Analysis of a nationwide sample of patients with low back pain shows an association between depression and higher rates of opioid prescribing.
Keywords: Low back pain, Opioid analgesics, Depression, Clinical trials
Abstract
Introduction:
Low back pain (LBP) is among the leading indications for the prescription of opioid analgesics in clinical practice. There is increasing evidence suggesting that these agents may have diminished efficacy in the treatment of LBP.
Objectives:
We evaluated the relationship between depression, the probability of receiving an opioid prescription, and the amount of morphine equivalent amounts prescribed per year among patients with LBP using nationwide data.
Methods:
A cross-sectional analysis was performed on existing data from the Medical Expenditure Panel Survey data set from the period 2004 to 2009. Demographic, medical condition, Patient Health Questionnaire-2 responses, and prescription drug information were obtained on 56,811,864 weighted person-years of data from individuals aged 18 to 65 with an ICD-9 code specific to LBP.
Results:
Increases in PHQ-2 score, as well a positive screen for depression, were associated with an increased probability of being prescribed opioid therapy and more morphine equivalents per year.
Conclusion:
Analysis of a nationwide sample of patients with LBP shows an association between depression and higher rates of opioid prescribing after controlling for several known cofounders. Clinicians prescribing opioids in LBP populations that rely on clinical trial results that exclude depressed patients may misjudge the risks and benefits of this class of therapy.
1. Introduction
Low back pain (LBP) remains a leading cause of disability and a major public health problem in the United States and worldwide. Rates of opioids prescribing for LBP indications have soared over the last 2 decades in the United States.4,18,19 Low back pain remains a leading indication for prescription of opioid analgesics in clinical practice.
It is well known that depression is highly comorbid with LBP syndromes across diagnostic groupings, both anatomic and nonspecific.16 Among patients with chronic noncancer pain (cNCP), the rate of opioid prescribing has been rising faster among patients with mental health diagnoses than among those without these diagnoses.1,9 This study examines the years 2004 to 2009 because a steep rise in opioid prescribing rates was observed during this interval.17 This 5-year period immediately predates the introduction of a new generation of reformulated opioids designed to deter abuse and the widespread implementation of a range of policies to address the epidemic of prescription opioid abuse. The 2004 to 2009 time frame will therefore serve as an important reference period for measuring the impact of subsequent public health interventions related to the use and abuse of opioid analgesics.
Regional studies and analyses of the worker's compensation population also demonstrate that cNCP patients with mental health disorders have an increased likelihood of receiving opioid therapy.1,23,25 This finding also extends to increased reporting of prescription opioid use for pain indications more broadly,24,25 including long-term use.1,25,31 Depressed patients with cNCP are more likely to receive higher opioid doses,10,20 despite evidence of concurrent dose-dependent increases in overdose-related events.6,11,17,21,34
Despite the comorbidity between depression and LBP, previous studies of the relationship between opioid prescribing and depression have had limited generalizability to the broader U.S. population.2,3,9,10,33 Regional studies are often subject to substantial state-to-state variation in opioid prescribing.15 Goesling et al demonstrated that among patients with cNCP in a single tertiary care center, those with symptoms of depression were more likely to take opioids at higher doses.10 Breckenridge et al showed an increased likelihood for depressed veterans to receive long-term opioid therapy vs nonsteroidal anti-inflammatory drug therapy for chronic LBP.2 Whether the results from these studies are applicable to patients with LBP among the national civilian population is unclear because of limitations of sample size, region or group studied, and the typical focus on cNCP instead of LBP.
Evaluation and confirmation of the relationship between opioid prescribing and depression in patients with LBP has clear relevance to clinical practice and policy because patients with LBP now constitute the primary study population in clinical trials leading to regulatory approval of opioid analgesics. Potential subjects with poorly controlled symptoms of depression have been known to be excluded from phase III clinical trials of opioids.12,25,27–30 Such exclusion may compromise the generalizability of clinical trial results to patients with mental illness and LBP.8 In this study, we sought to determine whether rates of opioid prescribing in depressed patients with LBP were increased relative to nondepressed patients using a nationally representative database during a key period of interest in the current U.S. epidemic of prescription opioid abuse.
