Abstract
Managing patients with dependence requires knowledge of pharmacology; an understanding of the diagnosis of dependence and recognition of withdrawal; skills in communication and collaborative working; and a nonjudgmental, empathic attitude.
Keywords: acute pain, opioid substitution therapy, analgesics, opioid, behavior, addiction
Key Points
Engaging in open and honest discussions with the patient and caregivers to agree to a management and discharge plan with clear, achievable goals.
Using strategies that both provide effective analgesia and prevent withdrawal syndrome, which are 2 separate goals.
The early recognition and treatment of symptoms and behavioral changes that might indicate withdrawal.
Using tamper-proof and secure analgesia administration procedures.
Using regional analgesia where possible, although it may be a challenge in patients who have depressed immunity or local or systemic sepsis from injections.
The patients we describe in this review are those who use illicit opioids, such as heroin, those who use diverted prescription opioids for nonmedical use, or those who take other illicit drugs. These drugs include stimulants (amphetamines, cocaine), depressants (alcohol, barbiturates, benzodiazepines), and others including cannabis, mescaline, and LSD.
1. Definitions
The nomenclature around the illicit use of drugs remains confusing, but the recent International Statistical Classification of Diseases and Related Health Problems (ICD-10, released by the World Health Organization in 2016) uses “dependence syndrome” as the preferred term.32 Other terms such as “addiction,” “substance use disorder,” and “substance misuse” relate to the same condition, but for the purpose of clarity and simplicity we use “dependence syndrome” in this review. Dependence syndrome is defined as follows:
A cluster of behavioral, cognitive, and physiological phenomena that develop after repeated substance use and that typically include a strong desire to take the drug, difficulties in controlling its use, persisting in its use despite harmful consequences, a higher priority given to drug use than to other activities and obligations, increased tolerance, and sometimes a physical withdrawal state.32
Patients may have active dependence, where they are currently using the drug, or controlled dependence, where they are on a clinically supervised maintenance or replacement regimen, or they may be currently abstinent. Those who are currently abstinent are no longer physically dependent on the drug but are particularly vulnerable to relapse, owing to the chronic changes induced by drug use. The American Society of Addiction Medicine defines “addiction” (or “dependence syndrome” in the newer nomenclature) as a chronic disease of brain reward, motivation, memory, and related circuitry.2
In the context of pain management, we also need to be aware of pseudoaddiction, a syndrome associated with the undertreatment of pain, characterized by problem behaviors around seeking more opioid analgesia. Pseudoaddiction can be distinguished from true addiction in that the behaviors cease when pain is effectively treated.
2. Background
Globally, the United Nations estimate that around 250 million people (5% of the world's adult population) used an illicit drug at least once in 2014, whereas the number of people classified as having a drug use disorder is estimated to be 29 million.29 Of the drugs abused, opioids are used less often than cannabis, cocaine, or amphetamine but contribute to 82% of fatal overdoses. Alcohol is one of the most frequently used drugs, with Germany citing 1 in 5 perioperative patients having an alcohol use disorder.14
Recent figures suggest that there are 1.3 million high-risk opioid users in the European Union. Approximately 435,000 people in the United States use heroin, whereas almost 5 times that number—1.9 million—meet criteria for prescription opioid use disorder.26 Australia has seen a similar increase in prescription opioid misuse, with 3.3% of Australians having used prescribed opioid analgesics for nonmedical purposes in the past year, compared with 0.1% using heroin.1 Those misusing prescription opioids may tamper with tablets to adapt them for inhalation, intravenous use, or smoking, leading manufacturers to investigate abuse-deterrent formulations.
3. Assessment and screening of patients
The stigma associated with addiction, often fueled by misinformation or prejudice on the part of health care professionals, is often a barrier to good medical care for those with a current or history of dependence. Competence in managing patients with dependence requires knowledge of pharmacology; an understanding of the diagnosis of dependence and recognition of withdrawal; skills in communication and collaborative working; and a nonjudgmental, empathic attitude. It is essential to establish good patient–clinician rapport to promote an atmosphere of trust and understanding. This will allow an open discussion to accurately ascertain which drugs the patient is currently taking, address the patient's anxieties, manage expectations, and plan care collaboratively, thus reducing discord between the patient and the health care team.
On or before admission, clinicians should reconcile the patient's medicines with the patient's primary care physician and/or drug and alcohol worker. In preparation for discharge, the team should arrange community support to provide follow-up and manage the process of analgesia reduction during the recovery phase.
