A retrospective review of 3,265,894 veterans seen over a 5-year period demonstrates dry eye is associated with numerous chronic pain conditions implying shared mechanisms.
Keywords: Dry eye, Chronic neuropathic ocular pain, Overlapping pain conditions
Abstract
Introduction:
Recent evidence suggests that dry eye (DE) may be comorbid with other chronic pain conditions.
Objectives:
To evaluate DE as a comorbid condition in the U.S. veteran population.
Methods:
Retrospective review of veterans seen in the Veterans Administration Healthcare System (Veteran Affairs) between January 1, 2010, and December 31, 2014. Dry eye and nonocular pain disorders were ascertained by International Classification of Diseases, Ninth Revision (ICD-9) codes. Dry eye was further separated into ICD-9 codes representing tear film dysfunction or ocular pain. χ2 and logistic regression analyses were used to examine frequency and risk of DE, ocular pain, and tear film dysfunction by pain disorders.
Results:
Of 3,265,894 veterans, 959,881 had a DE diagnosis (29.4%). Dry eye frequency increased with the number of pain conditions reported (P < 0.0005). Ocular pain was most strongly associated with headache (odds ratio [OR] 2.98; 95% confidence interval [CI] 2.95–3.01), tension headache (OR 2.64; 95% CI 2.58–2.71), migraine (OR 2.58; 95% CI 2.54–2.61), temporomandibular joint dysfunction (OR 2.39; 95% CI 2.34–2.44), pelvic pain (OR 2.30; 95% CI 2.24–2.37), central pain syndrome (OR 2.24; 95% CI 1.94–2.60), and fibromyalgia/muscle pain (OR 2.23; 95% CI 2.20–2.26), all P < 0.0005. Tear film dysfunction was most closely associated with osteoarthritis (OR 1.97; 95% CI 1.96–1.98) and postherpetic neuralgia (OR 1.95; 95% CI 1.90–2.00), both P < 0.0005.
Conclusions:
Dry eye, including both ocular pain and tear film dysfunction, is comorbid with pain conditions in this nationwide population, implying common mechanisms.
1. Introduction
Chronic pain conditions are estimated to affect at least 116 million adults in the United States alone, with an economic burden of $560–635 billion in medical expenditure because of treatment costs and loss of productivity.3 Pain conditions are generally considered to be chronic when they persist beyond a time of expected resolution, typically 3 months or longer. Certain chronic pain conditions, such as temporomandibular joint dysfunction (TMD), fibromyalgia, irritable bowel syndrome (IBS), back pain, and headaches, have been found to coexist with one another,8 and this concept has been referred to as chronic overlapping pain conditions (COPCs).27,46 Chronic overlapping pain conditions are further separated into categories based on the presence or absence of observable underlying pathology. So-called “functional disorders” describe conditions where pain exists without known underlying pathology (eg, TMD, fibromyalgia), whereas structural disorders occur in the setting of a well-defined pathology (eg, osteoarthritis, rheumatoid arthritis), infection (eg, postherpetic neuralgia), drug-induced (eg, opioids), or trauma (eg, persistent postoperative pain).55,56 Another way in which pain conditions have been subcategorized is using the International Association for Study of Pain (IASP) scheme for coding chronic pain which takes into account regions or the main site of the pain (eg, cervical region, thoracic region, lower limbs, etc.) for axis I, organ system involved (eg, nervous system, respiratory system, etc.) for axis II, characteristics of the pain (eg, continuous, irregularly, single episode, etc.) for axis III, intensity of pain and time since onset (eg, mild, medium, or severe, and number of months) for axis IV, and etiology (eg, genetic, trauma, inflammatory, toxic, etc) for axis V. Several mechanisms likely underlie the coexistence of pain conditions including peripheral and central sensitization54 and psychosocial vulnerabilities that have an effect on pain amplification,5,27 both of which are believed to be strongly influenced by genetic factors.9,27
Dry eye (DE) is a complex disorder that can present with ocular pain and/or tear film dysfunction. Dry eye affects an estimated 15% of the U.S. population and millions of people worldwide.2 Symptoms of DE include a wide range of dysesthesias described as unpleasant including “dryness,” “burning,” and “aching,”21 and many individuals report a chronic symptom course.32 Signs of DE are varied and can include decreased tear production, increased tear evaporation, and ocular surface disruption.1 Similar to nonocular chronic pain conditions, DE symptoms often do not correlate with clinical signs of DE.15,38 Furthermore, a subset of patients do not respond to therapies that target the ocular surface.11,41 Like other COPC, DE symptoms negatively impact the quality of life by affecting the ability to perform basic activities of daily living such as reading, watching television, driving, and using a computer.29,33
Several mechanisms common to COPC are implicated in the pathogenesis of chronic DE symptoms, including inflammation,39 nerve sensitization,16,36,48 and genetic susceptibility.50 Similar to COPC, psychosocial vulnerabilities likely play a role in ocular symptom amplification, as demonstrated by the close association between DE with posttraumatic stress disorder, depression, and insomnia.14,18 We previously showed that DE symptoms correlated with nonocular pain7 and that individuals with a greater number of chronic pain conditions had more severe DE symptoms.12 Interestingly, ocular surface signs of DE were comparable among those with and without chronic pain. The generalization of these data is limited by the fact that our study evaluated a sample population from a single center in South Florida. To build on our previous work, we re-examined associations between DE and nonocular pain conditions in a large nationwide population of veterans over a 5-year period. Specifically, we evaluated the association between DE subtypes (including ocular pain and tear film dysfunction) with nonocular pain conditions listed under the heading of COPC. Based on our previous work, we hypothesized that DE, specifically the subtype defined by ocular pain, would be more prevalent in individuals with pain conditions with functional conditions known to associate with central sensitization compared with individuals with a reported diagnosis of DE with tear film dysfunction.
