Abstract
Purpose
Retinal ischemic phenomena occur in several ocular diseases that share the degeneration and death of retinal ganglion cells (RGCs) as the final event. We tested the neuroprotective effect of azithromycin, a widely used semisynthetic macrolide antibiotic endowed with anti-inflammatory and immunomodulatory properties, in a model of retinal ischemic injury induced by transient elevation of intraocular pressure in the rat.
Methods
Retinal ischemia was induced in adult rats with transient elevation of intraocular pressure. RGCs were retrogradely labeled with Fluoro-Gold, and survival was assessed following a single dose of azithromycin given systemically at the end of the ischemia. The expression of death-associated proteins and extracellular signal-regulated kinase (ERK) activation was studied with western blotting. Expression and activity of matrix metalloproteinase-2 (MMP-2) and -9 were analyzed with gelatin zymography.
Results
Acute post-injury administration of azithromycin significantly prevented RGC death. This effect was accompanied by reduced calpain activity and prevention of Bcl-2-associated death promoter (Bad) upregulation. The observed neuroprotection was associated with a significant inhibition of MMP-2/-9 gelatinolytic activity and ERK1/2 phosphorylation.
Conclusions
Azithromycin provides neuroprotection by modifying the inflammatory state of the retina following ischemia/reperfusion injury suggesting potential for repurposing as a drug capable of limiting or preventing retinal neuronal damage.
Introduction
Retinal ischemia/reperfusion injury occurs in several ocular pathologies, including glaucoma, diabetic retinopathy, and anterior ischemic neuropathy [1]. Among the neuronal subtypes of the retina, retinal ganglion cells (RGCs) are highly vulnerable to ischemic insults [2,3]. Located in the innermost layer, RGCs represent the final cellular element of the retinal visual processing and the only output cells, transmitting the visual information to the brain throughout their axons forming the optic nerve [4]. Due to the function of RGCs, their damage and loss are associated with visual impairments and eventually blindness. Therefore, the identification and availability of neuroprotective therapeutic approaches aimed at preserving RGC survival are a relevant need for the clinical management of all ocular pathologies that share the death of RGCs as the final event [5].
Azithromycin is a semisynthetic, 15-membered lactone ring azalide related to the macrolide antibiotic class indicated for the treatment of a wide range of bacterial infections and chronic inflammatory disorders [6]. The drug has a good pharmacokinetic profile characterized by a large volume of distribution, a high tissue concentration, and a prolonged half-life allowing once-daily administration [7]. The broad spectrum and favorable pharmacokinetics make azithromycin routinely used for urogenital and airway infections [8,9], while current ophthalmological use is limited to topical treatment against ocular surface infections, such as bacterial conjunctivitis, trachoma, and blepharitis [10]. In addition to antimicrobial activity, azithromycin exerts immunomodulatory and anti-inflammatory effects [11] that contribute to the clinical outcome amelioration in the pathologies where azithromycin is currently used [6] and might suggest the application of the macrolide in other pathological contests where these phenomena are implicated.
For instance, activation of innate and adaptive immunity has recently been recognized as an important player in the pathogenesis and progression of glaucoma, and inflammatory responses have been identified as common features in clinical and experimental settings [12]. Similarly, increasing evidence identified inflammation as a key contributor to the vision loss that occurs in diabetic retinopathy [13]. Therefore, we tested the neuroprotective effect of azithromycin on RGCs using an experimental model of retinal ischemia/reperfusion injury induced by transient elevation of the intraocular pressure (IOP) in the rat.
Methods
Animals
Male Wistar rats (280–330 g) were purchased from Charles River (Lecco, Italy) and housed with a 12 h:12 h light-dark cycle with ad libitum access to food and water. Animal care and experimental procedures were performed in accordance with the guidelines of the Italian Ministry of Health (D.L. 26/2014), the European Communities Council Directive (2010/63/UE), and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All surgical procedures were performed under deep anesthesia, and all efforts were made to minimize the number of animals used and their suffering.
Retinal ischemia injury
Retinal ischemia was induced in the right eye of each rat by acutely increasing the IOP according to the method previously reported [14,15]. In this model, along with the ischemic damage, a transient mechanical deformation of the eye has also been recently reported [16].
Briefly, the rats were anesthetized with an intraperitoneal (i.p.) injection of chloral hydrate (400 mg/kg) and laid on a heating pad to maintain the body temperature at 37 °C. Topical anesthesia was induced with 0.4% Oxybuprocaine eye drops (Novesina, Novartis, Varese, Italy).
A 27-gauge infusion needle, connected to a 500 ml bottle of sterile saline, was inserted in the anterior chamber of the eye. The saline reservoir was elevated to produce an IOP of 120 mmHg for 50 min. Retinal ischemia was confirmed by whitening of the fundus. For each animal, the opposite eye served as non-ischemic control. Body temperature was monitored before, during, and after ischemia, and animals with body temperature lower than 35.5 °C were excluded from the study.
Rats were euthanized by cervical dislocation at 1 and 6 h of reperfusion or 7 days following the 50 min of ischemia. Both eyes were immediately removed, and the retinas quickly dissected, snap frozen in liquid nitrogen, and stored at −80 °C until use.
Immunoblot analysis
Retinas were homogenized in 130 µl of ice-cold lysis buffer (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% Triton-X100) containing 1 nM okadaic acid, protease inhibitor cocktail (Sigma-Aldrich, Milan, Italy), and phosphatases inhibitor cocktail (cod. 524,625, Calbiochem, La Jolla, CA). Tissue lysates were centrifuged for 15 min at 10,000 ×g at 4 °C, and supernatants were collected. Protein concentration was determined using a Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories, Milan, Italy) with BSA as standard. Equal amounts of total proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred on polyvinylidene difluoride (PVDF) membranes (Immobilon-P, Sigma-Aldrich).
