Abstract
Several nucleoporins in the nuclear pore complex (NPC) have been reported to be involved in abiotic stress responses in plants. However, the molecular mechanism of how NPC regulates abiotic stress responses, especially the expression of stress responsive genes remains poorly understood. From a forward genetics screen using an abiotic stress-responsive luciferase reporter (RD29A-LUC) in the sickle-1 (sic-1) mutant background, we identified a suppressor caused by a mutation in NUCLEOPORIN 85 (NUP85), which exhibited reduced expression of RD29A-LUC in response to ABA and salt stress. Consistently, the ABA and salinity induced expression of several stress responsive genes such as RD29A, COR15A and COR47 was significantly compromised in nup85 mutants and other nucleoporin mutants such as nup160 and hos1. Subsequently, Immunoprecipitation and mass spectrometry analysis revealed that NUP85 is potentially associated with HOS1 and other nucleoporins within the nup107-160 complex, along with several mediator subunits. We further showed that there is a direct physical interaction between MED18 and NUP85. Similar to NUP85 mutations, MED18 mutation was also found to attenuate expression of stress responsive genes. Taken together, we not only revealed the involvement of NUP85 and other nucleoporins in regulating ABA and salt stress responses, but also uncovered a potential relation between NPC and mediator complex in modulating the gene expression in plants.
Author summary
Nuclear pore complex (NPC) mediates the traffic between nucleus and cytoplasm. This work identified NUCLEOPORIN 85 (NUP85) as an important factor for the expression of stress-responsive luciferase reporter gene RD29A-LUC in response to ABA and salt stress from a forward genetics screen. Mutation in NUP85 and other NPC components such as NUP160 and HOS1 resulted in decreased expression of several stress responsive genes such as RD29A, COR15A and COR47. Proteomics data uncovered a list of putative NUP85 associated proteins. Furthermore, NUP85 was demonstrated to interact with MED18, a master transcriptional regulator, to control the expression of stress responsive genes. The study has added a new layer of knowledge about the diverse functions of NPC in abiotic stress responses.
Introduction
Nucleocytoplasmic transport plays vital roles in eukaryotic systems [1,2]. The exchange of macromolecules such as RNAs and proteins is predominantly regulated by highly conserved nuclear pore complexes (NPCs), which consist of multi-nucleoporins (Nups) arranged in distinct sub-complexes [3,4]. Although the composition and functions of NPCs have been extensively characterized in yeast and vertebrate systems, our knowledge on the functions of plant NPCs remains poor.
Recent genetic screens have identified the involvement of several Nups from Arabidopsis and Lotus japonicus (lotus) in a variety of developmental processes, hormone signaling pathways and environment adaptation [3,5,6]. For instance, NUP160 (SUPPRESSOR OF AUXIN RESISTANCE1) and NUP96 (SUPPRESSOR OF AUXIN RESISTANCE3) have been indicated to play a role in auxin signaling as they were identified as suppressors of auxin-resistant 1(axr1) mutants [7]. NUP160 was also shown to be involved in cold stress responses since the knock-out of NUP160 impaired cold-induced expression the CBF3-LUC reporter gene and several endogenous genes; and thus resulted in hypersensitivity to chilling and freezing stresses [8]. Another important NPC component [9], HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES1 (HOS1), which encodes a RING finger E3 ubiquitin ligase, is well-known as a negative regulator in the cold signaling [10]. Cold-responsive genes such as RESPONSIVE TO DESICCATION 29A (RD29A), COLD-REGULATED 47 (COR47), COLD-REGULATED 15A (COR15A) and KIN1 were reported to be induced to higher levels in hos1 mutants than in wild type plants [11,12]. HOS1 was further shown to attenuate cold signaling by ubiquitination and degradation of INDUCER OF CBF EXPRESSION 1 (ICE1), which encodes an important positive transcription factor critical for the cold-induction of C-REPEAT BINDING FACTORs (CBFs) [11–13]. Moreover, the mutation of NUP160, HOS1 and NUP96 caused early flowering phenotypes, indicating that NPCs are also involved in flowering time regulation [5]. Interestingly, only HOS1 was demonstrated to interact with specific chromatin such as the floral repressor FLOWERING LOCUS C (FLC) chromatin to regulate FLC transcription under low temperature [14–16].
Besides their involvement in hormonal and cold stress signaling and flowering time control, some Nups such as NUP160 and Seh1 are also important for disease resistance in Arabidopsis [17,18]. Similarly, mutation of either of NUP85, Seh1 and NUP133 led to defective responses to symbiotic microorganisms in Lotus Japonicus [19,20]. Most recent genetic studies have revealed NUP85 (also termed as SBB1, suppressor of bak1 bkk1-1) as a suppressor for BRI1-ASSOCIATED KINASE 1 (BAK1) and BAK1-LIKE 1 (BKK1)-mediated cell death control [21]. These studies suggested that Nups also participate in plant defense and cell death control. Nevertheless, the biological functions of many Nups remain elusive in plants.
Recently, a proline-rich protein gene, SICKLE-1 (SIC-1), was isolated because sic-1 mutants exhibited enhanced expression of stress-inducible RD29A-LUC reporter in response to abiotic stresses such as cold and salt treatments [22]. To identify new regulatory components involved in the response to abiotic stresses, we performed a forward genetic screen after EMS mutagenesis of the RD29Apro-LUC line in the sic-1 mutant background. NUP85 was identified as its mutation caused significantly reduced expression of RD29A-LUC in response to ABA and salt stress. Moreover, we discovered a list of putative NUP85 interacting proteins by affinity purification followed by mass spectrometry and further validated the interaction between NUP85 and MED18.
Results
The nup85 mutation attenuates proRD29A-LUC reporter gene expression in response to ABA and salt stress
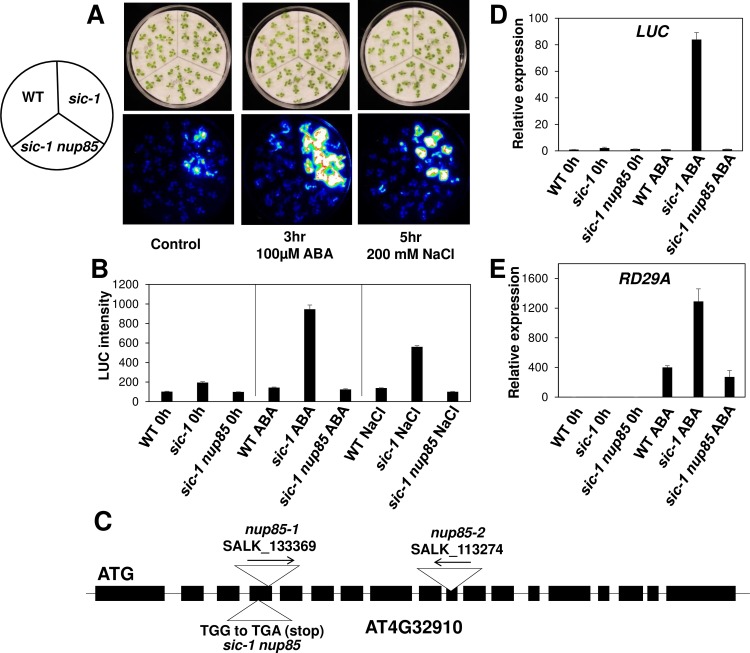
To identify new factors involved in abiotic stress responses, we performed a forward genetics screen using an EMS mutant population generated in the sic-1 mutant background with the stress-inducible proRD29A-LUC reporter gene. Putative mutants with altered luciferase (LUC) activities under abiotic stress treatments such as ABA treatment and salt stress were selected. One suppressor mutant was identified which exhibited reduced bioluminescence in response to ABA and salt stress compared to sic-1 plants (Fig 1A). As revealed in Fig 1A and 1B, the luminescence representing RD29A-LUC expression was highly induced in sic-1 by ABA and salt stress, whereas the luminescence was considerably weaker in the double mutant than sic-1 after either ABA or salt stress treatment. The luminescence intensities of the suppressor mutant were similar to those in the Col gl1 parental line harboring the proRD29A-LUC transgene (referred to as WT), which showed little LUC activity likely due to progressive transgene silencing [22,23]. After genetic mapping and whole genome re-sequencing, we discovered a mutation causing a premature stop codon in the 4th exon in NUP85 (AT4G32910), which encodes a nucleoporin protein (Fig 1C).