There is a high prevalence of psychiatric comorbidities in those with drug dependence, with more than 50% of patients showing evidence of significant psychopathology, particularly anxiety disorders and affective disorders, including depression.5 Such comorbidities may further complicate patients' behavior and their interaction with staff while in the hospital.
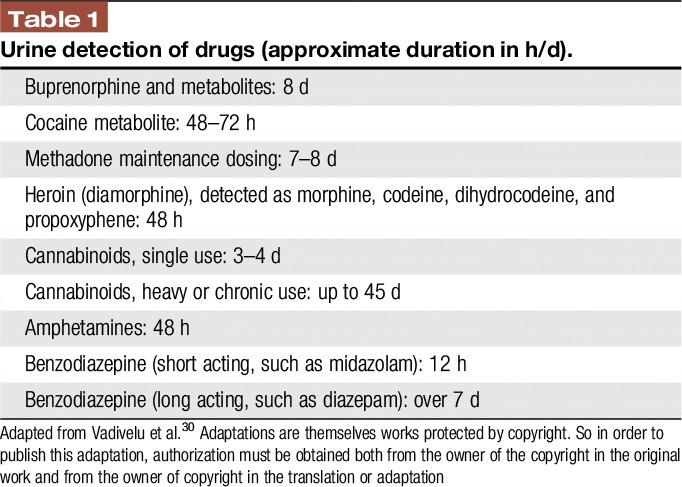
In some cases, urine screening could be considered to detect drugs and assist in formulating a treatment plan while in hospital. Table 1 lists the duration of time that drugs may be detected in urine.
Table 1.
Urine detection of drugs (approximate duration in h/d).
4. Concerns in treating acute pain in patients with drug dependence syndrome
On admission to hospital, patient concerns center on the following:
(1) The fear of withdrawal, such as when the usual opioids used in substitution therapy are not given promptly. These anxieties are most obvious after long waits in the emergency department or immediately after admission to the hospital, when the drug has not yet been prescribed or released by the pharmacy. If doses are omitted or delayed, there will be a re-emergence of withdrawal symptoms and drug cravings.
(2) The fear of pain not being taken seriously, with restricted access to analgesia and pain left unrelieved.
(3) The fear of discrimination, often based on previous poor hospital experiences, leading to clinician distrust.
(4) In those currently abstinent, the fear of relapse if re-exposed to opioids or untreated pain.
Clinician concerns center on the following:
(1) Mistrust of those with addiction.
(2) Overtreatment of pain, leading to opioid-induced ventilatory impairment.
(3) The possibility that reports of pain may be fabricated to acquire opioids for euphoria.
(4) The diversion of prescribed opioids.
(5) Fear that patients may leave the hospital against medical advice (elopement) and not completing essential medical care (eg, infection control).
5. Key issues to assess on hospital admission for dependence syndrome
(1) The appropriate use and dosage of opioid substitution therapies (OSTs) (methadone and buprenorphine); doses will need to be verified by the opioid prescriber and the dispensing pharmacist.12
(2) Other prescribed medications, over-the-counter drugs, or illicit substances including heroin, alcohol, nicotine, benzodiazepines, cannabis, and cocaine (polyabuse is common).
(3) Routes of administration—some patients may be injecting prescription drugs intended for oral, transdermal, or sublingual use.
(4) Medical comorbidities, eg, HIV/AIDS, hepatitis, cirrhosis, tuberculosis, endocarditis, cellulitis, abscesses, and chronic pain.
(5) Psychiatric comorbidities, eg, anxiety, depression, personality disorder, and posttraumatic stress disorder.
(6) Social factors, eg, abuse, interpersonal violence, and homelessness.
(7) Support systems after discharge.
6. Principles of acute pain management in opioid-dependent patients
The goals of treating acute pain in patients using opioids are to provide adequate analgesia, prevent withdrawal, and avoid triggering a relapse or worsening of the addiction disorder.
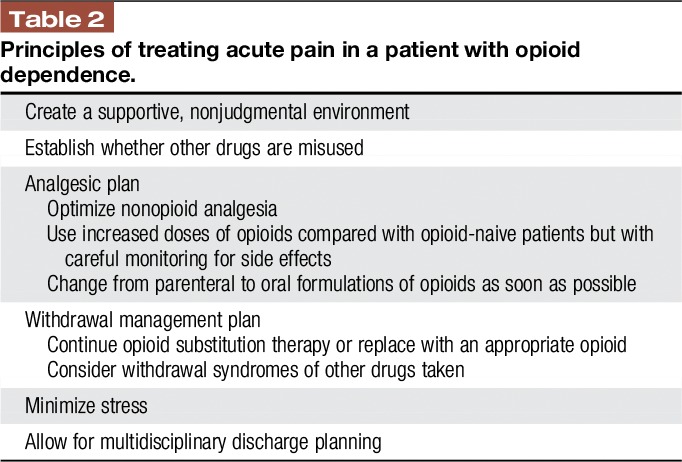
The principles of treating acute pain in a patient with opioid addiction are summarized in Table 2.