The membranes were blocked with 5% non-fat milk in Tris-buffered saline containing 0.05% Tween-20 (TBS-T) for 1 h at room temperature. Primary antibodies were incubated overnight at 4 °C followed by species-specific horseradish peroxidase–conjugated secondary antibody (Pierce Biotechnology, Rockford, IL) for 1 h at room temperature. Protein bands were visualized with enhanced chemiluminescent detection reagents (ECL, Amersham Biosciences, GE Healthcare, Milan, Italy) and exposed to X-ray films (Hyperfilm, Amersham Biosciences, GE Healthcare). Autoradiographic films were scanned and digitalized, and band quantification was performed using ImageJ software (National Institutes of Health [NIH], Bethesda, MD). The following primary antibodies and dilutions were used: anti-Bcl-2-associated death promoter (Bad) 1:1,000, anti-phospho-p44/p42 MAPK (pERK1/2; Thr202/Tyr204) 1:1,000 (Cell Signaling Technology, Beverly, MA); anti-spectrin (non-erythroid) 1:2,000 (Chemicon International Inc., Temecula, CA); and anti-actin 1:1,000 (Sigma-Aldrich).
Gel zymography
Matrix metalloproteinase-2 (MMP-2; gelatinase A9) and MMP-9 (gelatinase B) gelatinolytic activity was detected with gelatin zymography [17,18]. Individual retinas were homogenized in 180 µl of ice-cold TBS lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2, 0.05% Brij35 (pH 7.6), 1% Triton-X100, and 0.02% NaN3) containing complete mini EDTA-free protease inhibitor cocktail tablets (Roche, Mannheim, Germany) and phenylmethylsulfonyl fluoride (PMSF; 1:1,000; Fluka, Sigma-Aldrich). Tissue lysates were centrifuged for 20 min at 14,000 ×g at 4 °C, and supernatants were assayed for protein content using a Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories). Four hundred micrograms of protein for each sample were subjected to affinity precipitation with 40 μl of gelatin-conjugated Sepharose beads (Gelatin-Sepharose 4B, Amersham Bioscience, GE Healthcare) overnight at 4 °C. Bound proteins were eluted from the beads in TBS containing 10% dimethyl sulfoxide (DMSO) by shaking for 1 h at 4 °C. Ten microliters of each sample were diluted 1:1 with non-reducing SDS loading buffer (0.0625 M Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and 0.25% bromophenol blue) and separated on a 10% SDS-PAGE gel copolymerized with 0.1% gelatin from porcine skin (Sigma-Aldrich). Gels were washed twice (30 min each) in 2.5% Triton X-100 and incubated with developing buffer (49.5 mM Tris-HCl, 9.18 mM CaCl2, and 0.02% NaN3), at 37 °C for 48 h. Gels were then stained with 0.25% Coomassie brilliant blue solution (0.25% Coomassie brilliant blue, 10% acidic acid, and 50% methanol) for 4 h and destained in a solution of acetic acid:methanol:water (1:3:6). Gel images were scanned and digitalized, and band densitometry was performed using ImageJ software (NIH). Values of gelatinolytic activity are expressed as arbitrary units of optical density.
Retrograde labeling of RGCs
Cell loss evaluation was performed by retrograde labeling RGCs with the fluorescent tracer Fluoro-Gold (Fluka, Sigma-Aldrich). The tracer, injected into the superior colliculus, is taken up by the axon terminals of the RGCs and transported retrogradely to the soma in the retina [19-21].
Briefly, 4 days after the ischemic insult, rats were anaesthetized and immobilized in a stereotaxic device (Kopf 900, Analytical Control, Milan, Italy), and the position of superior colliculi was identified using the Paxinos and Watson atlas (1998). The skull was exposed, and 2 µl of 5% Fluoro-Gold (FG) solution was injected on both sides of the skull 6 mm posterior to the bregma, 1.2 mm lateral to the sagittal suture, and 4 mm deep from the bone surface using a 33-gauge Hamilton syringe (Hamilton Robotics Headquarters, Bonaduz, Switzerland). The skin was then sutured, and a 0.3% tobramycin ointment was applied (Alcon, Milan, Italy). Seven days after ischemia (3 days following FG injection), the animals were euthanized, and the eyeballs enucleated and fixed for 30 min in paraformaldehyde 4%. The timing of the FG injection (after injury) and the time elapsed between the dye application and the processing of the retina were chosen based on previous studies; this experimental setting also prevents the labeling of activated microglia [22,23]. The anterior segment of the eye was removed, and the posterior eyecup additionally fixed for 1 h. Isolated retinas were divided into four quadrants (nasal, temporal, upper, and lower) and mounted on the slide using Vectashield medium (Vector Laboratories, DBA, Milan, Italy). Twenty images per retina (two from the peripheral, two from the middle, and one from the central retina for each quadrant) were acquired using a deconvolution microscope (Leica Microsystems CMS EL6000, GBH, Mannheim, Germany) at 40X magnification and subjected to cell counting by a blinded investigator. The total number of labeled cells in the ischemic eye was compared with that in the contralateral eye and expressed as a percentage of RGC loss.
Drug administration
Azithromycin (Zitromax® 500 mg, azithromycin dehydrate injectable, Pfizer Inc., Pearl River, NY) was dissolved in sterile saline (0.9% NaCl). Based on our previous observations [24], a single dose of azithromycin (150 mg/kg) or vehicle (1 ml/kg) was i.p. at the end of the ischemia. The rats were randomly assigned to azithromycin or vehicle treatment. Animals were euthanized after 1 or 6 h of reperfusion.
Statistical analysis
Data are given as mean ± standard error (SEM) of three to four independent experiments and statistically evaluated for differences with the Student t test or with one-way ANOVA (ANOVA) followed by the Tukey-Kramer test for multiple comparisons. A p value of less than 0.05 was considered statistically significant.