Fig 1. Isolation of NUP85 as a suppressor of sic-1 in response to ABA and salt stress.
(A) Luminescence images of wild type, sic-1 and sic-1 nup85 double mutant seedlings under control, ABA and high salt treatments. (B) Quantification of the luminescence intensities of Fig 1A. (C) Diagram of NUP85 genomic sequence and the three nup85 mutants used in this study. The sic-1 nup85 double mutant was caused by a G to A substitution which led to a pre-mature stop codon. (D) The expression of luciferase (LUC) in WT, sic-1 and sic-1 nup85 seedlings under control and ABA treatment. (E) The expression of RD29A in WT, sic-1 and sic-1 nup85 seedlings under control and ABA treatment. Total RNA was extracted from 7-day-old seedlings with mock or 50 μM ABA treatment. The relative transcript levels were normalized to Arabidopsis Actin2 (ACT2) and the normalized expression level of WT at 0 h was set to 1. Data are present as mean value ± SD (n = 3).
Gene expression data show that the LUC expression was highly induced in sic-1 by ABA, but this induction was impaired in the suppressor (i.e. sic-1 nup85) mutant (Fig 1D). The transcript level of endogenous RD29A was induced by ABA treatment in WT, sic-1 and the double mutant. However, the induction was significantly lower in the WT and sic-1 nup85 double mutant compared to that in sic-1 (Fig 1E). We also tested the expressions of other stress-responsive genes and the results showed that ABA-induced expression of COLD-REGULATED 15A (COR15A) and RD29B was lower in sic-1 nup85 double mutants than that in sic-1 mutant plants (S1 Fig).
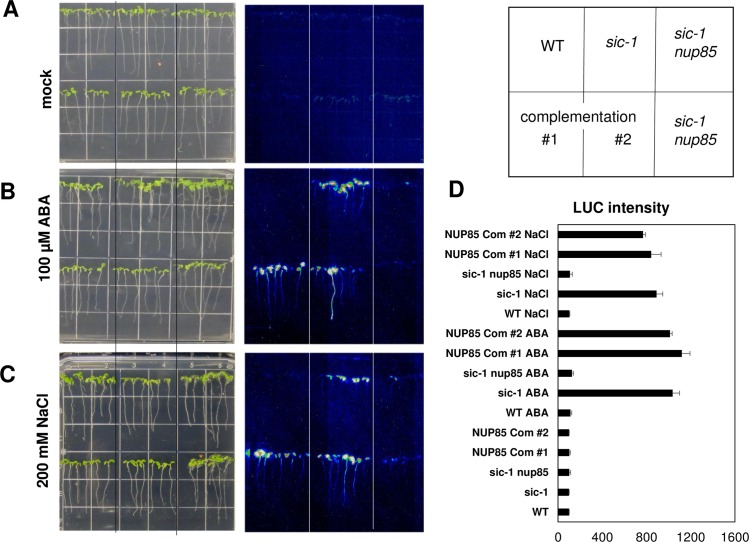
To verify if the suppressor phenotype was caused by the NUP85 mutation, we cloned the genomic sequence of NUP85 with its native promoter and generated two independent complementation lines by transforming sic-1 nup85 double mutants. As shown in S2 Fig, the leaves of sic-1 nup85 double mutant were bigger than sic-1. The wild type NUP85 gene rescued the bigger leave size phenotype of the double mutant. We also examined the expression of RD29A-LUC reporter gene in the complementation lines after ABA and NaCl treatments (Fig 2). The diminished luminescence in sic-1 nup85 double mutant was rescued in the NUP85 complementation lines, which exhibited comparable LUC activity to sic-1. These genetic evidences demonstrated that the NUP85 mutation is responsible for the double mutant phenotypes.
Fig 2. The reduced expression of RD29A-LUC in response to ABA and salt stress was rescued in NUP85 complementation lines.
The Luminescence images of WT, sic-1, sic-1 nup85 double mutant and two independent NUP85 complementation lines with mock treatment (A), 200 mM NaCl treatment (B) and 100 μM ABA treatment (C). At least 20 seedlings of each genotype were treated with 100 μM ABA or 200 mM NaCl. Quantification of the luminescence intensities in A to C is present in (D). Error bars indicate SD. The experiments were conducted three independent times with similar results.
The attenuated expression of stress responsive genes caused by NUP85 mutation
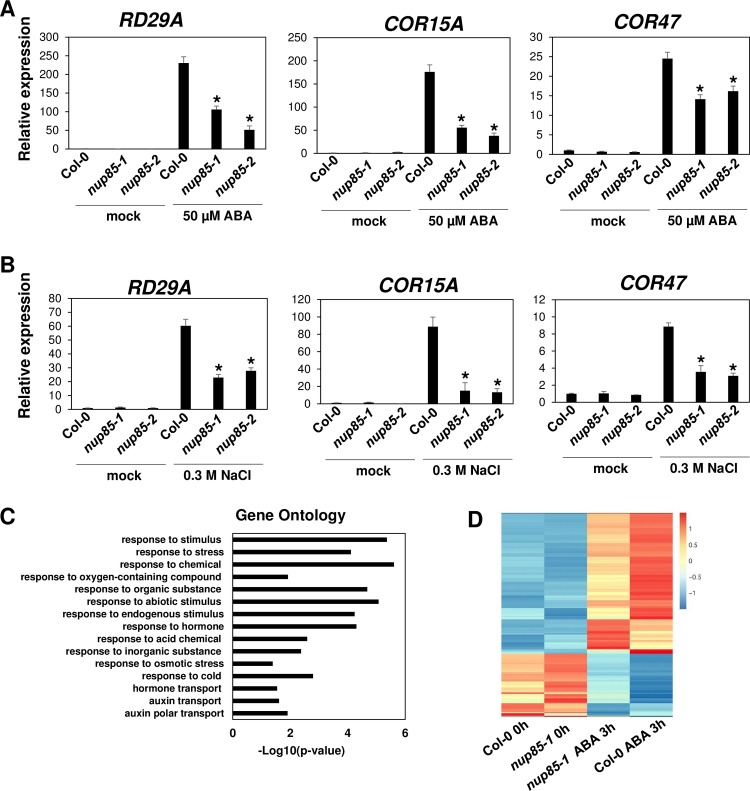
To investigate if NUP85 affects the expression of endogenous stress responsive genes in response to ABA and salt stress, we obtained two independent T-DNA insertion homozygous mutants, nup85-1 (SALK_133369) and nup85-2 (SALK_113274) (Fig 1C). PCR analysis confirmed that the two T-DNA insertion mutant lines are homozygous (S3A Fig). Gene expression data further showed that NUP85 expression was significantly lower in those two mutant lines compared to the Col-0 wild type (S3B Fig). We then treated the Col-0 wild type and two nup85 mutants with 50 μM ABA for 3h, and found that the expression of several stress responsive genes such as RD29A, COR15A and COR47 was significantly lower in the nup85 mutants than that in the wild type after ABA treatment (Fig 3A). In addition to ABA, we also investigated if high salinity induced expression of stress responsive genes was also altered by the NUP85 mutation. As shown in Fig 3B, salt stress induced expression of RD29A, COR15A and COR47 was evidently impaired in the two nup85 mutants after treated with 0.3M NaCl for 5h.