Table 2.
Principles of treating acute pain in a patient with opioid dependence.
Patients with an opioid dependency have 3 interlinked obstacles to effective pain management: (1) opioid-induced hyperalgesia (OIH), resulting in increased pain sensitivity; (2) opioid tolerance, leading to reduced effectiveness of opioids used to treat pain; and (3) opioid withdrawal, producing sympathetic stimulation and heightened stress responses if the usual opioids, such as those for OST, are not given.
6.1. Opioid-induced hyperalgesia
Quantitative sensory testing has shown that, similar to patients with pain taking long-term opioids, those abusing heroin and those on methadone and buprenorphine substitution therapy may develop hyperalgesia.23 Once opioids are stopped, it is unclear when this pain sensitivity reverses, but it would appear that heat and pain perception remain abnormal for months after cessation. It is wise, then, to consider patients who are currently abstinent to also be at risk of OIH.
6.1.1. Management of opioid-induced hyperalgesia
Multimodal analgesia should be optimized by adding opioid-sparing analgesics such as paracetamol (acetaminophen), nonsteroidal anti-inflammatory drugs, or cyclooxygenase-2 inhibitors, using local anesthetic regional techniques where appropriate, and considering opioid rotation.
In addition, adjuvants have an important role in pain management. Ketamine attenuates OIH in patients on long-term opioids. Gabapentin and pregabalin reduce OIH in animal models, human volunteers, and patients. There is some evidence that alpha-2 agonists—clonidine and dexmedetomidine—may decrease hyperalgesia, whereas experimental results indicate that COX- 2 inhibitors may also have a role.23
6.2. Opioid tolerance
Opioid tolerance is the decreased effectiveness of opioids over time, such that higher doses are needed to achieve the same effect. Given that opioids are the mainstay of treating severe acute pain, it is unfortunate that opioid tolerance and OIH combine so that opioids are least effective for those patients with, arguably, the most pain.
Opioid-tolerant patients report higher pain scores, have slower pain resolution, and experience a longer hospital stay with increased chance of readmission, compared with opioid-naive patients.7,10 They have higher opioid requirements, but interpatient variability is high, so doses need to be titrated to effect for each patient, with careful observation for signs of opioid toxicity.
6.2.1. Management of opioid tolerance
Where opioids are required, higher doses are needed in opioid-tolerant patients compared with opioid-naive patients.
The adjuvants used to reduce OIH also reduce opioid tolerance. Ketamine particularly has been found to improve postoperative pain in opioid-tolerant patients. In various experimental and clinical models, gabapentin and pregabalin, paracetamol, nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 inhibitors, and alpha-2 agonists all moderated tolerance.23
6.3. Opioid withdrawal
Opioid withdrawal symptoms are caused by sympathetic overactivity and include tachycardia, anxiety, restlessness, insomnia, sweating, diarrhea, rhinorrhea, muscle aches, yawning, and “gooseflesh.” Scoring systems such as the Subjective Opiate Withdrawal Scale (SOWS) or the more objective Clinical Opiate Withdrawal Scale (COWS)31 offer ways to assess the severity of withdrawal. These symptoms contribute to stress and anxiety, which in itself will increase pain sensitivity, reduce coping ability, and drive drug craving.
Some patients may be established on OST, also known as opioid maintenance treatment. This treatment involves providing controlled doses of long-acting opioids to maintain blood levels within a narrow range, such that patients experience minimal intoxication and minimal withdrawal, thus reducing the sensations driving opioid drug seeking and drug misuse.9 Sublingual buprenorphine and oral methadone are most commonly used for OST and have recognized benefit in reducing levels of drug use, offending, overdose risk, and the spread of blood-borne viruses, as well as providing stability for drug users and their families. Some people may subsequently achieve long-term sustained abstinence.
