There are signs of an opioid epidemic in Australia and Canada, but not in Germany. Implementation of opioid prescribing guidelines should ensure that physicians prescribe opioids only for appropriate indications in limited doses for selected patients for chronic noncancer pain.
Keywords: Chronic noncancer pain, Opioids, Abuse, Misuse, Addiction, Mortality, Guidelines
Abstract
Introduction:
A marked rise in opioid prescriptions for patients with chronic noncancer pain (CNCP) with a parallel increase in opioid abuse/misuse, and resulting deaths was noted in the Unites states in the past decade (opioid epidemic). In response, the US Center of Diseases Control (CDC) developed a guideline for prescribing of opioids for patients with CNCP.
Objectives:
To assess (1) if there is an opioid epidemic in Australia, Canada, and Germany (2) to compare Australian, Canadian, German, and Center of Diseases Control guidelines recommendations for long-term opioid therapy for CNCP.
Methods:
National evidence-based guidelines and PubMed were searched for recommendations for opioid prescriptions for CNCP.
Results:
There are signs of an opioid epidemic in Australia and Canada, but not in Germany. Guidelines in all 4 countries provide similar recommendations: opioids are not the first-line therapy for patients with CNCP; regular clinical assessments of benefits and harms are necessary; excessive doses should be avoided (recommended morphine equivalent daily doses range from 50 to 200 mg/d); stopping rules should be followed. All guidelines do not recommend the use of opioids in chronic pain conditions without an established nociceptive or neuropathic cause such as fibromyalgia and primary headache.
Conclusion:
Implementation of opioid prescribing guidelines should ensure that physicians prescribe opioids only for appropriate indications in limited doses for selected patients and advice patients on their safe use. These measures could contribute to reduce prescription opioid misuse/abuse and deaths.
1. Introduction
About 20 years ago, compassionate advocacy for better treatment of chronic pain, combined with aggressive marketing of opioid formulations, led to a sharp increase in the prescribing of opioid analgesics for patients with chronic noncancer pain (CNCP) in most developed countries.7 An unintended consequence of this approach was an “opioid epidemic” in the United States, namely an increase in opioid use and in parallel misuse/abuse and deaths.39 Prescription of opioid analgesics was responsible for more deaths than both suicide and motor vehicle accidents or deaths from cocaine and heroin abuse combined in the United States in 2010. It was estimated that the majority of deaths (60%) occurred in patients receiving prescriptions based on prescribing guidelines by medical boards, while 40% of deaths occurred in individuals with substance use disorders who obtained opioids through multiple prescriptions, doctor shopping, and drug diversion.39 In response to the opioid epidemic, hundreds of local, regional, state, and federal interventions have been implemented in the United States.11 To ensure that patients have access to safer, more effective chronic pain treatment while reducing the number of people who misuse, abuse, or overdose from these drugs, the US Center of Diseases Control (CDC) released a guideline in 2016 to provide recommendations for the prescribing of opioid pain medication for patients older than 18 years in primary care settings with CNCP.9
The “flood opioids and the rising tide of deaths”48 in the United States raised the question if there is a worldwide opioid epidemic32 and if the recommendations of the CDC can be applied to other countries. The aims of this review are to assess in 3 countries in 3 different continents (Australia, Canada and Germany)
(1) if there is an opioid epidemic;
(2) which courses of actions were taken by national bodies or guidelines to prevent or reduce an opioid epidemic.
In addition, the main recommendations of the guidelines in the 3 countries are compared with the recent US guideline to point out if there are some universal recommendations, which can be applied to most health care settings and countries.
2. Methods
The authors refer to national evidence-based guidelines on opioid therapy for CNCP of their countries8,18,25,26,31 and a selective search of literature in the database PubMed with the search terms “Analgesics, Opioid” (Mesh), “Chronic Pain” (Mesh) and “Guideline” (Publication Type) from 2000 to December 2016 without language restrictions.
3. Results
3.1. Consumption and prescriptions of opioids
The consumption of prescribed opioids in 2014 in Australia, Canada, Germany and United States was as follows [49]:
Australia: 2014: 358 mg Morphine Equivalence (ME) (minus methadone) per person and 481 mg ME including methadone (Methadone is frequently used for chronic pain management in Australia) (Fig. 1).
Figure 1.
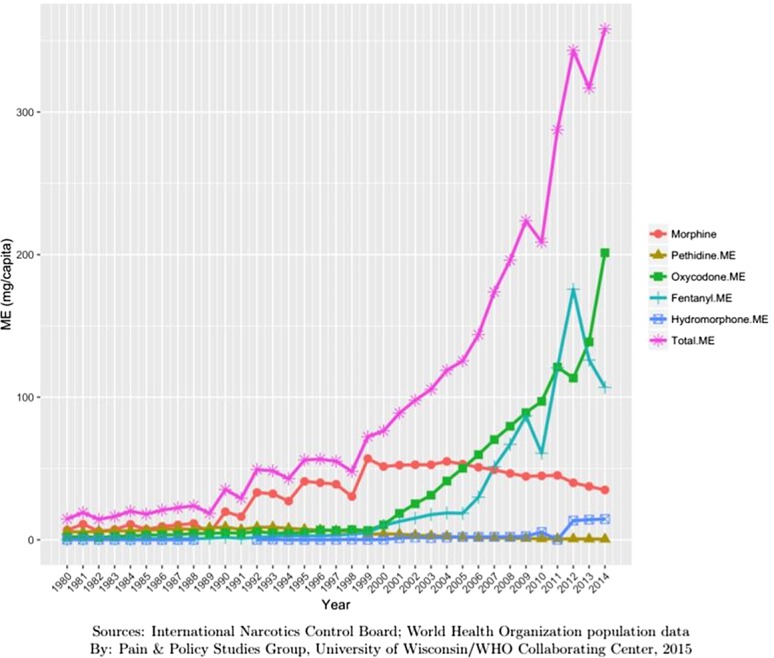
Opioid consumption in Morphine Equivalence minus methadone, mg per person in Australia from 1980 to 2014.49
Canada: 2014: 732 mg ME (minus methadone) per person (Fig. 2).
Figure 2.
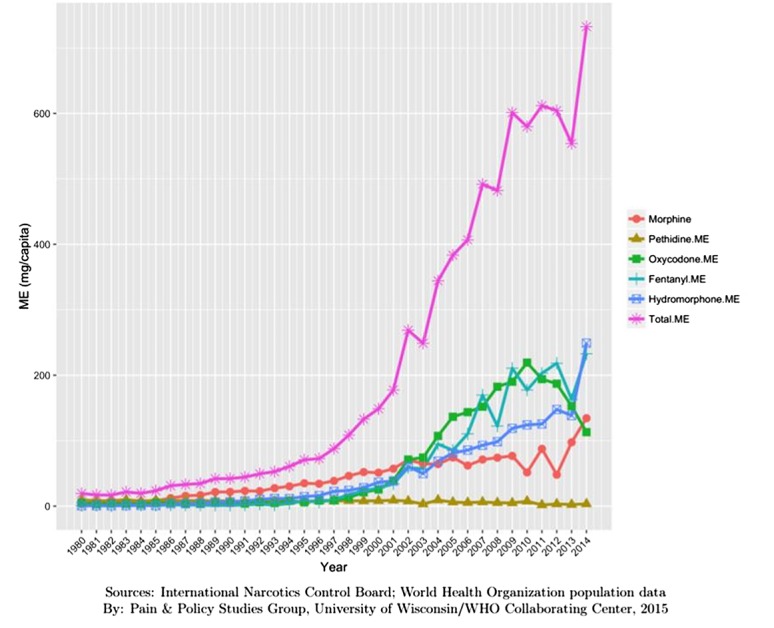
Opioid consumption in Morphine Equivalence minus methadone, mg per person in Canada from 1980 to 2015.49
Germany: 2014; 480 mg ME (minus methadone) per person (Fig. 3).
