Abstract
Difficulties in decision making are a core impairment in a range of disease states. For instance, both obsessive-compulsive disorder (OCD) and hoarding disorder (HD) are associated with indecisiveness, inefficient planning, and enhanced uncertainty intolerance, even in contexts unrelated to their core symptomology. We examined decision-making patterns in 19 individuals with OCD, 19 individuals with HD, 19 individuals with comorbid OCD and HD, and 57 individuals from the general population, using a well-validated choice task grounded in behavioral economic theory. Our results suggest that difficulties in decision making in individuals with OCD (with or without comorbid HD) are linked to reduced fidelity of value-based decision making (i.e. increase in inconsistent choices). In contrast, we find that performance of individuals with HD on our laboratory task is largely intact. Overall, these results support our hypothesis that decision-making impairments in OCD and HD, which can appear quite similar clinically, have importantly different underpinnings. Systematic investigation of different aspects of decision making, under varying conditions, may shed new light on commonalities between and distinctions among clinical syndromes.
1. Introduction
Individuals with obsessive compulsive disorder (OCD) and hoarding disorder (HD) often report difficulties with decision making, such as indecisiveness, pathological doubt, increased deliberation times, and general avoidance of decisions (Frost and Shows, 1993; Hunink et al., 2014). OCD and HD together impact over 5% of the population, causing great suffering and substantial economic burden (Koran et al., 1996; Tolin et al., 2008; Timpano et al., 2011; Pittenger, 2017). Those with primary hoarding symptoms were formerly diagnosed with OCD. However, recently, primary hoarding symptoms have led to development of HD as a distinct clinical diagnosis (Black and Grant, 2014). Pathological indecisiveness, doubt, and intolerance of uncertainty are often prominent sources of disability in individuals with OCD or HD (Reed, 1985,1985; Rasmussen and Eisen, 1992; Tolin et al., 2003; Taillefer et al., 2016). We employ the Risk & Ambiguity task (Levy et al., 2010), a behavioral task grounded in economic theory, to investigate value-based decision making under uncertainty in individuals with OCD and HD. Specifically, we examine whether the clinically similar abnormalities in decision making seen in OCD and HD relate to similar or to distinct basic sub-processes of decision formation (Rangel et al., 2008).
Converging empirical evidence suggest differences in decision formation during value-based decision making and perceptual decision making (Summerfield and Tsetsos, 2012; Polanía et al., 2014; Dutilh and Rieskamp, 2016). Perceptual decision making entails accumulation of sensory information towards a categorical choice between alternatives (e.g., a melon is bigger than an apple). In studies employing a range of behavioral paradigms, those with OCD tend to act more cautiously during perceptual decision making than healthy individuals (Beads Task: Fear and Healy, 1997; Pélissier and O’Connor, 2002, Random Dot Motion task: Banca et al., 2015; but also note negative results by Jacobsen et al. (2012) and Chamberlain et al. (2007). In contrast, value-based decisions depend on subjective goals (e.g., I like melons more than apples) and are assumed to follow several simple and intuitive rules (Rangel et al., 2008). Decision-makers aim to maximize some subjective measure of expected value across available options, choose one option over another if clearly more valuable, and be largely consistent choices unless the available alternatives are close in subjective value (Neumann and Morgenstern, 1944). Studies of value-based making have also employed a variety of behavioral tasks, but have produced less consistent results. For instance, using the Iowa Gambling task (Bechara et al., 1994), some studies found that individuals with OCD perform worse than controls (Da Rocha et al., 2011; Zhang et al., 2015) while others studies found no between-group differences (Nielen et al., 2002; Lawrence et al., 2006). On the Cambridge Gambling Task (Manes et al., 2002), Dittrich and Johansen (2013) found that individuals with OCD are less likely to choose an objectively more valuable option and take longer to decide, but Chamberlain et al. (2007) found no between-group differences. On the Game of Dice task, some studies (Brand et al., 2002; Admon et al., 2012) found that individuals with OCD are more risk averse than healthy individuals, while others (Zhang et al., 2015) found no difference These inconsistent findings may be attributed to OCD group size and composition, effects of medication, or lack of control for effects of anxiety, depression, and other comorbidities (Kuelz et al., 2004).
In light of largely conflicting evidence, the replication studies that carefully control for potential confounds are critically important. Note, however, that the Cambridge Gambling Task, the Game of Dice task, and the Iowa Gambling task all provide feedback and thus allow participants to learn. Thus, it is not clear whether impaired performance on these tasks should be attributed to value-based choice, feedback evaluation, strategy update, or ability to learn about the decision context. This limits the construct validity of these tasks (Buelow and Suhr, 2009). To characterize abnormal decision-making performance in clinical populations, it is vital to use tasks that avoid such confounds (as in Pushkarskaya et al., 2015, Sip et al., 2016, Aranovich et al., 2017).
In our recent work (Pushkarskaya et al., 2015), we employed the Risk and Ambiguity Task (Levy et al., 2010) to investigate value-based decision making in OCD. We found that individuals with OCD were more likely to make “noisy”, inconsistent choices than participants from the general population, suggesting impairments in basic value-based computations in OCD. Our study found no group differences in how they valued uncertain options whose outcome probabilities were known (risk) but that those with OCD were more likely than controls to avoid uncertain options whose outcome probabilities were imprecisely specified (ambiguity), perhaps reflecting the intolerance of uncertainty commonly reported in OCD.
The aim of the present study was to replicate and extend these previous findings (Pushkarskaya et al., 2015) in a larger sample of unmedicated individuals with OCD, HD, and comorbid OCD and HD (n = 19 in each clinical group), compared with 57 individuals from the general population. Given similarities in clinically observed difficulties in decision making in OCD and HD, we expected to find impaired basic value-based computations and higher levels of uncertainty avoidance in HD. Our alternative hypothesis was that OCD and HD are associated with distinct impairments in basic sub-processes of value-based decision formation, despite similarities in clinically observed difficulties in decision making.
2. Methods
2.1. Participants
All procedures were approved by the Yale University Human Investigation Committee and the Hartford Hospital Institutional Review Board. All participants provided written informed consent, completed a demographic questionnaire (Supplementary materials S.1), and were assessed using the Kaufman Brief Intelligence Test (Kaufman, 1979).
Fifty-seven patients, unmedicated for at least 8 weeks, were recruited through the Yale OCD Research Clinic and the Anxiety Disorders Center at the Institute of Living, Hartford Hospital. Nineteen patients had OCD but not HD symptoms, 19 had HD but not OCD, and 19 had both OCD and HD. Of these, 10 individuals with OCD and 10 individuals with comorbid OCD and HD participated in our prior study (Pushkarskaya et al., 2015). Diagnoses were established by doctoral-level clinicians and confirmed using the Structural Clinical Interview for DSM-IV Disorders (SCID-IV; First et al., 2012) or a structured diagnostic interview for DSM-5 anxiety, mood, and obsessive-compulsive and related disorders (DIAMOND; Tolin et al., 2016).
The three clinical groups did not differ significantly in terms of gender, IQ, income, or education (Table 1). However, they differed significantly on age (p < 0.001), with the HD group significantly older than the OCD group.
Table 1.