2. Methods
2.1. Study methodology
This retrospective review of medical records examined all patients seen in the nationwide Veterans Affairs Healthcare System between January 1, 2010, and December 31, 2014. Institution Review Board approval of Miami Veterans Affairs was obtained to allow the retrospective evaluation of charts. The study was conducted in accordance with the principles of the Declaration of Helsinki and complied with the requirements of the United States Health Insurance Portability and Accountability Act.
2.2. Study population
Between January 1, 2010, and December 31, 2014, 3,265,894 patients were evaluated within the Veterans Affairs Healthcare System.
2.3. Data collected
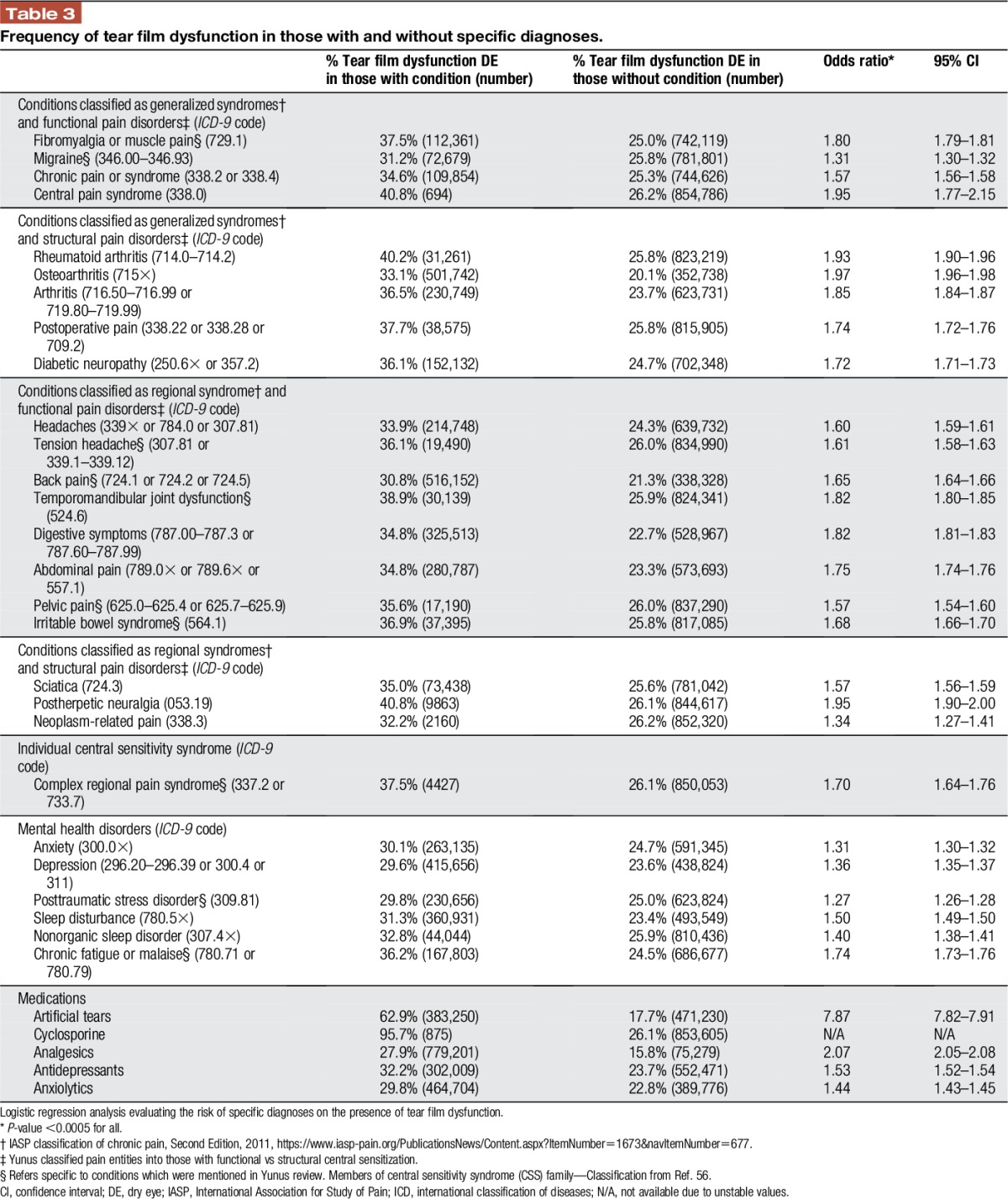
Data were extracted from the clinical charts by a programmer and arranged into an excel sheet so that each row represented a unique patient, identified by an assigned number, followed by information on demographics (sex, race, ethnicity, and age), comorbidities (DE and pain diagnoses by International Classification of Diseases, Ninth Revision [ICD-9] codes), and medication use (artificial tears, cyclosporine, analgesics, antidepressants, and anxiolytics). Dry eye was defined as the presence of an ICD-9 code for sicca syndrome, keratoconjunctivitis sicca, tear film insufficiency, visual discomfort, or pain in or around the eye. We further separated DE into 2 categories: tear film dysfunction (sicca syndrome, keratoconjunctivitis sicca, and tear film insufficiency) and ocular pain (visual discomfort and pain in or around the eye). We organized pain diagnoses in Tables 2 and 3 using “functional or structural” pain classification by Yunus and the IASP axis I regional classification of chronic pain.
Table 2.
Frequency of ocular pain in those with and without specific diagnoses.