Figure 3.
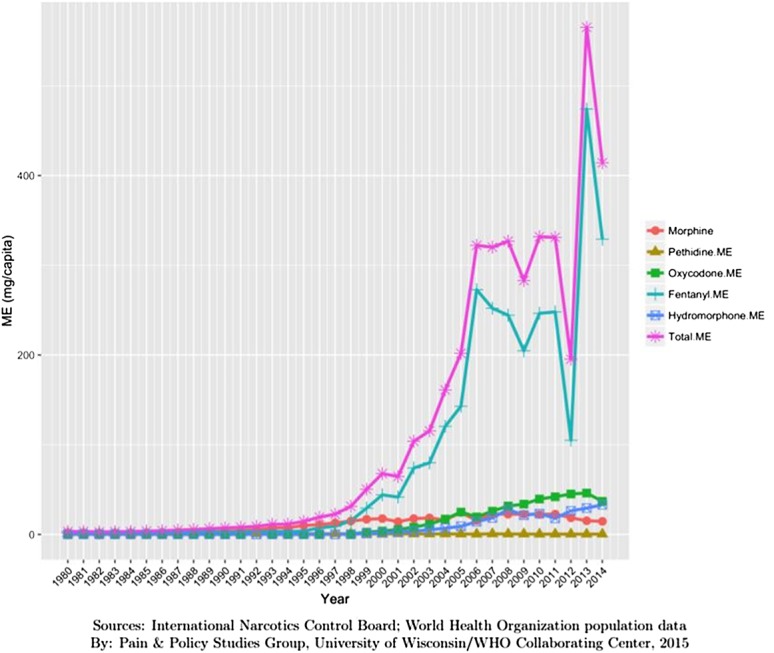
Opioid consumption in Morphine Equivalence minus methadone, mg per person in Germany from 1980 to 2015.49
United States 2014; 500 mg ME (minus methadone per person) (Fig. 4).
Figure 4.
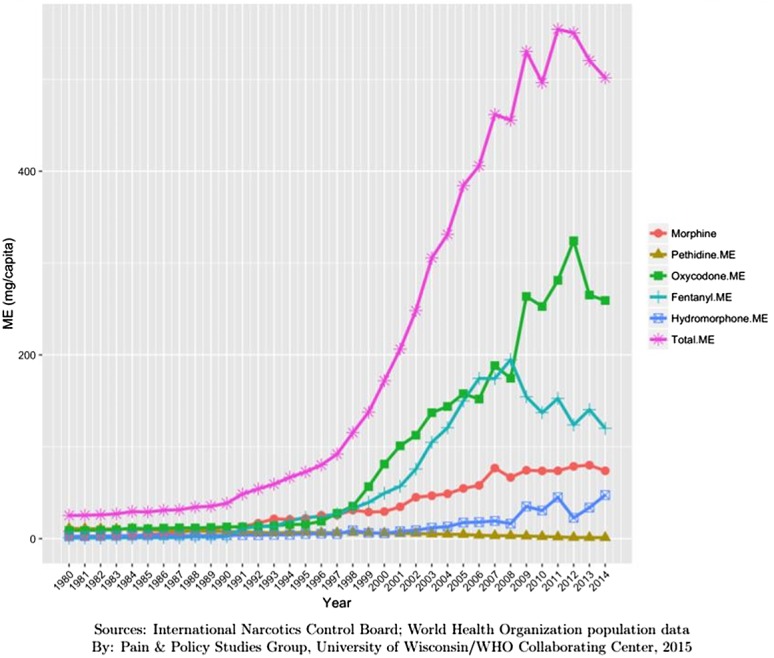
Opioid consumption in Morphine Equivalence minus methadone, mg per person in United States from 1980 to 2015.49
In Australia, 8 opioids were subsidized by the public health insurance system through the Pharmaceutical Benefit Scheme (PBS) in 2014: Buprenorphine, codeine, fentanyl, hydromorphone, methadone, morphine, oxycodone, and tramadol. Australia-wide opioid dispensing episodes covered by the PBS increased 15-fold (from 500,568 to 7,495,648) between 1992 and 2012. This was accompanied by a 32-fold increase of expenses for the Australian government from Au$ 8.5 million to Au$ 270.8 million.4 This study is based on PBS dispensing records available in aggregate (de-identified) form via Medicare Australia. Defined daily doses (DDDs) (The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults. A DDD can only be assigned for drugs that already have an Anatomical Therapeutic Chemical (ATC) code which is available from the WHO Collaborating Centre for Drug Statistics Methodology.) dispensed increased from 4.6 to 17.4 DDD/1000/d from 1990 to 2014.35 The pattern changed from primarily weak and short-acting opioids to strong and long-acting ones. The authors used dispensing claims processed through the PBS, and estimates of nonsubsidized medicine use from a survey of Australian pharmacies. These opioids were analysed: Buprenorphine, codeine, dextropropoxyphene, fentanyl, hydromorphone, methadone, morphine, oxycodone, pethidine, tapentadol, and tramadol. The DDD metric, established by the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology was used. The consumption figures were for outpatient care and did not allow a distinction between cancer vs noncancer pain and for acute vs long-term therapy. Oxycodone prescriptions increased from 35.3 to 89.2/1000 inhabitants from 2002/03 and 2007/08.54 This is confirmed for the Australian state of Victoria, where oxycodone use increased nine-fold from 7.5 to 67.5 mg per capita per year in the period 2000 to 2009.52 It is of interest that there was substantial geographic variation in the prescribing of opioids with rural areas having much higher use of all opioids; prescribing in these areas is more by general practitioners with limited access to pain clinics.12
All opioids require a prescription in Canada, except for Codeine 8 mg which can be dispensed with pharmacist control (ie, over the counter). Methadone requires a physician to have a special licence to prescribe, and buprenorphine/naloxone requires that the prescriber demonstrate a special training in how to prescribe it. Morphine and oxycodone are covered by either provincial or private insurers in almost all provinces. However the newer and most expensive agents such as tramadol, tapentadol and buprenorphine are almost universally not covered by provincial drug programs, but are covered by most health insurers. In Ontario (Canada) from 1991 to 2007, the annual prescriptions for opioids in general increased from 458 to 591 per 1000 individuals, by contrast, prescriptions of oxycodone increased by 850% during the same period, from 23 per 1000 individuals in 1991 to 197 per 1000 in 2007. The authors used prescribing data from IMS Health Canada, which collects monthly prescription records from nearly two-thirds of all Canadian pharmacies on all opioid-containing analgesics and cough suppressants prescribed on an outpatient basis. The consumption figures were for outpatient care and did not allow a distinction between cancer vs noncancer pain and for acute or long-term therapy. The authors did not report details on how the DDD were assessed.14
In Germany, every opioid needs a prescription by a physician. All opioids except oxymorphone are available and can be prescribed for any type of acute and chronic pain. All opioids except so-called weak opioids (codeine, tilidine, tramadol) can only be prescribed with a special prescription (so-called narcotic prescription) which has to be ordered by the physician from the federal opium agency. The prescriptions have to be locked in a safe. The costs for opioid prescriptions are reimbursed by statutory and private health insurance companies. In Germany, the percentage of patients insured by a large general statutory health insurance receiving at least one opioid prescription in outpatient care rose from 3.31% in 2000 to 4.53% in 2010, a relative gain of 37%. Opioids were mostly prescribed to patients with noncancer pain (2010: about 77% of opioid recipients). Opioids were selected via the ATC classification (subgroup N02A excluding codeine, levomethadone, and methadone). The current (2010) DDD of each agent was taken as the DDD for the whole study period. The data did not allow to making a distinction between short-term and long-term opioid therapy.58 The prevalence of LTOT prescriptions (defined by at least one opioid prescription per quarter for at least 3 consecutive quarters in outpatient care) for CNCP was 1.3% of all patients insured by a large German statutory health insurance. Oral opioid prescriptions of outpatient care were identified by the ATC Classification code N02A (WHO 2009). Prescriptions of the following opioids were included into the analyses: Buprenorphine, dihydrocodeine, fentanyl, hydromorphone, morphine, oxycodone, tapentadol, tilidine, and tramadol. Methadone and polamidone were excluded from analysis. The mean daily dosage of LTOT was 58 (SD 79; minimum 0.3, maximum 2010) mg morphine equivalent/day. The percentage of insured patients with high-dose opioid prescriptions (≥100 mg morphine equivalent/day) among LTOT insureds was 15.5%.40
3.2. Nonmedical use
3.2.1. Prevalence
Australia: A sample of 142 patients seeking treatment for opioid dependence was interviewed in 2008. Of these, 108 reported abusing prescription opioids. Of these, 56% used prescription opioids primarily and 86% saw this use as problematic; 75% injected prescription opioids; 46% bought their prescription opioids from a dealer.44