The central tenet of withdrawal management is to prevent its development, providing more stability for patients and reducing psychological and physiological stress. This is important both in the short term during hospital admission, and in the longer term to ensure that patients maintain their commitment to the OST program. An acute pain episode can precipitate disengagement from OST and a return to haphazard drug-taking, with poorly treated acute pain increasing that risk further.4
6.3.1. Methadone
When patients are taking methadone for OST, clinicians should continue this drug after confirming the dose with the usual prescriber or the dispensing pharmacy. Withdrawal suppression lasts around 24 hours, so the dose can be continued once a day as in the community, or given in 2 or 3 divided doses during hospital admission. It is important to understand that the patient will not gain any meaningful analgesia from this dose, and that withdrawal prevention and analgesia provision should be treated as separate entities.
Methadone can cause prolongation of the corrected electrocardiographic QT interval, which may predispose patients to the ventricular arrhythmia torsades de pointes. Acute illness and coprescription of acute medicines may increase the risk of cardiac arrhythmia in the hospital in patients who may have previously been stable. Circumstances include electrolyte abnormalities such as hypokalemia or hypomagnesemia; impaired liver function; and the use of drugs with corrected electrocardiographic QT–prolonging properties, for example antibiotics and benzodiazepines.8
6.3.2. Buprenorphine
Buprenorphine is a partial agonist at the mu-opioid receptor with a high-binding affinity. At high doses of 16–32 mg, such as those used in OST, there is little free receptor availability, such that additional pure opioid agonists including heroin would impart no benefit. For this reason, it had been thought that buprenorphine should be stopped during hospital admission to allow receptor accessibility for opioids used for analgesia. Studies are beginning to emerge to question that approach. It would now appear that, when given in divided doses, some patients on buprenorphine OST may do better if the buprenorphine is continued.16 The total dose should be split into 2 or 3 divided doses during hospital admission and then converted back to a daily dose on discharge into the community.23
6.3.3. Withdrawal prevention for patients not on opioid substitution therapy
If a patient with opioid dependence is not on an OST program, withdrawal remains a problem and can be assessed using the COWS measure alongside routine patient observations. Small doses of methadone (10–20 mg depending on the severity of withdrawal) can then be used on the basis of the COWS score.
Methadone is preferred over morphine for withdrawal prevention as it provides longer-lasting withdrawal prevention and reduces craving (the psychological need for more opioid).
6.3.4. Withdrawal prevention for patients on nil by mouth
For patients with unreliable enteral absorption, intravenous patient-controlled analgesia should be established with the bolus providing analgesia. A background infusion can then be added to the patient-controlled analgesia for opioid withdrawal prevention. Careful monitoring of withdrawal symptoms, sedation, and respiratory rate is essential to guide dose requirements. Although those with opioid dependence demonstrate tolerance to the analgesic effects of opioids, tolerance to the side effects will vary, so sedation and opioid-induced ventilatory insufficiency remain a risk. A tamper-proof housing should be used to reduce access to the programmable device and the analgesic medicine.
6.4. Risk of relapse in abstinent patients
The rate of absorption of an opioid and the peak concentration attained determine its abuse potential.22 Intravenous opioids given for analgesia will therefore have a stronger reinforcing effect and present more of a threat to relapse in an abstinent patient than oral opioids, with sustained-release preparations lowering the abuse potential further. Conversely, the severity of acute pain will fluctuate according to activity, so such pain is best treated with short-acting formulations. A balance should be found between the 2 with a plan to switch to oral opioids as soon as practicable.
Some patients who are currently abstinent will be very reluctant to have intravenous opioid boluses, and this preference should be respected, with other drugs given instead where possible. There is some evidence to suggest that the risk of relapse is small with the use of perioperative opioids, although unrelieved pain could be a trigger.23 If an abstinent patient relapses, he or she should be supported to engage with addiction services to aid recovery.
7. Nonopioid substance dependence in acute pain
An appreciation of the patterns of nonopioid substance misuse is necessary to reduce the risk of drug interactions and to identify and treat withdrawal. Commonly misused drugs are outlined below with information about the signs and symptoms of withdrawal and toxicity. Withdrawal from central nervous system (CNS) stimulants is predominantly affective rather than physical, whereas withdrawal from CNS-depressant drugs leads to CNS and autonomic hyperexcitability.
Substance misusers are one of the groups of patients at high risk of self-discharge against medical advice.11 They, like opioid-dependent patients, are at risk of pseudoaddiction if their acute pain is undertreated.
