Abstract
Renal myxoma is a very rare benign neoplasm seen almost exclusively in adults with only 16 reported cases in the literature. All of these cases have been reported in native kidneys with none being reported in a transplant kidney. We report the case of a renal myxoma in a 17-year-old boy’s transplant kidney that was found as an incidental mass on ultrasonography and further evaluated with CT and PET scans. PET findings of a renal myxoma are reported here for the first time, and imaging findings from previous cases are briefly reviewed. This case report highlights the fact that adult-predominant tumors and pathology should always be a consideration in pediatric patients who receive organ transplants from adult donors.
Keywords: myxoma, renal myxoma, kidney, transplant, children, computed tomography, CT, ultrasonography, positron emission tomography, PET
CASE REPORT
A 17-year-old male received a living related donor kidney transplant from his mother at age 2 secondary to congenital renal dysplasia. At the age of 5 years, he was treated for post-transplant lymphoproliferative disorder [PTLD - abdominal stage IV Burkitt Lymphoma] per Pediatric Oncology Group (POG) 9317 without complication. At the age of 12 years, the patient developed chronic transplant nephropathy and concomitant renal insufficiency. He received a second kidney transplant at the age of 15 years secondary to graft failure of the first transplant. The initial transplant kidney was left in place. The second transplanted kidney was removed 1 week later due to renal vein thrombosis. Six months later, the patient began hemodialysis and continued to be evaluated for another transplant. He underwent an abdominal ultrasound as part of his pre-transplant work-up and a mass was incidentally discovered in the original transplanted kidney. The ultrasound study showed an enlarged heterogeneous transplant kidney measuring 14.2 cm with a 4.2 × 5.4 × 3.0 centimeter nodular area in the midpole [Fig 1]. A subsequent CT scan was done pre- and post-intravenous contrast (CTDIvol = 20.26 mGy, DLP = 921.70 mGy-cm). The images showed an enlarged transplant kidney with heterogeneous enhancement and a hypodense mass [Fig 2]. Hypodense areas in the inferior portion of the kidney appeared to be infiltrating the kidney and extending into the renal pelvis and proximal ureter [Fig 3]. Initial concern was for the recurrence of PTLD, but a PET-CT scan done 2 weeks prior to the ultrasound and CT did not show any hypermetabolic areas in the mass [Fig 4]. The patient underwent a radical right nephrectomy of the transplanted kidney. The kidney was noted to have a neoplasm in the postero-lateral aspect which extended into and was adherent to the retroperitoneal tissues. The specimen consisting of the kidney, mass, and attached ureter measured 13.6 × 6 × 9 cm (craniocaudal × anteroposterior × transverse respectively). The mass had gelatinous, fluffy, nodular soft tissue that filled the hilum and measured 10 × 9 × 4 cm (Fig 5). A myxoid substance was noted to compress the renal medulla and cortex without infiltration and to encase the ureter down to the distal ureteral margin. The tumor was found to be chromosomally normal and analysis showed a 46XX karyotype, suggesting that the tumor was of donor origin. On histological analysis, the tumor had the appearance of a typical myxoma with myxoid stroma and fibroblastic-like spindle cells and stellate cells [Fig 6]. Immunohistochemical stains for Ki-67 and Human Melanoma Black (HMB)-45 were performed, showing a low proliferation rate (2%) on Ki-67 and an absence of staining with HMB-45.
Figure 1.
Renal ultrasound of a 17-year-old male with a renal myxoma in his transplanted kidney.
FINDINGS: There is an enlarged 14.2 cm transplant kidney with heterogeneous echogenicity. A 4.2 × 5.4 × 3.0 cm nodular area of similar echotexture to that of the surrounding parenchyma is seen in the midpole. There is no evidence of hydronephrosis or perinephric fluid.
TECHNIQUE: Longitudinal sonographic images were acquired utilizing a multihertz transducer.
Figure 2.
Contrast-enhanced axial CT image of a 17-year-old male with a renal myxoma in his transplanted kidney.
