Abstract
Objective
Behçet’s disease is heterogeneous with clinical variability across ethnicities and geographic locations. The goal of this study was to analyze the clinical characteristics of our multi-ethnic Behçet’s disease cohort at the University of Michigan.
Material and Methods
A detailed patient characterization was performed. Differences in disease characteristics between men and women, and between patients fulfilling the International Criteria for Behçet’s Disease (ICBD) and the International Study Group criteria (ISG) were determined in our cohort.
Results
A total of 114 patients with a male to female ratio of ~ 1:4 were included. All patients met the ICBD criteria, including 76 who also met the ISG criteria. Over 95% of patients had recurrent genital ulcers, which is higher than generally reported. Retinitis was 5.3 times more likely in men than in women (p=0.009), and arthralgia was 3.3 times more likely in women than men (p=0.048). When comparing cohorts derived from the two different criteria, the ISG cohort had more skin manifestations (OR=3.3, p=0.0006). Acneiform lesions were associated with ~8 times higher odds of developing retinitis in our patients (p=0.0008), and superficial thrombophlebitis was associated with a trend for higher odds of developing uveitis (OR=4.1, p=0.057). Using the ICBD criteria, 38 additional patients were identified compared to only using the ISG criteria. Of these patients, 28 presented with only mucosal ulceration with or without joint involvement.
Conclusion
We characterize Behçet’s disease in a multi-ethnic cohort from North America. Using ICBD criteria in the United States significantly increases the likelihood of identifying Behçet’s disease, particularly in patients with isolated mucosal involvement who constitute a substantial subset of patients in this region.
Keywords: Behçet’s disease, United States, Michigan, criteria, manifestations, sex bias
Introduction
Behçet’s disease is a rare, chronic, and multisystemic immune-mediated disease with incompletely understood etiology. While there are many different diagnostic criteria for Behçet’s disease, the most commonly used are the International Study Group (ISG) criteria. These criteria require the presence of oral ulcers, in addition to two or more of the following manifestations: genital ulceration, eye lesions (uveitis, retinitis), skin lesions (folliculitis, papulopustular lesions, acneiform nodules, erythema nodosum), and a positive pathergy test (1). In addition, patients with Behçet’s disease can present with other symptoms, such as thrombophlebitis, deep venous thrombosis, central nervous system involvement, arthralgia, arthritis, and gastrointestinal features (1). More recently, new International Criteria for Behçet’s Disease (ICBD) were proposed, which emphasize oral, genital, and ocular involvement, and allow for incorporating vascular and neurological involvement as a guide for diagnosis and classification of Behçet’s disease (2). The symptoms, severities, and prevalence of Behçet’s disease tend to differ between geographical locations and, although Behçet’s disease can be found all over the world, it is more prevalent around the ancient “Silk Road” (3, 4). This disease also has higher prevalence rates in countries such as Turkey, Iraq, Iran, Korea, Japan, and China (5–8). In Turkey, Behçet’s disease can be found in 80–370 people per 100,000; meanwhile, in the United States 5.2 per 100,000 people present with the disease (4, 9).
As a rare disease with numerous manifestations, the purpose of much of the research for Behçet’s disease has been to describe and characterize the clinical manifestations. These studies have been performed using different cohorts from a variety of geographical locations. Recently, the characteristics of Behçet’s disease were described in cohorts from the North Eastern United States and compared to patients from a center in Turkey. A much higher percentage of patients in the United States were reported to have neurologic and gastrointestinal manifestations compared to Behçet’s disease patients from Turkey (10).
To establish the heterogeneity in which Behçet’s disease can manifest, it is important to record the clinical findings of the disease around the world. Here we describe the characteristics of Behçet’s disease in the Michigan Behçet’s Disease Cohort, based at the University of Michigan. We examine associations between the clinical manifestations of the disease, and compare the ICBD and ISG criteria in our cohort.