The U.S. Preventive Services Task Force recommends the screening of all patients for alcohol misuse, but it does not recommend screening for other substance use disorders as there is limited evidence.18
7.1. Illicit drugs
A full medical history related to prescription and illicit drug use may identify a number of agents that could potentially require adjustment of in-patient analgesia for pain. These illicit drugs may include stimulants (amphetamines and cocaine); depressants (alcohol, barbiturates, and benzodiazepines); and others including cannabis, mescaline, and LSD. An overview of the origin and mode of action appears below for each subgroup of drugs together with the signs and symptoms of withdrawal or abstinence, along with considerations for a treatment plan.
7.2. Stimulants
7.2.1. Amphetamines (eye-openers, dexies, poppers, speed, or uppers)
Amphetamines are CNS stimulants commonly prescribed for narcolepsy and hyperactivity disorders, eg, attention deficit hyperactivity disorder. One common stimulant, methylphenidate (sold under the trade name “Ritalin” in some countries and often called the “Smart Drug” or “Study Drug”), is commonly abused by students as they perceive that it helps them concentrate.
The release of sympathetically active substances by the use of amphetamines, most commonly 3,4-methylenedioxymethamphetamine (MDMA), can lead to euphoria, aggression, and personality changes. Toxic MDMA levels lead to tachycardia, sweating, rhabdomyolysis, malignant hyperpyrexia, and hepatorenal failure. Long-term use is associated with psychosis, cardiac problems, malnutrition, and convulsions.
Abstinence or withdrawal from this class of drug within the first week produces a “crash” phase that lasts for a few days, characterized by severe dysphoria, irritability and melancholia, anxiety, hypersomnia (but with poor quality sleep) and marked fatigue, intense craving for the drug, and paranoia.24 Three components of amphetamine withdrawal have been described: (1) a hyperarousal factor involving drug craving, agitation, and vivid or unpleasant dreams; (2) a reversed vegetative factor involving decreased energy, increased appetite, and increased craving for sleep; and (3) an anxiety factor involving loss of interest or pleasure, anxiety, and slowing of movement.25
Aspects of the withdrawal syndrome might be mediated by different neurotransmitter systems including dopamine, norepinephrine, and serotonin.24
Trauma patients presenting to the emergency department with the use of cocaine and/or amphetamines before their acute injury had analgesic and sedation requirements (where ventilated) similar to those of a control cohort.15
7.2.2. Khat (kat, qat, and chat)
Khat contains an alkaloid stimulant (cathinone) and is derived from the shrub Catha edulis. The leaves are chewed like tobacco or, less frequently, are used to make tea. Khat is a euphoric stimulant less potent than amphetamines. Khat is widely used in the Arabian Peninsula (especially Yemen) and the Horn of Africa. World Health Organization has identified that khat has the ability to cause mild to moderate psychological dependence. In the developing world, khat is often used to relieve fatigue.13 Because physiological withdrawal is unknown with khat, no substitution is required.
7.2.3. Cocaine
The easy availability of cocaine coupled with a relative reduction in consumer cost has led to widespread use. It is available in 2 forms; hydrochloride (white powder), and free base (crack cocaine), which is made by combining the hydrochloride with an alkali. With a bioavailability of up to 90% by the inhalation or intranasal route, cocaine inhibits the presynaptic uptake of dopamine, serotonin, epinephrine, and norepinephrine, thus leading to an increased availability to act on the adrenergic receptors stimulating the cardiovascular, renal, and CNS.23 Excessive stimulation by cocaine can lead to seizures, myocardial infarction, stroke, respiratory depression, cardiac arrhythmias, and sudden death. The features of cocaine withdrawal include agitation, restlessness, depressed mood, fatigue, increased appetite, vivid dreams, extreme suspicion, and paranoia. Cocaine use is associated with particular types of acute pain, for example chest pain, including acute coronary syndrome.23
7.3 Depressants
7.3.1. Alcohol
A full discussion of inpatient management of patients with alcohol dependence is beyond the scope of this review, but it is important to identify patients with problem alcohol use. SBIRT (Screening, Brief Intervention, and Referral to Treatment) is a strategy used to determine those with risky drug use and to guide early intervention. It has become one of the major tools recommended for use in primary care by governments and expert panels, including the Joint Commission on Accreditation for Health Care Organizations (JCAHO), the major accrediting body for hospitals in the United States, which uses SBIRT as a quality indicator for general hospital care. The American Society of Addiction Medicine provides detailed guidance on managing acute alcohol withdrawal19 and has been updated by Makdissi et al in 2013.17 The summary of alcohol and acute pain in Acute Pain Management: Scientific Evidence reports no cross-tolerance between alcohol and opioids in animal studies and states that effective remifentanil concentrations do not differ between alcoholic and nonalcoholic patients.23 Thus, there is no need to use higher than standard doses of opioids in alcohol-dependent patients. Abstinence or withdrawal from alcohol may produce withdrawal seizures and delirium tremens.
