Noninvasive vagus nerve stimulation inhibited mechanical nociception and repressed the expression of proteins associated with sensitization of trigeminal neurons in a rodent model of episodic migraine.
Keywords: Vagus nerve stimulation, Glia, Nociception, Trigeminal, Neural modulation
Abstract
Introduction:
Although neck muscle tension is considered a risk factor for migraine, pungent odors can act as a trigger to initiate an attack in sensitized individuals. Although noninvasive vagus nerve stimulation (nVNS) is now an approved treatment for chronic migraine, how it functions to inhibit trigeminal nociception in an episodic migraine model is not known.
Objectives:
The objectives of this study were to determine if nVNS could inhibit trigeminal nociception in a novel model of episodic migraine and investigate changes in the expression of proteins implicated in peripheral and central sensitization.
Methods:
Sprague-Dawley male rats were injected with an inflammatory agent in the trapezius muscle before exposure to pungent volatile compounds, which was used to initiate trigeminal nociceptor activation. The vagus nerve was stimulated transdermally by a 1-ms pulse of 5 kHz sine waves, repeated at 25 Hz for 2 minutes. Nocifensive head withdrawal response to von Frey filaments was determined and immunoreactive protein levels in the spinal cord and trigeminal ganglion (TG) were investigated.
Results:
Exposure to the pungent odor significantly increased the number of nocifensive withdrawals in response to mechanical stimulation of sensitized TG neurons mediated by neck muscle inflammation. Noninvasive vagus nerve stimulation inhibited nociception and repressed elevated levels of P-ERK in TG, Iba1 in microglia, and GFAP in astrocytes from sensitized animals exposed to the pungent odor.
Conclusion:
Our findings demonstrate that nVNS inhibits mechanical nociception and represses expression of proteins associated with peripheral and central sensitization of trigeminal neurons in a novel rodent model of episodic migraine.
1. Introduction
Migraine is a common neurological disease43 that involves sensitization and activation of trigeminal neurons.19,54 Migraine patients often report the presence of several risk factors that lower the activation threshold of trigeminal nociceptive neurons,25,42 including unmanaged stress and anxiety,10 which are associated with neck muscle tension.49 Muscle tenderness and pain are reported to have a direct relationship with chronic migraine and frequency of episodic migraine.4,14,22,23,38 This strong association likely involves convergence of nociceptive neurons providing sensory innervation of neck muscles and trigeminal nerves in the upper spinal cord.6,50,61 Thus, sustained signaling from neck muscles could promote sensitization of trigeminal nociceptors, lowering their activation threshold to reported triggers such as pungent odors.8 Nassini et al.53 demonstrated that an extracted compound from leaves of the California Bay tree, also known as the headache tree, activated transient receptor potential ankyrin 1 (TRPA1) receptors and mediated calcitonin gene-related peptide (CGRP) release from trigeminal neurons. TRPA1 receptors are expressed by trigeminal ganglion neurons that provide sensory innervation of the nasal mucosa.52,53,62,63 Within the context of a migraine model, exposure to a pungent odor could trigger a migraine attack in an individual with a sensitized trigeminal nervous system mediated by neck muscle inflammation.
There is emerging evidence that electrical stimulation of the vagus nerve, which modulates pain transmission,56 is beneficial in the treatment of neurological diseases including migraine and cluster headache.30,38,39,46,59 The use of vagus nerve stimulation (VNS) has already received FDA approval for the treatment of seizures in refractory epilepsy and depression,48,58 and most recently for episodic cluster headache. With respect to migraine therapy, transdermal noninvasive VNS (nVNS) decreased the number of headache days and lowered reported pain intensity scores.38 Results from animal studies demonstrate that VNS inhibits nociceptive signaling within the trigeminal system in models of migraine pathology2,16,55 and orofacial pain.9,65 The inhibitory effects of VNS on trigeminal nerves likely involve the activation of the opioid system66 and induction of the serotonergic descending inhibition pathway.67 Release of 5-HT within the spinal cord stimulates inhibitory neurons by the activation of 5-HT3 receptors to mediate release of the inhibitory neurotransmitter GABA and hence decrease trigeminal nociception.3,45,65 Vagus nerve stimulation was also reported to decrease stimulated glutamate levels in cerebrospinal fluid in the trigeminal nucleus caudalis in a model of migraine allodynia.55
The mechanism by which neck muscle tension contributes to migraine pathology is not well understood, but is likely to involve sensitization of the trigeminal system. Toward this end, we have developed a model of episodic migraine that involves activation of neck muscle-mediated sensitization of trigeminal neurons by a pungent odor. Findings from our study provide evidence that nVNS is sufficient to inhibit trigeminal nociception and expression of proteins associated with sensitization of trigeminal neurons in a rodent model of episodic migraine.
2. Methods
2.1. Animals
All animal studies were conducted in agreement with approved protocols by the Institutional Animal Care and Use Committee at Missouri State University. Procedures were also conducted in compliance with all guidelines established in the Animal Welfare Act, National Institutes of Health, and ARRIVE. A concerted effort was made to minimize the number of animals used in this study and their suffering. On arrival, young adult male Sprague-Dawley rats (200–300 g; Charles River, Durham, NC) were housed in standard, clean plastic cages and allowed unrestricted access to water and food. All animals were isolated for 1 week before the initiation of the study to acclimate to the environment that included a 12-hour light/dark cycle.
