Abstract
Restoring T cell mediated anti-tumor immunity by targeting immune checkpoint inhibitors in head neck squamous cell carcinoma (HNSCC) results in immune modulation and durable remissions. However, the overall response rate to these immunotherapies in HNSCC is only ~20%. This raises the possibility that immunologic intervention earlier in the HNSCC continuum, such as in oral premalignant lesions (OPL) could elicit an increased therapeutic response. New experimental studies suggest that immune therapies can be used for HNSCC prevention rather than therapy. Given the current excitement for precision medicine, these findings support the future development of multimodality approaches for preventive immune oncology.
Oral premalignancy and chemoprevention
Every year more than 600,000 cases of head and neck squamous cell carcinomas (HNSCC) are diagnosed worldwide. Preinvasive tissue histologic abnormalities, such as dysplasia, often represent early to intermediate stages of the carcinogenic process during HNSCC progression. At a macroscopic level, these preinvasive changes present as oral premalignant lesions (OPL) - leukoplakia or erythroplakia (white or red patches, respectively) that have variable rates of progression to HNSCC. Unfortunately, there are still no effective therapeutic options to halt the progression of OPL into HNSCC. To address this clinical need, our laboratory has developed a series of chemically-induced oral carcinogenesis models by optimizing the delivery of a tobacco-mimetic, 4NQO, in the drinking water of C57Bl6 mice (1). This model recapitulate HNSCC progression, including the presence of easily recognizable OPL. Using this model system, we have recently shown that the administration of metformin, the most widely used antidiabetic drug, reduces >90% the conversion of OPL into HNSCC (1). This led to a currently open clinical trial evaluating the chemopreventive efficacy of metformin in patients with OPL (NCT02581137)
HNSCC and immunotherapies
Recent breakthrough discoveries have highlighted the importance of the tumor microenvironment and its associated immune cells in cancer development and therapeutic resistance. For example, HNSCCs deploy multiple mechanisms to avoid immune recognition and an anti-tumor immune response, including the recruitment of myeloid-derived suppressor cells (MDSC) and conditioning of the surrounding microenvironment to become highly immune suppressive by expressing cytokines, such as IL6, IL10 and TGFβ, leading to the accumulation of suppressive regulatory T cells (Tregs) and the polarization of macrophages toward an immune suppressive (M2) tumor associated macrophage (TAM) phenotype (2). A key emerging mechanism of tumor immunosuppression involves T cell exhaustion, whereby T cell reactivity is impaired due to activation of T cell checkpoints, including PD-1, by its ligand, PD-L1 that is expressed by macrophages and some HNSCC cells restraining T cell activation. Together, these conditions contribute to the suppression of cytotoxic CD8+ T lymphocytes (CTLs) recruitment, survival, and function, and ultimately to the loss of an effective anti-tumor immune response. Indeed, recent revolutionary therapeutic strategies restoring T cell mediated anti-tumor immunity in HNSCC by targeting immune checkpoint inhibitors, such a PD-L1 and PD-1, demonstrated immune modulation and durable remissions (3,4). This led to the recent approval by the FDA of anti-PD-1 antibodies, nivolumab and pembrolizumab, for HNSCC treatment. However, the overall response rate to these immunotherapies in HNSCC is only ~20% (3,4). Moreover, whether PD-L1/PD-1 acts at the OPL stage to inhibit antitumor immunity is not known.
HNSCC immunoprevention
Unfortunately, we cannot yet predict which HNSCC patients will respond best to these immune oncology (IO) agents, and we still do not know whether immunologic intervention earlier in the HNSCC continuum, such as the premalignant state or early HNSCC lesions, could elicit an increased therapeutic response. In this regard, a study in this issue of Cancer Prevention Research has begun to address the impact of treating mice harboring 4NQO-induced OPL with anti-PD1 inhibitors. They provide exciting evidence that PD1 blockade significantly diminishes the progression of low grade oral SCC lesions into high grade lesions. In this case, the authors have used heterozygous Tp53-/+ mice to accelerate OPL development in response to 4NQO. While this approach may have skewed the mutational landscape of the emerging tumors, this possibility is mitigated by our recent sequencing of multiple 4NQO-induced tumors, which show Tp53 mutations (unpublished observations) that may have been accelerated by the genetic deletion of one Tp53 allele. They also observed a trend toward a decrease in the conversion of OPL into HNSCC, which may need to be confirmed in larger groups of animals to achieve statistical significance.
The authors found that anti-PD1 treatment increased the number of CD4+, CD8+, and FOXP3+ T cells in low grade lesions. Although the effect is slight, giving rise to on average a two-fold increase in these populations, the findings were significant and certainly were correlated with prevention of cancer progression. The finding that FOX3+ cells were also increased suggests that the treatment overall increased infiltration of all types of T cells. It would be interesting to define the CD8+:FOXP3+ ratio, as this has been well documented to correlate with productive antitumor immunity. This increase in T cell infiltration correlated with increased production of known antitumor effector molecules, including IFNγ and granzyme B, but not OX40 and 41BB, suggesting that CD8+ cytotoxic activity was increased to a larger extent than conventional CD4+ activity. These correlative immune signatures will need to be causally linked to the preventive effects of the anti-PD1 therapy in future studies. Also, future studies could examine the role of PD1+ macrophages in this model system, given recent findings that PD1 inhibits macrophage antitumor immune responses. Nevertheless, these findings suggest that controlling the infiltration and activity of T cells into pre-malignant lesions could emerge as effective strategies in the prevention of cancer. These findings provided the rationale for a recently launched HNSCC immune prevention trial (NCT02882282).