Results
Azithromycin prevents RGC loss induced by retinal ischemia/reperfusion
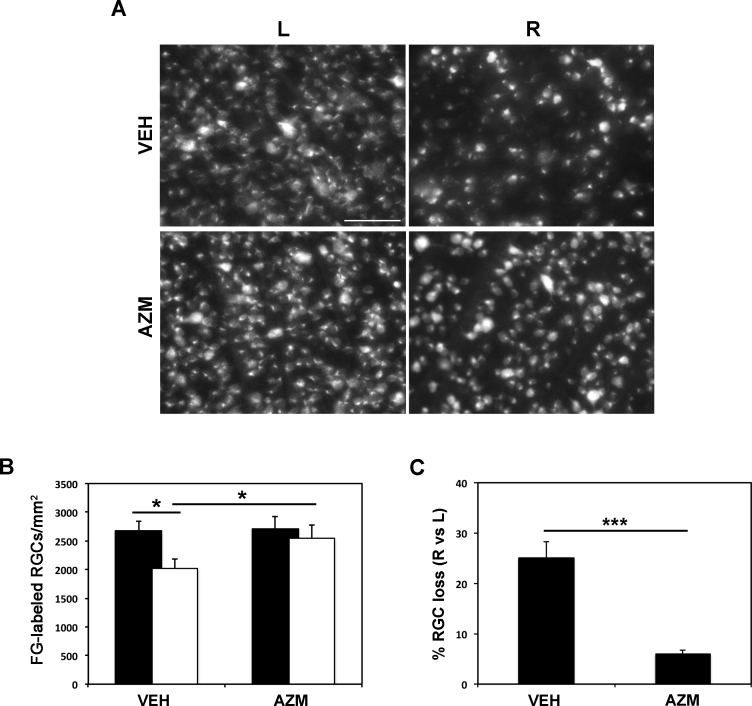
Previous studies have shown a significant reduction in RGC survival following retinal ischemia/reperfusion induced by transient increase in the IOP [15,25]. Accordingly, a significant loss of Fluoro-Gold-labeled RGCs was observed in the ischemic retina (R, right) of vehicle-treated animals, 7 days following the insult, compared to the contralateral, nonischemic retina (L, left; Figure 1A,B). To evaluate the effect of azithromycin on RGC survival, a single dose of the drug (150 mg/kg), which has been previously reported to be neuroprotective in a mouse model of transient cerebral ischemia [24], was intraperitoneally injected at the end of the ischemia. Seven days after the ischemic insult, a relevant and statistically significant reduction in RGC loss was reported in the ischemic retinas from the azithromycin-treated rats compared to the vehicle-treated rats (Figure 1A,B).
Figure 1.
Azithromycin reduces RGC death following retinal ischemia/reperfusion injury. Representative fluorescent photomicrograph of whole-mount retinas showing the effect of intraperitoneal injection of azithromycin (150 mg/kg) on retinal ganglion cell (RGC) survival following retina ischemia. Scale bar = 75 μm. A: Histogram shows the average number of surviving RGCs per retina and (B–C) the reduction of RGC death in azithromycin-treated retinas compared to vehicle. Twenty images per retina were acquired, and Fluoro-Gold (FG)-labeled cells were counted. The total number of labeled cells in the ischemic eye was compared with the contralateral, non-ischemic eye and expressed as absolute values per mm2 (B) and percentage of RGC loss (C). Results were reported as mean ± standard error of the mean (SEM; n = 4; *p<0.05; ***p<0.001, Student t test). AZM = azithromycin-treated rats; VEH = vehicle-treated rats; L = left, non-ischemic eye; R = right, ischemic/reperfused eye.
Azithromycin prevents calpain activation and reduces Bad expression following retinal ischemia/reperfusion
Excitotoxicity has been identified as a key component of RGC death triggered by ischemia/reperfusion [26]. The anoxic stimulus induces the pathological release of glutamate with overstimulation of N-methyl-D-aspartate (NMDA) receptor subtypes [25], calcium overload, and activation of calcium-dependent enzymes that contribute to neuronal damage [27]. Calpains, ubiquitously expressed calcium-dependent cysteine proteases, are involved in the execution of excitotoxic neuronal death, and their pharmacological inhibition has been associated with reduced RGC death following retina ischemia/reperfusion injury [28,29].
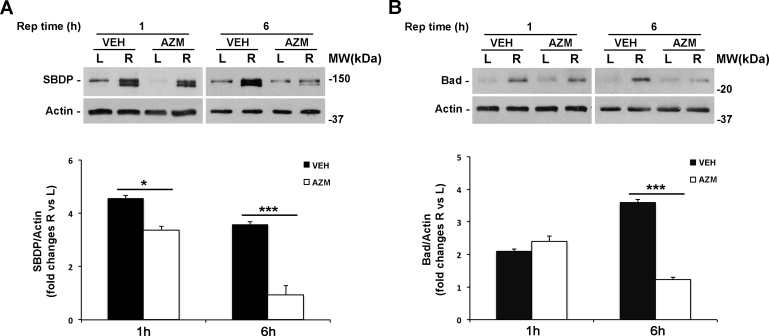
Non-erythroid α-spectrin is cleaved by calpains into two breakdown products (SBDP) of 150 and 145 kDa, while a 150 kDa and an apoptotic-specific 120 kDa fragment are generated by caspase-3 [30]. The neuroprotection provided by azithromycin is accompanied by a significant reduction of the 150/145 kDa doublet generated by calpain in the ischemic retina during the first hour of reperfusion, and this effect is maintained at 6 h of reperfusion suggesting reduced calpain activation in the treated eye (Figure 2A).
Figure 2.