Fig 3. The attenuated expression of stress responsive genes in nup85 mutants in response to ABA and salt stress.
(A) The expression of stress responsive genes RD29A, COR15A and COR47 in Col-0 wild type and two alleles of nup85 mutants under mock and 50 μM ABA treatments for 3 h. The RNA was extracted from 7-day-old seedlings which were treated with mock or 50 μM ABA for 3 h. Data are mean values ±SD of three independent replicates. Asterisks indicate significant differences compared to WT Col under the same treatments. (B) The expression of RD29A, COR15A and COR47 in Col-0 wild type and two mutant alleles of nup85. The RNA was extracted from 7-day-old seedlings which were treated with mock or 0.3 M NaCl for 5 h. Data are mean values ±SD of three independent replicates. Significance between mean values were analyzed by student’s t test (* P< 0.05). Asterisks indicate significant differences compared to WT Col under the same treatments. (C) Gene ontology enrichment analysis of the differentially expressed (DE) genes regulated by NUP85 under mock condition (p value < 0.05). (D) Heat map depiction of the NUP85 regulated ABA-responsive genes in Col-0 wild type and nup85-1 mutants under mock and ABA treatments.
To better understand the genome-wide effects of NUP85, we performed RNA-sequencing experiments. Col-0 wild type and nup85-1 mutant seedlings were treated with mock or 50 μM ABA for 3 hours. Under mock treatment, there were 197 differentially expressed (DE) genes which showed more than 1.5-fold changes in nup85-1 mutant compared to Col-0 wild type plants (S1 Table). Gene ontology (GO) analysis revealed that the DE genes regulated by NUP85 under mock conditions were enriched in categories such as responses to stimulus, response to stress and response to hormone, indicating an important role of NUP85 in plant responses to environmental stresses (Fig 3C). Upon ABA treatment, there were totally 1389 ABA-responsive genes (759 ABA induced genes and 630 ABA repressed genes), whose expressions were significantly induced or repressed more than 4-fold changes by ABA treatments in Col-0 wild type (S2 Table), whereas there were 1357 ABA-responsive genes in nup85-1 mutants after ABA treatments with 982 overlapping genes in Col-0 wild type (S3 Table). Out of 1389 ABA responsive genes in Col-0, 178 ABA responsive genes including RD29B exhibited significantly altered expressions in nup85-1 mutants in comparison with Col-0 wild type, suggesting that about 13% of ABA responsive genes are regulated by NUP85 (S4 Table). GO analysis further revealed that those NUP85 regulated genes were also enriched at categories such as response to stimulus, response to chemical and response to abiotic stimulus (S4 Fig). It is possible that Nups may have redundant function in regulating ABA responsive genes since nup85 single mutants did not alter most ABA-responsive genes. The heat map generated with NUP85 regulated ABA- responsive genes in both Col-0 wild type and nup85-1 mutant showed that the induction of a group of ABA-responsive genes was partially suppressed by the NUP85 mutation (Fig 3D). In contrast, a small portion of DE genes were up-regulated in nup85-1 mutant compared to Col-0 wild type. These results indicate that NUP85 is involved in regulating gene expressions in response to ABA.
The abiotic stress tolerance phenotypes of nup85 and other nucleoporin mutants
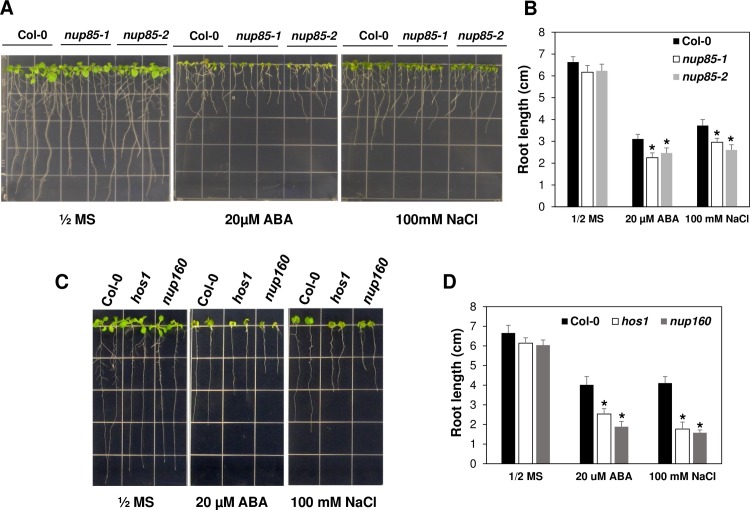
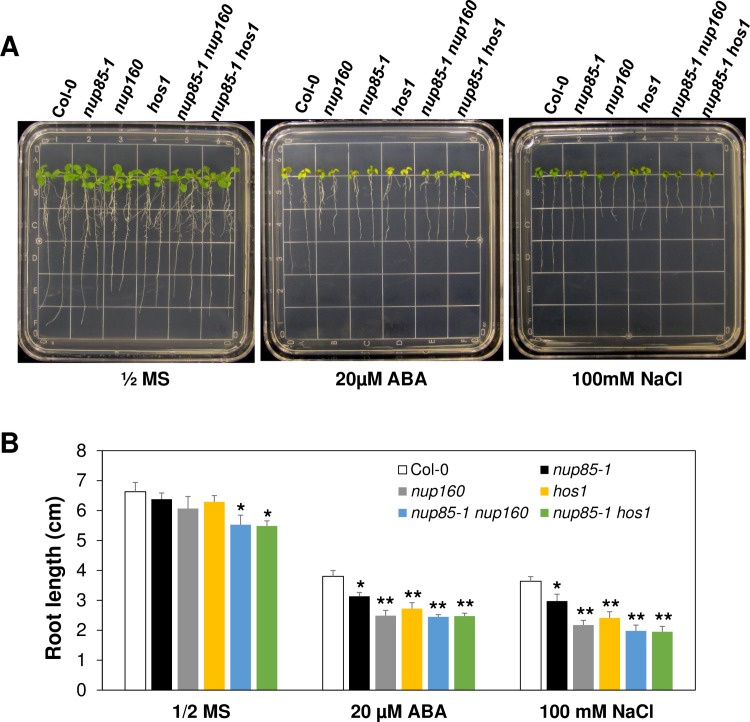
Since we have shown that NUP85 is important for the expression of several stress responsive genes in response to ABA and salt stress, we next examined if nup85 mutants may show altered stress phenotypes to ABA and salt stress. In plants, the NUP107–160 sub-complex consists of eight members, NUP160, NUP133, NUP107, NUP96, NUP85, NUP43, SEH1 and SEC13 [3,21,24]. Thus, we isolated homozygous mutant plants for the other seven nucleoporins and tested their stress phenotypes. As shown in S5 Fig, mutation of NUP43, NUP107, NUP133, NUP96, SEC13 and SEH1 did not cause obvious changes in their responses to ABA and salt stress when compared to Col-0 wild type control plants. The average root length of those mutants was similar to the Col-0 wild type control after being transferred to ABA and NaCl containing medium. Nevertheless, two alleles of nup85 mutants were slightly more sensitive to ABA and salt stress as their root length was shorter than the wild type control after being transferred to ABA- and NaCl-containing medium (Fig 4A and 4B). In contrast, the root length of Col-0 wild type and nup85 mutants was comparable when they were transferred to control medium. In addition to the nup85 mutants, hos1 and nup160 mutants also showed increased sensitivity to ABA and salt stress as their root growth was significantly less compared to the Col-0 wild type after being transferred to the ABA and NaCl containing medium (Fig 4C and 4D). We also examined the responses of nup85-1 nup160 and nup85-1 hos1 double mutants to ABA and salt stress. As illustrated in Fig 5A, nup85-1, nup160, hos1 single mutants and those two double mutants all displayed hypersensitivity to ABA and NaCl because their root length was shorter than Col-0 wild type after being transferred to the indicated ABA and NaCl containing medium. The root length of the double mutants was not evidently shorter than the single mutant (Fig 5B), indicating a lack of additive phenotypes in the double mutant, and thus suggesting that the Nups may function in the same genetic pathway in regulation of the tested stress responses. These genetic results suggest that NUP85, NUP160 and HOS1 are involved in ABA and salt stress responses.