Methods
We identified all patients with Behçet’s disease who presented to the University of Michigan Health System from October 2011 to June 2017. Only patients who met the International Criteria for Behçet’s Disease (ICBD) or the International Study Group (ISG) criteria were included in this study. Basic demographic information including age, sex, race, and ethnicity were recorded. Clinical manifestations and current medications used to treat Behçet’s disease in our cohort were also recorded. An experienced rheumatologist (AHS) either directly evaluated each patient or reviewed the medical records of each patient included in this study. Statistical analysis was completed using a chi square test and Yates’ correction to determine statistical significance of differences in disease characteristics between men and women, differences between patients who met the ICBD and the ISG criteria, and associations between major and minor disease manifestations.
Results
In total, 114 patients who met the ICBD criteria were included in this study, 76 of whom also met the ISG criteria. As most previous studies on Behçet’s disease have used the ISG criteria, we focused our initial analysis on the 76 patients who met the ISG criteria, allowing us to compare results with previous reports (Table 1). Our ISG criteria cohort consisted of 18 male and 58 female patients. The mean (S.D.) age of this cohort was 40.6 (13.9) years. We had a large majority of White patients, six Black patients, one American Indian and Alaskan Native, two Asian, and four patients whose ancestral background was unknown/other. In addition, the majority of our patients claimed ethnicities of non-Hispanic, 1 was Hispanic, and 5 patients had unknown ethnicities. Patients who met ICBD criteria consisted of 23 men and 91 women (Table 1). The mean (S.D) age of patients in the ICBD cohort was 41.4 (14.5) years. The ICBD cohort also had a large majority of White patients; in addition, there were six Black patients, one American Indian and Alaskan Native, three Asians, and five patients with unknown/other ancestral backgrounds. The ethnicities representing the ICBD cohort were as follows: a large majority of non-Hispanics, 1 Hispanic, and 6 patients with unknown ethnicities.
Table 1.
Demographics and disease characteristics of Behçet’s disease patients included in our study
| Demographics | ISG (n=76) | ICBD (n=114) | |||
|---|---|---|---|---|---|
| Mean age (years ± SD) | 40.6± 13.9 | 41.4 ± 14.5 | |||
| Sex (Male: Female) | 1:3.2 | 1:4.0 | |||
| Race n (%) | |||||
| White | 63 (82.9) | 99 (86.8) | |||
| Black | 6(7.9) | 6(5.3) | |||
| American Indian and Alaska Native | 1(1.3) | 1(0.9) | |||
| Asian | 2(2.6) | 3(2.6) | |||
| Unknown/Other | 4(5.3) | 5(4.4) | |||
| Ethnicity n (%) | |||||
| Non-Hispanic | 70(92.1) | 107(93.9) | |||
| Hispanic | 1(1.3) | 1(0.9) | |||
| Unknown | 5(6.6) | 6(5.3) | |||
|
| |||||
| ISG cohort | Total | Male | Female | Odds Ratios | p-value |
|
| |||||
| Disease Manifestations n (%) | |||||
| Oral Ulcers | 76 (100) | 18 (100) | 58 (100) | - | - |
| Genital Ulcers | 75 (98.7) | 18 (100) | 57 (98.3) | - | 0.5749 |
| Skin Manifestations | 60 (78.9) | 15 (83.3) | 45 (77.6) | 1.4 | 0.8481 |
| Folliculitis | 31 (40.8) | 8 (44.4) | 23 (39.7) | 1.2 | 0.9309 |