Azithromycin prevents calpain activation and Bad upregulation following retinal ischemia/reperfusion. A: Immunoblotting showing the reduced accumulation of calpain-specific 150/145 kDa SBDP and (B) Bcl-2-associated death promoter (Bad) expression levels after 1 and 6 h of reperfusion in the ischemic retina of azithromycin-treated rats compared to vehicle-treated rats. Histograms show the results of densitometric analysis of the bands normalized to the value of actin and expressed as mean ± standard error of the mean (SEM; n = 4 for each group; *p<0.05, ***p<0.001; ANOVA followed by the Tukey-Kramer test for multiple comparisons). AZM = azithromycin-treated rats; VEH = vehicle-treated rats; Rep time = reperfusion time; R = right ischemic eye; L = left eye; MW = molecular weight.
Bad is a proapoptotic protein involved in the regulation of the mitochondrial apoptotic pathway [31]. Overexpression of Bad correlates with neuronal cell death and RGC apoptosis in experimental models of glaucoma [32]. We have previously reported that retinal ischemia upregulates Bad expression in the right ischemic eye compared to the control eye [26]. Here, azithromycin treatment significantly reduced Bad induction reported after 6 h of reperfusion while no effect on Bad expression was evident following 1 h of reperfusion (Figure 2B).
Azithromycin reduces MMP-9 gelatinolytic activity
MMPs are a family of zinc-dependent proteolytic enzymes known as the physiologic mediators of extracellular matrix (ECM) remodeling [33]. In addition to their physiologic role, MMPs participate in the propagation of the inflammatory response, and their activation has been implicated in the pathogenesis and progression of several neurodegenerative disorders [34].
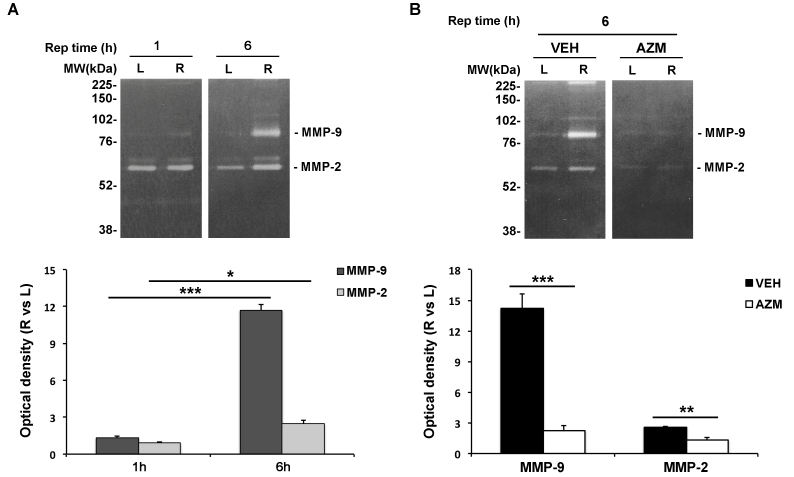
We analyzed the time-dependent profile of gelatinase (MMP-2 and MMP-9) expression and activity following retinal ischemia with gel zymography. Densitometric analysis of zymograms revealed a significant increase in active MMP-9 and MMP-2 after 6 h of reperfusion (Figure 3A) while no changes were found at an earlier time point (1 h; Figure 3A).
Figure 3.
MMP-2/-9 activation following retinal ischemia is abrogated with azithromycin treatment. A: Representative gel zymograms showing matrix metalloproteinase-2 (MMP-2)/-9 gelatinolytic activity in the ischemic (R) and contralateral (L) retina after 1 and 6 h of reperfusion and (B) reduction of MMP-2/-9 expression/activity after 6 h of reperfusion in the azithromycin-treated rats compared to the vehicle-treated rats. Active MMPs were identified on the zymogram based on their molecular weight and visualized as a white band against a saturated blue background. The histogram represents the densitometry analysis of the bands expressed as arbitrary units of optical density (mean ± standard error of the mean [SEM]; n = 4 for each group; ***p<0.001; ANOVA followed by Tukey-Kramer test for multiple comparisons). AZM = azithromycin-treated rats; VEH = vehicle-treated rats; Rep time = reperfusion time; R = right ischemic eye; L = left eye; MW = molecular weight.
In addition to antibiotic activity, azithromycin is able to modulate the inflammatory response under several experimental settings [24,35]. In an attempt to identify the contribution of the anti-inflammatory activity in azithromycin-mediated neuroprotection, we evaluated the effect of the treatment on MMP-2 and MMP-9 following retinal injury. Gel zymography revealed that systemic administration of azithromycin almost completely abrogated the upregulation of gelatinase expression and activity reported in the ischemic retinas following 6 h of reperfusion (Figure 3B).
Systemic treatment with azithromycin reduces ERK1/2 phosphorylation
Activation of ERKs following ischemic insult has been linked to retinal degeneration mechanisms, and many studies have reported that blockade of ERK1/2 activity prevents histologic damage, apoptotic cell death, and RGC loss [36,37]. Moreover, upregulation of ERK1/2 kinase is considered an early cellular marker of reactive Müller cells during retinal injury [38].
