Abstract
Risk stratification for the development chronic postsurgical pain is an important tool, which may permit preventive measures or appropriate advice for patients at high risk.
Keywords: Chronic postsurgical pain, Persistent postsurgical pain, Risk stratification, Genetics, Psychosocial, Surgery
Key Points
Chronic postsurgical pain (CPSP) is a common complication of surgery with important consequences for the individual patient and society as a whole.
Risk stratification is best defined as the grouping of patients based on factors measured at baseline (in this context before surgery), to determine an individual's risk of suffering a particular condition and thereby the likely level of need for preventive interventions.
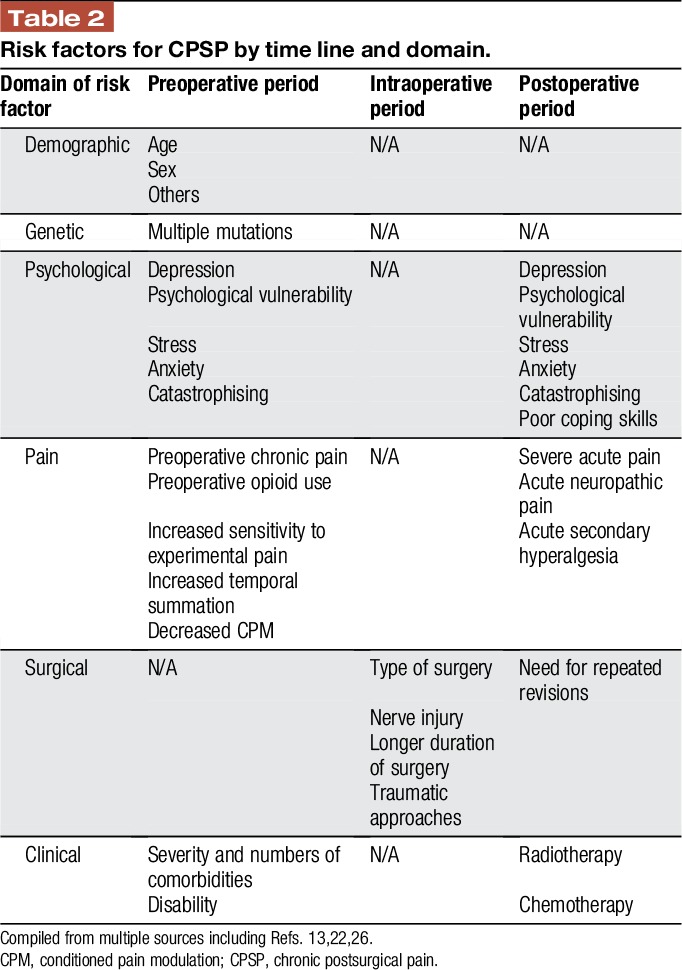
Risk factors for CPSP have been identified in the preoperative, intraoperative, and postoperative periods and cover 6 broad domains: genetic, demographic, psychosocial, pain, clinical, and surgical factors.
Risk stratification for CPSP enables clinicians to address these risk factors before surgery, to discuss the necessity of surgery or to change the surgical and anaesthetic/analgesic planning.