Canada: In 2009, the prevalence of nonmedical prescription opioid use in the general population was 4.8% (95% CI 4.1 to 5.5%) (60). In Ontario, the prevalence was 7.7% in 2010, but in 2011 it decreased to 4.0%. The difference between 2010 and 2011 was statistically significant (P < 0.001).21
Germany: In a parallel series of self-administered, cross-sectional, general population surveys conducted in 2014 with noninstitutionalized participants, aged 12 to 49 years in 5 European countries (Denmark, Germany, Great Britain, Spain, and Sweden) lifetime and past-year nonmedical use of prescription medications (self-treatment of a medical condition using medication without a prescriber's authorization as well as use to achieve euphoric states) such as stimulants, opioids, and sedatives were ascertained via a modified version of the World Health Organization's Composite International Diagnostic Interview. The reported prevalence of past year (0.2%) and lifetime (0.4%) nonmedical use of opioids was the lowest in Germany.45 Up to 25% of patients of inpatient treatment of opioid addiction report opioid analgesics as the primary drug.17
3.2.2. Deaths
Australia: 465 deaths related to oxycodone were reported from 2001 to 2009. Most of the deaths (82%) were due to multiple drug use, mainly including alcohol and benzodiazepines beside oxycodone. In 53% of cases, oxycodone was prescribed to the victim and 27% were known intravenous drug users.54 For the time frame 2002 to 2011, deaths due to opioids increased 1.7 fold from 151 to 266. Population based this is an increase from 0.78 to 1.19 deaths/100,000.4 Specifically for oxycodone in the state of Victoria, deaths with a detection of oxycodone increased 21 times from 2000 to 2009 (4 [0.08/100,000 population] to 97 [1.78/100,000 population]); death was caused by oxycodone in 54% of the 320 cases.52
Canada: Most published data come from the largest Canadian Province of Ontario. Opioid-related deaths in Ontario have increased markedly since 1991, and the majority was deemed unintentional. Opioid-related deaths doubled from 13.7 per million in 1991 to 27.2 per million in 2004.14 In 2010, the opioid-related deaths were 41.6 per million Ontarians.28 Nearly one of every 8 deaths (12.1%) among individuals aged 25 to 34 years was opioid-related in 2010.27
Germany: In 2015, the German police departments reported 1226 drug-associated deaths (0.002% of the population). Most deaths were caused by heroin use.15
3.2.3. Abuse and addiction
Australia: Treatment episodes for oxycodone abuse increased from 0.01 to 0.02 per 1000 population from 2002 and 2003 to 2007 and 2008.54
Canada: In Ontario, the number of individuals enrolled in methadone maintenance treatment has increased from 3000 in 1996, to 29,000 in 2010, and to just under 50,000 in 2014.21
Germany: The pooled 1-year prevalence of opioid prescription abuse (defined by hospital stays because of mental and behavioural disorders due to alcohol, opioids, tranquilizers, multiple substances, and intoxications by narcotic agents) in a large German medical health insurance organization during the fiscal year 2012 was 0.008%.40 The pooled 1-year prevalence of opioid prescription abuse (defined by codes of mental and behavioural disorders due to alcohol, opioids, tranquilizers, multiple substances, and intoxications by narcotic agents within inpatient care) in a large German sample representative of all statutory health insurance organizations during the fiscal year 2014 was 5.0% (Tölle, submitted). Two case series of patients at a German tertiary care pain center demonstrated that problematic use and concealed use of other drugs were rare: In 6 of 121 patients with a LtOT of more than 3 years, an opioid detoxification was performed because of concurrent use of benzodiazepines.36 The incidence of under-reported opioid use was 3% in 243 patients of a tertiary care pain clinic.38
3.3. Medical use
3.3.1. Hospital admissions because of adverse events except abuse/addiction
Australia: The pattern of hospitalization for opioid overdose changed from primarily heroin related in 1998 (65%) to primarily prescription opioids in 2009 (58%); in 2001 prescription opioids overtook heroin as the most common reason for the first time.4
Canada: In 2014, there were 3241 opioid-related emergency department visits and 1620 opioid-related hospital admissions in Ontario. The rate of opioid-related emergency department visits was 2.4 per 10,000 residents while the rate of hospital admissions was 1.2 per 10,000 residents in 2014. The rate of opioid-related emergency department visits was highest among residents aged 25 to 44 years (3.1 visits per 10,000 residents). By contrast, the rate of opioid-related hospital admissions was highest among residents aged 65 and older (1.9 admissions per 10,000 residents).63
Germany: In a multicenter observational study covering a hospital catchment area of approximately 500,000 inhabitants, self-medication–related adverse events leading to hospital admissions in internal medicine departments were analyzed between 2000 and 2008. None of the admissions was associated with the use opioid analgesics.56 The incidence of drug-related hospitalizations in a longitudinal population-based study including all patients admitted between October 1997 and March 2000 to departments of internal medicine and emergency departments in the urban regions of Jena and Rostock, Germany was 0.9%. None of the admissions was due to prescribed opioids.57 Unfortunately, no more up to date data on hospital admission associated with the use of prescribed or self-medication drug use are available.
A search in the database of the Federal Institute For Drugs and Medical Devices for reports of adverse events (Intended overdose; withdrawal syndrome; unintentional overdose) by buprenorphine, fentanyl, hydromorphone, oxycodone, morphine, tapentadol, tilidine, and tramadol demonstrated no increase from 2006 to 2015. Reports per year ranged from 36 to 110 cases (Häuser, personal communication).
3.4. Comparison of guidelines recommendations
Some major recommendations of the Australian,18 Canadian,26 German,31 and CDC9 guidelines are outlined in Table 1.
Table 1.
Comparison of the Australian, Canadian, German, and Center of Diseases Control guidelines on long-term opioid therapy for chronic noncancer pain on selected parameters.