Demographics.
| Matched groups
|
Pooled controls, N = 57 | ||||
|---|---|---|---|---|---|
| OCD | OCD/HD | HD | |||
| AGE | Patients, N = 19 | 33.3 ± 2.6 | 40.6 ± 3.0 | 51.3 ± 1.9 | 37.2 ± 2.0 |
| Controls, N = 19 | 29.1 ± 1.9 | 35.3 ± 3.4 | 47.4 ± 3.7 | ||
| p-value | 0.2 | 0.25 | 0.35 | ||
| Male | Patients, N = 19 | 0.37 ± 0.1 | 0.47 ± 0.1 | 0.32 ± 0.1 | 0.40 ± 0.1 |
| Controls, N = 19 | 0.37 ± 0.1 | 0.58 ± 0.1 | 0.37 ± 0.1 | ||
| p-value | 0.5 | 0.53 | 0.74 | ||
| IQ | Patients, N = 19 | 102.4 ± 3.2 | 114.9 ± 2.4 | 111.2 ± 3.7 | 111.8 ± 1.6 |
| Controls, N = 19 | 107.4 ± 2.3 | 110.9 ± 3.4 | 112.3 ± 3.2 | ||
| p-value | 0.13 | 0.17 | 0.83 | ||
| Income | Patients, N = 19 | 3.8 ± 0.5 | 3.0 ± 0.5 | 3.2 ± 0.5 | 4.3 ± 0.3 |
| Controls, N = 19 | 4.6 ± 0.6 | 4.2 ± 0.6 | 4.0 ± 0.5 | ||
| p-value | 0.27 | 0.12 | 0.22 | ||
| Education | Patients, N = 19 | 4.2 ± 0.3 | 4.3 ± 0.2 | 5.0 ± 0.2 | 4.9 ± 0.1 |
| Controls, N = 19 | 4.8 ± 0.2 | 4.8 ± 0.2 | 5.0 ± 0.2 | ||
| p-value | 0.12 | 0.12 | 0.86 | ||
Note: Significance of the between-group difference, p-value, for Age, IQ, Income and Education is based on the one-way ANOVA; significance of the between -group difference, p-value, for Male is based on the Pearson’s chi-squared test (χ2). Age is significantly different across patients groups; F(2,54) = 12.59, p < 0.001. Income is assessed on a 10 point scale (SM S.1.), where “3” = $25,000–34,999; “4” = $35,000–49,999”, and “5” = $50,000–74,999. Education is assessed on a 5-point scale (SM S.1) with “4” = College Graduate, and “5” = Advanced graduate or professional degree.
Severity of OCD was assessed by clinicians using the Yale-Brown Obsessive-Compulsive Scale (Goodman et al., 1989a, Goodman et al., 1989b). Assessment included a question about the degree of indecisiveness, from 0 = “None” to 4 = “Extreme”. Severity of hoarding symptoms was assessed using the Saving Inventory – Revised (SI-R; Frost et al., 2004). Since patients were recruited over 3 years at two different sites, data are not available for all patients on this scale. Severity of depression symptoms was assessed using the Hamilton Depression–17 scale (HAM-D17; Hamilton, 1960). Severity of anxiety was assessed using the Hamilton Anxiety scale (HAM-A; Hamilton, 1959).
Fifty-seven participants from the general population (Controls) were recruited in the New Haven, CT area using flyers. Controls did not self-identify as having a psychiatric illness but were not formally assessed using clinical measures and therefore represent the general population; comparison to such a group of individuals is more conservative than comparison to diagnosis-free healthy controls. Three subgroups of controls (N = 19 each) matched our clinical samples on age, gender, IQ, income, and education (Table 1).
2.2. Task
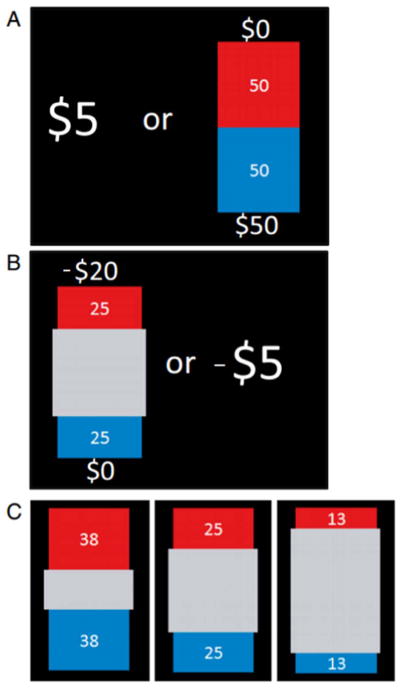
The Risk and Ambiguity Task (R & A; Levy et al., 2010) was developed to study value-based choices under uncertainty (Supplementary materials S.2). Briefly, participants made 320 sequential choices between certain and uncertain gains (presented on a computer screen) by pressing a corresponding button, without feedback but under time constraint (10 s); 160 of these choices were between certain and uncertain gains, and 160 of these choices were between certain and uncertain losses. Choices were grouped into 4 Gain blocks and 4 Loss blocks. Each trial entailed a choice between a certain payoff of $5 or −$5 and a gamble that offered some chance of a positive outcome (between $5 and $125) or negative outcome (between −$5 and −$125), and some chance of a zero outcome. On risky trials, the lottery payoff and outcome probability were known (Fig. 1A). On ambiguous trials, the potential payoff was known, but the outcome probability was imprecisely specified (Fig. 1B & C).
Fig. 1. Experimental design.
On each trial, participants chose between $5 during gain blocks or −$5 during loss blocks and a lottery. Lotteries varied in the amount they offered and in either the winning probability or the level of ambiguity around that probability. The lottery appeared on the screen as a bag containing a total of 100 red and blue plastic chips. The red and blue areas of the bag represented the relative numbers of red and blue chips. The numbers next to these areas represented the sums of money that could be won if a chip of that color were drawn ($5, $8, $20, $50, or $125, −$5, −$8, −$20, −$50, or −$125, depending on the block and the trial; SM, S.5). A: In risky trials, the lottery payoff and outcome probability were precisely specified. The number of chips associated with a winning color was 13, 25, 38, 50, or 75, depending on the trial (SM, S.5). B: In ambiguous trials, part of the bag was hidden by a gray occluder. Thus the number of chips associated with a winning color was uncertain, C: 3 levels of uncertainty were used. The number of chips associated with a winning color belonged to a small (between 38 and 62), medium (between 25 and 75), or wide (between 13 and 87) range, always centered around 0.5 probability (SM, S.5).
At the beginning of the experiment, participants received a $125 endowment; at the end of the experiment, one trial was chosen randomly and played for real money. Additional winnings or losses were added to or subtracted from the initial endowment to determine compensation for participating. Before beginning, participants answered a series of questions designed to assess how well they understood the task (Supplementary materials S.2.3) and had a chance to practice the task for no payment. Only after participants answered all questions and felt comfortable with the task did they proceed to the experiment.
2.3. Data analysis
Most statistical analyses were performed using SPSS v.21. Nonparametric ANOVAs were performed using R 3.3.1 (command “t2way”). All variables were tested for normality using the Shapiro-Wilk test. The primary analyses were of the pooled sample of 57 patients and the pooled sample of 57 controls, using a 2 × 2 ANOVA for normally distributed variables and a 2 × 2 nonparametric ANOVA for variables that were not normally distributed; the between factors were presence of clinically significant OCD and HD symptoms. Recall that OCD and HD differed significantly in age, which could potentially affect the results of the ANOVA-based analyses. Including age as a covariate in ANOVA-based analyses may also bias the results (Miller and Chapman, 2001,2001; Field, 2013). Thus, we also conducted secondary analyses that contrasted behaviors of each clinical group, individually, with those of the age-matched subgroup of 19 Controls: 1-way ANOVA for normally distributed variables, and Mann-Whitney U test for variables that were not normally distributed. Behavior under gains and under losses was analyzed separately.
2.3.1. Response time
For each participant we calculated the average of log-transformed response time (van der Linden, 2006), separately for risky and for ambiguous trials and gain and loss blocks, excluding omissions. We also calculated how often participants failed to make a choice within the allotted 10 s.
2.3.2. Choice-based measures of interest
As in our previous work (Pushkarskaya et al., 2015), we calculated five measures that describe behavior in the R & A task: three measures of fidelity of value-based decision making and two measures of uncertainty attitudes, using data from gain blocks and loss blocks separately. These are described in detail in the Supplementary materials (S.3).