FINDINGS: A predominantly hypodense non-enhancing right lower quadrant mass (arrows) is seen distorting the transplant kidney in the posterior aspect (CTDIvol = 20.26 mGy, DLP = 921.70 mGy-cm). This hypodense area appears to be infiltrating the transplant kidney. There is no evidence of lymphadenopathy. Vascular structures show no abnormalities.
TECHNIQUE: Contiguous 5 millimeter axial CT images were acquired in the nephrogenic phase after ingestion of oral contrast material and bolus intravenous administration of 100 cc of Omnipaque 300 (iohexol).
Figure 3.
Contrast-enhanced coronal CT images of a 17-year-old male with a renal myxoma in his transplanted kidney.
FINDINGS: A hypodense mass is seen in the medial aspect of the transplant kidney and appears to be infiltrating the kidney, extending from the renal pelvis (3A, arrows) to the transplant ureter and almost to the ureterovesical junction (3B, arrows). There are no areas of enhancement within the mass. The ureter and collecting system appear dilated (3A and 3B, asterisks).
There is no lymphadenopathy. The urinary bladder shows no wall thickening or filling defect.
TECHNIQUE: Contiguous 5 millimeter coronal CT images were acquired in the nephrogenic phase after ingestion of oral contrast material and bolus intravenous administration of 100 cc of Omnipaque 300 (iohexol).
Figure 4.
A PET-CT image of a 17-year-old male with a renal myxoma in his transplanted kidney.
FINDINGS: Coronal imaging shows the mass in the transplant kidney (arrow) with no discrete hypermetabolic foci. There is dilation of the collecting system and ureter.
TECHNIQUE: Coronal PET-CT images were acquired 60 minutes after intravenous administration of 13.73 millicuries of F-18 fluorodeoxyglucose.
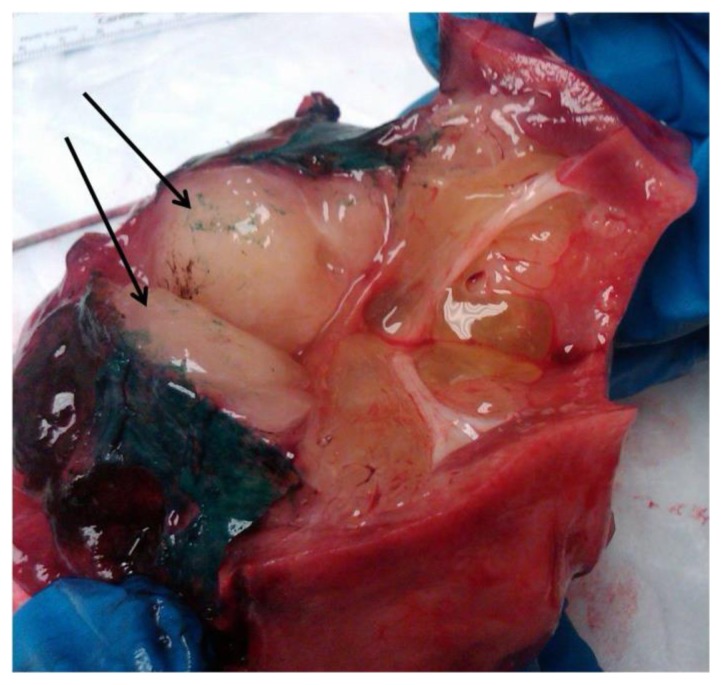
Figure 5.
The gross specimen image shows a well-circumscribed, glistening, gray-tan mass filling the hilum of the kidney (arrows). The dark green substance is a remnant of specimen preparation.
Figure 6.
Hematoxylin and eosin-stained slide of a renal soft tissue mass from a 17-year-old male with a renal myxoma. Original magnification is 40× (low power). The tumor is relatively acellular with an abundant slightly basophilic mucoid matrix (black box). Focal areas of more eosinophilic fibrous matrix are also present. Vessels are inconspicuous. The neoplastic cells are spindled or stellate with no mitotic figures (asterisk). Scattered mononuclear inflammatory cells are seen.