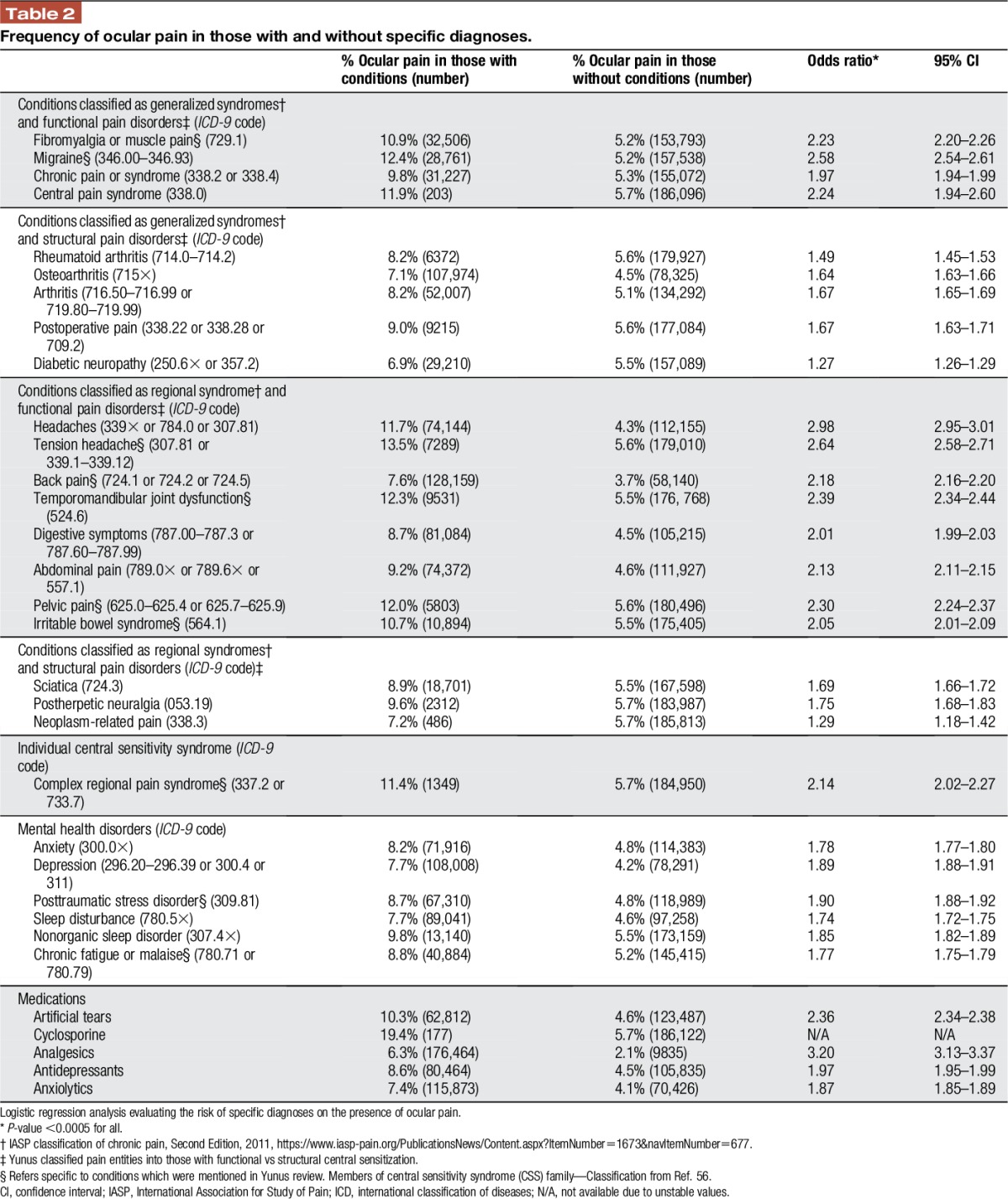
Table 3.
Frequency of tear film dysfunction in those with and without specific diagnoses.
2.4. Statistical analysis
Descriptive statistics were prepared for the study population. Frequencies were compared using χ2 tests; means were compared with 2-sample t tests. Binary logistic regression analysis was used to calculate odds ratios (ORs) between DE and chronic pain conditions. Multivariable analyses were performed to evaluate the influence of multiple variables (demographic and pain conditions) on DE. A sum score was generated representing the total number of chronic pain conditions carried by an individual.
3. Results
3.1. Study population
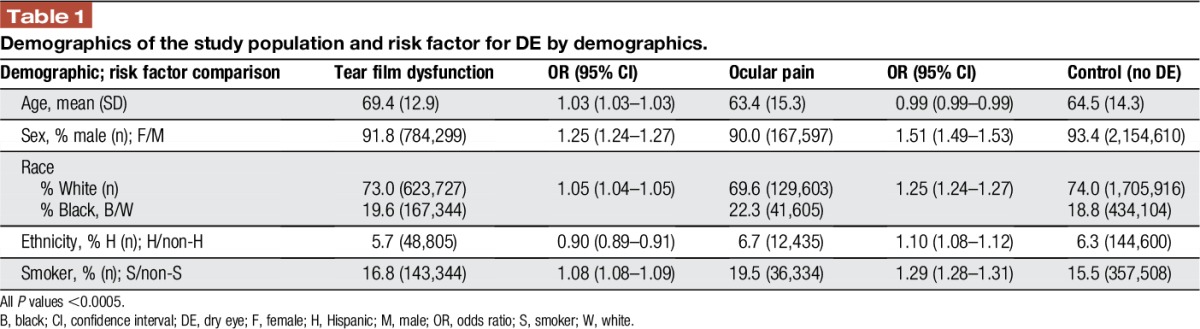
Over the 5-year period, 3,265,894 veterans were seen at a Veteran Affairs medical center, of which 959,881 veterans carried an ICD-9 diagnosis of DE (29.4%). Of those, 26.2% (854,480) had a diagnosis of tear film dysfunction and 5.7% (186,299) had a diagnosis of ocular pain. The 2 DE subtypes were not mutually exclusive with 2.5% (80,898) of veterans having both diagnoses. Patients with tear film dysfunction were older compared with their counterparts without DE (mean 69.4 years SD 12.9 vs 64.5 years SD 14.3, P < 0.0005), whereas individuals with ocular pain were younger (63.4 years SD 15.3, P < 0.0005). Examining age groups, the frequency of tear dysfunction DE increased with age (Fig. 1) while ocular pain occurred at a higher frequency in younger individuals (Fig. 2). Women were more likely to have diagnosis of both ocular pain and tear film dysfunction (OR 1.51 and 1.25, respectively, Table 1). Blacks had a higher risk of ocular pain compared with whites (OR 1.25); the risk of tear film dysfunction was also increased but to a lesser magnitude (OR 1.05). In a similar pattern, smokers had a higher risk of ocular pain (OR 1.29) and a slightly higher risk of tear film dysfunction (1.08) compared with nonsmokers, P < 0.0005 for both.