2.2. Muscle inflammation
Some animals (24 total) were anesthetized using 5% isoflurane by inhalation. To induce trigeminal sensitization, complete Freund adjuvant (CFA; Sigma-Aldrich, St. Louis, MO; 1:1 in 0.9% sterile saline) was injected at ten different sites in the trapezius muscle between C1 and C4 vertebras. The final volume injected was 100 μL (50 μL bilaterally) and was applied as microinjections of 10 μL each. Animals were allowed to recover in their cages and were monitored for 8 days for normal grooming and feeding behaviors.
2.3. California Bay leaf exposure
To prepare the oil extract, California Bay leaves (CBL; World Spice, Seattle, WA) were crushed and dried. The bulk, volatile oil extract was obtained through standard steam isolation (AOAC Official Method 962.17). From 12.5 g of leaves, approximately 1000 μL of oil was obtained, which was stored at 4°C. Sprague-Dawley rats (36 total) were placed in a 3.3-L box containing a tube that was used to expose the animals to the volatile compounds in the extracted oil. A cotton swab containing 20 μL of extracted oil was placed in the tube such that direct contact was not possible, and oxygen was allowed to flow through the tube for 10 minutes at a rate of 2 L/min. After exposure to the extracted oil, animals were returned to their normal cages.
2.4. Vagus nerve stimulation
Before exposure to the CBL extract, some animals (16 total) were lightly anesthetized using 3% isoflurane. Noninvasive vagus nerve stimulation was accomplished using a modified VNS device, supplied by electroCore (Basking Ridge, NJ). The electrodes of the stimulator were covered in electrolyte gel (electroCore) and placed on a shaved area of the neck lateral to the trachea, parallel to and over the vagus nerve. A 1-ms pulse of 5 kHz sine waves, repeated at 25 Hz, for 2 minutes was administered. After the initial stimulation and electrode removal, the animals were allowed to recover in their home cage for 2 hours before nocifensive behavior testing. Because an inhibitory effect on mechanical nociception was not observed after the first nVNS, which occurred before trigeminal activation with CBL exposure, a second nerve stimulation was delivered as described above, and 1 hour later mechanical sensitivity was determined (Fig. 1). The control animals were prepared in the same manner as the nVNS animals, except no current was applied.
Figure 1.

Timeline and experimental design. See text for specific details.
2.5. Assessment of nocifensive response to mechanical stimulation
All behavioral studies were performed between the hours of 6 am and 12 pm as described in previous published studies.11,17,26–28 Before testing, animals were acclimated to the Durham Animal Holder (Ugo Basile, Varese, Italy) for 5 minutes on 3 consecutive days. To reduce false positive responses during assessments, animals were conditioned to a mechanical stimulus by gently rubbing the hair follicles and epidermis located over the cutaneous region over the eyebrow with a pipette tip. Mechanical nocifensive thresholds were determined in response to a series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) applied in increasing force to the cutaneous tissue. Baseline thresholds were determined before experimental procedures. A positive response, which was defined by head withdrawal before the bending of the filament, was recorded by a second researcher. Each filament was applied 5 times, and data were reported as the average number of responses obtained from 5 applications of each specific calibrated filament. The 60-g filament was chosen for additional assessment over the eyebrow region because this force elicited an average of less than 1 response on either the right or left side at baseline measurements for all animals (0.29 ± 0.11 and 0.42 ± 0.15, respectively). The researcher responsible for directly testing the response to each filament was blinded to the experimental conditions. Data are reported as the average number of nocifensive responses to the designated force ± SEM. Furthermore, animals that responded on average 2.5 of 5 or more times to the 60-g filament over the eyebrow region were identified as “responders.” Animals that responded less then these parameters were considered “non-responders.” The percentage of responders in each group is also reported.
2.6. Tissue acquisition and processing
Spinal trigeminal nucleus (STN) and trigeminal ganglion (TG) tissues were acquired through cranial dissection 3 hours after exposure to the leaf extract. Animals were euthanized by CO2 asphyxiation and decapitation. Once extracted, tissues designated for immunohistochemical studies were placed in 4% paraformaldehyde overnight at 4°C. Samples were cryoprotected by placing tissues in a 12.5% sucrose solution for 1 hour at 4°C and then in a 25% sucrose solution overnight at 4°C. Samples were then mounted in Tissue-Tek Optimal Cutting Temperature mounting media (Sakura Finetek, Torrance CA), and 14 μm STN cross-sections and TG longitudinal sections were obtained using a Microm HM 525 (Richard-Allen Scientific, Kalamazoo, MI) set at −24°C. Slices were placed on Fisherbrand Superfrost Plus Microscope slides (Thermo Fischer Scientific) and stored at −20°C.