DISCUSSION
Etiology & Demographics
Renal myxomas are considered to be benign tumors with a good prognosis following removal, and they are exceedingly rare with only 16 cases previously reported in the literature [1–11]. As a general tumor class, myxomas have the highest prevalence between the ages of 40 and 70, they are very rare in patients under 20 years of age, and roughly two-thirds of the patients are female [12]. Renal myxomas, however, have not shown a gender preference. The age range for renal myxomas is between 27 and 68 years and is similar to the age range for other myxomas.
The origin of renal myxomas is not entirely clear, although different theories have been presented. Some authors have considered myxomas to be degenerative in origin, similar to the changes seen in adipose tissue during brown atrophy of the heart, while others argue that the uniform cellular component seen on ultrastructural analysis suggests that myxomas are neoplastic in origin, arising from a primitive mesenchymal cell with fibroblast-like features [1, 3]. These findings suggest that a renal myxoma can ultimately be defined as a benign fibroblastic tumor that may have differentiated to produce abundant mucopolysaccharide instead of mature collagen [2, 3, 7]. However, even the exclusively benign nature of a myxoma is controversial, and malignant transformations of primary myxomas have been previously reported [13–15].
In our case, the myxoma in the transplant kidney had a karyotype of 46XX. Therefore, the tumor likely originated from donor tissue (the patient’s mother). To the best of our knowledge, there have been no previously reported cases of renal myxoma in a transplant kidney. Even among cardiac myxomas, which are relatively much more common than renal myxomas, there has only been 1 reported case of a myxoma of donor origin found in a transplanted heart [16]. Because myxomas are so rare in children, this report highlights the fact that tumors and other pathologies that are adult-predominant must also be considered in children receiving adult transplant organs.
Clinical & Imaging Findings
The majority of previous cases were discovered incidentally on imaging for unrelated problems; one case presented with a pelvic mass and epigastric pain, and 2 others presented with dull flank pain [1–4, 6–11]. Myxomas in general occur at various sites including the heart, skeletal muscle, joints, pharynx, tonsils, bones, skin, retroperitoneum, intestines, subcutaneous tissue, aponeurotic tissue, eye, ovary, and in the genitourinary tract [1, 12]. The muscles of the legs and buttocks are the most common sites of origin [2, 12].
There is a paucity of information on the imaging characteristics of renal myxomas. Murphey et al. reported on the imaging characteristics of 45 intramuscular myxomas and perhaps some of their findings can be extrapolated to myxomas in the kidneys. Overall, they showed that the appearance of a myxoma was hypoechoic relative to skeletal muscle on ultrasound, showed low attenuation on CT with little to no enhancement post-contrast, and had low signal intensity on T1-W and high signal intensity on T2-W MRI [17].
Imaging characteristics of reported cases of renal myxomas [Table 1] are not dissimilar to soft tissue myxomas as they are both solid tumors with significant mucus content. Ultrasound is helpful in characterizing the lesion as a solid mass with heterogeneous echogenicity. CT findings in our case demonstrated the mass to be indistinguishable from surrounding kidney on non-contrasted CT and of relatively low density with poor enhancement after contrast administration. This is similar to the CT findings of others, with some variation in post-contrast enhancement [3, 4]. Although we did not perform an MRI, other reports suggest that renal myxomas are consistently of homogenous low-signal intensity on T1-W imaging and heterogeneous high signal intensity on T2-W imaging [1, 2, 4]. These findings are consistent with its gross characteristics of a mass with a high water content, which stems from its abundance of mucus. There have been no prior reports on the imaging characteristics of renal myxomas on PET. In our case, the absence of hypermetabolic activity on FDG-PET steered us away from the diagnosis of recurrent PTLD and was strongly suggestive of a benign lesion.
Table 1.