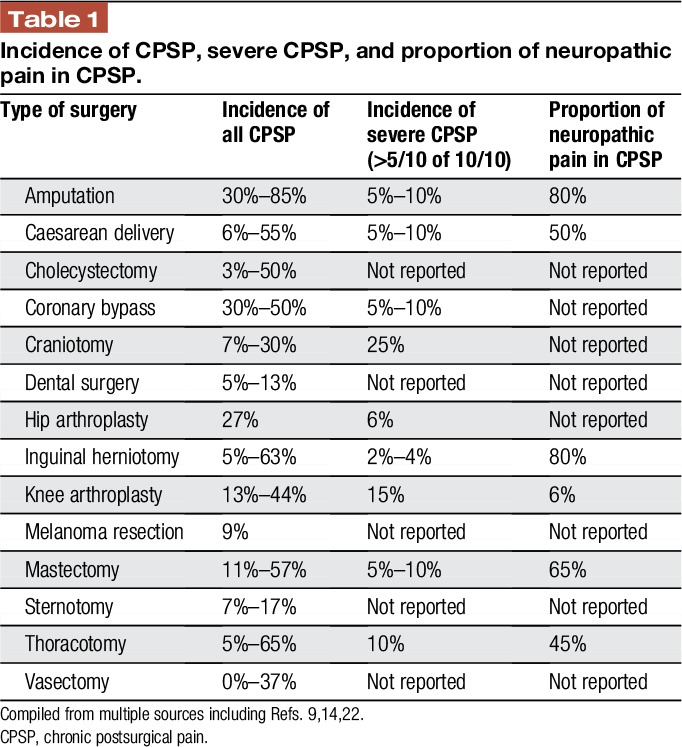
The past 20 years have seen an increasing recognition of the burden of chronic pain after surgery and other trauma. There is now good evidence that chronic postsurgical pain (CPSP) is by far more common and more severe than previously thought with far-reaching consequences for quality of life and function of those affected. There are also significant implications and costs for health care systems and society as a whole.14 Table 1 highlights this by showing the incidence of chronic pain after a number of surgical interventions, as well as the proportion of patients who experience severe pain and the contribution of neuropathic pain features to this presentation. The wide variability of these numbers is largely due to methodological differences, caused by the use of variable definitions for chronicity, in particular regarding the time frame applied for measurement (between 2 and 12 months). Other factors include differences in study design (eg, cross-sectional, prevalence surveys or prospective surgical cohort studies), as well as variable assessment of preoperative chronic pain and measurement of postoperative pain. Attempts to standardise a definition have been based on the initial proposal by Macrae and Davies,17 modified by Werner and Kongsgaard,27 which have now been presented by the IASP to the WHO for the upcoming revision of the International Classification of Diseases Eleventh Revision (ICD-11) as25: “Chronic postsurgical pain is pain developing after a surgical procedure and persisting beyond the healing process, ie, at least 3 months after surgery. The pain is either localized to the surgical field, projected to the innervation territory of a nerve situated in this area, or referred to a dermatome (after surgery/injury to deep somatic or visceral tissues). Other causes of pain including infection, malignancy etc. need to be excluded as well as pain continuing from a preexisting pain problem.”
Table 1.
Incidence of CPSP, severe CPSP, and proportion of neuropathic pain in CPSP.
These initial observations have resulted in a large research effort focussing on the topic of CPSP. This research effort has focussed initially on the epidemiology to establish the burden of disease but very soon expanded to attempts to identify predictive factors as well as preventive and treatment measures. This has led to the question of whether identification of risk factors can guide such preventive measures. Risk stratification is best defined as the grouping of patients based on factors measured at baseline (in this context before surgery), to determine an individual's risk of suffering a particular condition and thereby the likely level of need for preventive interventions. Although terms are often used interchangeably, prognostic factors refer to factors that influence the outcome in people who already have the condition. The following brief review summarises the current knowledge on predictive factors for chronic pain after surgery in an attempt to present strategies for risk stratification. It is based on the literature searches performed by the systematic reviews and meta-analyses quoted as well as an updated MEDLINE search of relevant keywords performed in line with a recent systematic review.26
1. Classification of putative risk factors
Risk factors for CPSP can be broadly classified by their temporal characteristics in relation to the time frame of surgery into preoperative, intraoperative, and postoperative factors (here early and late). Although the identification of preoperative and intraoperative risk factors is most desirable to identify those at greatest risk preoperatively and thus take preventive measures, risk factors captured in the acute postoperative period may still offer a chance for mitigation. For example, those identified as being at greater risk in the hours or days after surgery could be referred for early involvement of an acute pain service (APS) or early management and follow-up by a “transitional pain clinic” or “APS outpatient clinic.”12
Other than by temporal characteristics, recent publications suggest a classification in domains based on reviews of the multidimensional nature of these risk factors.26 These domains include innate risk factors such as genetic predisposition, demographic factors (eg, age and socioeconomic status), sensitivity to experimentally induced pain and pain history, as well as domains potentially amenable to interventions, such as psychological, medical comorbidities and surgical approaches. It is of note that 1 key surgical risk factor is the type of surgery, which means that risk factors may not be generalizable across all operations but are “procedure specific.”
Table 2 proposes a grid for these risk factors, which will be discussed in more detail in the following paragraphs.
Table 2.
Risk factors for CPSP by time line and domain.