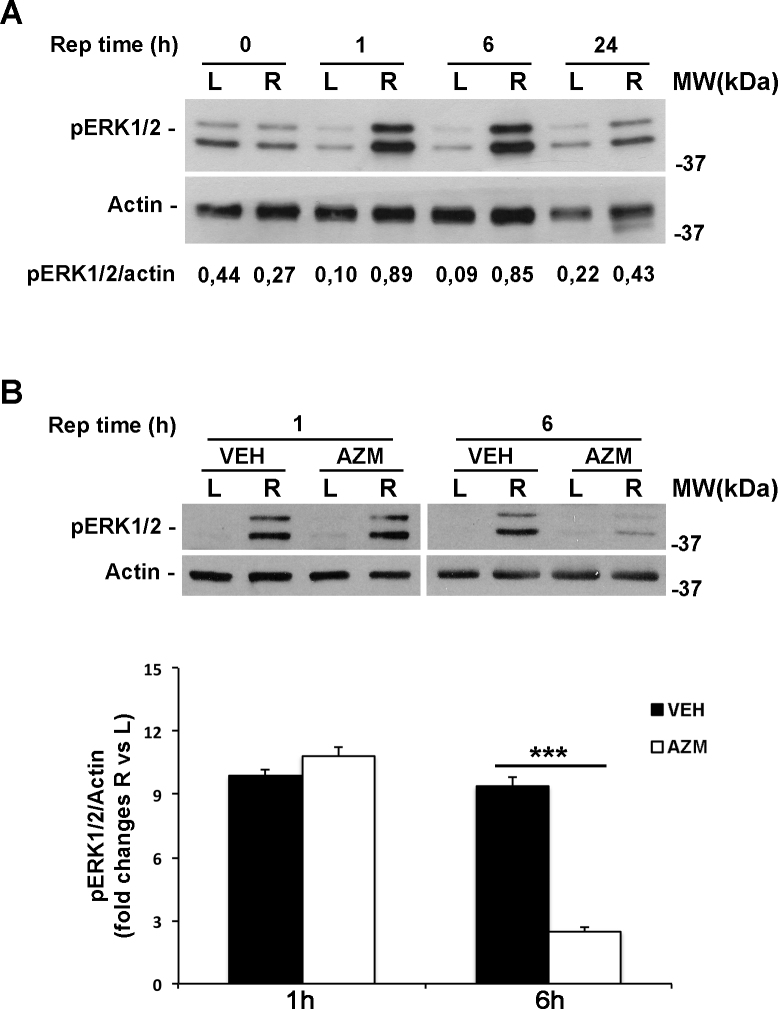
As shown in Figure 4A, phosphorylation of ERK1/2 (pERK1/2) significantly increased in the ischemic retina within 1 h of reperfusion and was maintained after 6 h; increased levels of the phosphorylated protein were still detectable after 24 h (Figure 4A). Azithromycin treatment did not affect ischemia-induced ERK activation at 1 h of reperfusion, while at 6 h, azithromycin treatment significantly reduced the pERK1/2 level in the ischemic retina compared to the contralateral and vehicle-treated eyes (Figure 4B).
Figure 4.
Erk1/2 activation induced by retinal ischemia is reduced by azithromycin administration. A: Representative immunoblot showing the expression of phospho-p44/p42 MAPK (pERK1/2) at 1 or 6 h of reperfusion. B: Systemic treatment with azithromycin significantly reduced ERK1/2 phosphorylation after 6 h of reperfusion in the ischemic eye (R) of azithromycin-treated rats compared to the vehicle-injected animals. Histograms show the results of densitometric analysis of the bands, normalized to the value of actin, and expressed as mean ± standard error of the mean (SEM; n = 4 for each group; **p<0.01, ***p<0.001; ANOVA followed by Tukey-Kramer test for multiple comparisons). AZM = azithromycin-treated rats; VEH = vehicle-treated rats; Rep time = reperfusion time; R = right ischemic eye; L = left eye; MW = molecular weight.
Discussion
The present study represents the first evidence of the neuroprotective effect of azithromycin in the retina showing that following ischemic insult induced by transient increase in IOP, acute administration of the macrolide, given systemically post-injury, prevents RGC death at 7 days after injury. The neuroprotective effect is associated with downregulation of gene products related to necrotic and apoptotic cell death, both occurring in the experimental model used in the study [39,40]. The downregulation of MMP-9/-2 activity, together with a significant reduction in ERK phosphorylation, suggests that the important reduction of neuronal loss might be, at least in part, mediated by anti-inflammatory mechanisms activated by azithromycin, a second-generation macrolide antibiotic. The latter is used for the treatment of a wide array of infectious diseases [41]. Compared to other macrolides, azithromycin is characterized by high stability in acidic pH, a longer half-life, and excellent tissue distribution [42]. In addition to antibacterial activity, azithromycin exerts anti-inflammatory and immunomodulatory effects in vitro and in vivo, which are independent from its antibacterial effects [11]. Moreover, its neuroprotective potential has been recently proven in a mouse model of transient middle cerebral artery occlusion [24,43]. The observed neuroprotection was ascribed to reduced brain infiltration of inflammatory myeloid cells and to the shift of macrophage polarization toward the M2 phenotype, peripherally and in the ischemic area [24].
The retinal neuroprotection afforded by azithromycin in our model is associated with reduced calpain activation that is evident after 1 h from the end of the ischemic insult; this effect is consolidated at a later time point (6 h) when the reduction of calpain activity, compared to untreated rats, is even more marked. Calpains are calcium-dependent enzymes activated by the overload of calcium through glutamate receptors under excitotoxic conditions [44]. The proteolysis mediated by activated calpains has been associated with the early necrotic phase of RGC death triggered by ischemia in the retina [45]. The causative role of calpain in retina neurodegeneration is supported by several studies; calpain inhibitors reduced ganglion cell loss and preserved retinal function in several models of retinal injury, including optic nerve crush [46], NMDA-intravitreal injection [47], elevated intraocular pressure, and transient retinal ischemia [29]. Based on these considerations, the effect on calpain activation reported following treatment with azithromycin in the retina represents biochemical proof that supports the neuroprotective effect observed on RGC survival. This is further corroborated by the outcome of the pharmacological treatment on the expression of Bad, a proapoptotic member of the Bcl-2 family [48]. In the retinas of rats treated with azithromycin, Bad induction was significantly reduced compared to the untreated rats.