Fig 4. The hypersensitivity of nup85, hos1 and nup160 plants in response to ABA and salt stress.
(A) The phenotypes of Col-0 wild type and two mutant alleles of nup85 in response to ABA and NaCl. (B) Quantification of the primary root length of wild type, nup85-1 and nup85-2 at 7 days after transfer to the indicated media. (C) The ABA and NaCl inhibition of seedling growth of wild type, hos1 and nup160 mutants. (D) Quantification of the primary root length of wild type, hos1 and nup160 at 7 days after transfer to the indicated media. Phenotypes were documented at 7 days after the seedling transfer to ½ MS plates, 20 μM ABA or 100 mM NaCl containing medium. The experiments were repeated at least three independent times with consistent results. Values indicate means ± SD (n = 36). Significance between the mean values were analyzed with Student’s t test (* P< 0.05). Asterisks indicate significant differences compared to WT Col under the same treatments.
Fig 5. The ABA and salt hypersensitivity of nup85-1 nup160 and nup85-1 hos1 double mutants.
(A)The root growth of Col-0 wild type, nup85-1, nup160, hos1 single mutants and nup85-1 nup160 as well as nup85-1 hos1 double mutants on control ½ MS and ABA or NaCl-containing MS medium. (B)Quantification of the primary root length of indicated genotypes at 7 days after transfer to control and ABA or NaCl- containing MS medium. Three-day-old seedlings from each genotype were transferred to the indicated media and primary root length was measured after 7 days. Values indicate means ± SD (n = 24). Asterisks indicate significant differences compared to WT Col under the same treatments. Significance between the mean values were analyzed with Student’s t test (* P< 0.05, ** P< 0.01).
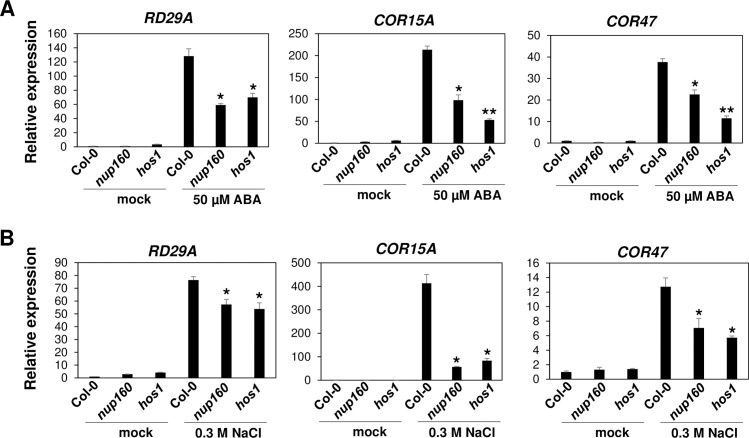
Expression of stress responsive genes is also attenuated in nup160 and hos1 mutants
To investigate if NUP160 and HOS1 affect the expression of stress responsive genes in response to ABA and salt stress, we treated Col-0 wild type, nup160 and hos1 mutants with mock, 50 μM ABA or 0.3 M NaCl. The results showed that ABA and salt stress induced expression of RD29A, COR15A and COR47 was expressively reduced in nup160 and hos1 mutants after ABA and salt treatment when compared to the Col-0 wild type (Fig 6A and 6B). Additionally, we also tested the expression of RD29B in the Col-0 wild type and those mutants. We found that the expression of RD29B was significantly inhibited in nup160, hos1 and nup85-1 mutants compared to Col-0 wild type (S6 Fig). These results suggest that HOS1 and NUP160 are also required for proper expression of stress responsive genes.
Fig 6. The impaired expression of stress responsive genes in nup160 and hos1 mutants in response to ABA and salt stress.
(A)The expression of stress responsive genes RD29A, COR15A and COR47 in Col-0 wild type, nup160 and hos1 mutants under mock and ABA treatment. (B)The expression of RD29A, COR15A and COR47 in Col-0 wild type, nup160 and hos1 mutants under mock and salt treatments. RNA was extracted from 7-day-old seedlings which were treated with mock, 50 μM ABA or 0.3 M NaCl. Data are mean values ±SD of three independent replicates. Significance between mean values were analyzed by student’s t test (* P< 0.05, ** P< 0.01). Asterisks indicate significant differences compared to WT Col under the same treatments.
Identification of putative NUP85 interacting proteins
Recent proteomics studies have revealed more than 22 Nups in plants [24]. To better understand the biological functions of NUP85 and its associating proteins, we performed anti-MYC immunoprecipitation and subsequent mass spectrometry (IP-MS) in WT and Nup85pro: NUP85-MYC transgenic plants. From two independent IP-MS experiments, we totally identified 55 putative NUP85 interacting proteins following two criteria to rule out the nonspecifically interacted proteins (1) proteins are present in the two independent replicates of IP-MS data; (2) proteins that have unique peptides or significantly more peptides (at least 5-fold more) identified in NUP85 transgenic plants compared to WT samples. The full list of putative NUP85 interacting proteins are listed in S5 Table, NUP160, NUP133, NUP43, NUP96, NUP107 and Seh1 as well as Sec13 were present in the NUP85 immuno-complex with abundant unique peptides, confirming the conserved configuration of NUP107-160 sub-complex in plants. HOS1 was also present in the NUP85 immuno-complex with comparable abundance to other Nups in NUP107-160 complex. Besides Nups, some Transducin/WD40 repeat-like superfamily proteins such as Sec13A, which are known to be involved in mRNA and protein transport, were found in the NUP85 immuno-complex. Interestingly, several mediator subunits including MED16, MED14 and MED18 were also precipitated by NUP85, suggesting that possible involvement of NUP85 and other Nups in transcriptional regulation. To validate some of the candidate interacting proteins, we cloned HOS1 and Sec13A, and tested their interactions with NUP85 in split-luciferase (LUC) complementation assays. The results indicated that NUP85 could directly interact with Sec13A but not HOS1 (S7 Fig).