Figure 1.
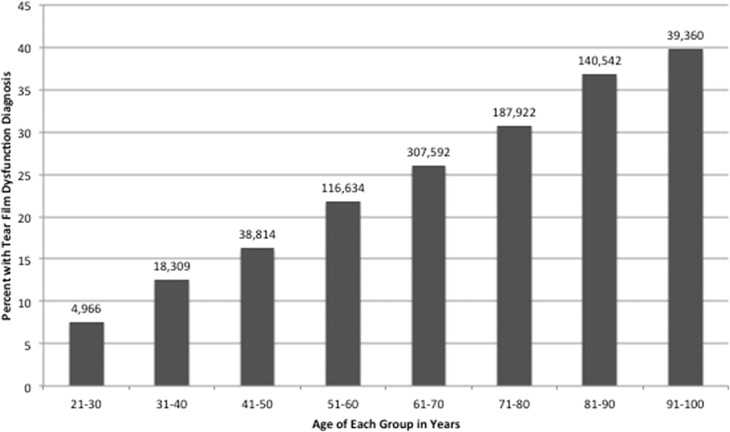
Frequencies of tear film dysfunction in each age group of veterans seen in the VA Healthcare System between January 1, 2010, and December 31, 2014. Total number of veterans in the study n = 3,265,894. VA, Veterans Affairs.
Figure 2.
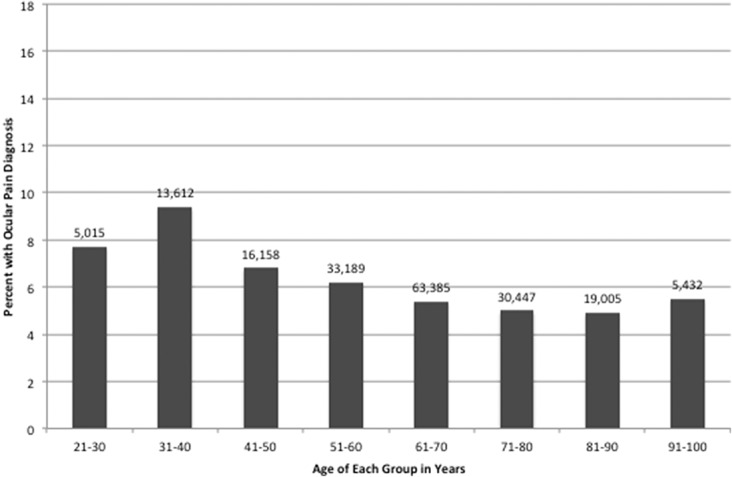
Frequencies of ocular pain in each age group of veterans seen in the VA Healthcare System between January 1, 2010, and December 31, 2014. Total number of veterans in the study n = 3,265,894. VA, Veterans Affairs.
Table 1.
Demographics of the study population and risk factor for DE by demographics.
3.2. Relationship between ocular pain and tear film dysfunction with nonocular pain diagnosis
We first added the number of pain diagnoses present per individual, of a total of 21 possible diagnoses (listed in Tables 2 and 3, excluding mental health disorders), to arrive at a sum score. We found that the mean number of pain diagnoses carried by an individual was 2.51 SD 2.10. Only 17.6% of individuals had no pain diagnosis, 54.6% had 1 to 3 pain diagnoses, 25.4% had 4 to 7 pain diagnoses, and 2.4% had 7 or more diagnoses. The frequency of DE in the population sample increased with an increasing number of pain diagnoses. Specifically, the frequency of DE was 16.3% in those with no pain diagnosis, 26.4% in those with 1 to 3 pain diagnoses, 42.0% in those with 4 to 7 pain diagnoses, and 59.7% in those with more than 7 pain diagnoses, (P < 0.0005). A similar pattern emerged when examining separating DE into ocular pain and tear film dysfunction (Figs. 3 and 4). As a control, we ran a similar analysis using ICD-9 codes for tobacco use disorder (305.1), tics (307.2×), and anorexia nervosa (307.1 or 307.5×) and found relatively stable frequencies of tobacco use disorder, tics, and anorexia nervosa as the number of pain diagnoses increased (data not shown).
Figure 3.