2. Methods
2.1. Study design and sample
This study was a cross-sectional secondary analysis of publicly available data from the Medical Expenditure Panel Survey (MEPS), a tool that has been previously used in spine research.19 The MEPS data do not distinguish between acute and chronic LBP syndromes. The MEPS is a longitudinal health care survey that provides a nationally representative sample of the noninstitutionalized, civilian population using an overlapping panel design, with oversampling of certain non-Caucasian racial groups. The MEPS obtains data on a subset of participants in the annual National Health Information Survey (NHIS) through 5 subsequent interviews over 2 years. Information obtained includes health care visit records and associated diagnoses, prescription data, and several standard scales for functional limitations and mental health issues.
MEPS data from 2004 to 2009 were collected for each person in the sample with separate data records for each person-year. Records were included for analysis if persons (1) were eligible for inclusion in the MEPS study for the entire year, (2) had a back condition defined by ICD-9 code 724, (3) were aged 18-65 inclusive, (4) were not identified as pregnant in years of study in which that information was available, and (5) had complete data for all variables of interest in the analysis, including smoking status, body mass index (BMI), physical activity status, and prescription information. The MEPS database specifies if subjects developed back pain secondary to acute injury. This designation was used to exclude subjects from this analysis. The unit of analysis was the person-year.
2.2. Study outcomes
Dependent variables were constructed based on prescription drug data that were summarized for the year. A person was deemed to have an opioid prescription if the person had at least 1 opioid prescription specifically recorded for a back pain condition. Data for persons with an opioid prescription also included (1) the total number of back-pain related opioid prescriptions in a study year and (2) the total number of morphine equivalents in a study year.
Prescriptions were classified as opioid analgesics were converted into the milligrams of morphine equivalent using the CONSORT (Consortium to Study Opioids Risks and Trends) equivalence conversion factors3
2.3. Independent variables and covariates
The Patient Health Questionnaire-2 (PHQ-2) is a short version of the standardized PHQ-9 Screening Questionnaire for depression. The PHQ-2 score is the sum of answers to 2 scored questions and has been shown to have good correlation with the longer PHQ-9, as well as with a standardized quality of life scale and health outcome measures such as disability days.14 Demographic covariates and data on individual's BMI, smoking status, and physical activity were collected from MEPS data and included in the final models. The final models controlled for these variables because these factors, along with depression, have been found to be associated with both incidence of LBP and likelihood of opioid prescription.
2.4. Analysis
Data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC). The sample included 5397 person-years after inclusion criteria were applied, with a weighted frequency of 56,811,864 person-years. MEPS individual weighting variables were used to obtain appropriate population estimates.
Descriptive statistics were obtained for the group with opioid prescriptions for LBP, the group without an opioid prescription, and the entire LBP group. Mean and SDs were presented for the continuous variables, and frequencies and weight frequencies were presented for categorical variables. Bivariate analyses were performed to compare variables across 3 different opioid prescription groupings based on the number of opioid prescriptions received. These analyses used the analysis of variance tests for continuous measures and the Rao-Scott χ2 tests for categorical measures.
Weighted logistic regression analysis was performed to test the association between the probability of opioid prescription and the PHQ-2 score. In addition, weighted linear regression analysis was performed to test the association between cumulative morphine equivalent amounts (MEAs) of opioid prescribed per year. Both models controlled for individual comorbidities (as a total number of comorbid conditions present), demographic characteristics, and other variables (BMI, smoking, and physical activity). For weighted multivariate models, the hypothesis was tested at the 2-tailed 0.05 level.