The interaction between NUP85 and MED18
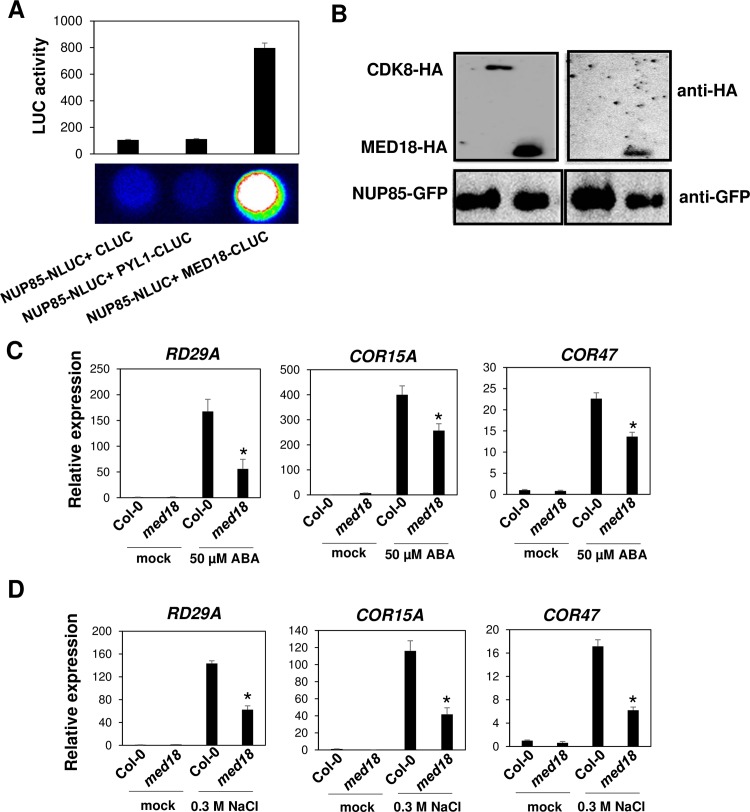
To investigate if there is an interaction between NUP85 and mediator subunits, we selected MED18, which has been reported to be involved in ABA signaling pathway [25,26]. As shown in Fig 7A, the co-expression of MED18-Cluc and NUP85-Nluc resulted in strong LUC activity in the split-LUC complementation assay. In contrast, the co-expression of NUP85-Nluc and empty Cluc or PYL1-Cluc did not cause any detectable LUC activities. Co-immunoprecipitation assay in protoplasts showed that MED18-HA was precipitated by NUP85-GFP (Fig 7B), further confirming the interaction between NUP85 and MED18. In addition, we also investigated the temporal and spatial expression patterns of NUP85 and MED18 in Arabidopsis electronic fluorescent pictograph (eFP) browser. As illustrated in S8 Fig, both NUP85 and MED18 are widely expressed in most of tissues such as seeds, leaves, and stems, especially in shoot apex and flowers with higher expression levels.
Fig 7. The interaction between NUP85 and MED18.
(A) Split luciferase complementation assay in Arabidopsis protoplasts showing the interaction between NUP85 and MED18. Empty Cluc and PYL1-Cluc were used as negative controls. Approximately 1×104 protoplasts per sample were co-transformed with indicated plasmids. The split-LUC complementation assay was repeated three independent times with consistent results. (B) Co-immunoprecipitation assays in Arabidopsis protoplasts confirming the interaction between NUP85 and MED18. CDK8-HA was used as a control. (C) The relative gene expression of RD29A, COR15A and COR47 in wild type and med18 mutants under mock or ABA treatments. (D)The relative gene expression of RD29A, COR15A and COR47 in wild type and med18 mutants under mock or salt treatments (0.3 M NaCl). In C and D, values indicate means ± SD (n = 3). Significance between the mean values were analyzed with Student’s t test (* P< 0.05).
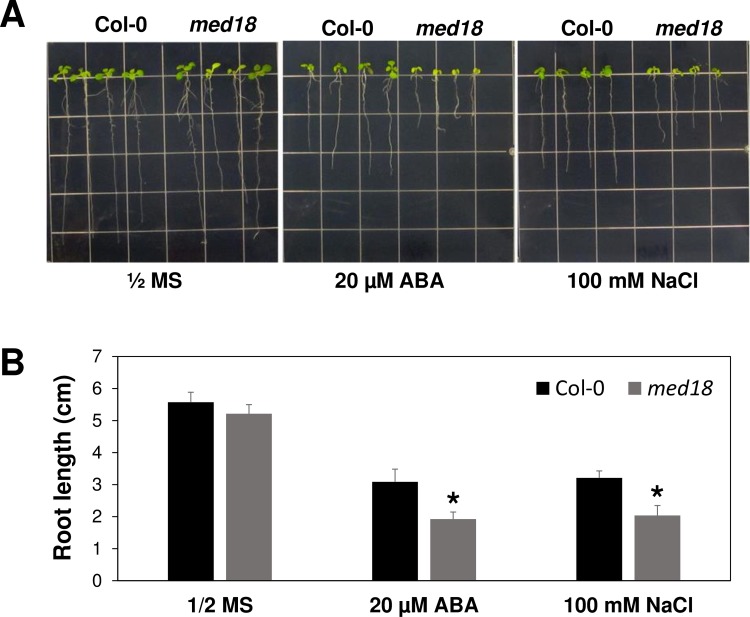
We next examined ABA and salt stress induced gene expression in Col-0 wild type and med18 mutants (Fig 7C). Similar to nup85 mutants, the ABA and salt stress induced expression of RD29A, COR15A and COR47 was also inhibited in med18 mutants relative to the wild type Col-0, signifying overlapping functions of MED18 and NUP85. To further explore the mechanism of how Nups regulate the expression of stress responsive genes, we examined ABI5 expression in wild type Col-0, nup85-1, nup160 and hos1 as well as med18 mutants under mock or ABA treatments. ABI5 is not only one of the MED18 targeted genes [25], but also a key regulator for the expression of stress responsive genes [27]. As illustrated in S9 Fig, ABI5 expression was obviously inhibited in those Nup mutants and med18 mutants after ABA treatments when compared to the wild type Col-0. Consistent with the interaction between NUP85 and MED18, med18 mutants also displayed hypersensitivities to ABA and salt stress because their primary root length was shorter than Col-0 wild type after transferred to MS medium containing indicated concentrations of ABA and NaCl (Fig 8).
Fig 8. The hypersensitivity of med18 in response to ABA and salt stress.
(A) The root growth of Col-0 wild type and med18 mutants on control ½ MS and ABA or NaCl-containing MS medium at 7days after transfer. (B) Quantification of the primary root length of Col-0 wild type and med18 mutants at 7 days after transfer to control and ABA or NaCl- containing MS medium. Three-day-old seedlings were transferred to the ½ MS or ABA/NaCl containing media and primary root length was measured after 7 days. Values indicate means ± SD (n = 20). Asterisks indicate significant differences compared to WT Col under the same treatments. Significance between the mean values were analyzed with Student’s t test (* P< 0.05).
Discussion
Although core factors in abiotic stress signaling and responses have been identified with extensive genetic and biochemical studies in plants [28], abiotic stress responses remain intricate as many other factors yet to be discovered. In our present study, we have identified NUP85 as a regulator of ABA and salt stress responsive genes. The mutation of NUP85 impaired the ABA- and salt stress-induced expression of RD29A and several other stress-responsive genes, suggesting that NUP85 may be a positive regulator for ABA and salt responses. Similarly, mutation of other Nups such as HOS1 and NUP160 also reduced the expression of stress responsive genes, thereby indicating that NUP85 and other Nups components have overlapping functions in regulating the expression of stress responsive genes. Consistent with the altered expression of stress responsive genes, nup85, nup160 and hos1 mutants are hypersensitive to ABA and salt stress. Thus, our results indicate the involvement of Nups in ABA signaling and salt stress responses.
Currently, one of the best characterized NPC components is HOS1, which has been shown to associate with the chromatin of FLOWERING LOCUS C (FLC) and the promoter region of MIR168b, to regulate flowering time and miRNA biogenesis [15,23]. Nevertheless, how other Nups regulate gene expression remains largely unknown. Based on previous and our present studies, NPC components were suggested to play a role in gene expression regulation through various mechanisms. In fact, a number of previous studies have revealed that mutations in NPC components resulted in the accumulation of mRNAs in the nuclei [3,7,8,29,30], which could subsequently affect the expression of genes such as stress responsive genes in plants. Moreover, increasing evidence supported NPC as gene-gating organelles which could recruit actively transcribed genes to them and regulate gene expression [31]. It is likely that transcription of group of stress responsive genes is dependent on certain NPC components in response to different environmental stresses. Our results showed that the expression of RD29A and several stress responsive genes was impaired at the transcriptional level in nup85, hos1 and nup160 mutants, indicating that these NPC components are required for high level transcription of the stress responsive genes in response to ABA and salt stress. Additionally, plant NPC was recently demonstrated to undergo conformational switch in response to pathogen infection, allowing significant activation of nucleocytoplasmic transport and multiple stress-related signaling pathways [32]. The study raised the possibility that NPC components may facilitate the transcription of stress responsive genes in response to various environmental stresses by controlling the transport of critical signaling components and transcriptional regulators between the nuclei and cytoplasm.