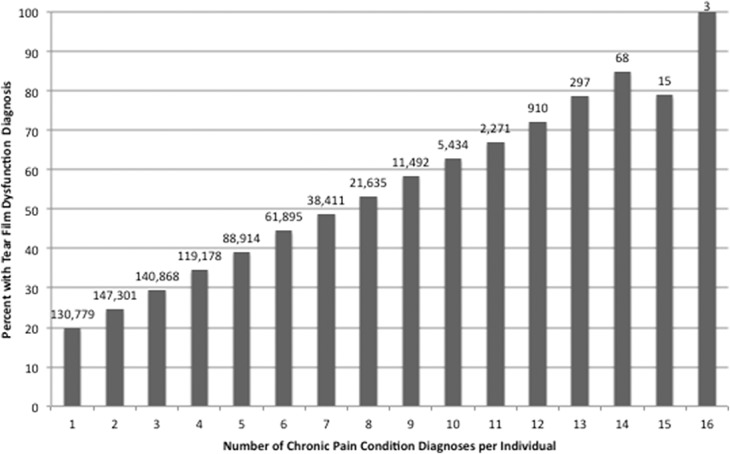
Frequencies of tear film dysfunction by number of pain diagnoses. Pain conditions included all those listed in Table 2 (other than mental health disorders).
Figure 4.
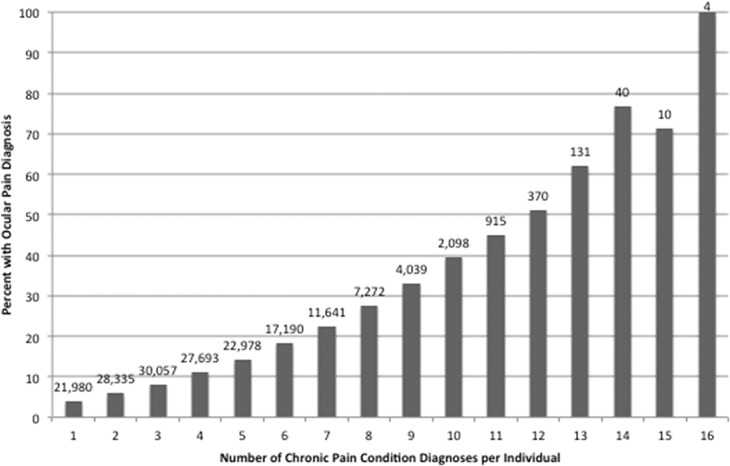
Frequencies of ocular pain by number of pain diagnoses. Pain conditions included all those listed in Table 2 (other than mental health disorders).
Tables 2 and 3 describe the relationship between DE subtypes and specific pain diagnoses. Ocular pain more closely associated with functional (as opposed to structural) pain conditions (Table 2) but associated to an equal magnitude with IASP axis I generalized and regional syndromes. Tear film dysfunction more strongly associated with structural pain conditions (Table 3) and IASP axis I generalized syndromes. Specifically, ocular pain most strongly associated with headache (OR 2.98; 95% confidence interval [CI] 2.95–3.01), tension headache (OR 2.64; 95% CI 2.58–2.71), migraine (OR 2.58; 95% CI 2.54–2.61), TMD (OR 2.39; 95% CI 2.34–2.44), pelvic pain (OR 2.30; 95% CI 2.24–2.37), central pain syndrome (OR 2.24; 95% CI 1.94–2.60), and fibromyalgia/muscle pain (OR 2.23; 95% CI 2.20–2.26), all P < 0.0005 (Table 2). The pain diagnoses most closely related to tear film dysfunction were osteoarthritis (OR 1.97; 95% CI 1.96–1.98) and postherpetic neuralgia (OR 1.95; 95% CI 1.90–2.00), both P < 0.0005 (Table 3). Dry eye also associated with mental health diagnoses but at magnitudes generally lower than with pain disorders. In addition, those using medications such as analgesics, antidepressants, and anxiolytics had a higher frequency of DE (both ocular pain and tear film dysfunction) compared with their counterparts who were not using these medications.
In a multivariable analysis, considering the effect of demographics, medications, and pain comorbidities on DE, we found that all variables examined associated with ocular pain except for sex, smoking status, tension headache, and neoplasm-related pain. In a similar manner, all variables associated with tear film dysfunction except for tension headache, migraine, and complex regional pain syndrome. The pain diagnoses most closely related to ocular pain were headache (OR 1.96; 95% CI 1.94–1.98), postherpetic neuralgia (OR 1.42; 95% CI 1.35–1.49), and migraine (OR 1.41; 95% CI 1.39–1.44), all P < 0.0005. The pain diagnoses most closely related to tear film dysfunction were postherpetic neuralgia (OR 1.41; 95% CI 1.36–1.45), osteoarthritis (OR 1.39; 95% CI 1.38–1.40), and diabetic neuropathy (OR 1.25; 95% CI 1.24–1.27), all P < 0.0005.
4. Discussion
To summarize, we found that within our population, 29.4% of individuals carried a diagnosis of DE, 5.7% of which were subtyped as ocular pain and 26.2% as tear film insufficiency; 2.5% carried both diagnoses. Consistent with other reports,19 the frequency of tear film deficiency steadily increased with age, and both DE types are more common in women. In support of our hypothesis, DE occurred at a higher frequency in those with pain conditions compared with those without pain conditions. In fact, the frequency of DE increased steadily with increased number of pain diagnoses. Our findings are important because they strongly support DE represents a COPC. Moreover, our data showed a stronger relationship between ocular pain and functional pain disorders as compared with tear film dysfunction in both generalized and regionalized syndromes under IASP axis I classification for chronic pain. These data are consistent with underlying somatosensory dysfunction as a shared mechanism among some DE subtypes and other COPC.17,21,48
We previously found that DE symptoms correlated more strongly with nonocular pain and chronic pain conditions than with tear film parameters in a predominantly male veteran population.13 In a complimentary fashion, Vehof et al.49 found that chronic pain syndromes were the most significant predictors of DE symptoms in a predominantly female population in the Netherlands. The current study expands on these findings by demonstrating that correlation between DE and other pain conditions remains strong when considering a large predominantly male population of veterans spanning the entire United States suggesting a mechanistic relationship.