3. Results
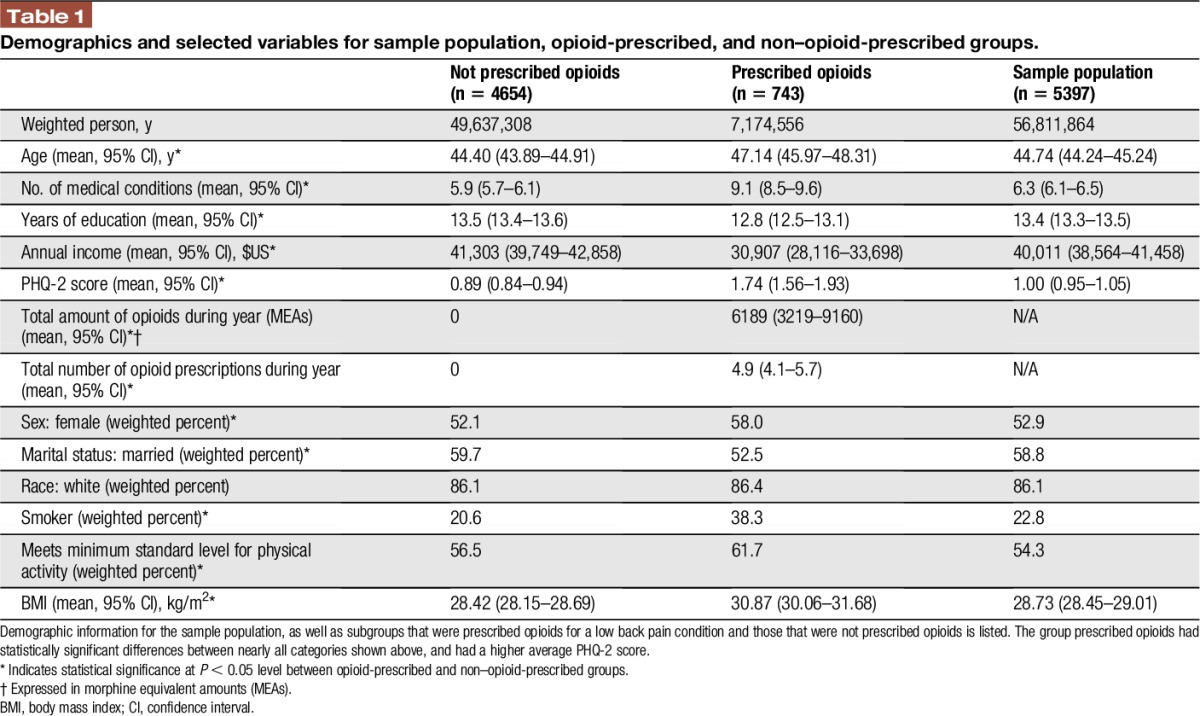
Demographic characteristics of the sample population (56,811,864 weighted person-years) are shown in Table 1. The average age was 44.7 years, and the average PHQ-2 score was 1.00, below the positive screening score of 3.14 Opioids were prescribed to 12.6% of the sample, and recipients averaged 4.9 prescriptions per year, amounting to 6,189 MEAs. Those in the opioid-prescribed group were older (P < 0.0001), were more likely to be women (P < 0.05), and had lower average annual income ($30,907 vs $41,303; P < 0.0001). The opioid-prescribed group also had a higher average PHQ-2 score (0.89 vs 1.74; P < 0.0001), although the average PHQ-2 scores for both groups were also below the recommended screening cut-off for depression. The opioid-prescribed group also had higher rates of smoking, lower rates of physical activity, and higher average BMI. To ensure that these variables would not bias multivariate analyses, we performed linear and logistic multivariate analyses between PHQ-2 score and BMI, smoking status, or physical activity to evaluate correlations between these variables. No significant correlations were found using either C or r-squared values where appropriate (data not shown).
Table 1.
Demographics and selected variables for sample population, opioid-prescribed, and non–opioid-prescribed groups.
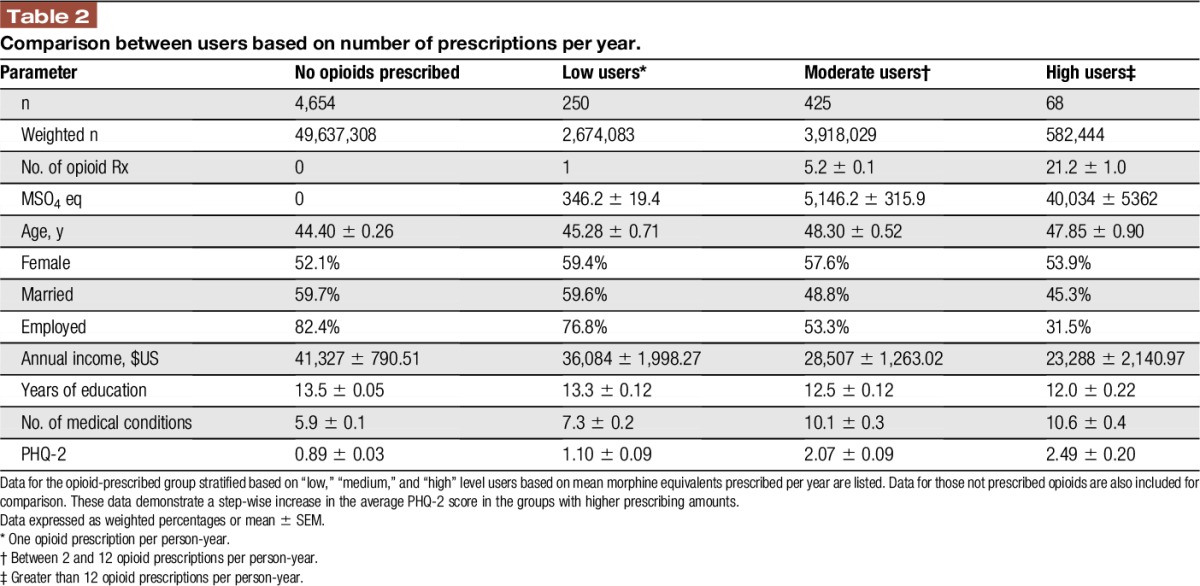
Opioid recipients were then stratified into “low” (1 prescription per year), “medium” (2–12 prescriptions per year), and “high” (more than 12 prescriptions per year) users, based on probable occasional, intermittent, or regular use of opioid medications (Table 2). This resulted in an average of 1, 5.2, and 21.2 prescriptions per year, respectively. Direct relationships between prescription frequency category and either sex or age were not significant, whereas the average income was lower in the higher prescription categories. In addition, the average PHQ-2 score was significantly greater in higher prescription categories, with PHQ-2 score increased most sharply between the low and medium prescription groups.