| Acneiform lesions | 27 (35.5) | 7 (38.9) | 20 (34.5) | 1.2 | 0.9527 |
| E. Nodosum | 24 (31.6) | 8 (44.4) | 16 (27.6) | 2.1 | 0.2912 |
| Arthralgia | 56 (73.7) | 10 (55.6) | 46 (79.3) | 0.3 | 0.0904 |
| Arthritis | 42 (55.3) | 7 (38.9) | 35 (60.3) | 0.4 | 0.1842 |
| Ocular Involvement | 37 (48.7) | 10 (55.6) | 27 (46.6) | 1.4 | 0.6908 |
| Uveitis | 35 (46.1) | 9 (50.0) | 26 (44.8) | 1.2 | 0.9093 |
| Retinitis | 14 (18.4) | 7 (38.9) | 7 (12.1) | 4.6 | 0.0267 |
| Positive Pathergy | 16 (21.1) | 1 (5.6) | 15 (25.9) | 0.2 | 0.1297 |
| Venous Thrombosis | 13 (17.1) | 4 (22.2) | 9 (15.5) | 1.6 | 0.7629 |
| Thrombophlebitis | 9 (11.8) | 2 (11.1) | 7 (12.1) | 0.9 | 0.9125 |
| Deep Thrombosis | 7 (9.2) | 4 (22.2) | 3 (5.2) | 5.2 | 0.0857 |
| CNS Behçet’s | 5 (6.6) | 1 (5.6) | 4 (6.9) | 0.8 | 0.8411 |
| GI Involvement | 4 (5.3) | 2 (11.1) | 2 (3.4) | 3.5 | 0.5043 |
|
| |||||
| ICBD cohort | Total | Male | Female | Odds Ratios | p-value |
|
| |||||
| Disease Manifestations n (%) | |||||
| Oral Ulcers | 114 (100.0) | 23 (100.0) | 91 (100.0) | - | - |
| Genital Ulcers | 109 (95.6) | 22 (95.7) | 87 (95.6) | 1.0 | 0.9920 |
| Skin Manifestations | 61 (53.5) | 15 (65.2) | 46 (50.5) | 1.8 | 0.3048 |
| Folliculitis | 32 (28.1) | 8 (34.8) | 24 (26.4) | 1.5 | 0.5877 |
| Acneiform lesions | 28 (24.6) | 7 (30.4) | 21 (23.1) | 1.5 | 0.6446 |
| E. Nodosum | 25 (21.9) | 8 (34.8) | 17 (18.7) | 2.3 | 0.1659 |
| Arthralgia | 81 (71.1) | 12 (52.2) | 69 (75.8) | 0.3 | 0.0480 |
| Arthritis | 58 (50.9) | 8 (34.8) | 50 (54.9) | 0.4 | 0.1350 |
| Ocular Involvement | 40 (35.1) | 11 (47.8) | 29 (31.9) | 2.0 | 0.2347 |
| Uveitis | 38 (33.3) | 10 (43.5) | 28 (30.8) | 1.7 | 0.3641 |
| Retinitis | 14 (12.3) | 7 (30.4) | 7 (7.7) | 5.3 | 0.0090 |
| Positive Pathergy | 16 (14.0) | 1 (4.3) | 15 (16.5) | 0.2 | 0.2456 |
| Venous Thrombosis | 19 (16.7) | 5 (21.7) | 14 (15.4) | 1.5 | 0.6763 |
| Thrombophlebitis | 11 (9.6) | 2 (8.7) | 9 (9.9) | 0.9 | 0.8624 |
| Deep Thrombosis | 12 (10.5) | 5 (21.7) | 7 (7.7) | 3.3 | 0.1139 |
| CNS Behçet’s | 6 (5.3) | 1 (4.3) | 5 (5.5) | 0.8 | 0.8258 |
| GI Involvement | 6 (5.3) | 2 (8.7) | 4 (4.4) | 2.1 | 0.7622 |
ISG: international study group criteria; ICBD: international criteria for Behçet’s disease; E. Nodusum: erythema nodosum; CNS: central nervous system; GI: gastrointestinal
All patients presented with oral ulcers in the ISG criteria cohort by definition, and all but one had oral ulcers in the ICBD cohort. A majority of the patients in both cohorts had genital ulcers, skin manifestations, and joint involvement (Table 1). Meanwhile, 48.7% of the patients fulfilling the ISG criteria had eye manifestations, 21.1% had a positive pathergy test/reaction, and 17.1% had venous thrombosis. However, when using the ICBD criteria, we noted that 35.1% of patients had ocular manifestations, 14.0% tested positive for pathergy, and 16.7% had venous thrombosis. Furthermore, in both cohorts, more patients had uveitis than retinitis, while nearly an equal number of patients who suffered vascular involvement had thrombophlebitis and deep venous thrombosis. Expectedly, very few patients presented with neurologic involvement or gastrointestinal manifestations in either cohort. Skin manifestations were documented as either folliculitis, acneiform lesions, or erythema nodosum.