2.7. Immunodetection and analysis
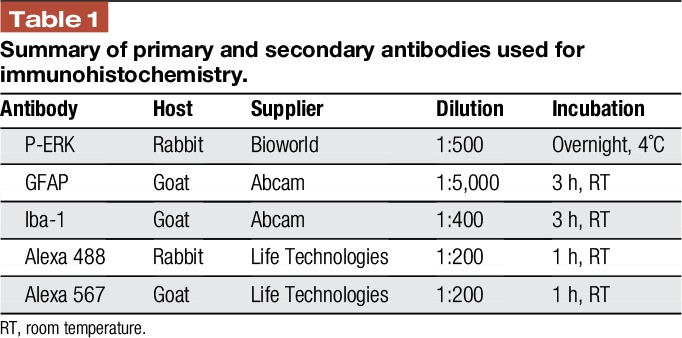
Immunostaining procedures and analysis were performed essentially as described in previous studies.11,17,27 Slides containing 1 tissue section from each experimental condition were covered with 1 × phosphate-buffered saline for 5 minutes before incubation for 20 minutes in 5% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA) in phosphate-buffered saline containing 0.1% Triton. Primary antibodies diluted in 5% normal donkey serum and Alexa Fluor secondary antibodies (Invitrogen; 1:200 dilution in PBS, Carlsbad, CA) were incubated with tissues as reported in Table 1. Tissues were mounted in the VECTASHIELD medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). A Zeiss AxioCam MRm camera (Carl Zeiss, Thornwood, NY) mounted on a Zeiss Imager Z1 fluorescence microscope was used to collect a single 4 × 3 tiled ×200 image of the dorsal medullary horn region and 3 × 3 tiled ×200 images of the V1/V2 area of the TG. Zen 2011 software (Carl Zeiss) was used to evenly balance the background of each image before densitometric analysis of gray scale JPEG images using ImageJ software. For spinal cord tissues, integrated densities were acquired from 3 independent experiments by measuring pixel densities in 10 nonoverlapping, rectangular regions of interest encompassing laminas I to III for each image. In addition, background measurements, which were acquired from acellular areas as determined by DAPI staining, were averaged and subtracted from the regions of interest values. Relative average mean values were determined for each condition and data were reported as average fold change ± SEM relative to the average mean for control animals, which was set equal to 1. Similar methods were used to quantitate P-ERK expression in primary trigeminal nociceptors using background measurements acquired from areas containing only Schwann cells and neural fibers in the ganglion as determined by DAPI staining. For quantification of nuclear P-ERK in the TG, the number of neuronal cells exhibiting nuclear localization of P-ERK was divided by the total number of visible neuronal nuclei as identified by DAPI staining. Results are reported as the average percent ± SEM of neurons with P-ERK nuclear staining.
Table 1.
Summary of primary and secondary antibodies used for immunohistochemistry.
2.8. Statistical analysis
For nociception experiments, we anticipated a medium effect size (η2 = 0.083). This study is a factorial design of 3 (Groups) × 4 (Time points). These groups included a set of animals without any additional experimental conditions (naive), a group involving trigeminal sensitization induced by neck muscle inflammation and later activated by an odor trigger (muscle + CBL) and a group that received both forms of stimulation and receiving transdermal VNS (muscle + CBL + VNS). Using G-Power (Heinrich-Heine-University, Düsseldorf, Germany) to conduct a power analysis and using a power of 0.80 at α = 0.05, the minimum sample size to detect expected main effect and interactions required at least 24 animals (8 naive, 8 M+CBL, 8 M+CBL+VNS). Outliers were determined by SPSS Statistics 21 software (IBM, North Castle, NY) using 3 as a multiplier. To determine the normality of behavioral data sets, a Shapiro-Wilk test was used, whereas a Levene test was used to determine equal or unequal variance. Because it was determined that data sets were in violation of these assumptions, a nonparametric statistical analysis was used to determine significant changes. To determine if the observed effects were statistically significant, a Kruskal-Wallis analysis of variance (ANOVA) was performed followed by a Mann-Whitney U post hoc test with a Bonferroni correction (αaltered = 0.05/3, P < 0.017) for pairwise comparisons at each time point. In addition, a Friedman ANOVA was performed within each group, followed by a Wilcoxon signed-rank test with a Bonferroni correction (αaltered = 0.05/3, P < 0.017) to evaluate comparisons made in a single group from baseline readings. All statistical tests were conducted using SPSS Statistics software.
For the immunohistochemical studies, we anticipated a large effect size (η2 = 0.702) based on previously published data.17 Using a power of 0.80 at α = 0.05 alpha, the minimum sample size needed to detect the expected main effect would require a minimum of 12 animals (4 naive, 4 M+CBL, 4 M+CBL+VNS). Any values detected as outliers by SPSS using 3 as a multiplier were removed from analysis. A Shapiro-Wilk test was used to determine data set normality, whereas a Levene test was used to determine equal or unequal variance. Data sets were found to adhere to the assumption of normality; however, not always to the assumption of equal variance. Therefore, statistical differences were determined using a 1-way ANOVA, supported by both Welch and Brown-Forsythe tests, with either a Tukey or a Games-Howell post hoc test in SPSS software, and considered different when P < 0.05.
3. Results
3.1. Repeated vagus nerve stimulation inhibited increased nociception induced by activation of trigeminal sensitization
To evaluate the potential therapeutic use of nVNS in orofacial pain conditions, nociception was evaluated over the cutaneous area of the eyebrow (V1) using von Frey filaments (Fig. 2). Basal nocifensive head withdrawals were not statistically different among the 3 groups assessed in this study in the cutaneous V1 dermatome (control: 0.38 ± 0.26, 0% responders, muscle + CBL: 0.38 ± 0.22, 0% responders, muscle + CBL + VNS: 0.31 ± 0.17, 0% responders, P = 0.994). In addition, values collected in control animals were not found to be statistically different from their basal values (D8: 0.38 ± 0.13, 0% responders, P = 0.527; 2 hours post CBL: 0.19 ± 0.10, 0% responders, P = 0.705; 3 hours post CBL: 0.38 ± 0.20, 0% responders, P = 0.666). Interestingly, neck muscle inflammation on its own did not elicit significantly different responses in the V1 dermatome as compared to control animals 8 days after injection (control: 0.38 ± 0.13, 0% responders, muscle + CBL: 0.50 ± 0.27, 0% responders, P = 0.505; muscle + CBL + VNS: 0.13 ± 0.09, 0% responders, P = 0.195). However, exposure of the CBL extract to animals that received neck muscle injections exhibited a significantly higher number of nocifensive responses as compared to control animals (2 hours post CBL: 3.25 ± 0.56, 75% responders, P < 0.001; 3 hours post CBL: 3.13 ± 0.40, 75% responders, P = 0.005). Initially, a single treatment with nVNS did not have a significant inhibitory effect on nocifensive responses 2 hours after CBL exposure as compared to M + CBL animals (3.44 ± 0.45, 87.5% responders, P = 0.721). However, animals treated with a second nVNS exhibited a significant decrease in the average number of nocifensive responses 3 hours after CBL exposure (0.63 ± 0.28, 0% responders, P = 0.005) that was similar to the level of response for control animals (P = 0.645).