The timing of calpain activation and Bad upregulation, both occurring within the first 6 h of reperfusion, might suggest that azithromycin exerts its neuroprotective effects by modulating molecular events that take place early after the end of the insult and is responsible for ischemia-induced RGC death. Although the prevention of calpain activation and Bad upregulation are important indicators of the effective neuroprotection mediated by azithromycin in the retina, the modulation of these proteins is probably the consequence more than the cause of a limited damage to the neuronal tissue.
In mice subjected to ischemic stroke, azithromycin reduced brain injury by minimizing blood–brain barrier (BBB) leakage that, in turn, reduced infiltration of inflammatory myeloid cells in the ischemic hemisphere [24]. BBB permeability and function during cerebral ischemia/reperfusion are closely dependent on MMP activity, given their ability to degrade the ECM and tight junction components [49]. Following stroke, the biphasic opening of the BBB correlates with altered expression and activity of gelatinases, MMP-2 and MMP-9 [50]. In particular, increased levels of MMP-2 have been linked to the early and reversible opening of the BBB [51] while elevated MMP-9 expression in the late phase of ischemic stroke was associated with the complete breakdown of the BBB leading to increased infarct volume, edema, and reduced neurologic outcomes in experimental animal models and in patients [52,53].
Similarly, in the retina subjected to ischemic insult overactivation of MMP-9 by resident and inflammatory cells has been associated with disruption of the blood–retinal barrier, proteolytic degradation of the extracellular matrix and alteration of β1-integrin survival signaling leading to detachment-induced RGC death [54]. In our experimental setting, azithromycin almost completely abrogated the MMP-9/-2 activation observed during the reperfusion phase. This result suggests a remarkable anti-inflammatory activity in the retina that is further supported by the observed reduction, following treatment with the macrolide, of ERK1/2 phosphorylation. ERK activation, as well as upregulation of the glial fibrillary acid protein (GFAP), has been indicated as a typical marker of activated Müller cells in the retina [38,55,56].
Upregulation of phospho-ERK has been previously reported in injured retinas, and more importantly, inhibition of ERK activation has been shown to prevent RGC degeneration induced by ischemic damage or axotomy [36,57-60]. This suggests that the reduced level of pERK observed following azithromycin treatment might play a role in the observed neuroprotective effect.
Following azithromycin treatment, inhibition of gelatinase activity reported in the ischemic retina might be instrumental to the RGC neuroprotection afforded by the macrolide. This hypothesis is supported by several reports showing the participation of MMPs in retinal damage following ischemic injury. MMP-9 deficiency and treatment with MMP inhibitors or drugs that are able to reduce MMP activity (i.e., minocycline) lead to retinal neuroprotection [61-64]. Furthermore, the detrimental role of this protease was recently confirmed in patients with glaucoma in whom altered expression of MMP-9 correlated with decreased retinal fiber layer thickness contributing to optic nerve head damage and RGC apoptosis [65].
The anti-inflammatory potential of azithromycin has been documented in animal models of spinal cord injury [66], lung ischemia/reperfusion injury [67], and patients following lung transplantation [68] or affected by cystic fibrosis [69]. Although the use of azithromycin in the eye is currently limited to ocular infections [10], there is evidence showing its anti-inflammatory activity in tissues of the anterior segment of the eye. Azithromycin suppressed zymosan-stimulated production of inflammatory cytokines (interleukin [IL]-1β, tumor necrosis factor alpha [TNF-α]), chemokines (IL-6, RANTES), and MMP activity (MMP-1, -3, and -9) in human corneal epithelial cell cultures (HCECs) [70]. Treatment with azithromycin reduced production of the adhesion molecule ICAM-1 and inhibited leukocytic infiltration in a mouse model of corneal inflammation [71] and following corneal transplantation in the rat, preventing rejection and prolonging graft survival [72].
Topical administration of the macrolide reduced expression of nuclear factor-kappa beta (NF-kB) and macrophage infiltration in conjunctival tissue following lipopolysaccharide (LPS)-induced conjunctivitis in the rat [35]. Furthermore, in patients with diagnosed blepharitis compared to healthy subjects, azithromycin inhibited the expression of proinflammatory mediators (IL-1β, IL-8, and MMP-9) and increased transforming growth factor beta 1 (TGF-β1) levels [73].
To the best of our knowledge, the experimental observations reported in the present study are the first evidence of the anti-inflammatory effect of a systemic treatment with azithromycin in the retina. Although the data reported in our study are not enough to clearly speculate on the mechanisms linking the observed molecular events with the reported neuroprotection, these findings suggest that the neuroprotection could be mediated by a reduced inflammatory state in the retina following ischemia/reperfusion injury.
Further experiments are needed to demonstrate that the neuroprotection observed with azithromycin after 7 days of reperfusion is extended for a longer period after injury, and it is associated with preservation of the visual function. Nevertheless, our experimental observation, together with the reported safety profile of azithromycin and the documented ability to achieve high tissue concentration following oral administration, suggests that the drug is a promising candidate for treating ocular conditions associated with RGC degeneration.
Acknowledgments
We thank Mr. Guido Fico for skillful technical support. Funding: this work was partially supported by University of Calabria (ex 60%); and by G.B. Bietti Foundation (Italian Ministry of Health and Fondazione Roma).