Table 2.
Comparison between users based on number of prescriptions per year.
Patients were then separated based on their PHQ-2 screening status using the clinical cut-off of a score greater than or equal to 3. Of those screening positive, 26.2% received an opioid prescription vs 11.4% of the screen-negative group (data not shown; P < 0.0001). The positive depression screen group also received over 5,000 more MEAs per year (4,675.5 vs 10,136.0; P < 0.0001). In summary, patients who were depressed were more likely to be prescribed an opioid and also were more likely to receive more of this class of therapy.
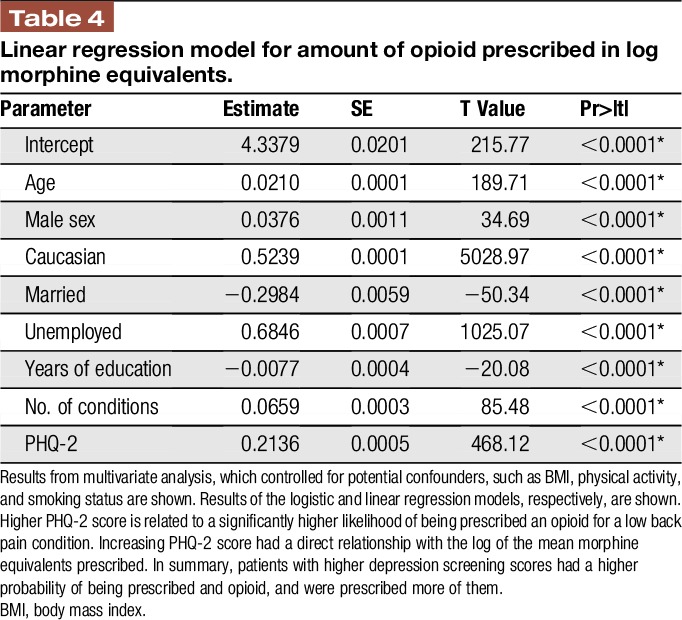
To assess if PHQ-2 score had a direct relationship on opioid-prescribing patterns, multivariate analyses were performed. Logistic regression analysis examined the probability of opioid prescription, and the linear regression model analyzed the amount of opioid prescribed per year. After accounting for potential cofounders, such as BMI, smoking status, minimum physical activity standards, and demographic covariates, a 1-point increase in the PHQ-2 score resulted in a significantly increased probability of being prescribed an opioid (Table 3; max likelihood estimate = 01050; P < 0.01). PHQ-2 score also demonstrated a direct relationship with log morphine equivalents prescribed (Table 4; P < 0.0001).
Table 3.
Logistic regression model for probability of opioid prescription.