Figure 2.

Vagus nerve stimulation (VNS) inhibits trigeminal nociception to mechanical stimuli. (A) Average number of nocifensive responses to the 60 g filament asserted over the cutaneous eyebrow region of the face is shown. Vertical dash arrows indicate when VNS was administered during this study. * denotes significance from naive levels where P < 0.017, # denotes significance from M+CBL group where P < 0.017. (B) Representation of the percentage of responders to the 60 g filament applied over the V1 dermatome. CBL, California Bay leaf.
3.2. Transdermal vagus nerve stimulation decreases expression of P-ERK in primary afferent neurons
To begin to understand the cellular mechanisms by which nVNS inhibits nociception, expression of P-ERK was evaluated in TG tissues. Tissues obtained from animals subjected to both conditions (muscle inflammation and CBL), but not receiving treatment exhibited a significantly higher percentage of P-ERK nuclear staining in neuronal cell bodies (38.93 ± 5.93, P = 0.016) when compared with control levels (Fig. 3; 14.18 ± 2.18). Similarly, in these same animals, the relative intensity value of cytoplasmic staining for P-ERK (2.73 ± 0.52, P = 0.032) was significantly greater than the value in control tissues (1.00 ± 0.24). Importantly, the percentage observed in animals that also received nVNS was similar to the expression level determined in control tissues (Fig. 3; nuclear: 20.71 ± 5.20, P = 0.567; cytoplasmic: 1.79 ± 0.66, P = 0.059).
Figure 3.

Stimulated P-ERK expression is repressed by vagus nerve stimulation (VNS). The percentage of P-ERK positive neurons and relative expression of cytoplasmic P-ERK was significantly increased in the V1/V2 region of the trigeminal ganglion in response to muscle inflammation and California Bay leaf (CBL). The stimulatory effect was inhibited by VNS. Representative ×200 images taken in V1/V2 regions of the trigeminal ganglion depicting DAPI and P-ERK staining in naive, M+CBL, and M+CBL+VNS samples taken 3 hours after CBL exposure. Scale bar = 100 μm. Blue boxes placed in P-ERK images indicate areas that were selected for enlarged images in the third and fourth columns. The table below the images summarizes the average percentage of positive labeled neurons ± SEM exhibiting nuclear localization and the change in relative intensity of P-ERK staining in the cytoplasm. * denotes significance from naive levels where P < 0.05.
3.3. Repeated vagus nerve stimulation significantly decreased activation of glia in the spinal trigeminal nucleus
To evaluate the effects of nVNS on glial cells in the STN, immunohistochemical analysis was performed for GFAP, a marker of activated astrocytes, and Iba1, a marker of activated microglia. Animals experiencing both muscle inflammation and CBL had significantly increased expression of Iba1 (Fig. 4; 2.97 ± 0.40, P = 0.007) as compared to control levels (1.00 ± 0.06). Importantly, animals that were also treated with nVNS exhibited levels of Iba1 expression that were similar to those observed in control tissues (Fig. 4; 1.67 ± 0.39, P = 0.360). In addition, animals that experienced both insults without treatment demonstrated significantly higher levels of GFAP expression (Fig. 5; 2.07 ± 0.2, P = 0.015) when compared with levels in control tissues (1.00 ± 0.14). In contrast, levels detected in animals that were also treated with nVNS were comparable to control tissues (Fig. 5; 1.58 ± 0.27, P = 0.137).
Figure 4.

Levels of Iba1 in microglia were repressed by vagus nerve stimulation (VNS). Representative ×200 images taken in laminas I to III of the medullary horn in the upper spinal cord depicting DAPI and Iba1 staining in naive, M+CBL, and M+CBL+VNS samples taken 3 hours after California Bay leaf (CBL) exposure. Scale bar = 100 μm. Blue boxes placed in Iba1 images indicate areas that were selected for enlarged images in the third and fourth columns. The table below the images summarizes the average fold change ± SEM as compared to levels detected in naive samples, in which the mean value was set equal to 1. * denotes significance from naive levels where P < 0.05.
Figure 5.
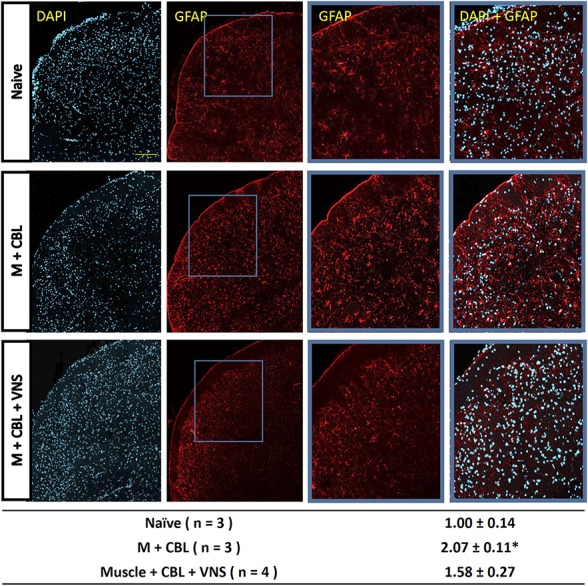
Vagus nerve stimulation (VNS) inhibits GFAP expression in astrocytes. Representative ×200 images taken in laminas I to III of the medullary horn in the upper spinal cord depicting DAPI and GFAP staining in naive, M+CBL, and M+CBL+VNS samples taken 3 hours after California Bay leaf (CBL) exposure. Scale bar = 100 μm. Blue boxes placed in GFAP images indicate areas that were selected for enlarged images in the third and fourth columns. The table below the images summarizes the average fold change ± SEM as compared to levels detected in naive samples, which the mean value was set equal to 1. * denotes significance from naive levels where P < 0.05.
