäckel C, Alter T, Janzcyk P, Stingl K, Knuver MT, et al. Reduction of Campylobacter jejuni in broiler chicken by successive application of group II and group III phages. PLoS One. 2014;9(12):e114785 doi: 10.1371/journal.pone.0114785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hertwig S, Hammerl JA, Appel B, Alter T. Post-harvest application of lytic bacteriophages for biocontrol of foodborne pathogens and spoilage bacteria. Berl Munch Tierarztl Wochenschr. 2013;126(9–10):357–69. [PubMed] [Google Scholar]
- 17.Humphrey T, O’Brien S, Madsen M. Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol. 2007;117(3):237–57. doi: 10.1016/j.ijfoodmicro.2007.01.006 [DOI] [PubMed] [Google Scholar]
- 18.Rosenquist H, Nielsen NL, Sommer HM, Norrung B, Christensen BB. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int J Food Microbiol. 2003;83(1):87–103. [DOI] [PubMed] [Google Scholar]
- 19.Shane SM. Campylobacter infection of commercial poultry. Rev Sci Tech. 2000;19(2):376–95. [DOI] [PubMed] [Google Scholar]
- 20.Fischer S, Kittler S, Klein G, Glunder G. Impact of a single phage and a phage cocktail application in broilers on reduction of Campylobacter jejuni and development of resistance. PLoS One. 2013;8(10):e78543 doi: 10.1371/journal.pone.0078543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kittler S, Fischer S, Abdulmawjood A, Glunder G, Klein G. Effect of bacteriophage application on Campylobacter jejuni loads in commercial broiler flocks. Appl Environ Microbiol. 2013;79(23):7525–33. doi: 10.1128/AEM.02703-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javed MA, Ackermann HW, Azeredo J, Carvalho CM, Connerton I, Evoy S, et al. A suggested classification for two groups of Campylobacter myoviruses. Arch Virol. 2014;159(1):181–90. doi: 10.1007/s00705-013-1788-2 [DOI] [PubMed] [Google Scholar]
- 23.Hammerl JA, Jäckel C, Reetz J, Beck S, Alter T, Lurz R, et al. Campylobacter jejuni group III phage CP81 contains many T4-like genes without belonging to the T4-type phage group: implications for the evolution of T4 phages. J Virol. 2011;85(17):8597–605. doi: 10.1128/JVI.00395-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loc Carrillo CM, Connerton PL, Pearson T, Connerton IF. Free-range layer chickens as a source of Campylobacter bacteriophage. Antonie Van Leeuwenhoek. 2007;92(3):275–84. doi: 10.1007/s10482-007-9156-4 [DOI] [PubMed] [Google Scholar]
- 25.Carvalho CM, Kropinski AM, Lingohr EJ, Santos SB, King J, Azeredo J. The genome and proteome of a Campylobacter coli bacteriophage vB_CcoM-IBB_35 reveal unusual features. Virol J. 2012;9:35 doi: 10.1186/1743-422X-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammerl JA, Jäckel C, Hertwig S. [Genetics of Campylobacter phages]. Berl Munch Tierarztl Wochenschr. 2015;128(3–4):148–54. [PubMed] [Google Scholar]
- 27.Jäckel C, Hammerl JA, Reetz J, Kropinski AM, Hertwig S. Campylobacter group II phage CP21 is the prototype of a new subgroup revealing a distinct modular genome organization and host specificity. BMC Genomics. 2015;16:629 doi: 10.1186/s12864-015-1837-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timms AR, Cambray-Young J, Scott AE, Petty NK, Connerton PL, Clarke L, et al. Evidence for a lineage of virulent bacteriophages that target Campylobacter. BMC Genomics. 2010;11:214 doi: 10.1186/1471-2164-11-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammerl JA, Jäckel C, Reetz J, Hertwig S. The complete genome sequence of bacteriophage CP21 reveals modular shuffling in Campylobacter group II phages. J Virol. 2012;86(16):8896 doi: 10.1128/JVI.01252-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen VM, Rosenquist H, Baggesen DL, Brown S, Christensen BB. Characterization of Campylobacter phages including analysis of host range by selected Campylobacter Penner serotypes. BMC Microbiol. 2007;7:90 doi: 10.1186/1471-2180-7-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loc Carrillo C, Atterbury RJ, el-Shibiny A, Connerton PL, Dillon E, Scott A, et al. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl Environ Microbiol. 2005;71(11):6554–63. doi: 10.1128/AEM.71.11.6554-6563.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gencay YE, Birk T, Sorensen MC, Brondsted L. Methods for isolation, purification, and propagation of bacteriophages of Campylobacter jejuni. Methods Mol Biol. 2017;1512:19–28. doi: 10.1007/978-1-4939-6536-6_3 [DOI] [PubMed] [Google Scholar]
- 33.Siringan P, Connerton PL, Cummings NJ, Connerton IF. Alternative bacteriophage life cycles: the carrier state of Campylobacter jejuni. Open Biol. 2014;4:130200 doi: 10.1098/rsob.130200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kropinski AM, Arutyunov D, Foss M, Cunningham A, Ding W, Singh A, et al. Genome and proteome of Campylobacter jejuni bacteriophage NCTC 12673. Appl Environ Microbiol. 2011;77(23):8265–71. doi: 10.1128/AEM.05562-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janez N, Kokosin A, Zaletel E, Vranac T, Kovac J, Vuckovic D, et al. Identification and characterisation of new Campylobacter group III phages of animal origin. FEMS Microbiol Lett. 2014;359(1):64–71. doi: 10.1111/1574-6968.12556 [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan L, Bolton D, McCauliffe O, Coffey A. Full genome sequencing and characterisation of a Campylobacter jejuni bacteriophage, vB_CjeM_Los1, isolated in the Republic of Ireland. EMBO Viruses of Microbes 2016. [Google Scholar]
- 37.Sambrook JF, Russel DW. Molecular Cloning: A Laboratory Manual (3-Volume Set). 3 ed: Cold Spring Harbor Laboratory Press, ISBN: 978-087969577-4; 2001. [Google Scholar]
- 38.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115 doi: 10.1093/nar/gks596 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.