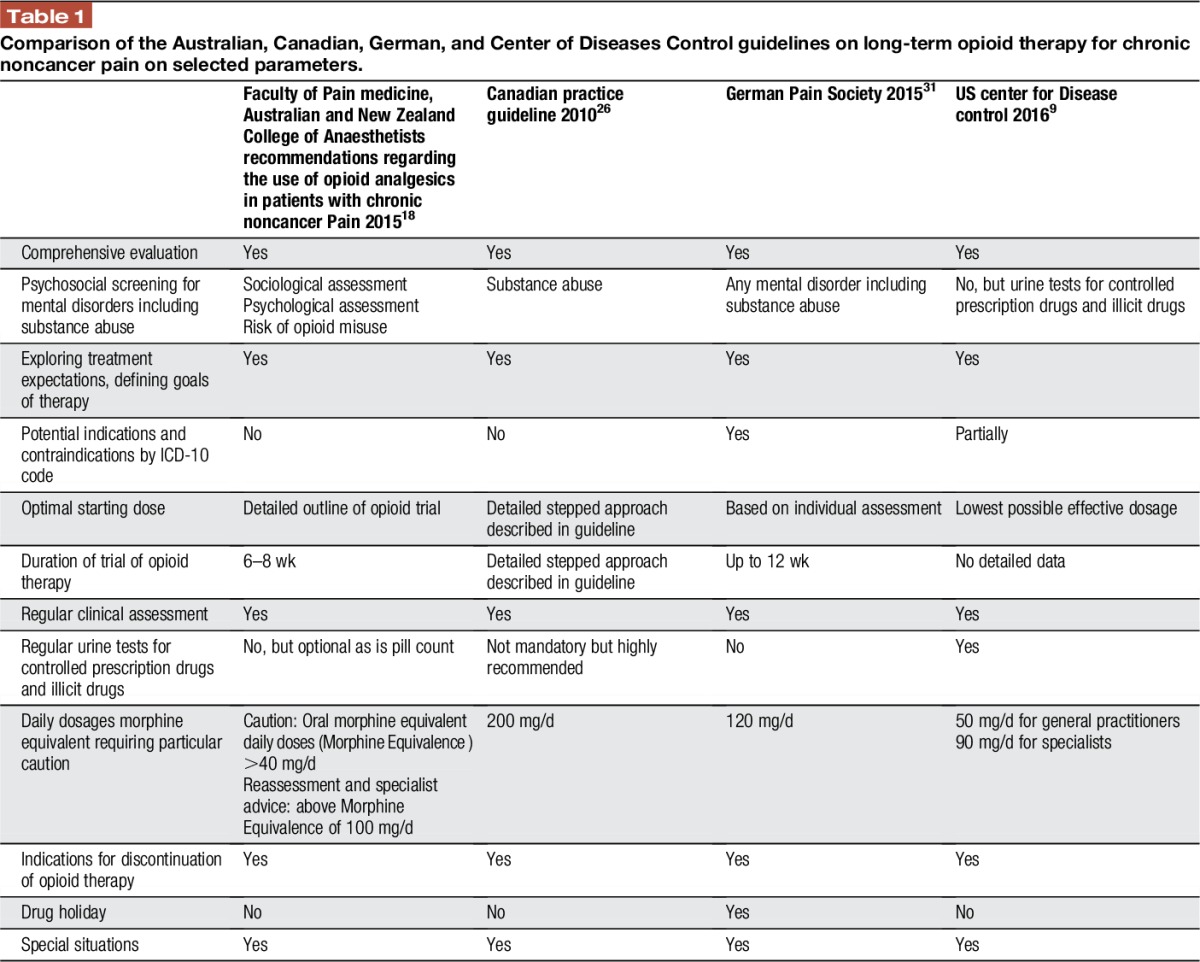
3.4.1. Patient selection and risk stratification
(1) A diligent medical assessment, including psychosocial risk factors of potential drug abuse, should be performed as part of the initial assessment of a patient with CNCP. The German guideline recommended a screening for any psychological distress as well; the Australian guidelines recommend a detailed socio-psycho-biomedical assessment and a risk assessment for problematic opioid use.
(2) Prior to initiating therapy, patients should be adequately informed about the benefits and risks of opioid therapy including driving ability. Obtaining written and signed informed consent prior to initiating therapy may be appropriate in some cases and is advised by the Australian guidelines to be done always and combined with a written treatment agreement (“opioid contract”), which is also required by some Australian state governments.
(3) All guidelines stressed that monotherapy with opioids should be avoided and opioids should be used as one component of multimodal and disciplinary pain management. The German, Australian, Canadian, and CDC guidelines emphasize that drugs in general and opioids in particular are not the first-line therapy for many chronic pain syndromes.
(4) Treatment expectations should be explored and individual goals of therapy should be defined together with the patient. The CDC, Canadian, and German guidelines define an at least 30% pain reduction and/or improvement of daily functioning relevant for the patient as a realistic treatment goal. The Australian guidelines expect achievement of goals based on improvement of function and state explicitly that a focus on pain relief alone via the passive receipt of opioid therapy can distract both patient and prescriber from active self-management strategies.
(5) The German guidelines specify potential indications and definite contraindications by ICD-10 codes and/or clinical pain diagnoses (Tables 2 and 3).
Table 2.
Potential indications for a short term (4 to 12 wk) and long term (>12 wk) with opioid analgesic according to the German guideline.31
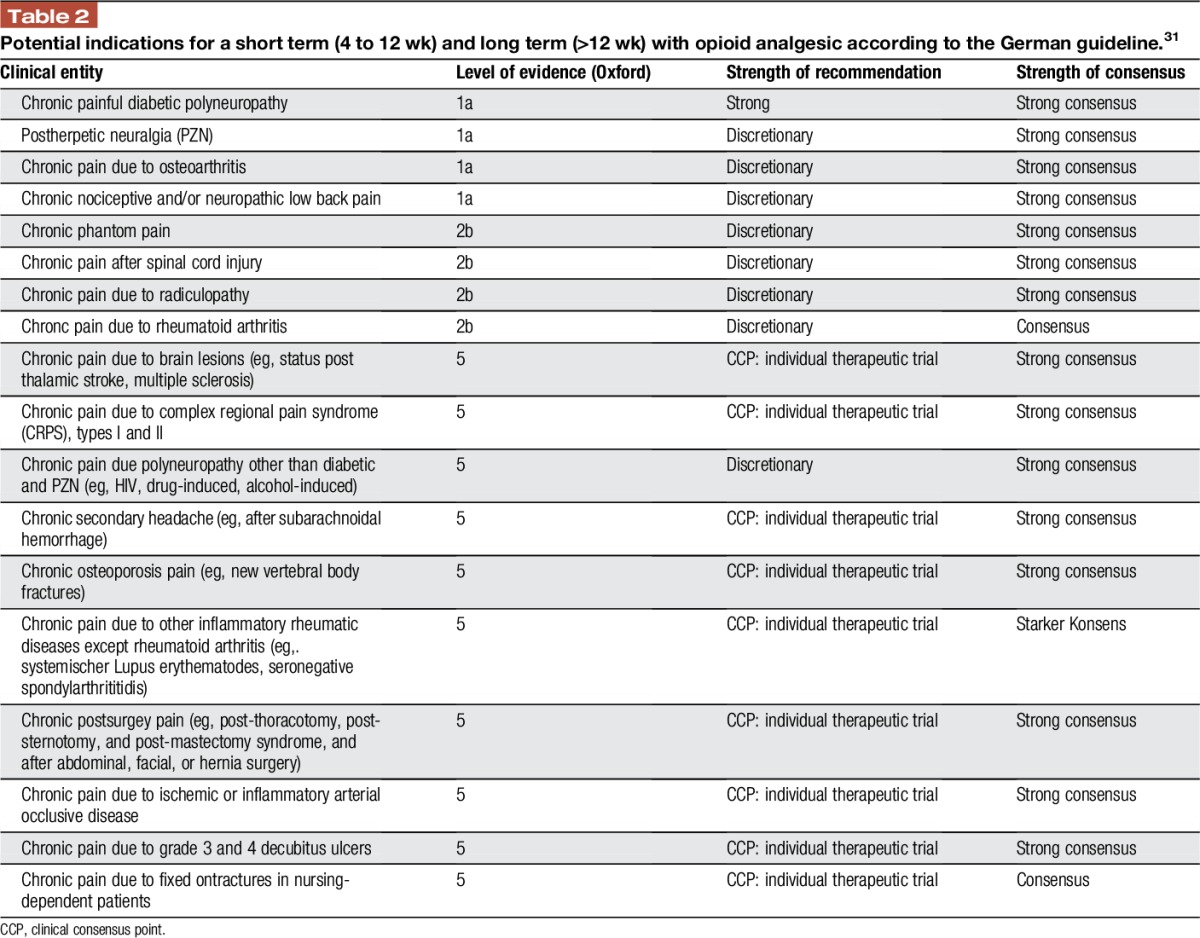
Table 3.