The clinical features of the men and women in our cohort were also examined to determine differences between male and female patients (Table 1). The only statistically significant difference noted between male and female patients in our ISG criteria cohort was the prevalence of retinitis, which was 4.6 times more prevalent in men than in women (p=0.0267). Significant differences between disease manifestations in men and women when using the ICBD criteria included arthralgia (p=0.048) and retinitis (p=0.009). Arthralgia was 3.3 times more likely in women than in men, and retinitis was 5.3 times more likely in men than in women.
The current systemic medications used to manage Behçet’s disease manifestations in our patients largely consisted of colchicine and prednisone (Table 2). These medications were used the most in this cohort, whereas cyclosporine, cyclophosphamide, and apremilast were not used in any of our patients. The biological medications we used to treat our patients were infliximab, adalimumab, and etanercept. In the ISG criteria cohort, nine patients received regular infusions of infliximab, eight received adalimumab, and one patient was on etanercept; in our ICBD criteria cohort, ten patients were on infliximab, ten were on adalimumab, and one was taking etanercept.
Table 2.
Systemic medications used in our Behçet’s disease patients
| ISG | ICBD | |||
|---|---|---|---|---|
|
|
|
|||
| Medications | number | Median dose | number | Median dose |
| Colchicine | 37 | 1.2 mg | 59 | 1.0 mg |
| Prednisone | 27 | - | 39 | - |
| Chronic | 22 | 10 mg | 30 | 10 mg |
| Azathioprine | 15 | 100 mg | 17 | 100 mg |
| Infliximab | 9 | 5.3 mg/kg/8wks | 10 | 5.4 mg/kg/8wks |
| Adalimumab | 8 | 40 mg/2wks | 10 | 40 mg/2wks |
| Methotrexate | 6 | 16.25 mg | 7 | 15 mg |
| Mycophenolate Mofetil | 5 | 2000 mg | 7 | 2000 mg |
| Etanercept | 1 | 50 mg/wk | 1 | 50 mg/wk |
| Cyclosporine (systemic) | 0 | - | 0 | - |
| Cyclophosphamide | 0 | - | 0 | - |
| Apremilast | 0 | - | 0 | - |
ISG: international study group criteria; ICBD: international criteria for Behçet’s disease
We examined if minor Behçet’s disease manifestations might correlate or predict other disease manifestations in our cohort. In all patients fulfilling the ICBD criteria, we found that patients with superficial thrombophlebitis had higher frequencies of deep vein thrombosis (OR=6.8; p=0.0155) and acneiform lesions (OR=4.4; p=0.0392), and there was a trend for higher odds of uveitis (OR=4.1; p=0.0566). Patients with acneiform lesions had significantly more retinitis; 32.1% of patients with acneiform lesions had retinitis, whereas only 5.8% of patients without acneiform lesions had retinitis (OR: 7.7; p=0.0008). No other minor manifestations showed correlative relationships with severe disease manifestations.