Imaging findings of Renal Myxoma to-date.
| Reference | Radiologic findings |
|---|---|
| 1 | MRI: Well-defined semisolid/semicystic mass. Homogenous low signal intensity on T1-W imaging and heterogeneous high signal intensity on T2-W imaging. |
| 2 | MRI: Well-defined mass with homogenous low signal intensity on T1-W and heterogeneous high signal intensity on T2-W imaging. CT: Well-defined mass with a heterogeneous center and a smooth attenuated rim. |
| 3 [first reported case] | IVP: large mass in the lower pole of the left kidney. US: heterogeneous lesion with the appearance of a solid tumor. CT: contrast-enhancing lesion of low density originating in the renal pelvis and extending below the lower pole of the left kidney. |
| 3 [second reported case] | US: Heterogeneous, solid, well-defined mass of medium density showing a sharp interface with adjacent kidney. CT: mass with a homogeneous center and a smooth attenuated rim. |
| 4 | MRI: low signal intensity on T1-W and high signal intensity on T2-W imaging, enhanced by gadolinium homogenously. CT: low-density lesion. |
| 5 | IVP: First exam showed a mass in the kidney, second exam showed medial displacement of the inferior calyx and infundibulum. Renal angiogram: splaying of vessels within the kidney with no abnormal vasculature. |
| 6 | CT [with contrast]: solid, low-density mass in the middle segment of the kidney, intruding the perirenal tissue |
| 7 | CT: low-density, well-defined solid mass occupying the upper portion of the kidney |
| 8 | US: solid mass with heterogeneous echogenicity in the renal pelvis CT: low-density mass |
| 9 [Myxoma with hemorrhage] | CT [without contrast]: round heterogeneous ill-demarcated lesion. CT [with contrast]: lesion with circular septal enhancement and ill-defined margin with the left psoas major muscle. |
| 10 | CT: large, heterogeneous solid mass, enhanced by iodinated contrast medium, not calcified, and with well-defined edges. |
| 11 | CT: hypodense, well-defined mass in the mid-portion of the kidney, with slight enhancement (61 HU) following IV contrast |
| Current case | US: Nodular lesion, heterogeneous in appearance, with similar echogenicity to the renal parenchyma. CT [with contrast]: Kidney showed heterogeneous enhancement with hypodense areas appearing to infiltrate the kidney PET/CT: No discrete hypermetabolic focus within the mass. |
Treatment & Prognosis
Renal myxomas are benign tumors with no known risk factors. Treatment involves removal of the tumor. Depending on the local tumor burden, a radical nephrectomy is often necessary, and previous cases of renal myxomas have been treated in this manner. More conservative treatment would be ideal, but there exists limited information on the imaging characteristics of renal myxomas that would allow clinicians to confidently distinguish this benign neoplasm from a malignant process. Prognosis following surgical resection has been excellent, with no known cases of recurrence.
Differential Diagnoses
Post-Transplant Lymphoproliferative Disorder
Given the clinical picture of our patient, this diagnosis is always possible following a transplant, especially in patients who are chronically immunosuppressed. Symptoms are usually nonspecific, but the absence of fever, malaise, or other signs of systemic illness makes this diagnosis less likely. A PET-CT scan would usually show hypermetabolic activity, which was also not seen in our case. CT imaging of PTLD usually shows scattered lymph node enlargement, which was also not seen in our case.
Nephroblastomatosis
This diagnosis should be considered in pediatric patients presenting with a renal mass. PET-CT imaging typically shows hypermetabolic activity, which was not observed in our case. Additionally, T2-W MRI usually shows low signal intensity foci, but Murphey et al. reported that myxomas have high signal intensity on T2-W MRI [17]. Nephroblastomatosis and renal myxomas can have similar nonspecific characteristics on both ultrasound and CT imaging.
Renal Carcinoma
Renal cell carcinoma and renal medullary carcinoma are both malignant tumors found in the kidney. As such, these malignancies would both show increased metabolic activity on PET-CT imaging, which was not seen in our case. On CT imaging, malignant tumors like renal cell carcinoma and renal medullary carcinoma might have associated involvement and enlargement of regional lymph nodes, whereas a benign tumor like a renal myxoma does not.