Contraindications to long-term treatment with opioid analgesics of the German guideline on opioids in chronic noncancer pain.31
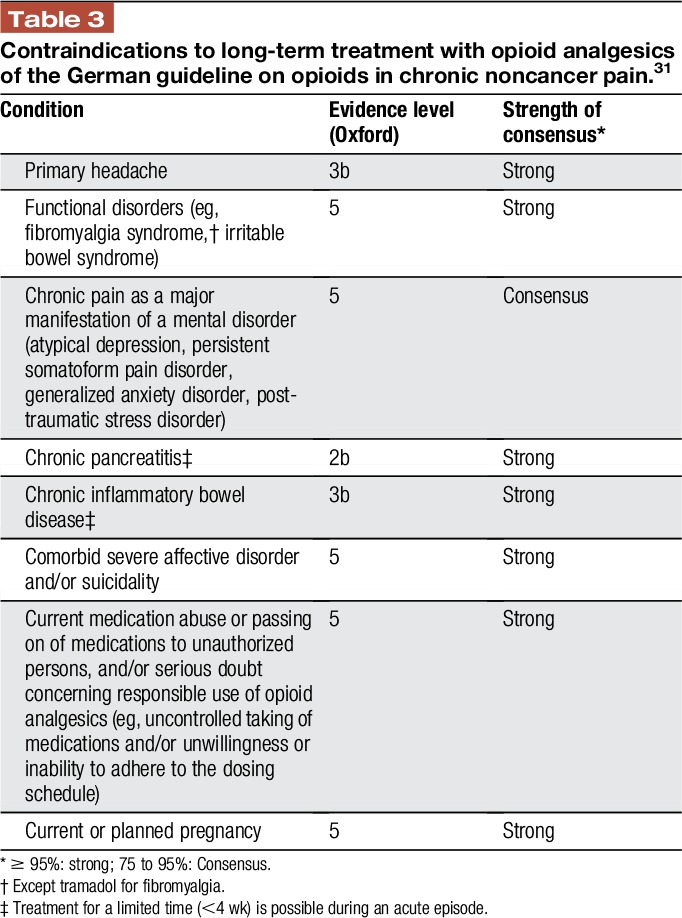
The CDC guidelines do not use the term “contraindications,” but outline that in some clinical contexts (eg, headache or fibromyalgia), expected benefits of initiating opioids are unlikely to outweigh risks regardless of previous nonpharmacologic and nonopioid pharmacologic therapies used.
3.4.2. Initiation and titration
(1) The selection and dosage of opioids should be tailored to the individual response of the patient. Therapy should be started with the minimum dose required to achieve relief of pain and/or improved functioning without clinically relevant adverse effects. The dose should be subsequently increased according to patient response and tolerability.
The Canadian guidelines provide detailed information on how to start and increase the dosage of the opioid.
(2) Daily dosages morphine equivalent requiring particular caution range from 90 to 200 mg ME/d.
(3) All guidelines recommend close monitoring of the patients for response and side effects in the first weeks of therapy.
3.4.3. Maintenance
(1) The German guideline clearly stated that LtOT should only be performed in “responders,” defined by the achievement of the individual treatment goals without or minor side effects. The Australian guidelines recommend tapered termination if treatment goals are not met, serious adverse outcomes or misuse occur or review appointments are not kept.
(2) All guidelines recommend regular reassessment (pain intensity and level of functioning, presence of adverse events, adherence to prescribed nonpharmacological therapies, aberrant drug behavior). The Australian guidelines request regular reassessment and documentation of 5As: analgesia, activity, adverse effects, affect, aberrant behaviour initially weekly then monthly. The German and CDC recommend that clinicians should evaluate benefits and harms of continued therapy with patients every 3 months or more frequently. Regular urine tests for controlled prescription drugs and illicit drugs are recommended by the United States and are suggested as an option as are pill counts in difficult situations by the Australian guidelines.
3.4.4. Discontinuation and stopping rules
(1) All guidelines give more or less detailed stopping rules such as serious or repeated aberrant drug-related behaviors or diversion, experience of intolerable adverse effects, or failure to maintain/achieve therapeutic goals. These are indications for discontinuation. The Australian guidelines advise on weaning strategies.
(2) The German guidelines recommend that after 6 months of opioid therapy, the possibility of dose reduction and/or a trial discontinuation should be discussed also with patients with a documented treatment response, to assess the indication for continuation of treatment and the potential response to the non-drug–based therapeutic measures initiated in parallel (eg, multimodal therapy).
3.4.5. Special situations
All guidelines contain more or less detailed information for special situations, eg, for patients with mental disorders including substance abuse; adolescents; pregnancy; seniors. The Canadian and CDC guidelines give advice for patients with obstructive sleep apnea and a specific recommendation to offer patients with opioid use disorder a therapy with buprenorphine or methadone in combination with behavioral therapies.
3.4.6. Practice tools
The Canadian8,25 and German13 guidelines offer online practice tools such as screening tools for mental disorders, opioid-equivalent tables, or treatment of nausea and constipation. There is a point-of-care tool based on the Canadian Opioid Guideline to help front-line prescribers and dispensers to remember and to document all important information related to the guideline.25,53
4. Discussion
4.1. Trends of opioid consumption in Australia, Canada, Germany, and United States
The trends of opioid consumption presented differ between the 4 countries. There is still an increase in Australia and Canada. By contrast, in Germany opioid consumption suddenly dropped in 2012, rose again in 2013 and dropped again in 2014. The total consumption of opioids decreased in the United States since 2012. We offer the following hypotheses for the different trends. In Australia and Canada, the overuse has not yet been stopped successfully. In Australia, oxycodone and fentanyl are so predominant as both got reimbursement registration for chronic nonmalignant pain and are actively promoted by the manufacturers to general practitioners. Fentanyl patches are the most frequently prescribed strong opioids in Germany (64). The Drug Commission of the German Medical Association has insistently warned against the uncritical use of fentanyl patches and recommended not to use these patches as first-line opioid therapy (2). The authors of a study with postmarketing surveillance data on trends of the diversion and abuse of prescription opioid medications between 2002 and 2013 concluded that the implementation of hundreds of local, regional, state, and federal interventions have started to work in the United States (11).