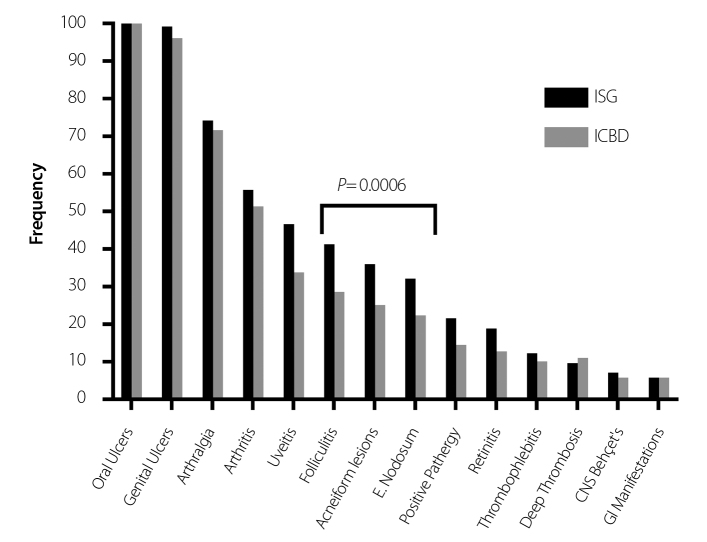
Next, we compared the ISG and ICBD cohorts to assess differences between the two sets of patients, as fewer studies to date have used the ICBD criteria. We noted that skin involvement (p=0.0006) was the only manifestation showing statistical significance, being more prevalent in the ISG cohort (Figure 1). Also of interest were the 38 patients who only met the ICBD criteria, but not the ISG criteria (Table 3). These patients had significantly less of the following clinical manifestations: folliculitis (p=0.0001), acneiform lesions (p=0.0003), erythema nodosum (p=0.001), uveitis (p=0.0001), retinitis (p=0.0117), and pathergy reaction (p=0.0057).
Figure 1.
The frequency of Behçet’s disease manifestations in patients fulfilling the ISG criteria compared to patients fulfilling the ICBD criteria in our study
ISG: international study group criteria; ICBD: international criteria for Behçet’s disease
Table 3.
Patients fulfilling the ISG criteria compared to patients fulfilling only the ICBD criteria
| Total Patients | ISG | ICBD only | p-value |
|---|---|---|---|
| Disease Manifestations n (%) | |||
| Oral Ulcers | 76 (100.0) | 38 (100) | - |
| Genital Ulcers | 75 (98.7) | 34 (89.5) | 0.0753 |
| Skin Manifestations | 60 (78.9) | 1 (2.6) | 0.0001 |
| Folliculitis | 31 (40.8) | 1 (2.6) | 0.0001 |
| Acneiform lesions | 27 (35.5) | 1 (2.6) | 0.0003 |
| E. Nodosum | 24 (31.6) | 1 (2.6) | 0.0010 |
| Arthralgia | 56 (73.7) | 25 (65.8) | 0.5111 |
| Arthritis | 42 (55.3) | 16 (42.1) | 0.2602 |
| Ocular Manifestations | 37 (48.7) | 3 (7.9) | 0.0001 |
| Uveitis | 35 (46.1) | 3 (7.9) | 0.0001 |
| Retinitis | 14 (18.4) | 0 (0.0) | 0.0117 |
| Positive Pathergy | 16 (21.1) | 0 (0.0) | 0.0057 |
| Venous Thrombosis | 13 (17.1) | 6 (15.8) | 0.8590 |
| Thrombophlebitis | 9 (11.8) | 2 (5.3) | 0.4324 |
| Deep Thrombosis | 7 (9.2) | 5 (13.2) | 0.7462 |
| CNS Behçet’s | 5 (6.6) | 0 (0.0) | 0.2577 |
| GI Manifestations | 4 (5.3) | 2 (5.3) | 1.0000 |
ICBD: international criteria for Behçet’s disease; ISG: international study group criteria; E. Nodosum: erythema nodosum; CNS: central nervous system; GI: gastrointestinal
Discussion
Our study describes the demographics and clinical characteristics of Behçet’s disease at a tertiary referral center in the United States. In addition, we compare the more recent International Criteria for Behçet’s Disease (ICBD) to the more established International Study Group criteria (ISG).
When compared to other multi-ethnic cohorts, the Behçet’s disease cohort at the University of Michigan shows an overall similar clinical pattern. In fact, when compared to a multi-ethnic cohort from France, the only manifestation showing a significant difference was the presence of genital ulceration, which was present in 98.7% of our patients fulfilling the ISG criteria compared to 79.7% of patients in the French cohort (p=0.0004) (11). It should be noted that such a high frequency of genital ulcers detected in our cohort is uncharacteristic of Behçet’s disease cohorts previously reported from the United States or elsewhere, and the reason for this difference in unclear.