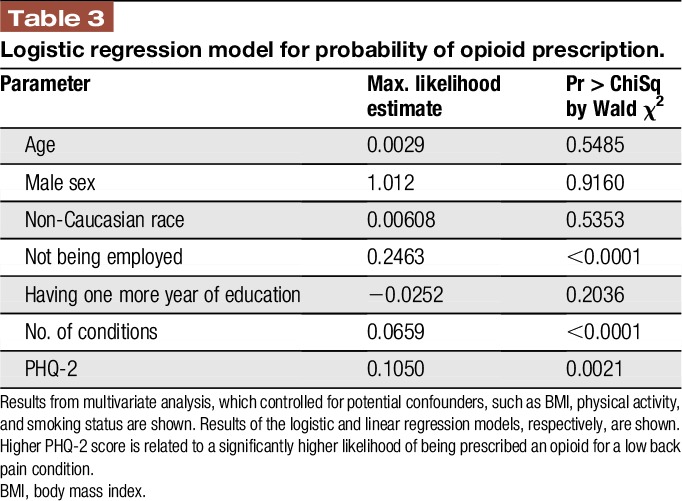
Table 4.
Linear regression model for amount of opioid prescribed in log morphine equivalents.
4. Discussion
This study of a nationwide sample demonstrates and reaffirms a strong association between depression symptoms and opioid use for LBP treatment. Patients prescribed opioids for LBP had higher average PHQ-2 scores, suggesting a higher prevalence of depressive symptoms among opioid recipients. Groups of opioid-treated patients with higher numbers of prescriptions per year also had more depressive symptoms. Individuals who screened positive for depression were more than twice as likely to be prescribed an opioid, and they received twice the cumulative dose of opioids per year. After adjusting for covariates and other variables that could be predictive of opioid prescription, logistic regression analysis demonstrated that a 1-point increase in the PHQ-2 score resulted in a higher probability of receiving an opioid prescription. Linear regression analysis also showed a direct relationship between the quantity of prescribed opioids and PHQ-2 score. Overall, this analysis consistently revealed that patients with LBP who are depressed are more likely to receive opioid prescriptions and to be prescribed higher amounts of them.
Depressed patients may be more likely to be prescribed opioids and receive higher dosages for a variety of clinical reasons. One potential explanation is that depression may be predictive of LBP chronicity and resulting disability,5,22 which may in turn prompt opioid prescribing. In addition, some studies have suggested that pain expression and behavior may also affect opioid-prescribing patterns.13,26 It is possible that patient suffering, both physical and psychological, may prompt clinicians to prescribe despite mounting evidence of a narrowed therapeutic index for opioids as an analgesic class.
The increased risk of misuse and overdose in depressed patients complicates the risk–benefit tradeoff of opioid analgesic therapy in patients with LBP.13 Because patients with depressive symptoms tend to experience reduced analgesia from using opioids for LBP32,33 and are apparently more likely to receive an opioid, it is critical to understand the extent to which these patients comprise the study populations of pivotal clinical trials. Multiple recent phase III trials testing opioid treatment in LBP populations, including hydrocodone bitartrate,5 transdermal buprenorphine,14 hydromorphone hydrochloride,3 and oxymorphone,6,22 that have supported regulatory approval have excluded potential subjects with poorly controlled or more severe depression (Table 5).
Table 5.
Examples of pivotal phase III clinical trials of long-acting opioids that exclude depressed patients.12,26–29
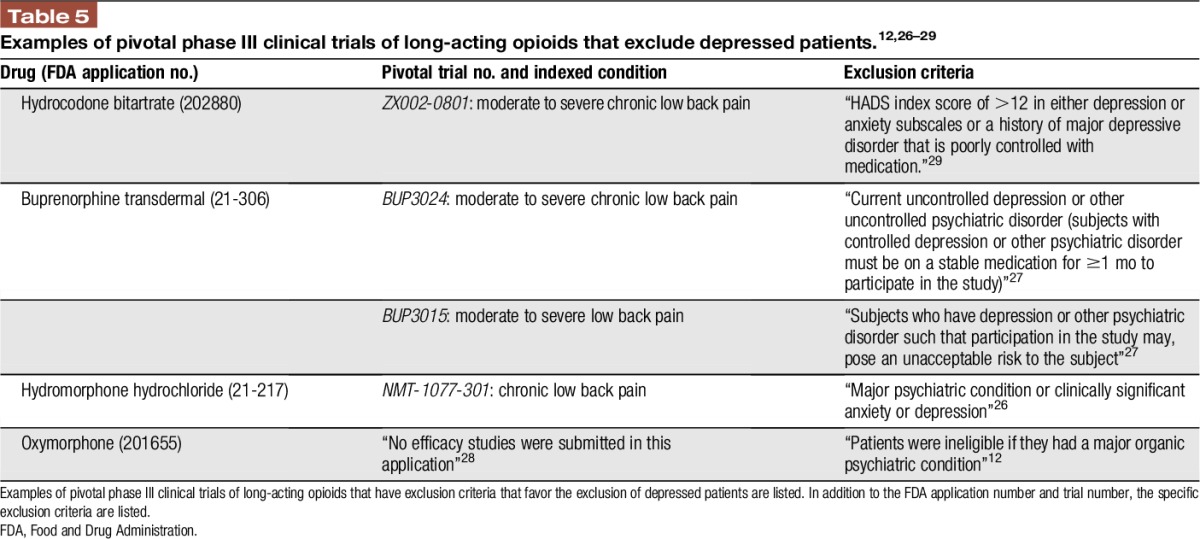
The extent to which mildly depressed patients are included in these pivotal trials of opioid analgesics is largely unknown because many studies do not specifically assess symptoms of depression using validated tools. Investigators may be inclined to exclude depressed subjects because of enhanced placebo response7 and diminished analgesic effect from opioids,32,33 thereby enhancing the assay sensitivity of a pivotal trial. It is important that removing these patients from a trial population may lead to inflation of expected analgesic benefit outside the clinical trial setting. Given the incentives to enroll compliant subjects in these studies and the sustained motivation needed of potential subjects to fulfill prolonged study requirements, multiple factors appear to favor exclusion of depressed patients. Depressed patients with LBP, at the very least, merit subgroup analysis to assess their analgesic benefit and unique risks with this class of therapy. For example, transdermal buprenorphine had been shown to have no distinct treatment benefit in a subgroup analysis of depressed patients.35