In addition to these evidence, proteomics data from our studies further revealed several putative NUP85 interactors, which not only broadened our understanding of NPC functions in plants, but also indicated some potential mechanisms of how NPC components regulate the gene expression. It is worth noting that all the proteins identified from our proteomics data are putative NUP85 interacting proteins until they are validated by further experiments. Our IP-MS results suggested that NUP85 forms a complex with HOS1 and seven other nucleoporins located in the NUP107-160 sub-complex in plants, thus confirming the conserved configuration of NUP107-160 sub-complex in eukaryotic cells. Additionally, some Transducin/WD40-repeat proteins were co-purified with NUP85 including Sec13A, which are involved in assembling NPC domains, signal transduction and mRNA and protein transport [24]. Another interesting discovery from our proteomics data was that several mediator subunits were also present in the NUP85 immuno-complex, providing an alternative way for NUP85 and other Nups to regulate transcription. Recent studies in yeast cells showed that nuclear pore-associated TREX-2 complex directly interacts with mediators to regulate gene expression through the RNA Pol II transcription machinery [33]. However, whether NPC could regulate transcription through the mediator complex remains unknown in plants. Our data suggested the possibility of NUP85 and other NPC components in accessing the core transcriptional machinery through modulating the mediator complex and RNA Pol II association. Among the mediator subunits co-purified by NUP85, MED18 has been known to be involved in ABA signaling, flowering control and plant defense [25,34]. The ABA and salt stress induced expression of several stress responsive genes is significantly attenuated in med18 mutants, which was similarly observed in nup85 mutants. Importantly, we further demonstrated that NUP85 and MED18 are both important for the expression of ABI5, which could activate the expression of downstream stress responsive genes [35]. In summary, our study illustrates that NUP85 and some Nups such as NUP160 and HOS1 regulate stress-responsive gene expression through cooperating with mediator complex. Thus, our findings provide new insights into the role of Nups in ABA signaling and salt responses.
Materials and methods
Plant materials, growth conditions and stress phenotypic analysis
To screen for new regulatory components involved in abiotic stresses, we first performed EMS mutagenesis on sic–1 mutants expressing RD29Apro-LUC as described in [23]. The mutants with significant reduced luminescence in response to 100μM ABA or 200 mM NaCl were selected. The wild type (WT) for LUC assays refers to Col–0 ecotype with the gl–1 mutation harboring the proRD29A-LUC transgene. For abiotic stress phenotypic analysis and gene expression, WT refers to Col-0. The T-DNA insertion mutants nup85-1 (SALK_133369C), nup85-2 (SALK_113274C), nup160 (SALK_126801C), hos1 (SALK_069312C), nup43 (SALK_095344C), nup107 (SALK_057072), nup96 (SALK_135920C), nup133 (SALK_092608C), sec13 (SALK_045825C), seh1 (SALK_022717C) and med18 (SALK_027178C) were in Col-0 background and were obtained from the Arabidopsis Information Resource Center (ABRC). nup85-1 nup160 double mutant was described in [36]. nup85-1 hos1 double mutant was generated by crossing the nup85-1 and hos1 single mutant. The seeds were surface sterilized and sown on half Murashige and Skoog (MS) medium containing 1% sucrose and 0.8% agar. After 2 days in 4°C, the plates were moved to growth chamber under photoperiod of 16 h light/8 h dark. Root growth inhibition assays were performed as described previously [37]. For post-germination root growth assays, 3 or 4-days-old seedlings were first germinated on vertical half MS medium and then were transferred to ABA-(20 μM) or NaCl (100 mM) containing medium and the primary root growth was documented and quantitatively measured at 7 days after transfer.
Generation of transgenic plants
To generate NUP85 complementation lines, the genomic sequence of NUP85 with about 2 kb upstream sequence before its start codon was amplified by high-fidelity DNA polymerase (PrimeSTAR HS DNA Polymerase, Clontech). The PCR product was first inserted into pENTR vector (Invitrogen), and then was transferred to destination vector pGWB-16 (MYC tag) via LR reaction. After verified by sequencing, the construct was introduced into Agrobacterium GV3101 for plant transformation. All indicated constructs were transformed by floral dip method [38]. The homozygous NUP85 complementation lines were used for affinity purification.
Luciferase imaging
Twelve-day-old seedlings of wild-type, sic–1 and sic–1 nup85 were sprayed with distilled water or indicated concentrations of ABA and NaCl solutions for 3h and 5h, respectively. The LUC images were captured using a low-light video imaging system with WinView software.
Map-based cloning and whole genome sequencing
Map-based cloning was performed as described [23]. The F2 mutants with suppressed LUC phenotype were selected by genotyping for sic-1 mutant. The results indicated that the mutation was located in chromosome 4 between 12,980 Kb and 16,500 Kb. Whole genome sequencing was performed, and a mutation was found in At4g32910 locus.
Quantitative real-time PCR
RNA was extracted from 7-day-old seedlings by RNeasy Plant mini kit (QIAGEN) followed by DNase (Turbo) digestion. For testing gene expressions, 1 μg total RNA was used for reverse transcription using M-MLV reverse transcriptase (Promega) according to the manufacturer's instructions. Real-time PCR was performed using iQ SYBR Green Supermix (Bio-Rad) on a CFX96 real-time PCR detection system (Bio-Rad). Actin 2 (ACT2) was used as the internal reference for all reactions. The relative gene expression value was calculated as described previously [39]. All primers were listed in S6 Table.
RNA-sequencing and data analysis
wild type and nup85 mutant seeds were germinated on 1/2 MS medium in growth chamber at 24°C for 7 days and were treated with mock or 3 hr of 50 μM ABA at room temperature. The total RNA was isolated with Trizol reagent (Invitrogen) according to the manufacturer’s instruction and RNA-sequencing were carried out by the Core Facility of Genomics in Shanghai Plant Stress Biology Center, China. Quality control was checked using FastQC (www.bioinformatics.babraham.ac.uk/projects/fastqc). RNA-Seq reads were trimmed using the fastx_trimmer command FASTX-Toolkit (hannonlab.cshl.edu/fastx_toolkit/index.html) with parameter “-f 11 -l 80” before alignment. Trimmed reads were mapped to Arabidopsis reference genome (TAIR10) using TopHat2 using “—b2-very-sensitive” option [40]. Read count for each gene were obtained using featureCounts [41]. Differentially expressed (DE) genes were identified using DESeq2 [42]. NUP85-regulated genes were defined under the criteria: 1) genes were differentially expressed after ABA in Col-0 with 4-fold change; 2) gene expressions in nup85 mutants were at least 1.5-fold higher or lower than those in wild type after ABA treatments. GO enrichment analysis were performed at http://geneontology.org/.
Affinity purification and mass spectrometry
For affinity purification of NUP85 and its associated proteins, about two grams of 12-day-old NUP85pro: NUP85-MYC transgenic seedlings were collected and same amount of WT seedlings was used as a negative control. Total protein extraction and affinity purification were performed as described previously [39]. The protein supernatants were incubated with 30 μL of anti-MYC agarose beads (Abcam), which had been pre-equilibrated with lysis buffer. After incubation at 4°C with rotation for 4 hours, the agarose beads were washed four times with lysis buffer followed by one time wash with 1 mL of PBS buffer. The agarose beads were finally resuspended in 100 μL of PBS buffer. After trypsin digestion, the mass spectrometer was operated in the data-dependent mode in which a full MS scan (from m/z 350–1500 with the resolution of 30,000 at m/z 400), followed by the 10 most intense ions being subjected to collision-induced dissociation (CID) fragmentation. CID fragmentation was performed and acquired in the linear ion trap (normalized collision energy (NCE) 30%, AGC 3e4, max injection time 100 ms, isolation window 3 m/z, and dynamic exclusion 60 s) according to [43]. The raw files were searched directly against the Arabidopsis thaliana database (TAIR10) with no redundant entries using Proteome Discover 2.1. Precursor mass tolerance was set at 10 ppm, and the MS/MS tolerance was set at 0.6 Da. The searches were performed with trypsin digestion and allowed a maximum of two missed cleavages on the peptides analyzed from the sequence database. The false discovery rates for proteins and peptides were set at 0.01, and the minimum peptide length was six amino acids. To identify significantly changed proteins from IP-MS results, we performed two biological replicates. The putative interacting proteins of NUP85 were selected based on two criteria:(1) proteins were identified in both replicates of IP-MS data or (2) proteins that have unique peptides or significantly more peptides in NUP85 transgenic plants than in control samples.