A previous collaboration between New York University (NYU), the National Institutes of Health (NIH), and the University of Istanbul in Turkey had shown higher rates of neurologic disease and gastrointestinal disease in American patients when compared to Turkish patients (10). NYU and the NIH reported prevalence of neurologic disease in 17.3% and 20.0% of their Behçet’s disease patients respectively, while also reporting that 37% of Behçet’s disease patients at NYU and 42.9% Behçet’s disease patients at the NIH had gastrointestinal manifestations (10). In contrast, 3.7% of Turkish patients from the University of Istanbul had neurologic disease, while none had gastrointestinal disease (10). Other Turkish cohort studies have shown similar patterns: gastrointestinal disease has been recorded in 0.4% to 2.7% of Turkish Behçet’s disease patients, whereas neuro-Behçet’s disease manifests in 1.8% to 3% of patients (12–14). Indeed, these previous studies suggest that a smaller proportion of Turkish Behçet’s disease patients present with these manifestations than in the United States. We observed a slightly higher frequency of gastrointestinal and neurological involvement in our patients when compared to Turkish cohorts; 5.3% and 6.6% of patients in our ISG cohort had gastrointestinal and neurological involvement, respectively. However, we do not see the drastic differences reported from patients at NYU and the NIH. It is important to note that in our cohort we required consistent MRI findings and colonoscopy features to diagnose neurological and gastrointestinal Behçet’s disease, respectively, which could explain this discrepancy.
In our cohort at the University of Michigan, the male to female Behçet’s disease ratio occurs similar to cohorts previously studied in the United States. Three other tertiary referral centers in the United States have shown a female predominance of Behçet’s disease. A Behçet’s disease cohort at the University of California Davis had a male to female ratio of 1:3.3 using the ICBD criteria, the NIH reported a male to female ratio of 1:4 using the ISG criteria, and NYU noted the largest female dominated cohort with a ratio of 1:11.8, once again using the ISG criteria (10, 15). In our cohort, we report male to female ratios of 1:3.2 (ISG) and 1:4 (ICBD), supporting a female predominance of Behçet’s disease in the United States. It is possible that there are significantly more women than men that present with Behçet’s disease in the United States. However, it is also possible that this female bias could be a consequence of studying tertiary referral centers. In fact, the Ambulatory and Hospital Care Statistics Branch of the Center of Disease Control and Prevention surveyed 847 hospital outpatient departments in the United States and found that, in 2011, 76,286 women and 49,435 men visited these participating outpatient clinics (16). These data suggest that women visit tertiary referral centers more frequently than men in the United States, which might contribute to the female sex bias reported in Behçet’s diseases. In France, a study performed in Paris in 2008 seemed to avoid this potential sex bias by including patients from hospitals, community physicians, and the National Health Insurance database. They observed a male to female ratio of 1.3:1 (11). Nonetheless, more analysis is required as differences in the frequency of tertiary referral center visits in the United States does not account for the entirety of female bias.
The presentation of Behçet’s disease manifestations can vary between geographic locations, but it can also vary with sex. In our cohort, we noticed that male and female patients present with Behçet’s disease differently (Table 1). Many previous studies have shown that men have a higher incidence of ocular involvement than women (17–21). A Turkish study at the University of Mersin followed 2,313 patients and found that 38.1% of men had ocular involvement, compared to 18.9% of women (17). While our own ISG criteria cohort data shows no significant difference in overall ocular manifestations between men and women (Men=55.6%, Women=46.6%, p=0.6908), retinitis was significantly more frequent in men (p=0.0267). Similarly, our patients fulfilling the ICBD criteria also showed a higher frequency of retinitis in men compared to women (p=0.009). Most studies reported uveitis and retinitis together as “eye involvement.” However, a study from the United Kingdom with 73 patients reported them separately, noting that 36% of men and only 11% of women had vascular retinitis, thus supporting our own findings (19).