Our study had limitations common to those relying on databases and surveys. For instance, using ICD-9 codes to select for LBP cases likely included a few patients with spinal pain beyond the lumbar region. Preliminary analysis performed at AHRQ Data Center found that code 724 provided the lowest percentage of back pain that was not from the lumbar region (2.58%). Subjects who developed back pain secondary to injury were not included in this sample. In addition, pregnancy-related ICD-9 codes were also used to estimate pregnancy status in some cases, as the MEPS Questionnaire did not capture this data before 2008. For 2008 and 2009, more than 84% of pregnancy cases could be identified using ICD-9 codes alone. Given the low prevalence of pregnancy in each year of the sample, the number of pregnancy women in our sample was likely to be insignificant. In addition, the nature of the MEPS Questionnaire made it impossible to distinguish acute vs chronic LBP, and tracking ICD-9 cases over time to include chronic LBP would have restricted the sample size beyond utility. Similarly, prescription data did not allow us to determine the time taken to consume all of an opioid prescription or how much of it was consumed. However, this limitation does not impact the rates of prescribing opioids, which if not used by the patients included in this sample, may be diverted and contribute to the high mortality associated with prescription opioid analgesics. Another key limitation is the lack of distinction in the MEPS data between acute and chronic LBP syndromes.
The models used in the analyses also have some limitations. First, measuring morphine equivalents as a pooled value did not capture the number of prescriptions or the time over which a prescription was taken, nor could analyses be performed on individual opioid drug types. Second, a cross-sectional study design does not allow us to evaluate causality. However, in terms of safety and efficacy, it matters most whether depressed patients are getting more opioids, rather than why. Third, the linear regression model's r-squared value (0.2005) indicated that 20% of the variability in morphine equivalents was due to the variables included in the model. Although this means that incremental changes should not be interpreted precisely, we were able to control for both demographics and lifestyle variables (BMI, smoking status, and physical activity) that also acted as predictors of increased opioid prescribing.
There are also various strengths of this study. MEPS data have allowed us to sample a truly representative national population. Unlike other studies, which use limited data sets or have unrepresentative populations, our study is more generalizable because of the broad scope of our data. In addition, we recorded other variables that could be predictors of opioid consumption themselves, including BMI, smoking status, and physical activity level. By finding that there were no correlations between these variables, we believed that the highest consumers did not fall into the extremes of all categories simultaneously.
Our results are consistent with previous studies that demonstrate an association between depression and opioid prescription for cNCP, particularly previous results by Goesling et al and Breckenridge et al.2,10 Breckenridge et al reported that 65% of veterans treated with opioids had depression diagnoses compared with 20% of those treated with nonsteroidal anti-inflammatory drugs. Goesling et al reported, among patients with cNCP, that opioid users reported more symptoms suggestive of depression, and depression moderated the probability of opioid prescription.10 However, in both the aforementioned studies, pain intensity either did not predict opioid use2 or its effect on prescribing was minimal on depressed patients.10
Because several pivotal clinical trials for opioid treatment of LBP have systematically excluded the most depressed patients,12,27–30 it is probable that clinicians and patients alike are drawing conclusions from a study group that may differ in important ways from likely opioid recipients. These clinical trial populations may underrepresent the patients most likely to receive opioids, especially those who are mostly likely to receive higher dosages for longer durations. Future studies should evaluate longitudinal changes in measures such as the PHQ-2 score over time.