The National Institute for Health and Care Excellence suggests following a symptom-triggered regimen for drug treatment21 and recommends using a tool such as the Clinical Institute Withdrawal Assessment–Alcohol, revised [CIWA–Ar] scale to supplement clinical judgment.27 The treatment for alcohol withdrawal seizures includes offering a benzodiazepine, carbamazepine, or chlormethiazole. Delirium tremens may be treated using oral lorazepam, escalating to parenteral lorazepam, haloperidol, or olanzapine. A short-acting benzodiazepine such as lorazepam may be used if the patient experiences seizures. Phenytoin should be avoided.21
7.3.2. Barbiturates
Derivatives of synthetic barbituric acid, barbiturates are sedative hypnotics; they include phenobarbitone and sodium thiopental. Although usually consumed in tablet form, barbiturates may be injected intravenously or intramuscularly. Most commonly, barbiturates are taken by misusers to counteract the effects of amphetamines and cocaine or to produce a state of euphoria (a “high”). Abstinence or withdrawal from this class of drug produces hallucinations, high temperatures, restlessness, and seizures. There is little high-quality evidence for the safe management of barbiturate withdrawal.
7.3.3. Benzodiazepines
The most commonly used anxiolytics and hypnotics, benzodiazepines act at gamma-aminobutyric acid receptors. Abrupt withdrawal of a benzodiazepine may produce confusion, toxic psychosis, convulsions, or a condition resembling delirium tremens.
Withdrawal syndrome may develop at any time up to 3 weeks after stopping a long-acting benzodiazepine (such as diazepam) but may occur within a day in the case of a short-acting one (such as lorazepam). Withdrawal is characterized by insomnia, anxiety, loss of appetite, loss of bodyweight, tremor, perspiration, tinnitus, and perceptual disturbances. Some symptoms may continue for weeks or months after stopping benzodiazepines. The dangers of coprescribing of benzodiazepines and opioids have been outlined in a previous issue of Pain: Clinical Up-dates.3 There is no need to use higher than standard opioid doses in benzodiazepine-dependent patients.23
7.4. Hallucinogens
7.4.1. Cannabis (marijuana, weed, and dope)
Derived from the Cannabis sativa plant, the extract from dried leaves is known as marijuana, whereas hashish is produced from a concentrated resin, resulting in a stronger drug effect. Delta-9-tetrahydrocannabinol is the psychoactive agent that inhibits the muscarinic receptor of the parasympathetic system, increasing the turnover of acetylcholine.30 Users report feelings of euphoria, enhanced mood, and reduction in nausea. Marijuana is usually smoked, but may be eaten or taken in tea. Withdrawal presents in chronic users as insomnia, craving, aggression, headaches, fatigue, hot/cold flushes, and aching muscles. There is limited evidence for the need for higher opioid doses for acute pain in recreational cannabis users.23
7.4.2. Synthetic cannabinoids (spice, K2, clockwork orange, and black mamba)
These drugs bear little relation to cannabis, but the synthetic cannabinoids are usually sprayed onto plant materials to make them look like marijuana. The varieties of chemicals used are far stronger8 than in naturally occurring cannabis, with a high potential for addiction and abuse and a risk of psychosis or CNS depression. Withdrawal states may mimic those for opioids. As these synthetic cannabinoids cause tachycardia, agitation, and nausea, supportive care will be needed for 8 hours after use.28
7.4.3. Mescaline (buttons, cactus, and mesc)
Mescaline (peyote) is a small, spineless cactus with button-like protrusions on its roots that contain mescaline, an hallucinogen. These cacti grow in the United States, Mexico, and Peru. The bitter protrusions are cut and allowed to dry, then ground into a powder, and soaked with tobacco or cannabis. Alternatively, they may be soaked in water, with the user drinking the liquid. The drug can also be chemically synthesized in the laboratory.6 Users report being in a pleasant dreamlike state that lasts 12–18 hours, although mescaline can cause vomiting, headaches, and a feeling of anxiety.