Briefly, the three measures of fidelity of value-based decision making were:
The choice of an uncertain $5 payoff in preference to a certain $5 payoff, or of a certain −$5 in preference to an uncertain −$5; such choices are always contrary to value-guided decision making.
Inconsistency when the same choice was repeatedly presented (SM S.4). Higher scores on this measure are indicative of greater inconsistency in choices.
The goodness of fit of the behavioral data to a quantitative model (Gilboa and Schmeidler, 1989), measured as R2; higher R2 values imply greater fidelity to a value-based decision-making framework.
Two measures of uncertainty attitudes were risk aversion and ambiguity aversion. A risky decision is one in which the outcome is uncertain, but the probabilities of the various possible outcomes are known. An ambiguous decision is one in which the outcome probabilities are themselves uncertain. Attitudes towards these two aspects of uncertainty have proven to be dissociable in previous studies (Camerer and Weber, 1992; Levy et al., 2010; Tymula et al., 2012, Tymula et al., 2013). A positive score on these measures implies risk (or ambiguity) aversion; a negative score implies risk (or ambiguity) seeking.
3. Results
3.1. Clinical symptoms
OCD and HD symptom severity correlates with self-reported indecisiveness.
Consistent with clinical diagnoses, individuals with OCD scored higher on OCD symptom severity scales (YBOCS: F(1,54) = 73.4, p < 0.001; OCI-R: F(1,32) = 13.8, p = 0.001; DOCs: F(1,42) = 31.7, p < 0.001), while individuals with HD scored higher on HD symptom severity scales (SI-R: F(1,31) = 32.7, p < 0.001). Severity of OCD symptoms did not differ between the OCD and OCD/HD groups (YBOCS: F(1,35) = 0.002, p = 0.96, OCI-R: F(1,16) = 1.1, p = 0.31; DOCs: F(1,26) = 0.05, p = 0.83); severity of HD symptoms did not differ between OCD/HD and HD groups (SI-R: F(1,22) = 2.6, p = 0.12) (Table 2). Individuals with OCD (with and without comorbid HD) scored higher on severity of both depression (Ham-D17: F(1,48) = 4.9, p = 0.03) and anxiety (Ham-A: F(1,46) = 4.9, p = 0.03) than did individuals with HD. An additional exploratory analysis conducted on a subsample of clinical participants revealed that severity of self-reported indecisiveness (a single question “Do you have trouble making decisions about little things that other people might not think twice about? Rated on the scale from 0 = ”None” to 4 = ”Extreme, unable to make any decisions”) correlated positively with severity of OCD (Y-BOCS: N = 39, Spearman’s r = 0.36, p = 0.02) and, at trend level, with severity of HD (SI-R: N = 18, Spearman’s r = 0.41, p = 0.09). This is consistent with the repeatedly reported association of both OCD and HD with self-reported difficulties in decision making (Frost and Shows, 1993,1993; Hunink et al., 2014,2014; Taillefer et al., 2016) and with previous findings showing that OCD and HD are clinically characterized by intolerance of uncertainty (Tolin et al., 2003; Mathes et al., 2017).
Table 2.
Clinical characteristics.
| Indecisiveness subscale of YBOCS
|
YBOCS
|
SI-R | OCI-R | DOCS | Ham -17 | HamAnx | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group mean | N | Group mean | N | Group mean | N | Group mean | N | Group mean | N | Group mean | N | Group mean | N | |
| OCD | 1.1 ± 0.3 | 12 | 26.3 ± 1.2 | 19 | 17.1 ± 11.4 | 9 | 27.6 ± 12.5 | 9 | 26.4 ± 11.4 | 14 | 10.4 ± 1.7 | 16 | 11.9 ± 1.8 | 16 |
| OCD/HD | 1.8 ± 0.3 | 15 | 25.8 ± 2.8 | 18 | 47.0 ± 20.9 | 9 | 36.4 ± 10.5 | 9 | 28.5 ± 9.5 | 14 | 11.7 ± 1.7 | 18 | 13.4 ± 1.8 | 16 |
| HD | 1.1 ± 0.4 | 12 | 5.2 ± 2.4 | 18 | 53.3 ± 12.9 | 15 | 15.8 ± 5.1 | 16 | 8.3 ± 6.5 | 16 | 6.5 ± 1.0 | 16 | 7.0 ± 2.3 | 16 |
Note: Participants from clinical populations were recruited over the period of 3 years at two different sites; consequently, the number of respondents varies across assessment instruments. Mean values that are significantly higher than mean values for other groups at p < 0.01 and p < 0.05 are in bold and in italic respectively. YBOCS – Yale Brown Obsessive Compulsive scales; SI-R – Saving Inventory revised; OCI-R – Obsessive Compulsive Inventory revised; DOCS – Dimensional Obsessive Compulsive scale; Ham-17 – Hamilton Depression scale; HamAnx - Hamilton Anxiety scale.
3.2. Replication of prior findings
We replicated previously observed negative effect of OCD diagnosis on fidelity of value-based decision-making under gains (Pushkarskaya et al., 2015)
20 individuals with OCD included in the current analysis, 10 with and 10 without comorbid HD symptoms, participated in our prior study (Pushkarskaya et al., 2015), which found unaffected risk aversion, enhanced ambiguity aversion, and diminished fidelity of value-based decision making in individuals with OCD. In the current study, 18 new individuals with OCD, 9 with and 9 without comorbid HD, performed the R & A task; we tested whether the results of our prior study replicated in this independent sample, as well as in the pooled sample of 38 participants (Table 3, Supplementary materials S.4). Medium to large effects of OCD diagnosis were seen on two measures of fidelity of value-based decision making, inconsistent choices and model fit, in both the independent and the pooled sample (Table 3). The effect of OCD diagnosis on the third measure of decision fidelity, the frequency of clearly suboptimal choices, remained small to medium in both new and pooled samples.
Table 3.
Replication of prior results.
| Effects of OCD Diagnosis | Statistical significance
|
||||||
|---|---|---|---|---|---|---|---|
|
Pushkarskaya et al. (2015) OCD = 10, OCD/HD = 10 |
Additional subjects OCD = 9, OCD/HD = 9 |
This study OCD = 19, OCD/HD = 19 |
|||||
| Effect size | p-value | Effect size | p-value* | Effect size | p-value | ||
| Risk aversion under gains | No effect | 0.09 | 0.57 | 0.10 | 0.56 | 0.08 | 0.21 |
| Ambiguity aversion under gains | Mixed results | 0.66 | 0.036 | 0.34 | 0.267 | 0.45 | 0.05 |
| Suboptimal choices under gains | More frequent | 0.32 | 0.042 | 0.27 | 0.10 | 0.38 | 0.018 |
| Inconsistent choice under gains | More inconsistent | 0.38 | 0.037 | 0.54 | 0.11 | 0.62 | 0.006 |
| Model fit, R2, under gains | Reduced fit | 0.73 | 0.016 | 0.68 | 0.04 | 0.75 | 0.001 |
Note: Ambiguity aversion, Proportion of inconsistent choices, and Model fit (R2) were distributed normally in all samples; means were compared using 1-way ANOVA, effect size is Cohen’s d. Risk aversion and proportion of suboptimal choices was not normally distributed in our samples; distributions were compared using Mann-Whitney U Test; effect size for Mann-Whitney U test is equal to .
2-tailed p-values are reported, although 1-tailed tests are justified for this replication cohort.
Effects on uncertainty measures were smaller and more variable. The effect of OCD on ambiguity aversion fell from medium to small and was not significant in the independent sample, but remained significant in a pooled sample (F(1,74) = 4.05, p = 0.05, Cohen’s d = 0.45). The effect of OCD on risk aversion remained small and nonsignificant in both independent and pooled samples. This suggests that the relationship between ambiguity aversion and OCD may be linked to only some of the subtypes of OCD and thus its detection may depend on the test group composition. Reduced fidelity of value-based decision making in OCD, on the other hand, appears to be robust across OCD samples.