4.2. Similarities and differences of the guidelines on opioid analgesic treatment for chronic noncancer pain reviewed
The common features of the 4 guidelines presented here on patient selection and risk stratification, initiating, monitoring, and finishing opioid therapy for CNCP and practice tools might assist those planning to develop guidelines in other countries. Of course, some recommendations have to be adapted according to the national situation. Regular urine tests for illicit drugs for any patient make sense in countries with high rates of legal and illicit drug abuse, whereas they can be limited to selected patients in countries with of low rates of legal and illicit drug abuse. The guidelines markedly differed in the daily dosages morphine equivalent requiring particular caution with lowest doses (50 mg ME/d) given in the CDC9 and highest dosages (200 mg ME/d) given in the Canadian guideline.26 There is evidence from studies conducted in North America that the risk of harms increases with opioid dosage. One large US retrospective cohort study found that recent opioid use was associated with increased risk for any overdose events and serious overdose events vs nonuse. It also found higher doses associated with increased risk. Relative to 1–19 ME/d, the adjusted hazard ratio for any overdose event (consisting of mostly nonfatal overdose) was 1.44 for 20 to 49 ME/d, 3.73 for 50–99 M E/d, and 8.87 for ≥100 M E/d.16 A Canadian population-based, nested case-control study also found a dose-dependent association with risk for overdose death. Relative to 1–19 ME/d, the adjusted odds ratio was 1.32 for 20–49 ME/d, 1.92 for 50–99 ME/d, 2.04 for 100–199 ME/d, and 2.88 for ≥200 ME/d.28 On the other hand, there is no evidence that the benefits of opioids increase with dosage. In a randomized trial, the effectiveness of a stable dose (40 mg ME/d) prescribing strategy for opioid medications with an escalating dose (on average 50 mg ME/d) approach in 135 patients with musculoskeletal pain referred to a specialty pain clinic at a Veterans Affairs Hospital was compared over 12 months. No group differences were found for primary outcomes of usual pain or functional disability, although the escalating dose group did show a small but significantly larger increase in self-rated pain relief from medications. About 27% of patients were discharged over the course of the study because of opioid misuse/noncompliance, but there were no group differences in rate of opioid misuse.43 The ME-dosages in placebo-controlled randomized controlled trials in chronic low back pain,51 osteoarthritis55 and neuropathic pain61 ranged from 60 to 240 mg/d. In studies with multiple dosage arms, no significant differences in pain reduction were found in studies with different dosage arms.51,55,61 The ME in open-label extension studies >26 weeks of opioids on CNCP did not exceed 120 mg/d in most studies. However, the average ME was up to 240 mg MME in some studies.30 In sum, the minimal effective ME for CNCP still needs to be determined and there may remain disagreement on the maximal useful dose.
4.3. Comparison with other guideline recommendations
Because of the paucity of direct head-to-head comparisons of drugs and drugs with nonpharmacological therapies in most CNCP-syndromes, recommendations for first, second, and third line therapies for CNCP can only be based on network-meta analyses or indirect evidence (eg, number of studies, consistency of study findings; balancing potential benefits and harms; ease of use) and expert consensus.20
Opioids are not recommended as first-line drug therapy for any CNCP by the 4 guidelines reviewed nor by other current guidelines. Using the Grading of Recommendations Assessment, Development, and Evaluation and the results of a systematic review and meta-analysis, the Special Interest Group on Neuropathic Pain gave a strong recommendation for use and proposal as first-line treatment in neuropathic pain for tricyclic antidepressants, serotonin-norepinephrinee reuptake inhibitors, pregabalin, and gabapentin, a weak recommendation for use and proposal as second line for lidocaine patches, capsaicin high-concentration patches, and tramadol; and a weak recommendation for use as third line for strong opioids and botulinum toxin A.20 The American College of Rheumatology recommended acetaminophen, oral, and topical NSAIDs (in combination with a proton pump inhibitor) and intra-articular corticosteroid injection were conditionally recommended for first-line pharmacological management of patients with knee osteoarthritis. Intra-articular hyaluronate injections, duloxetine and opioids were conditionally recommended for second-line therapy. Opioids were strongly recommended for patients who were either not willing to undergo or had contraindications for total joint arthroplasty after the failure of first and second-line drug therapies.33 The French guideline on strong opioids in CNCP concluded that strong opioids have been shown to be moderately effective against CNCP because of osteoarthritis of the lower limbs, and for back pain and neuropathic pain. Their introduction is advised only after the failure of first-line treatments.41
In conclusion, all recent guidelines on opioids in CNCP agree that opioids should only be considered in selected patients with a clearly defined nociceptive and/or neuropathic pain syndrome. The recommendation of the German guideline not to use strong opioids for chronic daily headache and fibromyalgia,31 is in line with the recommendation of the American Pain Society, the French guideline on strong opioids in CNCP41 and the CDC guidelines.9
4.4. Potential reasons of the opioid epidemic
The opioid epidemic can be approached as a dynamic system, composed of networks of interacting individuals including nonusers, users, legal prescribers, illicit suppliers, treatment providers, supporters (family and friends), and law enforcement officials.7 (1) Pain societies: The campaign “Pain relief as a human right and pain as a fifth vital sign” acknowledged that there are severe restrictions on the availability of opioids and other essential medications, critical to the management of pain in most of the world.5,34 However, it might have promoted high-dose and/or prolonged opioid therapy for acute postsurgical pain in North American hospitals.9,39 (2) Opinion leaders. Over the past 20 years, laws and regulations by state medical boards and other authorities governing the prescribing of opioids for the treatment of CNCP were liberalized. This approach was driven by arguments in favor of opioids based solely on traditions, expert opinion, practical experience, and uncontrolled anecdotal observations.39 Statements on under-treatment of chronic pain in general might have promoted the idea that “strong” opioids are the most effective medication for every chronic pain syndrome. Some opinion leaders even claimed that CNCP when severe requires a treatment with “strong pain killers”.42 (3) Pain physicians and other medical practitioners: Applying the treatment principles of palliative care (titrate opioids to the effect of pain relief) to CNCP is an easy and time-saving approach to CNCP. It is in particular easier and less time consuming than to educate a patient about the biopsychosocial model of chronic pain, to motivate to use self-management strategies and to increase physical activity and self-efficacy. Already in 2003, Ballantyne and Mao highlighted that physicians should make every effort to control indiscriminate prescribing, even when they are under pressure by patients to increase the dose of opioids.3 It is also the easier way for patients reluctant to change their life style and increases patient satisfaction, at least initially. (4) Medical and political system: Generally, high levels of psychotrophic drug use, dynamics of medical-professional culture (including patient expectations for “effective treatment”), as well as the more pronounced “for-profit” orientation of key elements of health care (including pharmaceutical advertising), may have boosted the opioid epidemic observed in North America.21
4.5. Guideline implementation
The methodology quality of guidelines can be assessed by instruments such as the Appraisal of Guidelines for Research and Evaluation II instrument6 and A Measurement Tool to Assess Systematic Reviews tool.59 The Canadian Guideline for Safe and Effective Use of Opioids for Chronic Non-cancer Pain8,26 received high quality marks with both instruments.46 Research indicates that clinical guidelines are often not implemented appropriately by clinicians. The success of their implementation depends on the consideration of personal, external, and guideline-related barriers. Structured implementation can improve adherence to guidelines.23 To the best of our knowledge, the implementation of the guidelines presented and their impact on the prescribing behavior of physicians has not been tested until now. A project to improve Canadian family physicians' knowledge and performance in safe prescribing of opioids using the Canadian Guideline for Safe and Effective Use of Opioids for Non-Cancer Pain is currently funded by Health Canada.37
4.6. Limitations
The topics discussed in this review are specifically issues that affect Western first-world countries with relatively easy access to a variety of opioid analgesics. It has to be acknowledged that in many parts of the world, the biggest problem with opioids is their lack of availability and difficulties with access for medical use.1 In these countries, opioids are often not or rarely available even for the treatment of acute pain after trauma or surgery or of cancer pain. This explains the extreme discrepancies in opioid consumption per capita in 2014 between the ones shown in this review for the countries discussed here (in the range of multiple hundred mg) and those for countries like India (0.56 mg), Zambia (0.8 mg), and Nicaragua (1.1 mg).49 However, even in this context, it is important to address the inappropriate use in first-world countries as the reports about this are used by governments with very restrictive opioid regulations as arguments in favor of the status quo.
The different time periods, type of opioids assessed, and population samples in the Australian, Canadian, and German studies on medical and nonmedical use forbids a direct comparison of the 3 countries. However, the data presented outline national trends in opioid consumption in the 3 countries.