Non-Hodgkin Lymphoma
This diagnosis is a consideration in chronically immunosuppressed transplant recipients. Symptoms are usually present and include fever, chills, night sweats, and bone pain, and there are usually enlarged lymph nodes on physical exam. Our patient did not have these symptoms. PET-CT imaging would also show increased metabolic activity, which was not seen in our case. Non-Hodgkin Lymphoma also demonstrates hypoattenuation on CT imaging with and without contrast. Although a renal myxoma can also demonstrate this finding, this pattern of hypoattenuation is less consistent among renal myxomas.
Metanephric Adenoma
This diagnosis is a benign mass that should be considered. Clinical presentation is usually asymptomatic just like in our case. However, ultrasound imaging of a metanephric adenoma usually shows a mass with well-defined borders and CT imaging usually shows a mass that is hyperdense relative to the surrounding parenchyma, neither of which were seen in our case. PET-CT imaging is not helpful when trying to differentiate this mass from a renal myxoma, as both show no hypermetabolic activity.
Renal Oncocytoma
This benign renal tumor should be considered in adult patients presenting with a renal mass. Most patients with this tumor are asymptomatic. Although this diagnosis can be difficult to distinguish from a malignancy, there are some imaging findings that help to differentiate it from a renal myxoma. On CT scan, oncocytomas are usually well-demarcated with sharp borders, and on ultrasound they are well-circumscribed. In contrast, renal myxomas are usually poorly demarcated with inconsistent borders. On PET-CT imaging, oncocytomas can show increased metabolic activity, whereas renal myxomas do not. On renal angiogram, renal myxomas usually show normal vasculature with splaying of vessels secondary to mass effect, whereas renal oncocytomas display neovascularization similar to renal cell carcinomas with a “spoke wheel” pattern, characterized by peripheral vessels that penetrate toward the center of the lesion.
Summary
Although considered a rare benign tumor, a renal myxoma should be in the differential diagnosis of a mass in a transplanted kidney regardless of the recipient’s age. This neoplasm does not have defining characteristics on CT or MRI, but shows little to no metabolic activity on PET. The combined findings of these modalities make other aggressive neoplastic and infectious processes less likely.
TEACHING POINT
Renal myxomas do not have defining characteristics on CT or MRI and show little to no metabolic activity on PET. In pediatric organ transplant recipients, adult-predominant pathology must be considered when the organ came from an adult donor.
Table 2.
Summary table for Renal Myxoma.
| Etiology | Benign fibroblastic tumor that may have differentiated to produce abundant mucopolysaccharide instead of mature collagen. Likely neoplastic and arising from primitive mesenchymal cells, but could be a degenerative process. |
| Incidence | 16 reported cases in literature (< 1 new case per year) |
| Gender Ratio | 1:1 |
| Age Predilection | Ages 40–70 |
| Risk Factors | None identified |
| Treatment | Tumor resection vs. radical nephrectomy |
| Prognosis | Excellent following removal |
| Findings on imaging | See Table 1 |
Table 3.
Differential diagnosis table for a renal mass, as well as the imaging findings for each diagnosis.