Dry eye has much in common with COPC including a higher incidence in women,46 strong associations with mood disorders like anxiety and depression,42,45 sleep impairment,10 and decreased quality of life.27 From a symptom perspective, many patients with DE with sensations of ocular dryness endorse other pain complaints that are frequently found in those with nonocular pain, namely hot-burning pain, hyperalgesia (eg, sensitivity to wind), and allodynia (eg, sensitivity to light).21 These symptoms are often chronic32 and do not completely respond to therapies targeting the tear film and ocular surface.11,41
Similar to other COPC, individuals with DE symptoms demonstrate evidence of somatosensory dysfunction.17,48 Specifically, in a large cohort of British women, those with DE symptoms had lower heat pain tolerance in an area remote from the eye (ie, the forearm).48 In a similar manner, in a predominantly male cohort, individuals with DE symptoms were found to have higher temporal summation scores and increased aftersensations to hot and cold pain on the forearm.17 These latter metrics are frequently abnormal in individuals with neuropathic pain and central sensitization.4,26 Furthermore, both conditions have been linked to inflammation and neuroendocrine dysregulation. Systemic inflammation has been implicated in the development and maintenance of central sensitization by means of proinflammatory cytokines like tumor necrosis factor–alpha, interleukin (IL)1 and IL6,22 and activation of glial cells25, with a resultant increase in nociceptor excitability and response. Circulating proinflammatory cytokines have been found elevated in complex regional pain syndrome,35,44 fibromyalgia,28,52 and some forms of chronic back pain.20,53 Ocular surface inflammation is an important component of some forms of DE,39,40 and in animal models, adoptive transfer of CD4+ cells was sufficient to induce disease in naive animals.31 Interestingly, hormonal disturbances have been found to influence maintenance of chronic pain, such as estradiol alterations in individuals TMD and IBS,43 and androgen alterations in DE,47 serving as a possible explanation of the higher frequency in women of DE and other COPC in women.7,27,34,37
Dry eye and other COPC have also been linked in previous genetic studies.51 Specifically DE, chronic widespread musculoskeletal pain, chronic pelvic pain, and IBS were linked by 2 latent genetic factors shared among these conditions.51 Furthermore, specific to inflammation, DNA variants in IL1 and IL6R have been associated with non-Sjogren DE in Korean patients.30 Genome wide association studies in European23 and Chinese24 populations have also found multiple loci associated with Sjogren syndrome. Interestingly, none of the identified genes encoded for salivary or lacrimal components but instead associated with immune activity and inflammation, such antibody production and clearance, cytokine production, and immune cell proliferation.6,23 It remains unclear if similar candidates will remain important in non–Sjogren syndrome DE.
As with all studies, our results must be considered bearing in mind the limitations of our research. In this study, data are arranged so that every unique patient seen during the period was placed on a row followed by a yes or no response for the items of interest. Given this format, in this article, we focus on the period prevalence of DE and pain conditions and cannot ascertain incidence or which diagnosis came first. This is an important avenue of future study. In a similar manner, only certain data could be automatically extracted from the database and as such we could not confirm the diagnosis by comparison with clinical notes, determine whether DE was the presenting diagnosis, ascertain the chronicity of DE and other pain diagnoses, or identify which physician made the diagnosis or the intention behind the diagnosis. Furthermore, ICD-9 coding does not capture the full complexity of DE, where manifestations can range from mostly symptoms, mostly signs, or by an overlap in symptoms and signs. Because of inherent limitation with ICD-9 coding methodology, we were unable to access information necessary to use IASP axis II, III, IV, and V classifications; as a result, this study primarily used IASP axis I regional classification of chronic pain. Finally, veterans represent a unique population with a male predominance and a high frequency of mental health disorders and chronic pain diagnoses. Despite these limitations, our study provides the benefit of using a very large nationwide database to evaluate our research questions. To conclude, this study provides further evidence that the frequency of DE increases with increasing numbers of nonocular pain conditions and should be considered a COPC group member. These findings suggest that pain specialists who screen for DE symptoms in those with COPC, and work with eye care providers to optimize both topical and systemic therapy, may improve patient outcomes.
Disclosures
The authors have no conflict of interest to declare.
Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006-15S (A. Galor), NIH Center Core Grant P30EY014801, R01EY026174 (A. Galor), Research to Prevent Blindness Unrestricted Grant, and NIDCR 1R01DE022903 (R. C. Levitt).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].The definition and classification of dry eye disease: report of the definition and classification subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:75–92. [DOI] [PubMed] [Google Scholar]
- [2].The epidemiology of dry eye disease: report of the epidemiology subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:93–107. [DOI] [PubMed] [Google Scholar]
- [3].Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington (DC): National Academies Press, 2011. [PubMed] [Google Scholar]
- [4].Backonja MM, Walk D, Edwards RR, Sehgal N, Moeller-Bertram T, Wasan A, Irving G, Argoff C, Wallace M. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain 2009;25:641–7. [DOI] [PubMed] [Google Scholar]
- [5].Benatti C, Blom JM, Rigillo G, Alboni S, Zizzi F, Torta R, Brunello N, Tascedda F. Disease-induced neuroinflammation and depression. CNS Neurol Disord Drug Targets 2016;15:414–33. [DOI] [PubMed] [Google Scholar]
- [6].Burbelo PD, Ambatipudi K, Alevizos I. Genome-wide association studies in Sjogren's syndrome: what do the genes tell us about disease pathogenesis? Autoimmun Rev 2014;13:756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Crane AM, Levitt RC, Felix ER, Sarantopoulos KD, McClellan AL, Galor A. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol 2016;101:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders–pathways of vulnerability. Pain 2006;123:226–30. [DOI] [PubMed] [Google Scholar]
- [9].Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet 2005;14:135–43. [DOI] [PubMed] [Google Scholar]
- [10].Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev 2013;17:173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Galor A, Batawi H, Felix ER, Margolis TP, Sarantopoulos KD, Martin ER, Levitt RC. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol 2016;100:745–9. [DOI] [PubMed] [Google Scholar]
- [12].Galor A, Covington D, Levitt AE, McManus KT, Seiden B, Felix ER, Kalangara J, Feuer W, Patin DJ, Martin ER, Sarantopoulos KD, Levitt RC. Neuropathic ocular pain due to dry eye is associated with multiple comorbid chronic pain syndromes. J Pain 2016;17:310–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Galor A, Felix ER, Feuer W, Shalabi N, Martin ER, Margolis TP, Sarantopoulos CD, Levitt RC. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol 2015;99:1126–9. [DOI] [PubMed] [Google Scholar]
- [14].Galor A, Feuer W, Lee DJ, Florez H, Faler AL, Zann KL, Perez VL. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative database. Am J Ophthalmol 2012;154:340–6. e342. [DOI] [PubMed] [Google Scholar]
- [15].Galor A, Feuer W, Lee DJ, Florez H, Venincasa VD, Perez VL. Ocular surface parameters in older male veterans. Invest Ophthalmol Vis Sci 2013;54:1426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond) 2015;29:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Galor A, Levitt RC, McManus KT, Kalangara JP, Seiden BE, Park JJ, Covington DB, Sarantopoulos CD, Felix ER. Assessment of somatosensory function in patients with idiopathic dry eye symptoms. JAMA Ophthalmol 2016;134:1290–8. [DOI] [PubMed] [Google Scholar]
- [18].Galor A, Seiden BE, Park JJ, Feuer WJ, McClellan AL, Felix ER, Levitt RC, Sarantopoulos CD, Wallace DM. The association of dry eye symptom severity and comorbid insomnia in US veterans. Eye Contact Lens 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol 2009;3:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Heffner KL, France CR, Trost Z, Ng HM, Pigeon WR. Chronic low back pain, sleep disturbance, and interleukin-6. Clin J Pain 2011;27:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kalangara JP, Galor A, Levitt RC, Covington DB, McManus KT, Sarantopoulos CD, Felix ER. Characteristics of ocular pain complaints in patients with idiopathic dry eye symptoms. Eye Contact Lens 2016;23:192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Laird BJ, McMillan DC, Fayers P, Fearon K, Kaasa S, Fallon MT, Klepstad P. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist 2013;18:1050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, Kelly JA, Dozmorov MG, Miceli-Richard C, Bowman S, Lester S, Eriksson P, Eloranta ML, Brun JG, Goransson LG, Harboe E, Guthridge JM, Kaufman KM, Kvarnstrom M, Jazebi H, Cunninghame Graham DS, Grandits ME, Nazmul-Hossain AN, Patel K, Adler AJ, Maier-Moore JS, Farris AD, Brennan MT, Lessard JA, Chodosh J, Gopalakrishnan R, Hefner KS, Houston GD, Huang AJ, Hughes PJ, Lewis DM, Radfar L, Rohrer MD, Stone DU, Wren JD, Vyse TJ, Gaffney PM, James JA, Omdal R, Wahren-Herlenius M, Illei GG, Witte T, Jonsson R, Rischmueller M, Ronnblom L, Nordmark G, Ng WF, Registry UKPSsS, Mariette X, Anaya JM, Rhodus NL, Segal BM, Scofield RH, Montgomery CG, Harley JB, Sivils KL. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren's syndrome. Nat Genet 2013;45:1284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li Y, Zhang K, Chen H, Sun F, Xu J, Wu Z, Li P, Zhang L, Du Y, Luan H, Li X, Wu L, Li H, Wu H, Li X, Li X, Zhang X, Gong L, Dai L, Sun L, Zuo X, Xu J, Gong H, Li Z, Tong S, Wu M, Li X, Xiao W, Wang G, Zhu P, Shen M, Liu S, Zhao D, Liu W, Wang Y, Huang C, Jiang Q, Liu G, Liu B, Hu S, Zhang W, Zhang Z, You X, Li M, Hao W, Zhao C, Leng X, Bi L, Wang Y, Zhang F, Shi Q, Qi W, Zhang X, Jia Y, Su J, Li Q, Hou Y, Wu Q, Xu D, Zheng W, Zhang M, Wang Q, Fei Y, Zhang X, Li J, Jiang Y, Tian X, Zhao L, Wang L, Zhou B, Li Y, Zhao Y, Zeng X, Ott J, Wang J, Zhang F. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjogren's syndrome at 7q11.23. Nat Genet 2013;45:1361–5. [DOI] [PubMed] [Google Scholar]