3.3. Response time
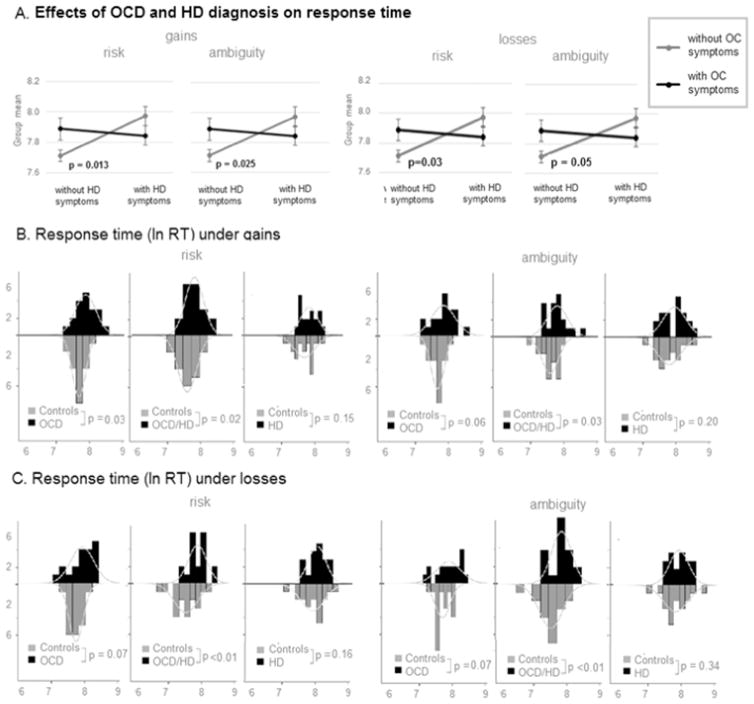
Individuals with OCD (with and without comorbid HD) but not individuals with HD took longer to make choices than nonclinical controls under both gains and losses.
If a response is not submitted within 10 s it is treated as missed; across all participants and conditions, < 1.5% of responses were missed (see Supplementary Materials S.5 for a detailed analyses of missed responses). Excluding missed responses, mean log-transformed response times (ln RT) were normally distributed under both risk and ambiguity in both gain and loss blocks in all clinical groups and in Controls. A 2 × 2 repeated measures ANCOVA (between subjects factors: presence or absence of clinical OCD/HD symptoms; repeated measures: risk and ambiguity conditions) revealed significant interaction between the presence of OCD and HD symptoms on ln RT in both gain (F(1,110) = 6.01, p = 0.016) and loss blocks (F(1,110) = 3.87, p = 0.05). Post hoc contrasts revealed that this interaction was driven by faster responses of Controls during both gain blocks (risk: t(113) = −2.24, p = 0.02, ambiguity: t (113) = −2.03, p = 0.04) and loss blocks (risk: t (113) = −3.36, p = 0.001, ambiguity: t (113) = −2.87, p = 0.005; Fig. 2A, Table 4). The three clinical groups did not differ significantly from one another in response time during both gain blocks (Fig. 2A, Table 4). Recall, however, that individuals with HD were older than individuals with OCD, which could influence these results.
Fig. 2. Response time in individuals with OCD and/or HD and controls.
Effect of diagnosis (OCD and/or HD vs. Controls) on response time and proportion of missed responses under RISK and under Ambiguity and during Gain and Loss blocks separately. Bars: histograms of respective distributions. Curves: empirically approximated normal curves.
Table 4.
Response time during gain and loss blocks, by clinical groups.
| Gain blocks
|
Loss blocks
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean, s | st. dev | Versus age-matched controls
|
Mean, s | st. dev | Versus age-matched controls
|
|||||
| F (1,36) | p-value | Cohen’s d | F (1,36) | p-value | Cohen’s d | |||||
| OCD | 2.7 | 0.30 | 4.67 | 0.037 | 0.64 | 2.7 | 0.36 | 3.69 | 0.06 | 0.6 |
| OCD/HD | 2.7 | 0.32 | 5.69 | 0.022 | 0.73 | 2.7 | 0.30 | 10.99 | 0.002 | 0.94 |
| HD | 3.0 | 0.29 | 1.08 | 0.31 | 0.47 | 3.0 | 0.28 | 0.66 | 0.42 | 0.33 |
Secondary pairwise comparisons (see Fig. 2BC, Table 4) revealed the tendency to make choices more slowly, during both gain and loss blocks, in individuals with OCD (gains: F(1,36) = 4.67, p = 0.037, Cohen’s d = 0.64; losses: F(1,36) = 3.69, p = 0.06, Cohen’s d = 0.60) and individuals with comorbid OCD and HD (gains: F(1,36) = 5.69, p = 0.022, Cohen’s d = 0.73; losses: F(1,36) = 10.99, p = 0.002, Cohen’s d = 0.94), but not in individuals with HD, as compared to age-matched controls. This result was unexpected, given consistent reports that individuals with HD tend to make decisions more slowly (Grisham et al., 2007, 2010; Saxena, 2007).
3.4. Fidelity to subjective value maximization
Fidelity to subjective value maximization was reduced in individuals with OCD (with and without comorbid HD) under gains but not under losses; it was unaffected in individuals with HD.
3.4.1. Inconsistent choices
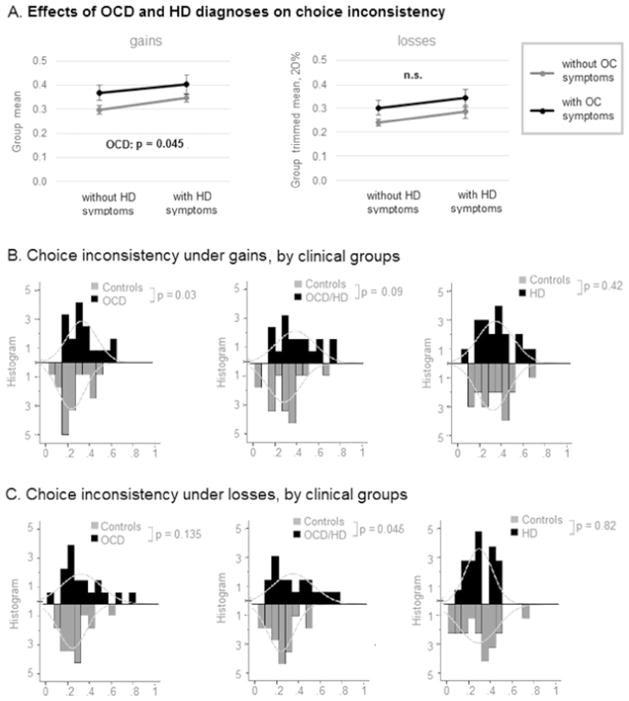
Proportion of inconsistent choices was normally distributed in all clinical groups and controls during gain blocks (Shapiro-Wilk, p > 0.10), but not during loss blocks (Shapiro-Wilk, p = 0.02 for controls). During gain blocks, 2 × 2 ANOVA revealed a significant main effect of OCD on choice inconsistency (F(1,110) = 4.104, p = 0.045, Cohen’s d = 0.37; Fig. 3A). During loss blocks, nonparametric 2 × 2 ANOVA (presence or absence of clinical OCD/HD symptoms) revealed no significant main or interaction effects.
Fig. 3. Proportion of inconsistent choices in individuals with OCD and/or HD and controls.