References
- 1.Zheng L, Gong B, Hatala DA, Kern TS. Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetes. Invest Ophthalmol Vis Sci. 2007;48:361–7. doi: 10.1167/iovs.06-0510. [DOI] [PubMed] [Google Scholar]
- 2.Lafuente MP, Villegas-Perez MP, Selles-Navarro I, Mayor-Torroglosa S, Miralles de Imperial J, Vidal-Sanz M. Retinal ganglion cell death after acute retinal ischemia is an ongoing process whose severity and duration depends on the duration of the insult. Neuroscience. 2002;109:157–68. doi: 10.1016/s0306-4522(01)00458-4. [DOI] [PubMed] [Google Scholar]
- 3.Kaur C, Foulds WS, Ling EA. Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol. 2008;2:879–89. doi: 10.2147/opth.s3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–57. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 5.Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119:979–86. doi: 10.1016/j.ophtha.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143:225–45. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990;25:73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 8.Slater M, Torr E, Harrison T, Forrester D, Knox A, Shaw D, Sayers I. The differential effects of azithromycin on the airway epithelium in vitro and in vivo. Physiol Rep. 2016;4 doi: 10.14814/phy2.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong FY, Tabrizi SN, Fairley CK, Vodstrcil LA, Huston WM, Chen M, Bradshaw C, Hocking JS. The efficacy of azithromycin and doxycycline for the treatment of rectal chlamydia infection: a systematic review and meta-analysis. J Antimicrob Chemother. 2015;70:1290–7. doi: 10.1093/jac/dku574. [DOI] [PubMed] [Google Scholar]
- 10.Luchs J. Azithromycin in DuraSite for the treatment of blepharitis. Clin Ophthalmol. 2010;4:681–8. doi: 10.2147/opth.s6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol. 2012;68:479–503. doi: 10.1007/s00228-011-1161-x. [DOI] [PubMed] [Google Scholar]
- 12.Russo R, Varano GP, Adornetto A, Nucci C, Corasaniti MT, Bagetta G, Morrone LA. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur J Pharmacol. 2016;787:134–42. doi: 10.1016/j.ejphar.2016.03.064. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y, Chen H, Su SB. Neuroinflammatory responses in diabetic retinopathy. J Neuroinflammation. 2015;12:141. doi: 10.1186/s12974-015-0368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchi ER, Suivaizdis I, Fu J. Pressure-induced retinal ischemia in rats: an experimental model for quantitative study. Ophthalmologica. 1991;203:138–47. doi: 10.1159/000310240. [DOI] [PubMed] [Google Scholar]
- 15.Russo R, Adornetto A, Cavaliere F, Varano GP, Rusciano D, Morrone LA, Corasaniti MT, Bagetta G, Nucci C. Intravitreal injection of forskolin, homotaurine, and L-carnosine affords neuroprotection to retinal ganglion cells following retinal ischemic injury. Mol Vis. 2015;21:718–29. [PMC free article] [PubMed] [Google Scholar]
- 16.Rovere G, Nadal-Nicolas FM, Wang J, Bernal-Garro JM, Garcia-Carrillo N, Villegas-Perez MP, Agudo-Barriuso M, Vidal-Sanz M. Melanopsin-Containing or Non-Melanopsin-Containing Retinal Ganglion Cells Response to Acute Ocular Hypertension With or Without Brain-Derived Neurotrophic Factor Neuroprotection. Invest Ophthalmol Vis Sci. 2016;57:6652–61. doi: 10.1167/iovs.16-20146. [DOI] [PubMed] [Google Scholar]
- 17.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–90. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 18.Russo R, Siviglia E, Gliozzi M, Amantea D, Paoletti A, Berliocchi L, Bagetta G, Corasaniti MT. Evidence implicating matrix metalloproteinases in the mechanism underlying accumulation of IL-1beta and neuronal apoptosis in the neocortex of HIV/gp120-exposed rats. Int Rev Neurobiol. 2007;82:407–21. doi: 10.1016/S0074-7742(07)82023-X. [DOI] [PubMed] [Google Scholar]
- 19.Selles-Navarro I, Villegas-Perez MP, Salvador-Silva M, Ruiz-Gomez JM, Vidal-Sanz M. Retinal ganglion cell death after different transient periods of pressure-induced ischemia and survival intervals. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:2002–14. [PubMed] [Google Scholar]
- 20.Nadal-Nicolas FM, Salinas-Navarro M, Jimenez-Lopez M, Sobrado-Calvo P, Villegas-Perez MP, Vidal-Sanz M, Agudo-Barriuso M. Displaced retinal ganglion cells in albino and pigmented rats. Front Neuroanat. 2014;8:99. doi: 10.3389/fnana.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadal-Nicolas FM, Salinas-Navarro M, Vidal-Sanz M, Agudo-Barriuso M. Two methods to trace retinal ganglion cells with fluorogold: from the intact optic nerve or by stereotactic injection into the optic tract. Exp Eye Res. 2015;131:12–9. doi: 10.1016/j.exer.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Galindo-Romero C, Valiente-Soriano FJ, Jimenez-Lopez M, Garcia-Ayuso D, Villegas-Perez MP, Vidal-Sanz M, Agudo-Barriuso M. Effect of brain-derived neurotrophic factor on mouse axotomized retinal ganglion cells and phagocytic microglia. Invest Ophthalmol Vis Sci. 2013;54:974–85. doi: 10.1167/iovs.12-11207. [DOI] [PubMed] [Google Scholar]
- 23.Lafuente Lopez-Herrera MP, Mayor-Torroglosa S, Miralles de Imperial J, Villegas-Perez MP, Vidal-Sanz M. Transient ischemia of the retina results in altered retrograde axoplasmic transport: neuroprotection with brimonidine. Exp Neurol. 2002;178:243–58. doi: 10.1006/exnr.2002.8043. [DOI] [PubMed] [Google Scholar]