Two different sets of Behçet’s disease criteria were evaluated in this study. Previous research suggests that the ICBD criteria are more sensitive than the ISG criteria, but slightly less specific (2). The sensitivity of the ICBD criteria is also apparent in our study. Using the ICBD criteria, we identified 38 additional patients compared to using the ISG criteria. Patients fulfilling the ICBD criteria compared to patients fulfilling the ISG criteria were statistically different in the frequency of only skin involvement (p=0.0006) (Figure 1). The ISG criteria cohort did not have higher proportions in any of the other manifestations, suggesting that the ISG criteria do not necessarily select patients with more severe disease compared to the ICBD criteria. However, the 38 patients who met the ICBD criteria but not the ISG criteria had experienced significantly fewer disease manifestations (Table 3). The ICBD criteria were able to more frequently identify patients with less severe disease than the ISG criteria.
In conclusion, we describe the clinical characteristics of a relatively large Behçet’s disease cohort in Michigan and compare the ISG and ICBD criteria for the first time using a cohort from the United States. This study complements previous literature to understand the heterogeneity of Behçet’s disease in different parts of the world and offers insight into the usefulness of the ICBD criteria for identifying Behçet’s disease patients in North America. Limitations of our study include the retrospective nature of collecting data from our cohort and the size of our patient population compared to larger cohorts from other parts of the world where Behçet’s disease is more prevalent. Despite these limitations, this study is an invaluable addition to the knowledge and understanding of Behçet’s disease in North America.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the institutional review board at the University of Michigan.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.H.S.; Design - A.H.S.; Supervision - A.H.S.; Resources - N.C.K., A.H.S.; Materials - N.C.K., A.H.S.; Data Collection and/or Processing - N.C.K., A.H.S.; Analysis and/or Interpretation - N.C.K., A.H.S.; Literature Search - N.C.K., A.H.S.; Writing Manuscript - N.C.K., A.H.S.; Critical Review - N.C.K., A.H.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Criteria for diagnosis of Behcet’s disease. International Study Group for Behcet’s Disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 2.International Team for the Revision of the International Criteria for Behcet’s D. The International Criteria for Behcet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28:338–47. doi: 10.1111/jdv.12107. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed Z, Rossi ML, Yong S, Martin DK, Walayat S, Cashman M, et al. Behcet’s disease departs the ‘Silk Road’: a case report and brief review of literature with geographical comparison. J Community Hosp Intern Med Perspect. 2016;6:30362. doi: 10.3402/jchimp.v6.30362. https://doi.org/10.3402/jchimp.v6.30362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yurdakul S, Hamuryudan V, Yazici H. Behcet syndrome. Curr Opin Rheumatol. 2004;16:38–42. doi: 10.1097/00002281-200401000-00008. https://doi.org/10.1097/00002281-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Chang HK, Kim JW. The clinical features of Behcet’s disease in Yongdong districts: analysis of a cohort followed from 1997 to 2001. J Korean Med Sci. 2002;17:784–9. doi: 10.3346/jkms.2002.17.6.784. https://doi.org/10.3346/jkms.2002.17.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida A, Kawashima H, Motoyama Y, Shibui H, Kaburaki T, Shimizu K, et al. Comparison of patients with Behcet’s disease in the 1980s and 1990s. Ophthalmology. 2004;111:810–5. doi: 10.1016/j.ophtha.2003.07.018. https://doi.org/10.1016/j.ophtha.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Yang P, Fang W, Meng Q, Ren Y, Xing L, Kijlstra A. Clinical features of chinese patients with Behcet’s disease. Ophthalmology. 2008;115:312–8. doi: 10.1016/j.ophtha.2007.04.056. https://doi.org/10.1016/j.ophtha.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 8.Yang P, Zhang Z, Zhou H, Li B, Huang X, Gao Y, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res. 2005;30:943–8. doi: 10.1080/02713680500263606. https://doi.org/10.1080/02713680500263606. [DOI] [PubMed] [Google Scholar]