| Differential Diagnosis | IVP Findings | US Findings | CT Findings | MRI Findings | PET Findings | Renal Angiogram |
|---|---|---|---|---|---|---|
| Renal Myxoma | A mass in the kidney, and medial displacement of inferior calyx and infundibulum. | Heterogeneous, solid, well-defined, or with poorly defined demarcations, and possibly nodular mass of medium density. | Usually hypodense mass with smooth attenuated rim, with slight enhancement post-contrast. May have septations with enhancement. | T1: Homogeneous low signal intensity, with post-contrast homogeneous enhancement T2: Heterogeneous high signal intensity with post-contrast homogeneous enhancement |
No increased metabolic activity | Splaying of vessels within kidney with no abnormal vasculature. |
| Post-transplant lymphoproliferative disorder | --- | Hypoechoic nodules in solid organs, circumferential wall thickening with or without aneurysmal dilatation, ulceration, or perforation in bowel. | Low-density nodules in solid organs, with non-specific nodal enlargement | T1 and T2: Iso- to hypointense masses relative to brain cortex. T2 might show hyperintensity when necrosis is present |
Increased metabolic activity | --- |
| Nephroblastomatosis | Increased parenchymal thickness, possibly with a lobulated appearance and a grossly normal renal pelvis. | Hypoechoic nodules | Low-attenuation peripheral nodules with poor enhancement relative to adjacent parenchyma. | T1 and T2: Low signal intensity foci | Increased metabolic activity | Avascular masses with stretching and narrowing of intrarenal arteries at the periphery |
| Renal Cell Carcinoma | Possible obstruction of renal pelvis | Usually too small for visualization | Nonspecific low-attenuation solid intrarenal mass with little enhancement, possibly areas of hemorrhage and necrosis. 25% have calcifications. | T1 and T2: Nonspecific solid intrarenal mass with little enhancement | Increased metabolic activity | Hypervascular mass with pooling of contrast, necrotic avascular areas, and possibly arteriovenous communications |
| Renal Medullary Carcinoma | Central renal mass, likely encasing renal pelvis | Heterogeneous echotexture, with possible loss of corticomedullary differentiation and hydronephrotic calices | Heterogeneous-enhanced mass, possibly associated with lymphadenopathy of retroperitoneal nodes. | T1 and T2: Infiltrative mass | Increased metabolic activity | Avascular mass |
| Non-Hodgkin Lymphoma | --- | Hypoechoic mass(es) with possible increased through transmission. | Homogeneous, hypoattenuating mass both with and without contrast. | T1: Low-intensity multicystic and medium-intensity solid masses with ill-defined margins and enhancement of solid components post-contrast. T2: Multicystic and solid heterogeneous high signal intensity masses. |
Increased metabolic activity | --- |
| Metanephric Adenoma | Hypovascular mass | Well-defined, expansile solid mass. Can be hypo- or hyper-echoic, and even possibly cystic with a mural nodule. Doppler shows hypovascularity. | Pre-contrast, mass may be iso- or hyper-attenuating, possibly with small calcifications. Post-contrast shows hypoattenuation compared with normal parenchyma. | T1: Mass with hypointense signal T2: Mass usually with a slightly hyperintense signal, however sometimes hypointense |
No increased metabolic activity | Hypovascular masses |
| Renal Oncocytoma | Large, well-demarcated, exophytic mass with enhancement | Well-circumscribed mass. Iso-echoic to adjacent kidney. Central scar possible. | Large, well-demarcated mass with heterogeneous enhancement post-contrast. Might have a central stellate nonenhancing scar. Associated renal vein thrombosis, perinephric fat stranding/edema, or calcification possible. | T1: hypointense mass relative to renal cortex. Homogeneous enhancement post-contrast. T2: hyperintense mass relative to renal cortex, with hypointense stellate scar possible. |
Increased metabolic activity. | “Spoke Wheel” pattern. |
ACKNOWLEDGEMENTS
We thank Dr. Sally Self and the Department of Pathology at our institution for assistance with the pathology illustrations.