1.1. Demographic risk factors
Younger age, but not very young age, ie, in paediatric age groups, seems to be a relatively consistent demographic risk factor in almost all studies.13,26 This is contrary to findings in other chronic pain diagnoses, where increased age can predispose to higher rates of chronic pain. Female sex, which is a risk factor for more severe acute and more common chronic pain states, is also a risk factor for CPSP,9,13 although not universally.26 Similarly, other sociodemographic risk factors such as educational, socioeconomic, or compensatory status have been identified in some studies, but inconsistently reported within systematic reviews and meta-analyses. Compensatory and employment-related factors have been mostly measured in studies of failed spinal surgery.23
1.2. Genetic risk factors
Chronic pain is currently regarded as a complex heritable trait with heritability in the range of 30% to 70%.5 It is therefore not surprising that CPSP has genetic risk factors. Limited evidence has been found for mutations of single candidate genes such as those encoding ion channels (potassium and calcium) and purinergic receptors influencing severity or frequency of CPSP, eg, after mastectomy or amputation.5 Furthermore, mutations of the gene encoding catechol-O-methyltransferase, an enzyme in the monoaminergic pathway influencing pain inhibition, and the OPRM1 gene encoding the μ-opioid receptor have been shown to affect CPSP.11 Limited findings have been reported with a number of other genes, including GCH1, CACNG, CHRNA6, P2X7R, cytokine-associated genes, human leucocyte antigens, DRD2, and ATXN1.11 Currently, genome-wide approaches are under way to identify further target genes.5
1.2.1. Sensitivity to experimentally induced pain
In a number of preoperative settings such as herniotomy and total knee joint replacement, rating experimental pain stimuli as being of increased intensity (hyperalgesia) has been identified as a risk factor for CPSP.9 Even more sensitive risk factors seem to be increased temporal summation as a measure of increased excitability and decreased conditioned pain modulation (CPM; previously diffuse noxious inhibitory control [DNIC]) as manifestation of decreased inhibitory function13; these findings are again in line with those in chronic pain states such as fibromyalgia.
1.3. Psychosocial risk factors
Chronic pain is now commonly regarded as a “sociopsychobiomedical” issue and not surprisingly; this is also true for CPSP.4 Psychosocial risk factors for its development have therefore been identified early, and these are both cognitive behavioural and emotional. In an early systematic review, depression, psychological vulnerability, stress, and late return to work were identified as the primary psychosocial risk factors.10 Recent work has focused on psychological factors, such as preoperative anxiety, including trait anxiety as well as fears specific to pending surgery, but even more so the concept of catastrophizing.24 Pain catastrophizing has been broadly defined as a tendency to magnify or exaggerate the threat value or seriousness of painful sensations.20
Other factors identified inconsistently include somatisation (hypochondriasis), avoidant coping, and low expectations of returning to work.26 Recent research has also focused on the protective role of positive psychological factors, such as resilience, optimism, and robustness, broadly interpreted as the ability to respond effectively to risk or adversity.3
Psychological factors in the postoperative period play a similar role as in the preoperative setting; identification by the Somatic Preoccupation and Coping (SPOC) questionnaire 6 weeks after surgery was a predictor of CPSP and pain interference at 1 year.15
1.4. Pain predicts pain
Pain itself is a risk factor: the strongest predictors of CPSP are chronic preoperative pain and the severity of acute postoperative pain.26 This risk factor is most complex, as chronic preoperative pain may well be a manifestation of the presence of many other risk factors for chronic pain in general and thereby a logical predictor of CPSP. Furthermore, acute postoperative pain of severe intensity could be the result of heightened pain susceptibility but also a consequence of poor postoperative pain management and then, thus, highly amenable to pain management strategies as well as preventive treatment. However, severe acute pain could also be an early manifestation of the subsequent CPSP, in particular, in the setting of acute neuropathic pain,9 and thereby not so much a predictive factor, but an early manifestation of the problem. Several well-designed prospective studies have found that early postoperative neuropathic symptoms are associated with higher rates of CPSP.
1.4.1. Preoperative pain
Regarding preoperative pain, as well as pain at the site of the operation, there is emerging evidence that general chronic pain states, such as chronic low back pain, irritable bowel syndrome, or fibromyalgia, increase the risk of CPSP. Furthermore, prospective studies have found that the longer the duration of preoperative chronic pain and more severe intensity of this pain are associated with higher likelihood of reporting pain persistence after surgery.9,13,26 Long-term opioid treatment of chronic pain may increase the risk of CPSP due to opioid-induced hyperalgesia as a further contributing relevant factor.16 Epidemiological studies have yet to explore the independent contribution of different factors, for example, untangling the relationship between experimentally induced pain sensitivity in those with or without chronic pain before surgery.