7.4.4. Lysergic acid diethylamide (acid, battery acid, blotter, elvis, loony tunes, and lucy in the sky with diamonds)
Lysergic acid diethylamide (LSD) is a synthetic drug that has been abused for its hallucinogenic properties since the 1960s. If consumed in a sufficiently large dose, LSD produces delusions and visual hallucinations that distort the user's sense of time and identity. Lysergic acid derives from the ergot fungus, found on grains such as rye. Lysergic acid diethylamide is manufactured by mixing the ergot fungus with other substances. It is hardened and crystalized, then liquefied, and finally sold on the streets, usually as gelatin squares or tablets. Lysergic acid diethylamide is not considered an addictive drug by the National Drug Intelligence Center,31 but LSD users may develop tolerance to the drug, meaning that they consume progressively larger doses of the drug to continue to experience the hallucinogenic effects that they seek. Unpleasant flashbacks can occur weeks after taking the drug.20
8. Discharge
Discharge planning begins at admission12 to ensure that the patient is discharged into a safe and supportive environment. Referral to a hospital social worker should be undertaken on awareness of a patient with a drug dependence syndrome to allow sufficient time to explore any community housing needs and to inform the discharge plan.
Good communication with primary care clinicians, dispensing pharmacists, and drug treatment services is essential to ensure that patients re-engage with support services and maintain their prehospital management.
Where opioids for analgesia are needed on discharge, decisions on how to provide that safely will be based on the services available in individual hospitals. Immediate-release formulations would be most appropriate for the ongoing treatment of acute pain, but they carry a higher risk of diversion or overdose. Some hospitals may be able to offer frequent outpatient appointments in the time immediately after discharge so that limited doses of short-acting opioids can be given and weaned under close supervision. Where this intense control is not available, supervised consumption of long-acting preparations may be the safest and most appropriate way of providing short-term pain relief and preventing diversion, with a clear and specific plan to guide dose reduction.
Disclosures
The authors have no conflict of interest to declare.
References
- [1].Alcohol and Drug Foundation. Infographic: alcohol and drugs affect everyone. 2017. Available at: http://adf.org.au/wp-content/uploads/2017/02/infographic-alcohol-and-drugs-affect-everyone.pdf. Accessed April 7, 2017.
- [2].American Society of Addiction Medicine. Available at: http://www.asam.org/quality-practice/definition-of-addiction. Accessed April 7, 2017.
- [3].Babalonis S, Walsh SL. Warnings unheeded: the risks of co-prescribing opioids and benzodiazepines. Pain: Clin Updates 2015;23:1–7. [PMC free article] [PubMed] [Google Scholar]
- [4].Bounes V, Palmaro A, Lapeyre-Mestre M, Roussin A. Long-term con- sequences of acute pain for patients under methadone or buprenorphine maintenance treatment. Pain Physician 2013;16:E739–47. [PubMed] [Google Scholar]
- [5].Callaly T, Trauer T, Munro L, Whelan G. Prevalence of psychiatric disorder in a methadone maintenance population. Aust N Z J Psychiatry 2001;35:601–5. [DOI] [PubMed] [Google Scholar]
- [6].Center for Substance Abuse Research, University of Maryland. Peyote/mescaline factsheet. 2016. Available at: http://www.cesar.umd.edu/cesar/drugs/peyote.pdf. Accessed April 7, 2017.
- [7].Chapman CR, Davis J, Donaldson GW, Naylor J, Winchester D. Postoperative pain trajectories in chronic pain patients undergoing surgery: the effects of chronic opioid pharmacotherapy on acute pain. J Pain 2011;12:1240–6. [DOI] [PubMed] [Google Scholar]
- [8].Chou R, Cruciani RA, Fiellin DA, Compton P, Farrar JT, Haigney MC, Inturrisi C, Knight JR, Otis-Green S, Marcus SM, Mehta D, Meyer MC, Portenoy R, Savage S, Strain E, Walsh S, Zeltzer L; American Pain Society; Heart Rhythm Society. Methadone safety: a clinical practice guideline from the American Pain Society and College on problems of drug dependence, in collaboration with the Heart Rhythm Society. J Pain 2014;15:321–37. [DOI] [PubMed] [Google Scholar]
- [9].Department of Health (England) and the devolved administrations. Drug misuse and dependence: UK guidelines on clinical management. London: Department of Health (England), the Scottish Government, Welsh Assembly Government and Northern Ireland Executive; 2007. [Google Scholar]
- [10].Gulur P, Williams L, Chaudhary S, Koury K, Jaff M. Opioid tolerance—a predictor of increased length of stay and higher readmission rates. Pain Physician 2014;17:E503–7. [PubMed] [Google Scholar]