- [25].Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, Wasan AD, Zurcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM. Evidence for brain glial activation in chronic pain patients. Brain 2015;138:604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede RD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. PAIN 2010;150:439–50. [DOI] [PubMed] [Google Scholar]
- [27].Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain 2016;17(9 suppl):T93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mendieta D, De la Cruz-Aguilera DL, Barrera-Villalpando MI, Becerril-Villanueva E, Arreola R, Hernandez-Ferreira E, Perez-Tapia SM, Perez-Sanchez G, Garces-Alvarez ME, Aguirre-Cruz L, Velasco-Velazquez MA, Pavon L. IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J Neuroimmunol 2016;290:22–5. [DOI] [PubMed] [Google Scholar]
- [29].Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol 2007;143:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Na KS, Mok JW, Kim JY, Joo CK. Proinflammatory gene polymorphisms are potentially associated with Korean non-Sjogren dry eye patients. Mol Vis 2011;17:2818–23. [PMC free article] [PubMed] [Google Scholar]
- [31].Niederkorn JY, Stern ME, Pflugfelder SC, De Paiva CS, Corrales RM, Gao J, Siemasko K. Desiccating stress induces T cell-mediated Sjogren's Syndrome-like lacrimal keratoconjunctivitis. J Immunol 2006;176:3950–7. [DOI] [PubMed] [Google Scholar]
- [32].Ong ES, Alghamdi YA, Levitt RC, McClellan AL, Lewis G, Sarantopoulos CD, Felix ER, Galor A. Longitudinal examination of frequency of and risk factors for severe dry eye symptoms in US veterans. JAMA Ophthalmol 2016;135:116–23. [DOI] [PubMed] [Google Scholar]
- [33].Pouyeh B, Viteri E, Feuer W, Lee DJ, Florez H, Fabian JA, Perez VL, Galor A. Impact of ocular surface symptoms on quality of life in a United States veterans affairs population. Am J Ophthalmol 2012;153:1061–6 e1063. [DOI] [PubMed] [Google Scholar]
- [34].Reed BD, Harlow SD, Sen A, Edwards RM, Chen D, Haefner HK. Relationship between vulvodynia and chronic comorbid pain conditions. Obstet Gynecol 2012;120:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ritz BW, Alexander GM, Nogusa S, Perreault MJ, Peterlin BL, Grothusen JR, Schwartzman RJ. Elevated blood levels of inflammatory monocytes (CD14+ CD16+) in patients with complex regional pain syndrome. Clin Exp Immunol 2011;164:108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol 2016;100:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol 2003;136:318–26. [DOI] [PubMed] [Google Scholar]
- [38].Schein OD, Tielsch JM, Munoz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology 1997;104:1395–401. [DOI] [PubMed] [Google Scholar]
- [39].Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocul Surf 2004;2:124–30. [DOI] [PubMed] [Google Scholar]
- [40].Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol 2013;32:19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stonecipher K, Perry HD, Gross RH, Kerney DL. The impact of topical cyclosporine A emulsion 0.05% on the outcomes of patients with keratoconjunctivitis sicca. Curr Med Res Opin 2005;21:1057–63. [DOI] [PubMed] [Google Scholar]
- [42].Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosom Med 2004;66:837–44. [DOI] [PubMed] [Google Scholar]
- [43].Traub RJ, Cao DY, Karpowicz J, Pandya S, Ji Y, Dorsey SG, Dessem D. A clinically relevant animal model of temporomandibular disorder and irritable bowel syndrome comorbidity. J Pain 2014;15:956–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Uceyler N, Eberle T, Rolke R, Birklein F, Sommer C. Differential expression patterns of cytokines in complex regional pain syndrome. PAIN 2007;132:195–205. [DOI] [PubMed] [Google Scholar]
- [45].Vassend O, Krogstad BS, Dahl BL. Negative affectivity, somatic complaints, and symptoms of temporomandibular disorders. J Psychosom Res 1995;39:889–99. [DOI] [PubMed] [Google Scholar]
- [46].Veasley C, Clare D, Clauw DJ, Cowley T, Nguyen RHN, Reinecke P, Vernon SD, Williams DA. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment: 2015 analysis and policy recommendations. Chronic Pain Res Alliance 2015;2016:5. [Google Scholar]
- [47].Vehof J, Hysi PG, Hammond CJ. A metabolome-wide study of dry eye disease reveals serum androgens as biomarkers. Ophthalmology 2017;124:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vehof J, Kozareva D, Hysi PG, Harris J, Nessa A, Williams FK, Bennett DL, McMahon SB, Fahy SJ, Direk K, Spector TD, Hammond CJ. Relationship between dry eye symptoms and pain sensitivity. JAMA Ophthalmol 2013;131:1304–8. [DOI] [PubMed] [Google Scholar]
- [49].Vehof J, Sillevis Smitt-Kamminga N, Nibourg SA, Hammond CJ. Predictors of discordance between symptoms and signs in dry eye disease. Ophthalmology 2017;124:280–6. [DOI] [PubMed] [Google Scholar]
- [50].Vehof J, Wang B, Kozareva D, Hysi PG, Snieder H, Hammond CJ. The heritability of dry eye disease in a female twin cohort. Invest Ophthalmol Vis Sci 2014;55:7278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vehof J, Zavos HM, Lachance G, Hammond CJ, Williams FM. Shared genetic factors underlie chronic pain syndromes. PAIN 2014;155:1562–8. [DOI] [PubMed] [Google Scholar]
- [52].Wallace DJ, Gavin IM, Karpenko O, Barkhordar F, Gillis BS. Cytokine and chemokine profiles in fibromyalgia, rheumatoid arthritis and systemic lupus erythematosus: a potentially useful tool in differential diagnosis. Rheumatol Int 2015;35:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang H, Ahrens C, Rief W, Gantz S, Schiltenwolf M, Richter W. Influence of depression symptoms on serum tumor necrosis factor-alpha of patients with chronic low back pain. Arthritis Res Ther 2010;12:R186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. PAIN 2011;152:S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum 2008;37:339–52. [DOI] [PubMed] [Google Scholar]
- [56].Yunus MB. Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev 2015;11:70–85. [DOI] [PubMed] [Google Scholar]