1.4.2. Acute postoperative pain
The severity of acute postoperative pain in the first days after surgery is highly predictive of CPSP.13 The analysis of pain trajectories in the acute postoperative period has revealed further that trajectories with constant higher pain intensity carry the most risk.2,19 As outlined in section 1.4., this may be a reflection of severe unrelieved pain being a manifestation of acute neuropathic pain, by itself a risk factor for subsequent CPSP.9 This is in line with observations of increased secondary hyperalgesia after surgery as another risk factor, which might be an early manifestation of nerve injury.
1.5. Surgical risk factors
The type of operation itself is predictive of CPSP; as suggested from Table 1, different operations have different risks of CPSP, its severity, and the incidence of neuropathic pain among those patients affected. This suggests that CPSP is to an extent procedure specific, and thereby, consideration of different risk factors and their relevance for different operations is worthy of investigation. Procedure-specific issues during axillary clearance in breast cancer surgery include nerve localisation, intercostobrachial nerve involvement, either from nerve handling or from complete or from partial dissection, and treatment-related factors, such as radiotherapy.3
The variety of risk factors identified in studies of various procedures supports this assumption. However, even minor surgery such as superficial melanoma resection or dental implant carries the risk of CPSP development.7
In many studies, longer duration of surgery, more traumatic or extensive approaches (eg, laparoscopic vs open hernia repair), or need for repeated revisions increase the risk of CPSP, possibly as a consequence of inflammatory response.26 In addition, intraoperative nerve injury is a risk factor, possibly explaining the linkage to acute neuropathic pain in the early postoperative pain and neuropathic pain characteristics in chronic states. However, studies on the intraoperative handling of nerves, ie, identifying nerves deliberately, preserving these nerves, or dissecting them, have led to inconclusive results.9
1.6. Medical comorbidities
Preoperative medical comorbidities, their severity and numbers, and preoperative disability have been identified in reviews of risk factors for CPSP.26 There is overlap with the earlier pain domain, eg, for painful conditions such as irritable bowel syndrome, rheumatoid arthritis, Raynaud syndrome, etc. History of peripheral neuropathy was found to increase the risk of chronic postoperative neuropathic pain in a large French survey.8 The effects of obesity and increased body mass index may be further risk factors, although findings here again are contradictory.
1.7. Chronic postsurgical pain in paediatric patients
Data on CPSP in children are limited and are summarized in a recent systematic review.21 The median prevalence of CPSP at 12 months after surgery over a range of operations was 20%. Similarly to adults, preoperative pain intensity, child anxiety, and child pain coping efficacy, but interestingly also parental pain catastrophizing, have been identified as risk factors; no biological or medical preoperative risk factors have yet been identified.
1.8. Integration of multiple risk factors to risk prediction tools
This brief overview of evidence suggests that the risk factors listed here are not independent of each other, but interlinked. For example, preoperative chronic pain is more common in females, while also sensitivity to experimental pain stimuli is often accompanied by mood disorders such as depression and anxiety. It is therefore not surprising that patients with established chronic pain and pain-related behaviours have a greater chance of reporting increased acute postoperative pain, which is often difficult to treat because of tolerance and opioid-induced hyperalgesia from their treatment of chronic pain with high-dose opioids. Similar interactions and correlations between others of these risk factors exist, as outlined for nerve injury above, thereby also explaining why certain operations with a significant risk of nerve injury (eg, thoracotomy) or even deliberate severing of nerves (eg, amputation) carry an increased risk of CPSP.
On the basis of the risk factors presented here, there have been a few attempts to develop prediction tools for CPSP. An early attempt was focused on the prediction of postherniotomy pain.1 Of the multiple risk factors identified by logistic regression, those that were available preoperatively were chosen to predict overall risk: severity of preoperative pain–related impairment by the value on the Activity Assessment Scale (AAS) and preoperative rating of pain caused by a tonic heat stimulus at 47°C. The simple tool had fair predictive and discriminatory ability. The authors suggested using this approach to direct patients at high risk (severe preoperative impairment and high preoperative pain sensitivity) away from open surgery (70% risk of CPSP for this group) to laparoscopic hernia repair (30% risk of CPSP). However, this tool has not been widely used or further validated.