4. Discussion
We found that nVNS significantly inhibited the nocifensive response to mechanical stimulation of primary trigeminal neurons in a novel rodent model of migraine pathology. In our model, neck muscle tension/inflammation was used to promote sensitization of trigeminal neurons that become activated on stimulation of nasal/sinus TRPA1 receptors by the pungent stimulus derived from California Bay leaves (CBLs). Neck muscle inflammation was chosen as a means to facilitate sensitization of the trigeminal system because neck/shoulder tension is often cited as a risk factor and a comorbid symptom of migraine.4,14,21,22 The stimulus, complete Freund adjuvant, was used to induce prolonged inflammation by eliciting a sustained immune response for up to 2 weeks.11,26,68 The amount of adjuvant used in our study did not negatively affect the normal feeding and eating behavior of the animals or cause excessive grooming. However, multiple injections of 10 μL CFA resulted in low-grade inflammation and muscle tenderness that mediated sensitization of primary trigeminal nociceptors rather than nociception, which was observed with a single 50 μL injection (data not shown). This sensitizing effect is likely attributable to the fact that afferent processes of the nerves providing sensory innervation of the muscle tissue terminate in the upper spinal cord where there is convergence with the trigeminal system.5,6,50 To cause activation of sensitized trigeminal nociceptive neurons, we used an oil extract containing volatile, stimulatory chemicals that was prepared from the leaves of the California Bay tree. The rationale for choosing this type of stimulus was based on pungent odors being a reported migraine trigger,35 and the seminal work by Nassini et al. that demonstrated application of umbellulone, a chemical isolated from CBL, could elicit a nocifensive trigeminal response on topical application of this compound to the nasal mucosa.53 In addition, umbellulone was shown to cause activation of the TRPA1 receptors expressed on trigeminal neurons and mediate the release of CGRP, a molecule strongly implicated in migraine pathology.7 Our goal was to develop a clinically relevant rodent migraine model that involved inducing or triggering trigeminal activation without having to directly apply or inject the stimulus, and hence why we chose to expose the animals to volatile compounds by inhalation, a more natural and less-invasive process. It should be noted that trigeminal nociceptor activation was induced on activation of V1/V2 neurons that provide sensory innervation of the nasal and sinus mucosa,62,63 which led to an enhanced nocifensive response in V1 (eyebrow) neurons. Taken together, results from our rodent model mimic several key features of episodic migraine pathology including increased mechanical nociception and temporally correlated changes in the expression of several proteins implicated in peripheral and central sensitization.
After the activation of trigeminal neurons, as determined by an increase in nocifensive withdrawal response from mechanical stimulation, elevated levels of the active, phosphorylated form of the MAP kinase P-ERK were observed primarily in trigeminal neurons in the V1/V2 regions of the ganglion. Increased P-ERK was seen in the cytoplasm and nucleus of both larger A-delta and smaller diameter C fiber neuronal cell bodies. P-ERK is regarded as a marker of activated nociceptive neurons by promoting cellular changes implicated in excitation and release of inflammatory mediators.32 For example, elevated P-ERK levels in the cytoplasm could mediate increased secretion by phosphorylation of ion channels and proteins involved in vesicle docking, fusion, and neuropeptide release.1 In addition, enhanced nuclear expression of P-ERK could lead to stimulation of promoter activity and thus contribute to the production of neuropeptides such as CGRP20 and other known MAP-kinase–responsive genes including cytokines and nitric oxide.24,29,37 Increased expression and release of inflammatory mediators from the neuronal cell body would stimulate satellite glial cells that function to modulate the excitability state of the neurons.40,64 In addition to cellular changes within the ganglion, activation of sensitized trigeminal neurons by a pungent odor resulted in enhanced expression of proteins associated with activated astrocytes (GFAP) and microglia (Iba1) within the upper spinal cord.33 Elevated levels of these proteins in glial cells, in conjunction with changes in second order neurons, are known to play an important role in the development and maintenance of central sensitization,18,31 which is a pathophysiological event reported in migraine that contributes to enhanced pain sensation (hyperalgesia) and allodynia.19 Interestingly, activated astrocytes in the dorsal medullary horn are involved in d-serine modulation of NMDAR activity and mechanical allodynia.47 Thus, the activation of sensitized trigeminal neurons leads to changes in neurons in the ganglion and glial cells in the spinal cord, which temporally correlate with increased trigeminal nociception.