The figures of opioid consumption presented may not be reliable as they are based on the import of opioids to various countries. The figures do not to differentiate between in-hospital and outpatient prescriptions and or for perioperative, cancer, or noncancer management. In addition, caution is required when interpreting the data on opioid consumption between countries because of differences in the collection and reporting of data.29 Furthermore, the DDD for opioids may not reflect their relative clinical potencies. Oral morphine equivalent reflects clinical dosing better than DDD and can also lead to different conclusions in opioid consumption studies compared with using DDD alone.62
5. Conclusions
There is a broad consensus in the pain community that the opioid epidemic in North America and Australia needs to be addressed immediately and that Europe and most importantly low and middle income countries should be protected from similar negative outcomes. In our opinion, this goal should not be achieved by critically disputing the long-term efficacy and safety of opioids in carefully selected patients with defined nociceptive and neuropathic pain conditions. The CDC required long-term (>1 year) outcomes related to pain, function, or quality of life for opioids compared to placebo or any other treatment.9 These data are likewise not available for any other drug treatment options in CNCP. It is important to emphasize the risks of long-term opioid therapy. However, long-term therapy with antidepressants, anticonvulsants, and NSAIDs is associated with frequent and sometimes severe side effects, too. LtOT of CNCP should not be expanded without caution and uncritically, but in view of the existing data it should not be categorically rejected, either.19,32 Opioid analgesics remain one option as a component of multimodal pain management of some carefully selected patients for example with chronic osteoarthritis pain, nociceptive and/or neuropathic low back pain, and other neuropathic pain states.
Overall, the primary culprit causing the North American and Australian opioid epidemic seems not be opioids per se, but the way in which many patients are treated with opioids, ie, without appropriate indication, appropriate precautions, and with excessive doses, often as a monotherapy for chronic pain.
Disclosures
Winfried Häuser received a honorarium by Grünenthal for 1 eductional lecture.
The Anaesthesiology Unit of the University of Western Australia, chaired by Stephan Schug, but not him personally, has received research funding, consultation fees, travel grants, and lecture honoraria from Pfizer Pharmaceuticals, bioCSL/Seqirus, iX Biopharma, and Mundipharma.
Andrea D. Furlan acknowledges the Canadian Institutes for Health Research (CIHR)—Drug Safety and Effectiveness Network for salary funding.
Some of the data were presented in a topical workshop at the 16th World Congress on Pain on September 27, Yokohama.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Anderson T. The politics of pain. BMJ 2010;341:c3800. [DOI] [PubMed] [Google Scholar]
- [2].Arzneimittelkommission der deutschen Ärzteschaft (Drug Commission of the German Medical Association). UAW-News International Die unkritische Anwendung von Fentanylpflastern erhöht das Risiko für schwerwiegende Nebenwirkungen (The uncritical use of fentanyl patches increases the risk of serious adverse events. Dtsch Ärztebl 2012;109:A724–725. [Google Scholar]
- [3].Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med 2003;349:1943–3. [DOI] [PubMed] [Google Scholar]
- [4].Blanch B, Pearson SA, Haber PS. An overview of the patterns of prescription opioid use, costs and related harms in Australia. Br J Clin Pharmacol 2014;78:1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brennan F, Cousins MJ. Pain relief as a human right. Pain Clinical Updates 2004;12:1–4. [Google Scholar]
- [6].Brouwers M, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium. On behalf of the AGREE Next Steps Consortium: AGREE II: Advancing guideline development, reporting and eval uation in healthcare. J Clin Epidemol 2010;63:1308–11. [DOI] [PubMed] [Google Scholar]
- [7].Burke DS. Forecasting the opioid epidemic. Science 2016;35:529. [DOI] [PubMed] [Google Scholar]
- [8].Canadian Guideline for Safe and Effective Use of Opioids for Chronic Non-Cancer Pain. Practicetoolkits. Available at: www.nationalpaincentre.mcmaster.ca/documents/practicetoolkit.pdf. Accessed December 1, 2016. [Google Scholar]
- [9].CDC guideline for prescribing opioids for chronic pain—United States, 2016. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed December 1, 2016. [DOI] [PubMed] [Google Scholar]
- [10].Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O'Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009;10:113–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 2015;372:241–8. [DOI] [PubMed] [Google Scholar]
- [12].Degenhardt L, Gisev N, Cama E, Nielsen S, Larance B, Bruno R. The extent and correlates of community-based pharmaceutical opioid utilisation in Australia. Pharmacoepidemiol Drug Saf 2016;25:521–38. [DOI] [PubMed] [Google Scholar]
- [13].Deutsche Schmerzgesellschaft. Guideliens initiated by the German Pain Society. Long-term opioid therapy for chronic non-cancer pain. Available at: www.dgss.org/versorgung/leitlinien-zur-schmerzbehandlung/von-der-deutschen-schmerzgesellschaft-ev-initiierte-leitlinien. Accessed December 1, 2016. [Google Scholar]
- [14].Dhalla IA, Mamdani MM, Sivilotti ML, Kopp A, Qureshi O, Juurlink DN. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ 2009;18:891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Drug commissary of the German government. Drug report 2015. Available at: www.drogenbeauftragte.de. Accessed December 1, 2016. [Google Scholar]
- [16].Dunn KM, Saunders KW, Rutter CM. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85– 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].European Monitoring Center for Drugs and addiction. European drug report 2016. Available at: http://www.emcdda.europa.eu/system/files/publications/2637/TDAT16001DEN.pdf. Accessed December 1, 2016. [Google Scholar]
- [18].Faculty of Pain Medicine, Australian and New Zealand College of Anaesthetists (FPMANZCA). Recommendations regarding the use of opioid analgesics in patients with chronic non-cancer pain. Available at: http://fpm.anzca.edu.au/documents/pm1-2010.pdf and http://fpm.anzca.edu.au/documents/4462_001.pdf. Accessed December 1, 2016. [Google Scholar]
- [19].Fanelli G, Tölle TR, DE Andrés J, Häuser W, Allegri M, Montella S, Kress HG. Opioids for chronic non-cancer pain: a critical view from the other side of the pond. Minerva Anestesiol 2016;82:97–102. [PubMed] [Google Scholar]
- [20].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fischer B, Keates A, Bühringer G, Reimer J, Rehm J. Non-medical use of prescription opioids and prescription opioid-related harms: why so markedly higher in North America compared to the rest of the world? Addiction 2014;109:177–81. [DOI] [PubMed] [Google Scholar]
- [22].Deleted in proof. [Google Scholar]
- [23].Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and strategies in guideline implementation-a scoping review. Basel: Healthcare, 2016;4:pii E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Deleted in proof. [Google Scholar]
- [25].Furlan AD, Reardon R, Salach L. The opioid manager: a point-of-care tool to facilitate the use of the Canadian Opioid Guideline. J Opioid Manag 2012;8:57–61. [DOI] [PubMed] [Google Scholar]
- [26].Furlan AD, Reardon R, Weppler C; National Opioid Use Guideline Group. Opioids for chronic noncancer pain: a new Canadian practice guideline. CMAJ 2010;182:923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gomes T, Mamdani MM, Dhalla IA, Cornish S, Paterson JM, Juurlink DN. The burden of premature opioid-related mortality. Addiction 2014;109:1482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011;171:686–91. [DOI] [PubMed] [Google Scholar]