Another research group developed a composite risk index for the development of CPSP after a wide range of surgical interventions.2 They started with 14 risk factors similar to the ones described in this review. The final model of multivariate analyses, through logistic regression, identified 4 predictive risk factors (2 factors related to preoperative pain ratings and 2 psychosocial factors), which had a sensitivity of 74% and specificity of 65%. Again, there are no reports of further application of this tool.
Pain after breast cancer surgery is well recognised, with 15% to 20% of women experiencing moderate to severe pain 1 year after breast cancer surgery. A prediction model for persistent pain after breast cancer was recently developed and tested using 3 different prospective data sets.18 Prediction models were firstly developed using data from 860 patients in Finland, then, models were tested in 2 independent cohorts from Denmark and Scotland. Identified risk factors included younger age, body mass index, preoperative pain intensity, and acute postoperative pain intensity on the first and seventh postoperative days. These prediction tools will require further refinement but can be used to identify those most at risk and potentially inform the targeting of preventive interventions.
2. Conclusions
Research over the past 20 years has identified a large number of risk factors for the development of CPSP. Risk factors have been identified in the preoperative, intraoperative, and postoperative periods and cover 6 broad domains: genetic, demographic, psychosocial, pain, clinical, and surgical factors.
Risk stratification for CPSP is possible looking at preoperative risk factors with the options to address these risk factors before surgery, discuss the necessity of surgery with the surgeon and patient, or changing the surgical and anaesthetic/analgesic planning to reduce exposure to intraoperative and postoperative risk factors. Genetic and demographic determinants are given, but all other factors can be addressed to reduce risk. Even postoperative risk factors can be addressed when identified and may thereby change the long-term prognosis of CPSP by good postoperative pain control and early management of potentially developing chronic pain.
In practical terms, the current data suggest that in the preoperative setting, patients at risk should be identified in particular by asking for longstanding painful conditions. In the postoperative period, early identification of severe pain intensity, increasing pain trajectories, and neuropathic pain states may enable APSs to address these conditions, and, in particular if not resolving, justify early ongoing care by systems such as the recently suggested “Transitional Pain Services.”12
Finally, it is of note that many of the risk factors are those identified as factors of “pain vulnerability”6 or of, as described by Wolfe in a more specific setting, “fibromyalgianess.”28 These terms describe factors underlying vulnerability towards chronic pain and are thereby the opposite constructs to resilience to chronic pain. Chronic postsurgical pain is not only a condition, which we need to prevent and manage, but it is also a model for the development of many other chronic pain states, such as back pain, which represent a progression of acute to chronic pain.
Disclosures
The authors have no conflicts of interest to declare.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Aasvang EK, Gmaehle E, Hansen JB, Gmaehle B, Forman JL, Schwarz J, Bittner R, Kehlet H. Predictive risk factors for persistent postherniotomy pain. Anesthesiology 2010;112:957–69. [DOI] [PubMed] [Google Scholar]
- [2].Althaus A, Hinrichs-Rocker A, Chapman R, Arranz Becker O, Lefering R, Simanski C, Weber F, Moser KH, Joppich R, Trojan S, Gutzeit N, Neugebauer E. Development of a risk index for the prediction of chronic post-surgical pain. Eur J Pain 2012;16:901–10. [DOI] [PubMed] [Google Scholar]
- [3].Bruce J, Thornton AJ, Powell R, Johnston M, Wells M, Heys SD, Thompson AM, Cairns Smith W, Chambers WA, Scott NW; Recovery Study Group. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. PAIN 2014;155:232–43. [DOI] [PubMed] [Google Scholar]
- [4].Carr DB, Bradshaw YS. Time to flip the pain curriculum? Anesthesiology 2014;120:12–4. [DOI] [PubMed] [Google Scholar]
- [5].Clarke H, Katz J, Flor H, Rietschel M, Diehl SR, Seltzer Z. Genetics of chronic post-surgical pain: a crucial step toward personal pain medicine. Can J Anaesth 2015;62:294–303. [DOI] [PubMed] [Google Scholar]