A major finding from our study was that nVNS was sufficient to significantly inhibit nocifensive head withdrawal response from mechanical stimulation of V1 trigeminal nociceptors. Our finding is similar to the results from other studies in which VNS inhibited trigeminal activation in novel models involving trigeminal allodynia, primary headache, and cortical spreading depression.2,16,55 Interestingly, a single nVNS before trigeminal activation did not block the nocifensive response to the pungent odor in our study. This finding is similar to the reported effect of the antimigraine triptan drugs, which are most effective when administered at the onset of trigeminal activation. In agreement with the change in nociception, nVNS resulted in an inhibitory effect on the expression of proteins associated with peripheral and central sensitization. The elevated level of P-ERK expression in the cytoplasm and nucleus of trigeminal neurons was significantly inhibited by nVNS as were the elevated levels of Iba1 and GFAP. Thus, nVNS seems to function at multiple levels within the trigeminal system to repress the activation of sensitized primary trigeminal neurons and repress the activation of microglia and astrocytes. The exact mechanism by which nVNS modulates nociception and regulates protein expression in our model is not known but may involve increased expression of IL-10 and MAP kinase phosphatases in the TG, activation of the descending inhibitory pathway, and suppression of glia activation. In support of a possible role for IL-10 in mediating the inhibitory effects of nVNS on P-ERK levels, VNS has been shown to cause an increase in peripheral levels of the anti-inflammatory cytokine IL-10.44 Elevated levels of IL-10 could suppress the stimulatory effects of proinflammatory cytokines on P-ERK expression36 in the TG to inhibit trigeminal sensitization and activation.15 Given the key role of MAP kinase phosphatases (MKPs) in regulating activity of the MAP kinases including ERK,34,60 increased levels of the phosphatases MKP-1 and MKP-3 are candidates for dephosphorylation and hence decreasing ERK levels. We have previously reported that increased levels of MKP-1 are associated with the inhibition of stimulated expression of signaling proteins in trigeminal neurons and satellite glial cells.12,13 Enhanced descending modulation mediated by VNS could lead to increased release of the inhibitory neurotransmitter GABA and inhibition of second-order nociceptive neurons, astrocytes, and microglia.41,56 Although evidence for direct activation of this pathway was not demonstrated in our study, enhanced descending modulation provides a possible mechanism to help explain the inhibitory effects of VNS in the STN on glial cell activation.51,57
In summary, results from our study provide evidence that nVNS can inhibit nociception and cellular changes associated with peripheral and central sensitization in a novel rodent model of episodic migraine. Future studies will focus on determining the mechanism of action of nVNS and optimizing the temporal parameters required to achieve maximum inhibitory effects of the trigeminal system.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgments
The authors thank Angela Goerndt for her assistance in the care and maintenance of the animals used in our study. Funding for this study, the noninvasive vagus nerve stimulator adapted for rodent use, was provided by electroCore.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Ait-Ali D, Turquier V, Grumolato L, Yon L, Jourdain M, Alexandre D, Eiden LE, Vaudry H, Anouar Y. The proinflammatory cytokines tumor necrosis factor-alpha and interleukin-1 stimulate neuropeptide gene transcription and secretion in adrenochromaffin cells via activation of extracellularly regulated kinase 1/2 and p38 protein kinases, and activator protein-1 transcription factors. Mol Endocrinol 2004;18:1721–39. [DOI] [PubMed] [Google Scholar]
- [2].Akerman S, Simon B, Romero-Reyes M. Vagus nerve stimulation suppresses acute noxious activation of trigeminocervical neurons in animal models of primary headache. Neurobiol Dis 2017;102:96–104. [DOI] [PubMed] [Google Scholar]
- [3].Alhaider AA, Lei SZ, Wilcox GL. Spinal 5-HT3 receptor-mediated antinociception: possible release of GABA. J Neurosci 1991;11:1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ashina S, Bendtsen L, Lyngberg AC, Lipton RB, Hajiyeva N, Jensen R. Prevalence of neck pain in migraine and tension-type headache: a population study. Cephalalgia 2015;35:211–19. [DOI] [PubMed] [Google Scholar]
- [5].Bartsch T. Migraine and the neck: new insights from basic data. Curr Pain Headache Rep 2005;9:191–6. [DOI] [PubMed] [Google Scholar]
- [6].Bartsch T, Goadsby PJ. The trigeminocervical complex and migraine: current concepts and synthesis. Curr pain headache Rep 2003;7:371–6. [DOI] [PubMed] [Google Scholar]
- [7].Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache 2013;53:1230–44. [DOI] [PubMed] [Google Scholar]
- [8].Borkum JM. Migraine triggers and oxidative stress: a narrative review and synthesis. Headache 2016;56:12–35. [DOI] [PubMed] [Google Scholar]
- [9].Bossut DF, Maixner W. Effects of cardiac vagal afferent electrostimulation on the responses of trigeminal and trigeminothalamic neurons to noxious orofacial stimulation. PAIN 1996;65:101–9. [DOI] [PubMed] [Google Scholar]
- [10].Buse DC, Silberstein SD, Manack AN, Papapetropoulos S, Lipton RB. Psychiatric comorbidities of episodic and chronic migraine. J Neurol 2013;260:1960–9. [DOI] [PubMed] [Google Scholar]