Effect of diagnosis (OCD and/or HD vs. Controls) on proportion of inconsistent choices during gain blocks and loss blocks. Bars: histograms of respective distributions. Curves: empirically approximated normal curves. Since proportion of inconsistent choices under losses was not normally distribute, instead of group mean the corresponding figure presents the group trimmed mean at 20% - a statistical measure of central tendency that involves the calculation of the mean after discarding 10% of a sample at both the high and low end.
Secondary pairwise comparisons (see Fig. 3BC, Table 5) confirmed a medium to large effect of OCD on frequency of inconsistent choices during gain blocks: in our sample, they were significantly more common among individuals with OCD (F(1,36) = 5.08, p = 0.03, Cohen’s d = 0.69) and at a trend level were more common among individuals with comorbid OCD and HD (F(1,36) = 3.02, p = 0.09, Cohen’s d = 0.55). No difference was observed in proportion of inconsistent choices between individuals with HD and matched on age controls. During loss blocks, the medium to large effect of OCD on frequency of inconsistent choices was significant only in individuals with OCD and comorbid HD (F(1,36) = 4.31, p = 0.045, Cohen’s d = 0.64), but not in individuals with OCD. No difference was observed in proportion of inconsistent choices between individuals with HD and matched on age controls.
Table 5.
Fidelity of value-based choice, by clinical groups.
| OCD
|
OCD/HD
|
HD
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean diff. | Test statistics | Effect size | p-value | Mean diff. | Test statistics | Effect size | p-value | Mean diff. | Test statistics | Effect size | p-value | |
| Gains | ||||||||||||
| Suboptimal choices | 0.12 | U = 143.5 | 0.17 | 0.28 | 0.12 | U = 101.5 | 0.37 | 0.02 | −0.03 | U = 164.5 | 0.07 | 0.65 |
| Inconsistent choices | 0.10 | F(1,36) = 5.08 | 0.69 | 0.03 | 0.09 | F(1,36) = 3.02 | 0.55 | 0.09 | 0.04 | F(1,36) = 0.67 | 0.26 | 0.42 |
| Model fit, R2 | −0.15 | U = 97.5 | 0.39 | 0.02 | −0.15 | F(1,36) = 5.96 | 0.76 | 0.02 | 0.03 | F(1,36) = 0.02 | 0.05 | 0.88 |
| Losses | ||||||||||||
| Suboptimal choices | 0.08 | U = 133.5 | 0.22 | 0.17 | 0.02 | U = 169 | 0.05 | 0.75 | 0.00 | U = 140 | 0.19 | 0.24 |
| Inconsistent choices | 0.08 | F(1,36) = 2.34 | 0.49 | 0.14 | 0.11 | F(1,36) = 4.31 | 0.64 | 0.05 | 0.01 | F(1,36) = 0.05 | 0.07 | 0.82 |
| Model fit, R2 | −0.07 | F(1,36) = 0.96 | 0.32 | 0.33 | −0.13 | F(1,36) = 3.99 | 0.63 | 0.05 | 0.03 | F(1,36) = 0.17 | 0.13 | 0.69 |
Note: For normally distributed variables means were compared using 1-way ANOVA, effect size is Cohen’s d; for variables that were not normally distributed distributions were compared using Mann-Whitney U Test; effect size for Mann- Whitney U test is equal to . In bold are effects of diagnoses that are significant at p = 0.05.
3.4.2. Value-based model fit, R2
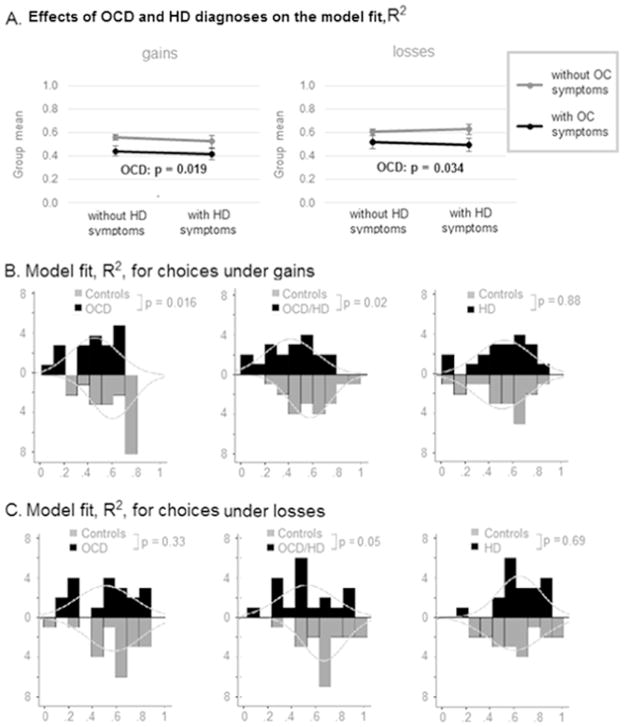
We fit the choice data of each individual participant with a theoretical model of subjective expected value (Gilboa and Schmeidler, 1989), and calculated model fit (R2) for each participant (see Methods and Supplementary materials S.3.3, Pushkarskaya et al., 2015). R2 was normally distributed during both gain and loss blocks in all clinical groups and controls (Shapiro-Wilk p > 0.10). 2×2 ANOVA (presence or absence of clinical OCD/HD symptoms) revealed a significant main effect of OCD on model fit both during gain (F(1,110) = 5.58, p = 0.019, Cohen’s d = 0.39) and loss blocks (F(1,110) = 4.62, p = 0.034, Cohen’s d = 0.46; Fig. 4A).
Fig. 4. Value-based model fit, R2, in Individuals with OCD and/or HD and controls.
Effect of diagnosis (OCD and/or HD vs. Controls) on the value-based model fit, R2, during gain blocks and loss blocks. Bars: histograms of respective distributions. Curves: empirically approximated normal curves.
Secondary pairwise comparisons (see Fig. 4BC, Table 5) confirmed a significant effect of OCD on model fit in individuals with OCD (Mann Whitney U = 97.5, p = 0.016, effect size = 0.39) and in individuals with OCD and comorbid HD (F(1,110) = 5.96, p = 0.02, Cohen’s d = 0.76) during gain blocks. During loss blocks, the effect of OCD on value-based model fit in our sample was significant in individuals with OCD and comorbid HD (F(1,110) = 3.99, p = 0.05, Cohen’s d = 0.63), but not in individuals with OCD. No difference was observed in value-based model fit between individuals with HD and age-matched controls.
3.4.3. Suboptimal choices
The proportion of clearly suboptimal choices was not normally distributed during either gain or loss blocks (Shapiro-Wilk p < 0.01). Nonparametric 2 × 2 ANOVA (presence or absence of clinical OCD/HD symptoms) revealed no significant main or interactive effects (Supplementary Materials S.6.A). Secondary pairwise comparisons (Supplementary Materials S.6.BC) revealed a tendency to make clearly suboptimal choices only in individuals with OCD and comorbid HD, compared to age-matched controls, only under gains (gains: Mann Whitney U = 101.5, p = 0.02; losses: Mann Whitney U = 169, p = 0.75).
Overall, diminished fidelity of value-based decision making in OCD appeared to be more pronounced under gains than under losses (but see discussion of potential limitations of the experimental design in Supplementary Materials S.7). Unexpectedly, fidelity of value-based decision making appeared to be intact in HD.
3.5. Uncertainty attitudes
Uncertainty avoidance was enhanced in individuals with OCD without comorbid HD under gains but not under losses. Uncertainty attitudes in individuals with HD (with and without comorbid OCD) were intact.