Emerging opportunities for mutimodality precision immunoprevention
The authors’ data also suggest that the fight against cancer could begin prior to transformation and could involve a broad swath of patients. In this regard, the current excitement for neoantigen discovery, whether for use in therapeutic vaccination or in the generation of neopeptide specific T cell therapy, represents a ‘holy grail’ in precision immunology. It remains to be seen whether OPL possess enough mutations to warrant a ‘preventive personalized vaccine.’ Even if OPL have antigens, it is not clear that they are heavily infiltrated with immune cells. In this context, it is possible that the use of adjuvants that activate the innate immune system, such as TLR or STING agonists, could initiate immune responses in OPL. For example, TLR7/8 ligands promote the recruitment and activation of innate immune cells and local antigen presenting cells, leading to local accumulation of IFNγ and induction of long lasting adaptive immune responses against tumors (5). STING agonists can induce the production of type I IFNs and initiate self-sustaining immune responses (6). When considering the use of innate immune agonists in pre-malignancy, it will be important to consider the potential paradoxical tumor-promoting effects of innate cell activation. In fact, the activation of innate immune responses without antigen-specific immunity during carcinogenesis may promote an inflammatory milieu that hastens cancer progression (7). In this context, one may speculate that combining anti-PD1 with TLR agonists at a pre-malignant stage may actually provide an optimal antitumor response. The increased accumulation of IFNγ downstream of TLR activation would increase PDL1 expression in the microenvironment, and thus adding anti-PD1 would overcome this suppressive effect.
Another exciting possibility is to combine anti-PD1 therapy with metformin or cyclooxygenase (COX) inhibitors. Besides its impact on OPL cells, metformin suppresses the polarization of pro-tumorigenic M2-like tumor associated macrophages and protects CD8+ tumor-infiltrating lymphocytes from functional exhaustion in the tumor microenvironment (8,9). Classical COX inhibitors, including aspirin, have been recently shown to override the tumor suppressive effect of the inflammatory mediator, PGE2, thereby synergizing with anti-PD1 blockade in inducing the eradication of tumors (10). This raises the exciting possibility of using metformin and/or COX inhibitors to boost the activity of immune checkpoint inhibitors, and to enhance the efficacy of anticancer prophylactic vaccines.
In summary, the authors findings could be interpreted to represent the innovative use of immune therapy for prevention rather than therapy. In addition, given the current excitement for precision medicine, these findings provide a much-needed spark of enthusiasm for the development of multimodality approaches for preventive immune oncology.
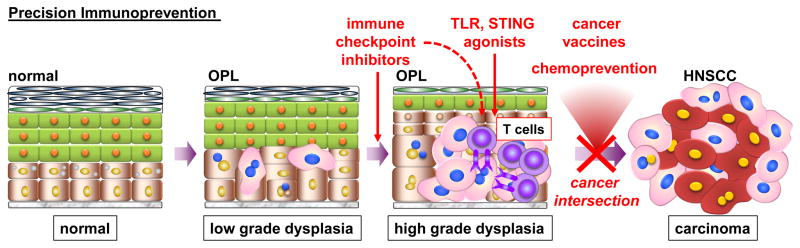
Figure 1.
During the progression of the normal oral epithelium to head and neck squamous cell carcinoma (HNSCC), preinvasive tissue histologic abnormalities, such as low and high grade dysplasia, often represent early to intermediate stages of the carcinogenic process. At a macroscopic level, these preinvasive changes present as oral premalignant lesions (OPL). New findings suggest that immunologic intervention with immune check point inhibitors in OPL can prevent the progression of low grade dysplasia to high grade dysplasia, and likely to HNSCC. Given the current excitement for precision medicine, these findings support the future development of multimodality approaches as part of new preventive immune oncology strategies to halt HNSCC progression. These include the use of immune check point inhibitors, cancer vaccines and adjuvants that activate the innate immune system, such as TLR or STING agonists, alone and in combination, as well as combined with promising chemopreventive agents that have a beneficial impact in the tumor immune environment, such as metformin and COX inhibitors.
Acknowledgments
The authors thank Dr. Zhiyong Wang and Michael Allevato for helping with graphic used in this article. J.S.G. is supported by a grant from the NIH (DE026644) J.D.B. is supported by grants from The Hartwell Foundation and the NIH (CA157885).
References
- 1.Vitale-Cross L, Molinolo AA, Martin D, Younis RH, Maruyama T, Patel V, et al. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev Res (Phila) 2012;5(4):562–73. doi: 10.1158/1940-6207.CAPR-11-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. J Clin Oncol. 2015;33(29):3293–304. doi: 10.1200/JCO.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehra RSTY, Mahipal A, Weiss J, Berger R, Eder JP, Burtness B, Tahara M, Keam B, Le DT, Muro K, Geva R, Chung HC, Lin CC, Meister A, Hille D, Cheng JD, Chow LQM, Haddad RI. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC): Pooled analyses after long-term follow-up in KEYNOTE-012. J Clin Oncol. 2016;34 (suppl; abstr 6012) [Google Scholar]
- 4.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corrales L, McWhirter SM, Dubensky TW, Jr, Gajewski TF. The host STING pathway at the interface of cancer and immunity. The Journal of clinical investigation. 2016;126(7):2404–11. doi: 10.1172/JCI86892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19(2):203–8. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Ding L, Liang G, Yao Z, Zhang J, Liu R, Chen H, et al. Metformin prevents cancer metastasis by inhibiting M2-like polarization of tumor associated macrophages. Oncotarget. 2015;6(34):36441–55. doi: 10.18632/oncotarget.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(6):1809–14. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zelenay S, van der Veen AG, Bottcher JP, Snelgrove KJ, Rogers N, Acton SE, et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162(6):1257–70. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]