- [6].Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci 2014;17:192–200. [DOI] [PubMed] [Google Scholar]
- [7].Devine M, Taylor S, Renton T. Chronic post-surgical pain following the placement of dental implants in the maxilla: a case series. Eur J Oral Implantol 2016;9(suppl 1):179–86. [PubMed] [Google Scholar]
- [8].Duale C, Ouchchane L, Schoeffler P, Group EI, Dubray C. Neuropathic aspects of persistent postsurgical pain: a French multicenter survey with a 6-month prospective follow-up. J Pain 2014;15:24.e1–20. [DOI] [PubMed] [Google Scholar]
- [9].Gerbershagen HJ. Transition from acute to chronic postsurgical pain. Physiology, risk factors and prevention [in German]. Schmerz 2013;27:81–93. [DOI] [PubMed] [Google Scholar]
- [10].Hinrichs-Rocker A, Schulz K, Jarvinen I, Lefering R, Simanski C, Neugebauer EA. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP)—a systematic review. Eur J Pain 2009;13:719–30. [DOI] [PubMed] [Google Scholar]
- [11].Hoofwijk DM, van Reij RR, Rutten BP, Kenis G, Buhre WF, Joosten EA. Genetic polymorphisms and their association with the prevalence and severity of chronic postsurgical pain: a systematic review. Br J Anaesth 2016;117:708–19. [DOI] [PubMed] [Google Scholar]
- [12].Huang A, Azam A, Segal S, Pivovarov K, Katznelson G, Ladak SS, Mu A, Weinrib A, Katz J, Clarke H. Chronic postsurgical pain and persistent opioid use following surgery: the need for a transitional pain service. Pain Manag 2016;6:435–43. [DOI] [PubMed] [Google Scholar]
- [13].Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother 2009;9:723–44. [DOI] [PubMed] [Google Scholar]
- [14].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- [15].Khan JS, Devereaux PJ, LeManach Y, Busse JW. Patient coping and expectations about recovery predict the development of chronic post-surgical pain after traumatic tibial fracture repair. Br J Anaesth 2016;117:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lavand'homme P, Thienpont E. Pain after total knee arthroplasty: a narrative review focusing on the stratification of patients at risk for persistent pain. Bone Joint J 2015;97-B(10 suppl A):45–8. [DOI] [PubMed] [Google Scholar]
- [17].Macrae WA, Davies HTO. Chronic postsurgical pain. In: Crombie IK, Linton S, Croft P, Von Korff M, LeResche L, editors. Epidemiology of pain. Seattle: International Association for the Study of Pain, 1999. p. 125–42. [Google Scholar]
- [18].Meretoja TJ, Andersen KG, Bruce J, Haasio L, Sipila R, Scott NW, Ripatti S, Kehlet H, Kalso E. Clinical prediction model and tool for assessing risk of persistent pain after breast cancer surgery. J Clin Oncol 2017;35:1660–7. [DOI] [PubMed] [Google Scholar]
- [19].Page MG, Katz J, Romero Escobar EM, Lutzky-Cohen N, Curtis K, Fuss S, Clarke HA. Distinguishing problematic from nonproblematic postsurgical pain: a pain trajectory analysis after total knee arthroplasty. PAIN 2015;156:460–8. [DOI] [PubMed] [Google Scholar]
- [20].Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother 2009;9:745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rabbitts JA, Fisher E, Rosenbloom BN, Palermo TM. Prevalence and predictors of chronic postsurgical pain in children: a systematic review and meta-analysis. J Pain 2017;18:605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schug SAPG, Scott DA, Halliwell R, Trinca J; ANZCA, FPM. Acute Pain Management: Scientific Evidence. 4th ed Melbourne: ANZCA & FPM, 2015. [Google Scholar]
- [23].Taylor RS, Desai MJ, Rigoard P, Taylor RJ. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysis. Pain Pract 2014;14:489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain 2012;28:819–41. [DOI] [PubMed] [Google Scholar]
- [25].Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JW, Wang SJ. A classification of chronic pain for ICD-11. PAIN 2015;156:1003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].VanDenKerkhof EG, Peters ML, Bruce J. Chronic pain after surgery: time for standardization? A framework to establish core risk factor and outcome domains for epidemiological studies. Clin J Pain 2013;29:2–8. [DOI] [PubMed] [Google Scholar]
- [27].Werner MU, Kongsgaard UE. I. Defining persistent post-surgical pain: is an update required? Br J Anaesth 2014;113:1–4. [DOI] [PubMed] [Google Scholar]
- [28].Wolfe F. Fibromyalgianess. Arthritis Rheum 2009;61:715–6. [DOI] [PubMed] [Google Scholar]