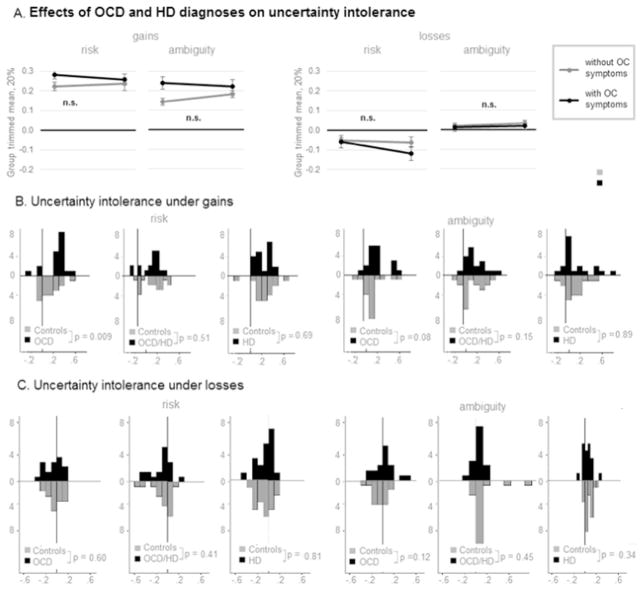
Distributions of risk aversion violated an assumption of normality in all clinical groups (Shapiro-Wilk p < 0.05); distributions of ambiguity aversion violated an assumption of normality in individuals with OCD and individuals with HD (Shapiro-Wilk p < 0.05). Nonparametric 2 × 2 ANOVA (presence or absence of clinical OCD/HD symptoms) revealed neither significant main nor significant interaction effects (Fig. 5A).
Fig. 5. Uncertainty aversion in individuals with OCD and/or HD and controls.
Effect of diagnosis (OCD and/or HD vs. Controls) on risk and ambiguity attitudes during gain blocks and loss blocks. Bars: histograms of respective distributions. Curves: empirically approximated normal curves. Since proportion of inconsistent choices under losses was not normally distribute, instead of group mean the corresponding figure presents the group trimmed mean at 20% - a statistical measure of central tendency that involves the calculation of the mean after discarding 10% of a sample at both the high and low end.
Secondary pairwise comparisons (Fig. 5B–C) revealed significantly enhanced risk aversion (Mann Whitney U = 93, p = 0.01, Mann Whitney U effect size = 0.41) and a trend towards enhanced ambiguity aversion (Mann Whitney U = 121, p = 0.08, Mann Whitney U effect size = 0.28) in individuals with OCD during gain blocks. No other significant effects were found (Supplementary materials S.8, Fig. 5BC). This may indicate that the relationship between ambiguity aversion and OCD is linked to only some of the subtypes of OCD and thus its detection may depend on the test group composition. The lack of risk and ambiguity intolerance in HD was unexpected.
3.6. Correlation with symptom severity
OCD severity correlated with measures of reduced fidelity of value-based under gains but not under losses.
To explore the effect of OCD diagnosis on fidelity of value-based choices further, we correlated the proportion of inconsistent choices and the value-based model fit, R2, with severity of OCD symptoms (total Y-BOCS score; we excluded 16 individuals with HD with YBOCS = 0 from this analysis to offset a potential binary effect of OCD diagnosis). During gain blocks, severity of OCD correlated with both the proportion of inconsistent choices (r = 0.37, p = 0.017, N = 40) and the value-based model fit, R2 (r = −0.35, p = 0.028, N = 40); we did not find similar relations during loss blocks (inconsistent choices: r = 0.11, p = 0.50; R2: r = −0.17, p = 0.31; N = 40).
For completeness, we also correlated the proportion of inconsistent choices and the value-based model fit, R2, with severity of HD symptoms (total SI-R score). Model fit, R2, correlated at trend level with severity of HD symptoms during gain blocks (N = 33, r = 0.31, p = 0.07), but not during loss blocks (N = 33, r = 0.11, p = 0.53). Inconsistency of choices did not correlate with severity of HD during either gain blocks (r = −0.17, p = 0.34) or loss blocks (r = −0.09, r = 0.61).
Severity of anxiety and depression did not correlate significantly with any of the behavioral measures (Supplementary Materials S.9).
4. Discussion
We examined the behavior of unmedicated individuals with OCD, comorbid OCD and HD, and HD and control participants using a decision task that tests several sub processes of value-based decision formation (valuation and value-based choice) in the presence of uncertainty, under gains and losses separately. In a larger sample we replicate our prior findings (Pushkarskaya et al., 2015) that when making choices between gains, OCD (with and without comorbid HD) were less compliant with the assumptions of subjective value maximization than Controls; we did not observe the same effect under losses. OCD severity correlated with measures of reduced fidelity of value-based under gains but not under losses. We found evidence of enhanced uncertainty avoidance only in individuals with OCD (without comorbid HD), under gains but not under losses. We also found that individuals with OCD (with and without comorbid HD) take more time to make choices both under gains and under losses. Unexpectedly, we found that choices of individuals with HD did not differ from those of age-matched Controls.
Our results indicate that value-based decision making is impaired in individuals with OCD (with and without HD). These individuals make choices more slowly and are less likely to follow simple and intuitive rules of value-based decision making. Under gains, this tendency correlated with OCD symptom severity. This suggests that clinically observed difficulties with decision making are linked to impairments in basic sub-processes of value-based decision formation. This result is consistent with prior findings by Dittrich and Johansen (2013), who found that individuals with OCD take more time and are less likely to choose objectively more valuable options during the Cambridge Gambling Task. Recall, however, that the Cambridge Gambling Task provides feedback to the participants, which complicates interpretation of their results.
In contrast, value-based decision making during a simple laboratory task appears to be intact in individuals with HD. Indeed, we observe positive correlation between HD severity and value-based model fit, at trend level. This suggests that, despite their clinical similarity, difficulties in decision making in OCD and HD relate to impairments in distinct sub-processes. Individuals with HD may have difficulties processing information in complex real word scenarios but be able to cope with the demands of a simple laboratory task. This is consistent with a number of studies reporting very limited evidence of decision making impairments in HD in a variety of other laboratory tasks (Grisham et al., 2010; Frost et al., 2011; Morein-Zamir et al., 2014; Mackin et al., 2015; Sumner et al., 2015).
While the link between OCD and measures of fidelity of value-based decision making appears to be robust, the relationship of OCD to intolerance of uncertainty is less so, and may depend on the test group composition (Kuelz et al., 2004). This is consistent with mixed results reported by prior studies of uncertainty intolerance in OCD that used various paradigms, including those that allow feedback evaluation and learning (Nielen et al., 2002; Lawrence et al., 2006; Da Rocha et al., 2011; Admon et al., 2012; Zhang et al., 2015). For instance, Sohn et al. (2014) utilized the Balloon Analogue Risk Task and found lower levels of risk taking in OCD relative to healthy individuals. Several studies have found enhanced ambiguity but not risk aversion in OCD (Starcke et al., 2010; Pushkarskaya et al., 2015; Zhang et al., 2015). Sip et al. (2016), using a gambling task without feedback, under different framing (gains versus losses) found that individuals with OCD are more risk averse when facing losses that are framed as gains, compared to explicit losses. A recent study decomposes risk attitudes into sensitivity to rewards and sensitivity to probabilities (Aranovich et al., 2017). They found reduced sensitivity to rewards in both OCD and HD, and explained it as enhanced risk aversion in these clinical populations. This is in contrast to our findings that uncertainty intolerance was unaffected in individuals with HD.
The findings of Aranovich et al. (2017) are particularly important given recent controversy about interpretation of the curvature of the utility function in the behavioral economics literature. While most studies interpret this parameter as a measure of risk aversion (Gilboa and Schmeidler, 1989), Rabin (2000) has demonstrated that diminishing sensitivity to rewards and risk aversion are largely independent concepts. We have suggested that reduced sensitivity to rewards in individuals with OCD may be consistent with reduced fidelity in value-based decision making that we observe in this population (see Pushkarskaya et al., 2015 for this discussion).