Split Luciferase (LUC) complementation
The full-length coding sequences of NUP85, HOS1, Sec13A and MED18 were amplified by PCR using primers listed in S2 Table. The PCR products were first cloned into pENTER vector (Invitrogen) and then transferred to nLUC/cLUC vectors via LR reactions. Split-LUC complementation assay was performed in Arabidopsis protoplasts per [39]. The reconstitute LUC activity was detected in the dark by cooling camera. The image and quantification of LUC activities were analyzed with Winview software.
Co-Immunoprecipitation assay
The NUP85-GFP and MED18-HA or CDK8-HA were transiently co-expressed in Arabidopsis protoplasts per [39]. After transformation, the protoplasts were collected and suspended in 1 mL lysis buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM DTT, 0.1 (v/v) Triton X-100 and Protease Inhibitor Cocktail (Sigma-Aldrich) in ice for 20 min. The lysis was then centrifuge at 13000 rpm for 15 min at 4°C and was incubated with pre-equilibrant GFP-Trap beads (Chromo Tek) for at least 4 hours with continuous rotation. The beads were washed at least four times with lysis buffer at 4°C and boiled in 4× SDS loading buffer for 10 min. Protein samples were separated by SDS-PAGE and were further detected with polyclonal anti-HA (Abcam) and anti-GFP antibody (Roche).
Supporting information
RT-qPCR analysis showed that the expressions of ABA responsive genes COR15A and RD29B were significantly lower in sic-1 nup85 double mutants when compared to sic-1. Values represent means ± SD (n = 3).
(TIF)
(A) The typical leaves detached from indicated 5-week-old plants grown in soil. (B) The morphology of indicated genotypes grown in soil under normal growth conditions.
(TIF)
(A) genomic DNA PCR showing the homozygous of nup85 mutants. (B) The relative gene expression levels of NUP85 in Col-0 wild type and two lines of nup85 mutants. The RNA was extracted from leaves of 4-week-old plants in soil. Data represent means value ± SD (n = 3). Significance between mean values were analyzed by student’s t test (* P< 0.05). Significance between mean values were analyzed by student’s t test (* P< 0.05). Asterisks indicate significant differences compared to WT Col under the same treatments.
(TIF)
(TIF)
The root length was documented at 7 days after seedlings transfer to ½ MS plates, 20 μM ABA or 100 mM NaCl MS plates At least twelve 3-day-old seedlings from each genotype were transferred and root length was measured after 7 days. The experiments were repeated two independent times. Values indicate means ± SD (n = 24).
(TIF)
Values represent means ± SD (n = 3). Asterisks indicate significant differences compared to WT Col under the same treatments. Significance between mean values were analyzed by student’s t test (* P< 0.05).
(TIF)
Split-LUC complementation assays showing the interactions between NUP85 and Sec13A or HOS1 in Arabidopsis protoplasts. Approximately 1×104 protoplasts per sample were co-transformed with indicated plasmids. The split-LUC complementation assay was repeated three independent times with similar results.
(TIF)
The snapshot of tissue specific expression patterns of MED18 and NUP85 from Arabidopsis eFP Browser.
(TIF)
Values indicate means ± SD (n = 3). Asterisks indicate significant differences compared to WT Col under the same treatments. Significance between the mean values were analyzed with Student’s t test (* P< 0.05).
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Dr. Leelyn Chong for editing manuscript. We also thank Dr. Gaobo Yu, Dr. Youben Yu, Shenyu Zhang, Sydney Clark and Ludia Yori Hong for their technical assistance. nup85 nup160 double mutant seeds were generously provided by Dr. Geraint Parry.
Data Availability
RNA-seq data to NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE99677 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE99677). The secure token is azyhoiqsnzmlpqx.
Funding Statement
This work was supported by the Chinese Academy of Sciences and NIH R01GM059138 to Jian-Kang Zhu. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gorlich D, Kutay U (1999) Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol 15: 607–660. doi: 10.1146/annurev.cellbio.15.1.607 [DOI] [PubMed] [Google Scholar]
- 2.Weis K (2003) Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 112: 441–451. [DOI] [PubMed] [Google Scholar]
- 3.Parry G (2015) The plant nuclear envelope and regulation of gene expression. J Exp Bot 66: 1673–1685. doi: 10.1093/jxb/erv023 [DOI] [PubMed] [Google Scholar]
- 4.Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, et al. (2007) The molecular architecture of the nuclear pore complex. Nature 450: 695–701. doi: 10.1038/nature06405 [DOI] [PubMed] [Google Scholar]
- 5.Xu XM, Meier I (2008) The nuclear pore comes to the fore. Trends Plant Sci 13: 20–27. doi: 10.1016/j.tplants.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 6.Meier I, Brkljacic J (2009) The nuclear pore and plant development. Curr Opin Plant Biol 12: 87–95. doi: 10.1016/j.pbi.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Parry G, Ward S, Cernac A, Dharmasiri S, Estelle M (2006) The Arabidopsis SUPPRESSOR OF AUXIN RESISTANCE proteins are nucleoporins with an important role in hormone signaling and development. Plant Cell 18: 1590–1603. doi: 10.1105/tpc.106.041566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong CH, Hu X, Tang W, Zheng X, Kim YS, et al. (2006) A putative Arabidopsis nucleoporin, AtNUP160, is critical for RNA export and required for plant tolerance to cold stress. Mol Cell Biol 26: 9533–9543. doi: 10.1128/MCB.01063-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacGregor DR, Penfield S (2015) Exploring the pleiotropy of hos1. J Exp Bot 66: 1661–1671. doi: 10.1093/jxb/erv022 [DOI] [PubMed] [Google Scholar]
- 10.Ishitani M, Xiong L, Lee H, Stevenson B, Zhu JK (1998) HOS1, a genetic locus involved in cold-responsive gene expression in arabidopsis. Plant Cell 10: 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H, Xiong L, Gong Z, Ishitani M, Stevenson B, et al. (2001) The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo—cytoplasmic partitioning. Genes Dev 15: 912–924. doi: 10.1101/gad.866801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong CH, Agarwal M, Zhang Y, Xie Q, Zhu JK (2006) The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc Natl Acad Sci U S A 103: 8281–8286. doi: 10.1073/pnas.0602874103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, et al. (2003) ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 17: 1043–1054. doi: 10.1101/gad.1077503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung JH, Park JH, Lee S, To TK, Kim JM, et al. (2013) The cold signaling attenuator HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE1 activates FLOWERING LOCUS C transcription via chromatin remodeling under short-term cold stress in Arabidopsis. Plant Cell 25: 4378–4390. doi: 10.1105/tpc.113.118364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung JH, Seo PJ, Park CM (2012) The E3 ubiquitin ligase HOS1 regulates Arabidopsis flowering by mediating CONSTANS degradation under cold stress. J Biol Chem 287: 43277–43287. doi: 10.1074/jbc.M112.394338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazaro A, Mouriz A, Pineiro M, Jarillo JA (2015) Red Light-Mediated Degradation of CONSTANS by the E3 Ubiquitin Ligase HOS1 Regulates Photoperiodic Flowering in Arabidopsis. Plant Cell 27: 2437–2454. doi: 10.1105/tpc.15.00529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth C, Wiermer M (2012) Nucleoporins Nup160 and Seh1 are required for disease resistance in Arabidopsis. Plant Signal Behav 7: 1212–1214. doi: 10.4161/psb.21426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiermer M, Cheng YT, Imkampe J, Li M, Wang D, et al. (2012) Putative members of the Arabidopsis Nup107-160 nuclear pore sub-complex contribute to pathogen defense. Plant J 70: 796–808. doi: 10.1111/j.1365-313X.2012.04928.x [DOI] [PubMed] [Google Scholar]