- [11].Cady RJ, Denson JE, Sullivan LQ, Durham PL. Dual orexin receptor antagonist 12 inhibits expression of proteins in neurons and glia implicated in peripheral and central sensitization. Neuroscience 2014;269:79–92. [DOI] [PubMed] [Google Scholar]
- [12].Cady RJ, Durham PL. Cocoa-enriched diets enhance expression of phosphatases and decrease expression of inflammatory molecules in trigeminal ganglion neurons. Brain Res 2010;1323:18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cady RJ, Hirst JJ, Durham PL. Dietary grape seed polyphenols repress neuron and glia activation in trigeminal ganglion and trigeminal nucleus caudalis. Mol Pain 2010;6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Calhoun AH, Ford S, Millen C, Finkel AG, Truong Y, Nie Y. The prevalence of neck pain in migraine. Headache 2010;50:1273–7. [DOI] [PubMed] [Google Scholar]
- [15].Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain 2009;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen SP, Ay I, de Morais AL, Qin T, Zheng Y, Sadeghian H, Oka F, Simon B, Eikermann-Haerter K, Ayata C. Vagus nerve stimulation inhibits cortical spreading depression. PAIN 2016;157:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cornelison LE, Hawkins JL, Durham PL. Elevated levels of calcitonin gene-related peptide in upper spinal cord promotes sensitization of primary trigeminal nociceptive neurons. Neuroscience 2016;339:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Davies AJ, Kim YH, Oh SB. Painful neuron-microglia interactions in the trigeminal sensory system. Open Pain J 2010;3:14–28. [Google Scholar]
- [19].Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache 2006;46(suppl 4):S182–91. [DOI] [PubMed] [Google Scholar]
- [20].Durham PL, Russo AF. Serotonergic repression of mitogen-activated protein kinase control of the calcitonin gene-related peptide enhancer. Mol Endocrinol 1998;12:1002–9. [DOI] [PubMed] [Google Scholar]
- [21].Fernandez-de-Las-Penas C, Cuadrado ML, Pareja JA. Myofascial trigger points, neck mobility and forward head posture in unilateral migraine. Cephalalgia 2006;26:1061–70. [DOI] [PubMed] [Google Scholar]
- [22].Florencio LL, Chaves TC, Carvalho GF, Goncalves MC, Casimiro EC, Dach F, Bigal ME, Bevilaqua-Grossi D. Neck pain disability is related to the frequency of migraine attacks: a cross-sectional study. Headache 2014;54:1203–10. [DOI] [PubMed] [Google Scholar]
- [23].Florencio LL, de Oliveira AS, Carvalho GF, Tolentino Gde A, Dach F, Bigal ME, Fernandez-de-las-Penas C, Bevilaqua Grossi D. Cervical muscle strength, muscle coactivation during isometric contractions in patients with migraine: a cross-sectional study. Headache 2015;55:1312–22. [DOI] [PubMed] [Google Scholar]
- [24].Freeman SE, Patil VV, Durham PL. Nitric oxide-proton stimulation of trigeminal ganglion neurons increases mitogen-activated protein kinase and phosphatase expression in neurons and satellite glial cells. Neuroscience 2008;157:542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Friedman DI, De ver Dye T. Migraine and the environment. Headache 2009;49:941–52. [DOI] [PubMed] [Google Scholar]
- [26].Garrett FG, Hawkins JL, Overmyer AE, Hayden JB, Durham PL. Validation of a novel rat-holding device for studying heat- and mechanical-evoked trigeminal nocifensive behavioral responses. J Orofac Pain 2012;26:337–44. [PMC free article] [PubMed] [Google Scholar]
- [27].Hawkins JL, Denson JE, Miley DR, Durham PL. Nicotine stimulates expression of proteins implicated in peripheral and central sensitization. Neuroscience 2015;290:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hawkins JL, Durham PL. Prolonged jaw opening promotes nociception and enhancedcytokine expression. J Oral Facial Pain Headache 2016;30:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hoffmeyer A, Grosse-Wilde A, Flory E, Neufeld B, Kunz M, Rapp UR, Ludwig S. Different mitogen-activated protein kinase signaling pathways cooperate to regulate tumor necrosis factor alpha gene expression in T lymphocytes. J Biol Chem 1999;274:4319–27. [DOI] [PubMed] [Google Scholar]
- [30].Hord ED, Evans MS, Mueed S, Adamolekun B, Naritoku DK. The effect of vagus nerve stimulation on migraines. J Pain 2003;4:530–4. [DOI] [PubMed] [Google Scholar]
- [31].Ikeda H, Kiritoshi T, Murase K. Contribution of microglia and astrocytes to the central sensitization, inflammatory and neuropathic pain in the juvenile rat. Mol Pain 2012;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ji RR. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allergy 2004;3:299–303. [DOI] [PubMed] [Google Scholar]
- [33].Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? PAIN 2013;154(suppl 1):S10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev 2009;60:135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007;27:394–402. [DOI] [PubMed] [Google Scholar]
- [36].Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Arch Toxicol 2015;89:867–82. [DOI] [PubMed] [Google Scholar]
- [37].Kim SH, Smith CJ, Van Eldik LJ. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1 beta production. Neurobiol Aging 2004;25:431–9. [DOI] [PubMed] [Google Scholar]
- [38].Kinfe TM, Pintea B, Muhammad S, Zaremba S, Roeske S, Simon BJ, Vatter H. Cervical non-invasive vagus nerve stimulation (nVNS) for preventive and acute treatment of episodic and chronic migraine and migraine-associated sleep disturbance: a prospective observational cohort study. J Headache Pain 2015;16:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lenaerts ME, Oommen KJ, Couch JR, Skaggs V. Can vagus nerve stimulation help migraine? Cephalalgia 2008;28:392–5. [DOI] [PubMed] [Google Scholar]