- 9.Calamia KT, Wilson FC, Icen M, Crowson CS, Gabriel SE, Kremers HM. Epidemiology and clinical characteristics of Behcet’s disease in the US: a population-based study. Arthritis Rheum. 2009;61:600–4. doi: 10.1002/art.24423. https://doi.org/10.1002/art.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibley C, Yazici Y, Tascilar K, Khan N, Bata Y, Yazici H, et al. Behcet syndrome manifestations and activity in the United States versus Turkey -- a cross-sectional cohort comparison. J Rheumatol. 2014;41:1379–84. doi: 10.3899/jrheum.131227. https://doi.org/10.3899/jrheum.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarica-Kucukoglu R, Akdag-Kose A, Kayabal IM, Yazganoglu KD, Disci R, Erzengin D, et al. Vascular involvement in Behcet’s disease: a retrospective analysis of 2319 cases. Int J Dermatol. 2006;45:919–21. doi: 10.1111/j.1365-4632.2006.02832.x. https://doi.org/10.1111/j.1365-4632.2006.02832.x. [DOI] [PubMed] [Google Scholar]
- 12.Dervis E, Geyik N. Sensitivity and specificity of different diagnostic criteria for Behcet’s disease in a group of Turkish patients. J Dermatol. 2005;32:266–72. doi: 10.1111/j.1346-8138.2005.tb00760.x. https://doi.org/10.1111/j.1346-8138.2005.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz S, Karadag O, Yazisiz V, Altun B, Gezer M, Karaman M, et al. Systemic involvements and preferred treatments in a large cohort of Behcet’s disease. Rheumatol Int. 2013;33:3025–30. doi: 10.1007/s00296-013-2830-0. https://doi.org/10.1007/s00296-013-2830-0. [DOI] [PubMed] [Google Scholar]
- 14.Davari P, Rogers RS, Chan B, Nagler TH, Fazel N. Clinical features of Behçet’s disease: A retrospective chart review of 26 patients. J Dermatolog Treat. 2016;27:70–4. doi: 10.3109/09546634.2015.1054781. https://doi.org/10.3109/09546634.2015.1054781. [DOI] [PubMed] [Google Scholar]
- 15.Branch TAaHCS. National Hospital Ambulatory Medical Care Survey: 2011 Outpatient Department Summary Tables. Center for Disease Control and prevention; 2011. [Google Scholar]
- 16.Mahr A, Belarbi L, Wechsler B, Jeanneret D, Dhote R, Fain O, et al. Population-based prevalence study of Behcet’s disease: differences by ethnic origin and low variation by age at immigration. Arthritis Rheum. 2008;58:3951–9. doi: 10.1002/art.24149. https://doi.org/10.1002/art.24149. [DOI] [PubMed] [Google Scholar]
- 17.Tursen U, Gurler A, Boyvat A. Evaluation of clinical findings according to sex in 2313 Turkish patients with Behcet’s disease. Int J Dermatol. 2003;42:346–51. doi: 10.1046/j.1365-4362.2003.01741.x. https://doi.org/10.1046/j.1365-4362.2003.01741.x. [DOI] [PubMed] [Google Scholar]
- 18.Bang DS, Oh SH, Lee KH, Lee ES, Lee SN. Influence of sex on patients with Behcet’s disease in Korea. J Korean Med Sci. 2003;18:231–5. doi: 10.3346/jkms.2003.18.2.231. https://doi.org/10.3346/jkms.2003.18.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ames PR, Steuer A, Pap A, Denman AM. Thrombosis in Behcet’s disease: a retrospective survey from a single UK centre. Rheumatology (Oxford) 2001;40:652–5. doi: 10.1093/rheumatology/40.6.652. https://doi.org/10.1093/rheumatology/40.6.652. [DOI] [PubMed] [Google Scholar]
- 20.Kural-Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V, et al. The long-term mortality and morbidity of Behcet syndrome - A 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine. 2003;82:60–76. doi: 10.1097/00005792-200301000-00006. https://doi.org/10.1097/00005792-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Yazici H, Tuzun Y, Pazarli H, Yurdakul S, Ozyazgan Y, Ozdogan H, et al. Influence of age of onset and patient’s sex on the prevalence and severity of manifestations of Behcet’s syndrome. Ann Rheum Dis. 1984;43:783–9. doi: 10.1136/ard.43.6.783. https://doi.org/10.1136/ard.43.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]