- [29].Hamunen K, Laitinen-Parkkonen P, Paakkari P, Breivik H, Gordh T, Jensen NH, Kalso E. What do different databases tell about the use of opioids in seven European countries in 2002? Eur J Pain 2008;12:705–15. [DOI] [PubMed] [Google Scholar]
- [30].Häuser W, Bernardy K, Maier C. Long-term opioid therapy in chronic noncancer pain. A systematic review and meta-analysis of efficacy, tolerability and safety in open-label extension trials with study duration of at least 26 weeks. Schmerz 2015;29:96–108. Erratum in: Schmerz 2015;29:309. [DOI] [PubMed] [Google Scholar]
- [31].Häuser W, Bock F, Engeser P, Hege-Scheuing G, Hüppe M, Lindena G, Maier C, Norda H, Radbruch L, Sabatowski R, Schäfer M, Schiltenwolf M, Schuler M, Sorgatz H, Tölle T, Willweber-Strumpf A, Petzke F. Recommendations of the updated LONTS guidelines. Long-term opioid therapy for chronic noncancer pain. Schmerz 2015;29:109–30. [DOI] [PubMed] [Google Scholar]
- [32].Häuser W, Petzke F, Radbruch L, Tölle TR. The opioid epidemic and the long-term opioid therapy for chronic noncancer pain revisited: a transatlantic perspective. Pain Manag 2016;6:249–63. [DOI] [PubMed] [Google Scholar]
- [33].Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–74. [DOI] [PubMed] [Google Scholar]
- [34].International Association for the Study of Pain. Declaration of Montréal. Declaration that access to pain management is a fundamental human right. Available at: www.iasp-pain.org/DeclarationofMontreal?navItemNumber=582. Accessed December 1, 2016. [DOI] [PubMed] [Google Scholar]
- [35].Karanges EA, Blanch B, Buckley NA, Pearson SA. Twenty-five years of prescription opioid use in Australia: a whole-of-population analysis using pharmaceutical claims. Br J Clin Pharmacol 2016;82:255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kipping K, Maier C, Bussemas HH, Schwarzer A. Medication compliance in patients with chronic pain. Pain Physician 2014;17:81–94. [PubMed] [Google Scholar]
- [37].Leece P. Update on health Canada project: family physicians & opioid prescribing. Available at: http://www.cpso.on.ca/CPSO/media/documents/Methadone/Presentations/16Nov25_Leece-Pamela.pdf. Accessed February 18, 2016. [Google Scholar]
- [38].Maier C, Schaub C, Willweber-Strumpf A, Zenz M. Long-term efficiency of opioid medication in patients with chronic non-cancer-associated pain. Results of a survey 5 years after onset of medical treatment. Schmerz 2005;19:410–17. [DOI] [PubMed] [Google Scholar]
- [39].Manchikanti L, Helm S, II, Fellows B, Janata JW, Pampati V, Grider JS, Boswell MV. Opioid epidemic in the United States. Pain Physician 2012;15:ES9–38. [PubMed] [Google Scholar]
- [40].Marschall U, L'hoest H, Radbruch L, Häuser W. Long-term opioid therapy for chronic non-cancer pain in Germany. Eur J Pain 2016;20:767–76. [DOI] [PubMed] [Google Scholar]
- [41].Moisset X, Trouvin AP, Tran VT, Authier N, Vergne-Salle P, Piano V, Martinez V. Use of strong opioids in chronic non-cancer pain in adults. Evidence-based recommendations from the French Society for the Study and Treatment of Pain. Presse Med 2016;45:447–62. [DOI] [PubMed] [Google Scholar]
- [42].Müller-Schwefe G. Aktionsprogramm Schmerz. Z Angew Schmerzth 1998;4:1 Available at: www.schmerz-therapie-deutschland.de/pages/zeitschrift/z4_98/art_401.htm. Accessed December 1, 2016. [Google Scholar]
- [43].Naliboff BD, Wu SM, Schieffer B, Bolus R, Pham Q, Baria A, Aragaki D, Van Vort W, Davis F, Shekelle P. A randomized trial of 2 prescription strategies for opioid treatment of chronic nonmalignant pain. J Pain 2011;12:288–96. [DOI] [PubMed] [Google Scholar]
- [44].Nielsen S, Bruno R, Degenhardt L, Stoove MA, Fischer JA, Carruthers SJ, Lintzeris N. The sources of pharmaceuticals for problematic users of benzodiazepines and prescription opioids. Med J Aust 2013;199:696–9. [DOI] [PubMed] [Google Scholar]
- [45].Novak SP, Håkansson A, Martinez-Raga J, Reimer J, Krotki K, Varughese S. Nonmedical use of prescription drugs in the European Union. BMC Psychiatry 2016;16:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nuckols TK, Anderson L, Popescu I, Diamant AL, Doyle B, Di Capua P, Chou R. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med 2014;160:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Deleted in proof. [Google Scholar]
- [48].Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med 2010;363:1981–5. [DOI] [PubMed] [Google Scholar]
- [49].Pain & Policy Studies Group, University of Wisconsin/WHO Collaborating Center, International Narcotics Control Board. World Health Organization population data 2015. Available at: http://www.painpolicy.wisc.edu/countryprofiles. Accessed December 1, 2016. [Google Scholar]
- [50].Deleted in proof. [Google Scholar]
- [51].Petzke F, Welsch P, Klose P, Schaefert R, Sommer C, Häuser W. Opioids in chronic low back pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration. Schmerz 2015;29:60–72. Erratum in: Schmerz 2015;29:308. [DOI] [PubMed] [Google Scholar]
- [52].Rintoul AC, Dobbin MD, Drummer OH, Ozanne-Smith J. Increasing deaths involving oxycodone, Victoria, Australia, 2000-09. Inj Prev 2011;17:254–9. [DOI] [PubMed] [Google Scholar]
- [53].Robertson A, Hitzig SL, Furlan AD. An evaluation of the performance of the Opioid Manager clinical tool in primary care: a qualitative study. J Opioid Manag 2014;10:187–99. [DOI] [PubMed] [Google Scholar]
- [54].Roxburgh A, Bruno R, Larance B, Burns L. Prescription of opioid analgesics and related harms in Australia. Med J Aust 2011;195:280–4. [DOI] [PubMed] [Google Scholar]
- [55].Schaefert R, Welsch P, Klose P, Sommer C, Petzke F, Häuser W. Opioids in chronic osteoarthritis pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration. Schmerz 2015;29:47–59. [DOI] [PubMed] [Google Scholar]
- [56].Schmiedl S, Rottenkolber M, Hasford J, Rottenkolber D, Farker K, Drewelow B, Hippius M, Saljé K, Thürmann P. Self-medication with over-the-counter and prescribed drugs causing adverse-drug-reaction-related hospital admissions: results of a prospective, long-term multi-centre study. Drug Saf 2014;37:225–35. [DOI] [PubMed] [Google Scholar]
- [57].Schneeweiss S, Hasford J, Göttler M, Hoffmann A, Riethling AK, Avorn J. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. Eur J Clin Pharmacol 2002;58:285–91. [DOI] [PubMed] [Google Scholar]
- [58].Schubert I, Ihle P, Sabatowski R. Increase in opiate prescription in Germany between 2000 and 2010: a study based on insurance data. Dtsch Arztebl Int 2013;110:45–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, Henry DA, Boers M. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62:1013–20. [DOI] [PubMed] [Google Scholar]
- [60].Shield KD, Jones W, Rehm J, Fischer B. Use and nonmedical use of prescription opioid analgesics in the general population of Canada and correlations with dispensing levels in 2009. Pain Res Manag 2013;18:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sommer C, Welsch P, Klose P, Schaefert R, Petzke F, Häuser W. Opioids in chronic neuropathic pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration. Schmerz 2015;29:35–46. [DOI] [PubMed] [Google Scholar]
- [62].Svendsen K, Borchgrevink P, Fredheim O, Hamunen K, Mellbye A, Dale O. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med 2011;25:725–32. [DOI] [PubMed] [Google Scholar]
- [63].The Ontario Drug Policy Research Network. Opioid use and related adverse events in Ontario. 2016. Available at: http://odprn.ca/wp-content/uploads/2016/11/ODPRN-Opioid-Use-and-Related-Adverse-Events-Nov-2016.pdf. Accessed February 20, 2017.
- [64].Werber A, Marschall U, L'hoest H, Hauser W, Moradi B, Schiltenwolf M. Opioid therapy in the treatment of chronic pain conditions in Germany. Pain Physician 2015;18:E323–31. [PubMed] [Google Scholar]