- [40].Li J, Vause CV, Durham PL. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res 2008;1196:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lin Q, Peng YB, Willis WD. Inhibition of primate spinothalamic tract neurons by spinal glycine and GABA is reduced during central sensitization. J Neurophysiol 1996;76:1005–14. [DOI] [PubMed] [Google Scholar]
- [42].Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 2005;45(suppl 1):S3–13. [DOI] [PubMed] [Google Scholar]
- [43].Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343–9. [DOI] [PubMed] [Google Scholar]
- [44].Majoie HJ, Rijkers K, Berfelo MW, Hulsman JA, Myint A, Schwarz M, Vles JS. Vagus nerve stimulation in refractory epilepsy: effects on pro- and anti-inflammatory cytokines in peripheral blood. Neuroimmunomodulation 2011;18:52–6. [DOI] [PubMed] [Google Scholar]
- [45].Matsumoto S, Takeda M, Tanimoto T. Effects of electrical stimulation of the tooth pulp and phrenic nerve fibers on C1 spinal neurons in the rat. Exp Brain Res 1999;126:351–8. [DOI] [PubMed] [Google Scholar]
- [46].Mauskop A. Vagus nerve stimulation for refractory cluster headaches. Headache 2014;54:170. [DOI] [PubMed] [Google Scholar]
- [47].Miraucourt LS, Peirs C, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through astrocyte-derived D-serine. PAIN 2011;152:1340–8. [DOI] [PubMed] [Google Scholar]
- [48].Mitsikostas DD, Edvinsson L, Jensen RH, Katsarava Z, Lampl C, Negro A, Osipova V, Paemeleire K, Siva A, Valade D, Martelletti P. Refractory chronic cluster headache: a consensus statement on clinical definition from the European Headache Federation. J Headache Pain 2014;15:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mongini F, Ciccone G, Ceccarelli M, Baldi I, Ferrero L. Muscle tenderness in different types of facial pain and its relation to anxiety and depression: a cross-sectional study on 649 patients. PAIN 2007;131:106–11. [DOI] [PubMed] [Google Scholar]
- [50].Morch CD, Hu JW, Arendt-Nielsen L, Sessle BJ. Convergence of cutaneous, musculoskeletal, dural and visceral afferents onto nociceptive neurons in the first cervical dorsal horn. Eur J Neurosci 2007;26:142–54. [DOI] [PubMed] [Google Scholar]
- [51].Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One 2008;3:e3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol 2014;167:1–43. [DOI] [PubMed] [Google Scholar]
- [53].Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, la Marca G, Andre E, Preti D, Avonto C, Sadofsky L, Di Marzo V, De Petrocellis L, Dussor G, Porreca F, Taglialatela-Scafati O, Appendino G, Nilius B, Geppetti P. The “headache tree” via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2012;135:376–90. [DOI] [PubMed] [Google Scholar]
- [54].Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. PAIN 2013;154(suppl 1):S44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Oshinsky ML, Murphy AL, Hekierski H, Jr, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. PAIN 2014;155:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Randich A, Gebhart GF. Vagal afferent modulation of nociception. Brain Res Brain Res Rev 1992;17:77–99. [DOI] [PubMed] [Google Scholar]
- [57].Ren K, Dubner R. Central nervous system plasticity and persistent pain. J Orofac Pain 1999;13:155–63; discussion 164–171. [PubMed] [Google Scholar]
- [58].Ryvlin P, Gilliam FG, Nguyen DK, Colicchio G, Iudice A, Tinuper P, Zamponi N, Aguglia U, Wagner L, Minotti L, Stefan H, Boon P, Sadler M, Benna P, Raman P, Perucca E. The long-term effect of vagus nerve stimulation on quality of life in patients with pharmacoresistant focal epilepsy: the PuLsE (open prospective randomized long-term effectiveness) trial. Epilepsia 2014;55:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sadler RM, Purdy RA, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia 2002;22:482–4. [DOI] [PubMed] [Google Scholar]
- [60].Seger R, Krebs EG. The MAPK signaling cascade. FASEB J 1995;9:726–35. [PubMed] [Google Scholar]
- [61].Sessle BJ. Neural mechanisms and pathways in craniofacial pain. Can J Neurol Sci 1999;26(suppl 3):S7–11. [DOI] [PubMed] [Google Scholar]
- [62].Shankland WE. The trigeminal nerve. Part II: the ophthalmic division. Cranio 2001;19:8–12. [DOI] [PubMed] [Google Scholar]
- [63].Shankland WE., II The trigeminal nerve. Part III: the maxillary division. Cranio 2001;19:78–83. [DOI] [PubMed] [Google Scholar]
- [64].Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, Matsumoto S. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. PAIN 2007;129:155–66. [DOI] [PubMed] [Google Scholar]
- [65].Takeda M, Tanimoto T, Nishikawa T, Ikeda M, Yoshida S, Ito M, Matsumoto S. Volume expansion suppresses the tooth-pulp evoked jaw-opening reflex related activity of trigeminal neurons in rats. Brain Res Bull 2002;58:83–9. [DOI] [PubMed] [Google Scholar]
- [66].Takeda M, Tanimoto T, Ojima K, Matsumoto S. Suppressive effect of vagal afferents on the activity of the trigeminal spinal neurons related to the jaw-opening reflex in rats: involvement of the endogenous opioid system. Brain Res Bull 1998;47:49–56. [DOI] [PubMed] [Google Scholar]
- [67].Tanimoto T, Takeda M, Matsumoto S. Suppressive effect of vagal afferents on cervical dorsal horn neurons responding to tooth pulp electrical stimulation in the rat. Exp Brain Res 2002;145:468–79. [DOI] [PubMed] [Google Scholar]
- [68].Zhou Q, Imbe H, Dubner R, Ren K. Persistent Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: implications for persistent orofacial pain. J Comp Neurol 1999;412:276–91. [DOI] [PubMed] [Google Scholar]