Disclosures
J.D.M. has consulted to Pfizer, Teva, Quark, Biogen, Nektar, ENDO, Immune Pharma, Chromocell, Collegium, Purdue, Quartet, Novartis, Depomed, Allergan, Egalet, Sanofi, Aptinyx, Diaachi Sankyo, Plasmasurgical, Grunenthal, Kempharm, and Inspirion. He has received research grant funding from Pfizer and Depomed and participated on clinical trial data safety monitoring committees for Novartis and Allergan. The remaining authors have no conflicts of interest to declare.
Supported by a Supporting Providers in Comparative Effectiveness Fellowship from the Center for Translational Science at the University of Rochester. Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
Author Contributions: Guarantor: J. A. Smith; Study concept and design: J. A. Smith, I. Pesis-Katz, X. Cai, B. Powers, and J. D. Markman; Acquisition of data: J. A. Smith, R. L. Fuino, M. Frazer, and J. D. Markman; Analysis and interpretation of data: J. A. Smith, R. L. Fuino, I. Pesis-Katz, X. Cai, and J. D. Markman; Drafting of the manuscript: R. L. Fuino, M. Frazer, and J. D. Markman; Critical revision of the manuscript for important intellectual content: All authors; Statistical analysis: J. A. Smith and X. Cai; Administrative, technical, and material support: All authors; Study supervision: I. Pesis-Katz, X. Cai, B. Powers, and J. D. Markman
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry 2009;31:564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain 2003;4:344–50. [DOI] [PubMed] [Google Scholar]
- [3].Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med 2009;22:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Deyo RA, Smith DH, Johnson ES, Donovan M, Tillotson CJ, Yang X, Petrik AF, Dobscha SK. Opioids for back pain patients: primary care prescribing patterns and use of services. J Am Board Fam Med 2011;24:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dionne CE, Koepsell TD, Von Korff M, Deyo RA, Barlow WE, Checkoway H. Predicting long-term functional limitations among back pain patients in primary care settings. J Clin Epidemiol 1997;50:31–43. [DOI] [PubMed] [Google Scholar]
- [6].Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dworkin RH, Katz J, Gitlin MJ. Placebo response in clinical trials of depression and its implications for research on chronic neuropathic pain. Neurology 2005;65(12 suppl 4):S7–19. [DOI] [PubMed] [Google Scholar]
- [8].Dworkin RH, Turk DC, Peirce-Sandner S, Baron R, Bellamy N, Burke LB, Chappell A, Chartier K, Cleeland CS, Costello A, Cowan P, Dimitrova R, Ellenberg S, Farrar JT, French JA, Gilron I, Hertz S, Jadad AR, Jay GW, Kalliomaki J, Katz NP, Kerns RD, Manning DC, McDermott MP, McGrath PJ, Narayana A, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Reeve BB, Rhodes T, Sampaio C, Simpson DM, Stauffer JW, Stucki G, Tobias J, White RE, Witter J. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. PAIN 2010;149:177–93. [DOI] [PubMed] [Google Scholar]
- [9].Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain 2010;26:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goesling J, Henry MJ, Moser SE, Rastogi M, Hassett AL, Clauw DJ, Brummett CM. Symptoms of depression are associated with opioid use regardless of pain severity and physical functioning among treatment-seeking patients with chronic pain. J Pain 2015;16:844–51. [DOI] [PubMed] [Google Scholar]
- [11].Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011;171:686–91. [DOI] [PubMed] [Google Scholar]
- [12].Hale ME, Dvergsten C, Gimbel J. Efficacy and safety of oxymorphone extended release in chronic low back pain: results of a randomized, double-blind, placebo- and active-controlled phase III study. J Pain 2005;6:21–8. [DOI] [PubMed] [Google Scholar]
- [13].Hirsh AT, Hollingshead NA, Bair MJ, Matthias MS, Wu J, Kroenke K. The influence of patient's sex, race and depression on clinician pain treatment decisions. Eur J Pain 2013;17:1569–79. [DOI] [PubMed] [Google Scholar]
- [14].Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284–92. [DOI] [PubMed] [Google Scholar]