ABBREVIATIONS
- CT
Computed tomography
- CTDIvol
Computed tomography dose index volume
- DLP
Dose length product
- IVP
Intravenous Pyelogram
- HMB-45
Human Melanoma Black-45, a monoclonal antibody
- Ki-67
a protein marker for cellular proliferation
- MRI
Magnetic Resonance Imaging
- PET
Positron Emission Tomography
- POG
Pediatric Oncology group
- PTLD
Post-Transplant Lymphoproliferative Disorder
REFERENCES
- 1.Bolat F, Turunç T, Kayaselçuk F, Ulusan S, Bal N. Primary renal myxoma: A case report. [Accessed May 28, 2017];Turkish J Pathol. 2007 23(3) Available at: http://www.turkjpath.org/text.php3?id=194. [Google Scholar]
- 2.Owari Y, Konda R, Omori S, Seo T, Suzuki K, Fujioka T. Myxoma of the kidney. Int J Urol. 2006;13:987–989. doi: 10.1111/j.1442-2042.2006.01453.x. [DOI] [PubMed] [Google Scholar]
- 3.Melamed J, Reuter V, Erlandson R, Rosai J. Renal Myxoma. A report of two cases and review of the literature. Am J Surg Pathol. 1994;18(2):187–194. [PubMed] [Google Scholar]
- 4.Nishimoto K, Sumitomo M, Kakoi N, Asano T, Hayakawa M. Case of renal myxoma. Int J Urol. 2007;14(3):242–244. doi: 10.1111/j.1442-2042.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 5.Shenasky JH, Gillenwater JY. Myxoma of the kidney. Urology. 1973;1(3):240–242. doi: 10.1016/0090-4295(73)90744-9. [DOI] [PubMed] [Google Scholar]
- 6.Val-Bernal JF, Aguilera C, Villagrá NT, Correas MA. Myxoma of the renal capsule. Pathol Res Pract. 2005;200(11–12):835–840. doi: 10.1016/j.prp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Hakverdi S, Gorur S, Yaldiz M, Kiper AN. Renal myxoma: a case report and review of the literature. [Accessed May 28 2017];Turkish J Urol. 2010 36(3) Available at: http://www.turkishjournalofurology.com/sayilar/10/buyuk/318-3211.pdf. [Google Scholar]
- 8.Yildirim U, Erdem H, Kayikci A, Uzunlar AK, Tekin A, Kuzey MA. Myxoma of the renal sinus: case report and literature review. Turkish J Pathol. 2012;28(1):76–79. doi: 10.5146/tjpath.2012.01102. [DOI] [PubMed] [Google Scholar]
- 9.Shah A, Sun W, Cao D. Myxoma of the kidney associated with hemorrhage. Indian J Surg. 2013;75(1 SUPPL):480–483. doi: 10.1007/s12262-013-0862-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souza CHC, Carneiro KS, Leite KRM, Junior A, Costa FS. Renal myxoma: a case report. [Accessed May 28, 2017];J Bras Patol e Med Lab. 2015 51(2) Available at: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1676-24442015000200113&lng=en&nrm=iso. [Google Scholar]
- 11.Tenkorang S, Kharbach Y, Omana J, et al. Myxoma of the kidney - an unusual benign renal tumor: a case report. J Med Case Rep. 2017;11:41. doi: 10.1186/s13256-016-1194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen PW. Myxoma is not a single entity: a review of the concept of myxoma. Ann Diagn Pathol. 2000;4(2):99–123. doi: 10.1016/s1092-9134(00)90019-4. [DOI] [PubMed] [Google Scholar]
- 13.Amano J, Kono T, Wada Y, et al. Cardiac myxoma: its origin and tumor characteristics. Ann Thorac Cardiovasc Surg. 2003;9(4):215–221. [PubMed] [Google Scholar]
- 14.Kusumi T, Minakawa M, Fukui K, et al. Cardiac tumor comprising two components including typical myxoma and atypical hypercellularity suggesting a malignant change. Cardiovasc Pathol. 2009;18(6):369–374. doi: 10.1016/j.carpath.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Noffke CEE, Raubenheimer EJ, Chabikuli NJ, Bouckaert MMR. Odontogenic myxoma: review of the literature and report of 30 cases from South Africa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(1):101–109. doi: 10.1016/j.tripleo.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Dufková B, Málek I, Vym?talová Y, et al. Myxoma of Donor Origin in a Transplanted Heart. J Hear Lung Transplant. 2007;26(8):865–867. doi: 10.1016/j.healun.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Murphey MD, McRae GA, Fanburg-Smith JC, Temple HT, Levine AM, Aboulafia AJ. Imaging of soft-tissue myxoma with emphasis on CT and MR and comparison of radiologic and pathologic findings. Radiology. 2002;225(1):215–224. doi: 10.1148/radiol.2251011627. [DOI] [PubMed] [Google Scholar]