Our results suggest that the effect of OCD diagnosis on both fidelity of value-based decision making and uncertainty intolerance is stronger during gain blocks than during loss blocks. A similar effect was seen by Sip et al. (2016), who report risk aversion only when a gambling task is framed in terms of gains, not when it is framed in terms of losses. This raises an interesting question: is the effect of OCD diagnosis on uncertainty attitudes reduced by the objective possibility of losses, or simply by loss framing? Enhanced sensitivity to negative information and slowed reaction times in response to negative information have been repeatedly demonstrated in individuals with OCD (Foa et al., 1993; Lavy et al., 1994; Williams et al., 1996; Hinds et al., 2012). Simply framing a task in term of losses may prompt these individuals to pay more attention and be more careful with choices, which could both improve fidelity of value-based decision-making and attenuate abnormal uncertainty intolerance. This could mean that reduced sensitivity to rewards may be compensated by enhanced sensitivity to losses in individuals with OCD. Future studies need to investigate this possibility further.
Overall, our results support the proposition that testing decision making across clinical populations, and especially across traditional diagnostic categories, in controlled laboratory experiments can inform on the nature of clinically observed impairments in decision making. This approach may shed new light on commonalties between and distinctions among clinical syndromes.
Supplementary Material
Acknowledgments
Funding sources
This research was supported by National Institutes of Mental Health Grants K01 MH101326-01 (to H.P.) and R01MH095790 (to C.P.), and National Institute on Aging, Grant R01AG033406 (to I.L.).
We thank Suzanne Wasylink, Eileen Billingslea, Stephen Kichuk, and Amber Billingsley for their support in subject recruitment and data collection.
This research was supported by National Institutes of Mental Health Grants K01 MH101326-01 (to H.P.) and R01MH095790 (to C.P.), and National Institute on Aging, Grant R01AG033406 (to I.L.).
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.psychres.2017.08.058.
Footnotes
Financial disclosures
Dr. Tolin receives research support from Palo Alto Health Sciences and Pfizer. Dr. Pittenger receives research support from Hoffman F. La Roche, Ltd., and has received unrestricted educational grants from F. Hoffman La Roche, Ltd., and Medtronic, Inc. Drs. Pushkarskaya, Levy, and Ruderman, Mrs. Kelly and Henick reported no biomedical financial interests or potential conflicts of interest.
References
- Admon R, Bleich-Cohen M, Weizmant R, Poyurovsky M, Faragian S, Hendler T. Functional and structural neural indices of risk aversion in obsessive–compulsive disorder (OCD) Psychiatry Res: Neuroimaging. 2012;203:207–213. doi: 10.1016/j.pscychresns.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Aranovich GJ, Cavagnaro DR, Pitt MA, Myung JI, Mathews CA. A model-based analysis of decision making under risk in obsessive-compulsive and hoarding disorders. J Psychiatr Res. 2017;90:126–132. doi: 10.1016/j.jpsychires.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banca P, Vestergaard MD, Rankov V, Baek K, Mitchell S, Lapa T, Castelo-Branco M, Voon V. Evidence accumulation in obsessive-compulsive disorder: the role of uncertainty and monetary reward on perceptual decision-making thresholds. Neuropsychopharmacology. 2015;40:1192–1202. doi: 10.1038/npp.2014.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Black DW, Grant JE. DSM-5® Guidebook: the Essential Companion to the Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Pub; 2014. [Google Scholar]
- Brand M, Greco R, Schuster A, Kalbe E, Fujiwara E, Markowitsch H, Kessler J. The game of dice—a new test for the assessment of risktaking behavior. Neurorehabilitation Neural Repair. 2002;16:142–143. [Google Scholar]
- Buelow MT, Suhr JA. Construct validity of the Iowa gambling task. Neuropsychol Rev. 2009;19:102–114. doi: 10.1007/s11065-009-9083-4. [DOI] [PubMed] [Google Scholar]
- Camerer C, Weber M. Recent developments in modeling preferences: uncertainty and ambiguity. J Risk Uncertain. 1992;5:325–370. [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. A neuropsychological comparison of obsessive–compulsive disorder and trichotillomania. Neuropsychologia. 2007;45:654–662. doi: 10.1016/j.neuropsychologia.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Da Rocha F, Malloy-Diniz L, Lage N, Correa H. The relationship between the Met allele of the BDNF Val66Met polymorphism and impairments in decision making under ambiguity in patients with obsessive–compulsive disorder. Genes Brain Behav. 2011;10:523–529. doi: 10.1111/j.1601-183X.2011.00687.x. [DOI] [PubMed] [Google Scholar]
- Dittrich WH, Johansen T. Cognitive deficits of executive functions and decision-making in obsessive-compulsive disorder. Scand J Psychol. 2013;54:393–400. doi: 10.1111/sjop.12066. [DOI] [PubMed] [Google Scholar]
- Dutilh G, Rieskamp J. Comparing perceptual and preferential decision making. Psychon Bull Rev. 2016;23:723–737. doi: 10.3758/s13423-015-0941-1. [DOI] [PubMed] [Google Scholar]
- Fear CF, Healy D. Probabilistic reasoning in obsessive–compulsive and delusional disorders. Psychol Med. 1997;27:199–208. doi: 10.1017/s0033291796004175. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering Statistics Using IBM SPSS Statistics. ISM; London, England: 2013. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Pub; 2012. [Google Scholar]
- Foa EB, Ilai D, McCarthy PR, Shoyer B, Murdock T. Information processing in obsessive—compulsive disorder. Cogn Ther Res. 1993;17:173–189. [Google Scholar]
- Frost RO, Shows DL. The nature and measurement of compulsive indecisiveness. Behav Res Ther. 1993;31:683-IN682. doi: 10.1016/0005-7967(93)90121-a. [DOI] [PubMed] [Google Scholar]
- Frost RO, Steketee G, Grisham J. Measurement of compulsive hoarding: saving inventory-revised. Behav Res Ther. 2004;42:1163–1182. doi: 10.1016/j.brat.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Frost RO, Steketee G, Tolin DF. Comorbidity in hoarding disorder. Depress Anxiety. 2011;28:876–884. doi: 10.1002/da.20861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa I, Schmeidler D. Maxmin expected utility with non-unique prior. J Math Econ. 1989;18:141–153. [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR. The Yale-brown obsessive-compulsive scale-II-validity. Arch Gen Psychiatry. 1989a;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-brown obsessive compulsive scale: I. development, use, and reliability. Arch General Psychiatry. 1989b;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Grisham JR, Brown TA, Savage CR, Steketee G, Barlow DH. Neuropsychological impairment associated with compulsive hoarding. Behav Res Ther. 2007;45:1471–1483. doi: 10.1016/j.brat.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Grisham JR, Norberg MM, Williams AD, Certoma SP, Kadib R. Categorization and cognitive deficits in compulsive hoarding. Behav Res Ther. 2010;48:866–872. doi: 10.1016/j.brat.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds AL, Woody EZ, Van Ameringen M, Schmidt LA, Szechtman H. When too much is not enough: obsessive-compulsive disorder as a pathology of stopping, rather than starting. PLoS One. 2012;7:e30586. doi: 10.1371/journal.pone.0030586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunink MM, Weinstein MC, Wittenberg E, Drummond MF, Pliskin JS, Wong JB, Glasziou PP. Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge University Press; Cambridge, England: 2014. [Google Scholar]