- [15].Kuo YF, Raji MA, Chen NW, Hasan H, Goodwin JS. Trends in opioid prescriptions among Part D medicare recipients from 2007 to 2012. Am J Med 2016;129:221.e21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Levy HI, Hanscom B, Boden SD. Three-question depression screener used for lumbar disc herniations and spinal stenosis. Spine (Phila Pa 1976) 2002;27:1232–7. [DOI] [PubMed] [Google Scholar]
- [17].Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic- prescribing rates by specialty in US, 2007–2012. Am J Prev Med 2015;49:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luo X, Pietrobon R, Hey L. Patterns and trends in opioid use among individuals with back pain in the United States. Spine (Phila Pa 1976) 2004;29:884–90; discussion 891. [DOI] [PubMed] [Google Scholar]
- [19].Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD. Expenditures and health status among adults with back and neck problems. JAMA 2008;299:656–64. [DOI] [PubMed] [Google Scholar]
- [20].Merrill JO, Von Korff M, Banta-Green CJ, Sullivan MD, Saunders KW, Campbell CI, Weisner C. Prescribed opioid difficulties, depression and opioid dose among chronic opioid therapy patients. Gen Hosp Psychiatry 2012;34:581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paulozzi LJ, Kilbourne EM, Shah NG, Nolte KB, Desai HA, Landen MG, Harvey W, Loring LD. A history of being prescribed controlled substances and risk of drug overdose death. Pain Med 2012;13:87–95. [DOI] [PubMed] [Google Scholar]
- [22].Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976) 2002;27:E109–120. [DOI] [PubMed] [Google Scholar]
- [23].Seal KH, Shi Y, Cohen G, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA 2012;307:940–7. [DOI] [PubMed] [Google Scholar]
- [24].Sullivan MD, Edlund MJ, Steffick D, Unutzer J. Regular use of prescribed opioids: association with common psychiatric disorders. PAIN 2005;119:95–103. [DOI] [PubMed] [Google Scholar]
- [25].Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med 2006;166:2087–93. [DOI] [PubMed] [Google Scholar]
- [26].Turk DC, OOkifuji A. What factors affect physicians' decisions to prescribe opioids for chronic noncancer pain patients? Clin J Pain 1997;13:330–6. [DOI] [PubMed] [Google Scholar]
- [27].U. S. Food and Drug Administration CfDEaR. Exalgo (Hydromorphone ER) NDA 21-217 medical review. 2009; Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/021217s000MedR.pdf. Accessed April 1, 2014. [Google Scholar]
- [28].U. S. Food and Drug Administration CfDEaR. Butrans (Buprenorphine transdermal system) medical review. 2010; Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/021306Orig1s000MedicalR.pdf. Accessed April 1, 2014. [Google Scholar]
- [29].U. S. Food and Drug Administration CfDEaR. Opana ER (Oxymorphone ER) NDA 201655 medical review. 2011; Available at: www.accessdata.fda.gov/drugsatfda_docs/nda/2011/201655Orig1s000MedR.pdf. Accessed June 3, 2014. [Google Scholar]
- [30].U. S. Food and Drug Administration CfDEaR. Zohydro ER (Hydrocodone bitartrate extender release capsules) medical review. 2012; Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/202880Orig1s000MedR.pdf. Accessed April 1, 2014. [Google Scholar]
- [31].Von Korff M, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C. De facto long-term opioid therapy for noncancer pain. Clin J Pain 2008;24:521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wasan AD, Davar G, Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. PAIN 2005;117:450–61. [DOI] [PubMed] [Google Scholar]
- [33].Wasan AD, Michna EF, Edwards RR, Edwards RF, Katz JN, Nedekjkovic SS, Dolman AJ, Janfaza D, Isaac Z, Jamison RN. Psychiatric comorbidity is associated Prospectively with diminished opioid analgesia and increased opioid misuse in patients with chronic low back pain. Anesthiology 2015;123:861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine (Phila Pa 1976) 2007;32:2127–32. [DOI] [PubMed] [Google Scholar]
- [35].Yarlas A, Miller K, Wen W, Lynch SY, Munera C, Dain B, Pergolizzi JV, Raffa R, Ripa SR. A subgroup analysis found no diminished response to buprenorphine transdermal system treatment for chronic low back pain patients classified with depression. Pain Pract 2016;16:473–85. [DOI] [PubMed] [Google Scholar]