- [11].Hines S, Theodorou S, Williamson A, Fong D, Curry K. Management of acute pain in methadone maintenance therapy in-patients. Drug Alcohol Rev 1998;27:519–23. [DOI] [PubMed] [Google Scholar]
- [12].Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care 2011;39:804–23. [DOI] [PubMed] [Google Scholar]
- [13].Kopf A. Abdominal cancer, constipation and anorexia. In: Kopf A, Patel NB, editors. IASP guide to pain management in low-resource settings. Seattle: IASP; 2010. [Google Scholar]
- [14].Kork F, Neumann T, Spies C. Perioperative management of patients with alcohol, tobacco and drug dependency. Curr Opin Anaesthesiol 2010;23:384– 90. [DOI] [PubMed] [Google Scholar]
- [15].Kram B, Kram SJ, Sharpe ML, James ML, Kuchibhatia M, Shapiro ML. Analgesia and sedation requirements in mechanically ventilated trauma patients with acute, preinjury use of cocaine and/or amphetamines. Anesth Analg 2017;124:782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Macintyre PE, Russell RA, Usher KA, Gaughwin M, Huxtable CA. Pain relief and opioid requirements in the first 24 hours after surgery in patients taking buprenorphine and methadone opioid substitution therapy. Anaesth Intensive Care 2013;41:222–30. [DOI] [PubMed] [Google Scholar]
- [17].Makdissi R, Stewart SH. Care for hospitalized patients with unhealthy alcohol use: a narrative review. Addict Sci Clin Pract 2013;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moyer V; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. Ann Intern Med 2013;159:210–8. [DOI] [PubMed] [Google Scholar]
- [19].Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of ad- diction medicine working group on Pharmacological management of alcohol withdrawal. JAMA 1997;278:144–51. [DOI] [PubMed] [Google Scholar]
- [20].National Drug Intelligence Center. LSD factsheet. 2008. Available at: https://www.justice.gov/archive/ndic/pubs4/4260/index.htm. Accessed April 7, 2017.
- [21].National Institute for Health and Care Excellence. Alcohol-use disorders: Diagnosis and management of physical complications clinical guideline [CG100]. Available at: https://www.nice.org.uk/guidance/cg100. Accessed April 7, 2017. [PubMed] [Google Scholar]
- [22].Schaeffer T. Abuse-deterrent formulations, an evolving technology against the abuse and misuse of opioid analgesics. J Med Toxicol 2012;8:400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schug SA, Palmer GM, Scott DA, Halliwell R, Trinca J; APM: SE Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. Acute pain management: Scientific evidence. 4th ed Melbourne: Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine; 2015. Available at: http://fpm.anzca.edu.au/documents/apmse4_2015_final. Accessed April 7, 2017. [Google Scholar]
- [24].Shoptaw SJ, Kao U, Heinzerling K, Ling W. Treatment for amphetamine withdrawal. Cochrane Database Syst Rev 2009;2:CD003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Srisurapanont M, Jarusuraisin N, Jittiwutikan J. Amphetamine withdrawal: II. A placebo-controlled, randomised, double-blind study of amineptine treatment. Aust N Z J Psychiatry 1999;33:94–8. [DOI] [PubMed] [Google Scholar]
- [26].Substance Abuse and Mental Health and Mental Health Services Administration. National survey on drug use and health. 2014. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf. Accessed April 7, 2017. [Google Scholar]
- [27].Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical Institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 1989;84:1353–7. [DOI] [PubMed] [Google Scholar]
- [28].Tait RJ, Caldicott D, Mountain D, Hill SL, Lenton S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila) 2016;54:1–13. [DOI] [PubMed] [Google Scholar]
- [29].United Nations Office on Drugs and Crimes. World drug report 2016. Available at: https://www.unodc.org/unodc/en/frontpage/2016/June/number-of-drug-dependent-adults-up-for-first-time-in-six-years–now-at- 29-million_-unodc-world-drug-report-2016.html. Accessed April 7, 2017. [Google Scholar]
- [30].Vadivelu N, Singh-Gill H, Kodumudi G, Kaye AJ, Urman RD, Kaye AD. Practical guide to the management of acute and chronic pain in the presence of drug tolerance for the healthcare practitioner. Ochsner J 2014;14:426–33. [PMC free article] [PubMed] [Google Scholar]
- [31].Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs 2003;35:253–9. [DOI] [PubMed] [Google Scholar]
- [32].World Health Organization. International statistical classification of diseases and related health problems, 10th revision. Available at: http://apps.who.int/classifications/icd10/browse/2016/en. Accessed April 7, 2017. [Google Scholar]