- Jacobsen P, Freeman D, Salkovskis P. Reasoning bias and belief conviction in obsessive-compulsive disorder and delusions: jumping to conclusions across disorders? Br J Clin Psychol. 2012;51:84–99. doi: 10.1111/j.2044-8260.2011.02014.x. [DOI] [PubMed] [Google Scholar]
- Kaufman A. Intelligent Testing. W/SC-R. Wiley-Interscience; New York: 1979. [Google Scholar]
- Koran LM, Thienemann ML, Davenport R. Quality of life for patients with obsessive-compulsive disorder. Am J Psychiatry. 1996;153:783. doi: 10.1176/ajp.153.6.783. [DOI] [PubMed] [Google Scholar]
- Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 2004;65:185–236. doi: 10.1016/j.biopsycho.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Lavy E, Van Oppen P, Van Den Hout M. Selective processing of emotional information in obsessive compulsive disorder. Behav Res Ther. 1994;32:243–246. doi: 10.1016/0005-7967(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Wooderson S, Mataix-Cols D, David R, Speckens A, Phillips ML. Decision making and set shifting impairments are associated with distinct symptom dimensions in obsessive-compulsive disorder. Neuropsychology. 2006;20:409. doi: 10.1037/0894-4105.20.4.409. [DOI] [PubMed] [Google Scholar]
- Levy I, Snell J, Nelson AJ, Rustichini A, Glimcher PW. Neural representation of subjective value under risk and ambiguity. J Neurophysiol. 2010;103:1036–1047. doi: 10.1152/jn.00853.2009. [DOI] [PubMed] [Google Scholar]
- Mackin RS, Vigil O, Insel P, Kivowitz A, Kupferman E, Hough CM, Fekri S, Crothers R, Bickford D, Delucchi KL. Patterns of clinically significant cognitive impairment in hoarding disorder. Depression Anxiety. 2015 doi: 10.1002/da.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Mathes BM, Oglesby ME, Short NA, Portero AK, Raines AM, Schmidt NB. An examination of the role of intolerance of distress and uncertainty in hoarding symptoms. Compr Psychiatry. 2017;72:121–129. doi: 10.1016/j.comppsych.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Morein-Zamir S, Papmeyer M, Pertusa A, Chamberlain SR, Fineberg NA, Sahakian BJ, Mataix-Cols D, Robbins TW. The profile of executive function in OCD hoarders and hoarding disorder. Psychiatry Res. 2014;215:659–667. doi: 10.1016/j.psychres.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann Jv, Morgenstern O. Theory of Games and Economic Behavior. Princeton University Press; Princeton: 1944. [Google Scholar]
- Nielen M, Veltman D, De Jong R, Mulder G, Den Boer J. Decision making performance in obsessive compulsive disorder. J Affect Disord. 2002;69:257–260. doi: 10.1016/s0165-0327(00)00381-5. [DOI] [PubMed] [Google Scholar]
- Pélissier MC, O’Connor KP. Deductive and inductive reasoning in obsessive-compulsive disorder. Br J Clin Psychol. 2002;41:15–27. doi: 10.1348/014466502163769. [DOI] [PubMed] [Google Scholar]
- Pittenger C. Obsessive-Compulsive Disorder: Phenomenology, Pathophysiology, and Treatment. Oxford University Press; New York: 2017. [Google Scholar]
- Polanía R, Krajbich I, Grueschow M, Ruff CC. Neural oscillations and synchronization differentially support evidence accumulation in perceptual and value-based decision making. Neuron. 2014;82:709–720. doi: 10.1016/j.neuron.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Pushkarskaya H, Tolin D, Ruderman L, Kirshenbaum A, Kelly JM, Pittenger C, Levy I. Decision-making under uncertainty in obsessive–compulsive disorder. J Psychiatr Res. 2015;69:166–173. doi: 10.1016/j.jpsychires.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin M. Diminishing Marginal Utility of Wealth Cannot Explain Risk Aversion. Department of Economics, UCB; Vancouver, Canada: 2000. [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Eisen JL. The epidemiology and clinical features of obsessive compulsive disorder. Psychiatr Clin North Am. 1992;15:743–758. [PubMed] [Google Scholar]
- Reed GF. Obsessional Experience and Compulsive Behaviour: a Cognitive-structural Approach. Academic Press; New York: 1985. [Google Scholar]
- Saxena S. Is compulsive hoarding a genetically and neurobiologically discrete syndrome? Implications for diagnostic classification. Am Psychiatr Assoc. 2007 doi: 10.1176/ajp.2007.164.3.380. [DOI] [PubMed] [Google Scholar]
- Sip KE, Muratore AF, Stern ER. Effects of context on risk taking and decision times in obsessive-compulsive disorder. J Psychiatr Res. 2016;75:82–90. doi: 10.1016/j.jpsychires.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Sohn SY, Kang JI, Namkoong K, Kim SJ. Multidimensional measures of impulsivity in obsessive-compulsive disorder: cannot wait and stop. PloS One. 2014;9:e111739. doi: 10.1371/journal.pone.0111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K, Tuschen-Caffier B, Markowitsch HJ, Brand M. Dissociation of decisions in ambiguous and risky situations in obsessive–compulsive disorder. Psychiatry Res. 2010;175:114–120. doi: 10.1016/j.psychres.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Tsetsos K. Building bridges between perceptual and economic decision-making: neural and computational mechanisms. Front Neurosci. 2012;6:70. doi: 10.3389/fnins.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JM, Noack CG, Filoteo JV, Maddox WT, Saxena S. Neurocognitive performance in unmedicated patients with hoarding disorder. 2015 doi: 10.1037/neu0000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillefer SE, Liu JJ, Ornstein TJ, Vickers K. Indecisiveness as a predictor of quality of life in individuals with obsessive and compulsive traits. J Obsessive-Compuls Relat Disord. 2016;10:91–98. [Google Scholar]
- Timpano KR, Exner C, Glaesmer H, Rief W, Keshaviah A, Brähler E, Wilhelm S. The epidemiology of the proposed DSM-5 hoarding disorder: exploration of the acquisition specifier, associated features, and distress. J Clin Psychiatry. 2011;72:780–786. doi: 10.4088/JCP.10m06380. (quiz 878–789) [DOI] [PubMed] [Google Scholar]
- Tolin DF, Abramowitz JS, Brigidi BD, Foa EB. Intolerance of uncertainty in obsessive-compulsive disorder. J Anxiety Disord. 2003;17:233–242. doi: 10.1016/s0887-6185(02)00182-2. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Frost RO, Steketee G, Gray KD, Fitch KE. The economic and social burden of compulsive hoarding. Psychiatry Res. 2008;160:200–211. doi: 10.1016/j.psychres.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Gilliam C, Wootton BM, Bowe W, Bragdon LB, Davis E, Hannan SE, Steinman SA, Worden B, Hallion LS. Psychometric properties of a structured diagnostic interview for DSM-5 anxiety, mood, and obsessive-compulsive and related disorders. Assessment. 2016 doi: 10.1177/1073191116638410. (1073191116638410) [DOI] [PubMed] [Google Scholar]
- Tymula A, Belmaker LAR, Roy AK, Ruderman L, Manson K, Glimcher PW, Levy I. Adolescents’ risk-taking behavior is driven by tolerance to ambiguity. Proc Natl Acad Sci. 2012 doi: 10.1073/pnas.1207144109. (201207144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymula A, Belmaker LAR, Ruderman L, Glimcher PW, Levy I. Like cognitive function, decision making across the life span shows profound age-related changes. Proc Natl Acad Sci. 2013;110:17143–17148. doi: 10.1073/pnas.1309909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden WJ. A lognormal model for response times on test items. J Educ Behav Stat. 2006;31:181–204. [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional stroop task and psychopathology. Psychol Bull. 1996;120:3. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dong Y, Ji Y, Zhu C, Yu F, Ma H, Chen X, Wang K. Dissociation of decision making under ambiguity and decision making under risk: a neurocognitive endophenotype candidate for obsessive–compulsive disorder. Progress Neuro-Psychopharmacol Biol Psychiatry. 2015;57:60–68. doi: 10.1016/j.pnpbp.2014.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.