- 19.Saito K, Yoshikawa M, Yano K, Miwa H, Uchida H, et al. (2007) NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell 19: 610–624. doi: 10.1105/tpc.106.046938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanamori N, Madsen LH, Radutoiu S, Frantescu M, Quistgaard EM, et al. (2006) A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc Natl Acad Sci U S A 103: 359–364. doi: 10.1073/pnas.0508883103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du J, Gao Y, Zhan Y, Zhang S, Wu Y, et al. (2016) Nucleocytoplasmic trafficking is essential for BAK1- and BKK1-mediated cell-death control. Plant J 85: 520–531. doi: 10.1111/tpj.13125 [DOI] [PubMed] [Google Scholar]
- 22.Zhan X, Wang B, Li H, Liu R, Kalia RK, et al. (2012) Arabidopsis proline-rich protein important for development and abiotic stress tolerance is involved in microRNA biogenesis. Proc Natl Acad Sci U S A 109: 18198–18203. doi: 10.1073/pnas.1216199109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B, Duan CG, Wang X, Hou YJ, Yan J, et al. (2015) HOS1 regulates Argonaute1 by promoting transcription of the microRNA gene MIR168b in Arabidopsis. Plant J 81: 861–870. doi: 10.1111/tpj.12772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K, Fukao Y, Iwamoto M, Haraguchi T, Hara-Nishimura I (2010) Identification and characterization of nuclear pore complex components in Arabidopsis thaliana. Plant Cell 22: 4084–4097. doi: 10.1105/tpc.110.079947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai Z, Schluttenhofer CM, Bhide K, Shreve J, Thimmapuram J, et al. (2014) MED18 interaction with distinct transcription factors regulates multiple plant functions. Nat Commun 5: 3064 doi: 10.1038/ncomms4064 [DOI] [PubMed] [Google Scholar]
- 26.Liao CJ, Lai Z, Lee S, Yun DJ, Mengiste T (2016) Arabidopsis HOOKLESS1 Regulates Responses to Pathogens and Abscisic Acid through Interaction with MED18 and Acetylation of WRKY33 and ABI5 Chromatin. Plant Cell 28: 1662–1681. doi: 10.1105/tpc.16.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelstein RR, Lynch TJ (2000) The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu JK (2016) Abiotic Stress Signaling and Responses in Plants. Cell 167: 313–324. doi: 10.1016/j.cell.2016.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Germain H, Qu N, Cheng YT, Lee E, Huang Y, et al. (2010) MOS11: a new component in the mRNA export pathway. PLoS Genet 6: e1001250 doi: 10.1371/journal.pgen.1001250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chinnusamy V, Gong Z, Zhu JK (2008) Nuclear RNA export and its importance in abiotic stress responses of plants. Curr Top Microbiol Immunol 326: 235–255. [DOI] [PubMed] [Google Scholar]
- 31.Blobel G (1985) Gene gating: a hypothesis. Proc Natl Acad Sci U S A 82: 8527–8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Y, Zebell SG, Liang Z, Wang S, Kang BH, et al. (2016) Nuclear Pore Permeabilization Is a Convergent Signaling Event in Effector-Triggered Immunity. Cell 166: 1526–1538 e1511. doi: 10.1016/j.cell.2016.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider M, Hellerschmied D, Schubert T, Amlacher S, Vinayachandran V, et al. (2015) The Nuclear Pore-Associated TREX-2 Complex Employs Mediator to Regulate Gene Expression. Cell 162: 1016–1028. doi: 10.1016/j.cell.2015.07.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Z, Guan H, Leal F, Grey PH, Oppenheimer DG (2013) Mediator subunit18 controls flowering time and floral organ identity in Arabidopsis. PLoS One 8: e53924 doi: 10.1371/journal.pone.0053924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brocard IM, Lynch TJ, Finkelstein RR (2002) Regulation and role of the Arabidopsis abscisic acid-insensitive 5 gene in abscisic acid, sugar, and stress response. Plant Physiol 129: 1533–1543. doi: 10.1104/pp.005793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parry G (2014) Components of the Arabidopsis nuclear pore complex play multiple diverse roles in control of plant growth. J Exp Bot 65: 6057–6067. doi: 10.1093/jxb/eru346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujii H, Zhu JK (2009) Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc Natl Acad Sci U S A 106: 8380–8385. doi: 10.1073/pnas.0903144106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Y, Schluttenhoffer CM, Wang P, Fu F, Thimmapuram J, et al. (2014) CYCLIN-DEPENDENT KINASE8 differentially regulates plant immunity to fungal pathogens through kinase-dependent and -independent functions in Arabidopsis. Plant Cell 26: 4149–4170. doi: 10.1105/tpc.114.128611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, et al. (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36 doi: 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao Y, Smyth GK, Shi W (2014) featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930. doi: 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- 42.Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550 doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang P, Du Y, Hou YJ, Zhao Y, Hsu CC, et al. (2015) Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc Natl Acad Sci U S A 112: 613–618. doi: 10.1073/pnas.1423481112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RT-qPCR analysis showed that the expressions of ABA responsive genes COR15A and RD29B were significantly lower in sic-1 nup85 double mutants when compared to sic-1. Values represent means ± SD (n = 3).
(TIF)
(A) The typical leaves detached from indicated 5-week-old plants grown in soil. (B) The morphology of indicated genotypes grown in soil under normal growth conditions.
(TIF)
(A) genomic DNA PCR showing the homozygous of nup85 mutants. (B) The relative gene expression levels of NUP85 in Col-0 wild type and two lines of nup85 mutants. The RNA was extracted from leaves of 4-week-old plants in soil. Data represent means value ± SD (n = 3). Significance between mean values were analyzed by student’s t test (* P< 0.05). Significance between mean values were analyzed by student’s t test (* P< 0.05). Asterisks indicate significant differences compared to WT Col under the same treatments.
(TIF)
(TIF)
The root length was documented at 7 days after seedlings transfer to ½ MS plates, 20 μM ABA or 100 mM NaCl MS plates At least twelve 3-day-old seedlings from each genotype were transferred and root length was measured after 7 days. The experiments were repeated two independent times. Values indicate means ± SD (n = 24).
(TIF)
Values represent means ± SD (n = 3). Asterisks indicate significant differences compared to WT Col under the same treatments. Significance between mean values were analyzed by student’s t test (* P< 0.05).
(TIF)
Split-LUC complementation assays showing the interactions between NUP85 and Sec13A or HOS1 in Arabidopsis protoplasts. Approximately 1×104 protoplasts per sample were co-transformed with indicated plasmids. The split-LUC complementation assay was repeated three independent times with similar results.
(TIF)
The snapshot of tissue specific expression patterns of MED18 and NUP85 from Arabidopsis eFP Browser.
(TIF)
Values indicate means ± SD (n = 3). Asterisks indicate significant differences compared to WT Col under the same treatments. Significance between the mean values were analyzed with Student’s t test (* P< 0.05).
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
RNA-seq data to NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE99677 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE99677). The secure token is azyhoiqsnzmlpqx.