- 24.Amantea D, Certo M, Petrelli F, Tassorelli C, Micieli G, Corasaniti MT, Puccetti P, Fallarino F, Bagetta G. Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype. Exp Neurol. 2016;275:116–25. doi: 10.1016/j.expneurol.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Nucci C, Tartaglione R, Rombola L, Morrone LA, Fazzi E, Bagetta G. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)-induced retinal ganglion cell death in rat. Neurotoxicology. 2005;26:935–41. doi: 10.1016/j.neuro.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Russo R, Cavaliere F, Berliocchi L, Nucci C, Gliozzi M, Mazzei C, Tassorelli C, Corasaniti MT, Rotiroti D, Bagetta G, Morrone LA. Modulation of pro-survival and death-associated pathways under retinal ischemia/reperfusion: effects of NMDA receptor blockade. J Neurochem. 2008;107:1347–57. doi: 10.1111/j.1471-4159.2008.05694.x. [DOI] [PubMed] [Google Scholar]
- 27.Russo R, Rotiroti D, Tassorelli C, Nucci C, Bagetta G, Bucci MG, Corasaniti MT, Morrone LA. Identification of novel pharmacological targets to minimize excitotoxic retinal damage. Int Rev Neurobiol. 2009;85:407–23. doi: 10.1016/S0074-7742(09)85028-9. [DOI] [PubMed] [Google Scholar]
- 28.Ono Y, Sorimachi H. Calpains: an elaborate proteolytic system. Biochim Biophys Acta. 2012;1824:224–36. doi: 10.1016/j.bbapap.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki R, Oka T, Tamada Y, Shearer TR, Azuma M. Degeneration and dysfunction of retinal neurons in acute ocular hypertensive rats: involvement of calpains. J Ocul Pharmacol Ther. 2014;30:419–28. doi: 10.1089/jop.2013.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:59. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- 31.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 32.Levkovitch-Verbin H, Dardik R, Vander S, Melamed S. Mechanism of retinal ganglion cells death in secondary degeneration of the optic nerve. Exp Eye Res. 2010;91:127–34. doi: 10.1016/j.exer.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–64. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005;46:175–82. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Robredo P, Recalde S, Moreno-Orduna M, Garcia-Garcia L, Zarranz-Ventura J, Garcia-Layana A. Azithromycin reduces inflammation in a rat model of acute conjunctivitis. Mol Vis. 2013;19:153–65. [PMC free article] [PubMed] [Google Scholar]
- 36.Roth S, Shaikh AR, Hennelly MM, Li Q, Bindokas V, Graham CE. Mitogen-activated protein kinases and retinal ischemia. Invest Ophthalmol Vis Sci. 2003;44:5383–95. doi: 10.1167/iovs.03-0451. [DOI] [PubMed] [Google Scholar]
- 37.Yokota H, Narayanan SP, Zhang W, Liu H, Rojas M, Xu Z, Lemtalsi T, Nagaoka T, Yoshida A, Brooks SE, Caldwell RW, Caldwell RB. Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Invest Ophthalmol Vis Sci. 2011;52:8123–31. doi: 10.1167/iovs.11-8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Joo CK, Choi JS, Ko HW, Park KY, Sohn S, Chun MH, Oh YJ, Gwag BJ. Necrosis and apoptosis after retinal ischemia: involvement of NMDA-mediated excitotoxicity and p53. Invest Ophthalmol Vis Sci. 1999;40:713–20. [PubMed] [Google Scholar]
- 40.Fujita R, Ueda M, Fujiwara K, Ueda H. Prothymosin-alpha plays a defensive role in retinal ischemia through necrosis and apoptosis inhibition. Cell Death Differ. 2009;16:349–58. doi: 10.1038/cdd.2008.159. [DOI] [PubMed] [Google Scholar]
- 41.Champney WS, Burdine R. Azithromycin and clarithromycin inhibition of 50S ribosomal subunit formation in Staphylococcus aureus cells. Curr Microbiol. 1998;36:119–23. doi: 10.1007/s002849900290. [DOI] [PubMed] [Google Scholar]
- 42.Girard AE, Girard D, English AR, Gootz TD, Cimochowski CR, Faiella JA, Haskell SL, Retsema JA. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987;31:1948–54. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrelli F, Muzzi M, Chiarugi A, Bagetta G, Amantea D. Poly(ADP-ribose) polymerase is not involved in the neuroprotection exerted by azithromycin against ischemic stroke in mice. Eur J Pharmacol. 2016;791:518–22. doi: 10.1016/j.ejphar.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 44.Chiu K, Lam TT, Ying Li WW, Caprioli J, Kwong Kwong JM. Calpain and N-methyl-d-aspartate (NMDA)-induced excitotoxicity in rat retinas. Brain Res. 2005;1046:207–15. doi: 10.1016/j.brainres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Pignol B, Auvin S, Carre D, Marin JG, Chabrier PE. Calpain inhibitors and antioxidants act synergistically to prevent cell necrosis: effects of the novel dual inhibitors (cysteine protease inhibitor and antioxidant) BN 82204 and its pro-drug BN 82270. J Neurochem. 2006;98:1217–28. doi: 10.1111/j.1471-4159.2006.03952.x. [DOI] [PubMed] [Google Scholar]
- 46.Ryu M, Yasuda M, Shi D, Shanab AY, Watanabe R, Himori N, Omodaka K, Yokoyama Y, Takano J, Saido T, Nakazawa T. Critical role of calpain in axonal damage-induced retinal ganglion cell death. J Neurosci Res. 2012;90:802–15. doi: 10.1002/jnr.22800. [DOI] [PubMed] [Google Scholar]
- 47.Shimazawa M, Suemori S, Inokuchi Y, Matsunaga N, Nakajima Y, Oka T, Yamamoto T, Hara H. A novel calpain inhibitor, ((1S)-1-((((1S)-1-Benzyl-3-cyclopropylamino-2,3-di-oxopropyl)amino)carbonyl)-3-me thylbutyl)carbamic acid 5-methoxy-3-oxapentyl ester (SNJ-1945), reduces murine retinal cell death in vitro and in vivo. J Pharmacol Exp Ther. 2010;332:380–7. doi: 10.1124/jpet.109.156612. [DOI] [PubMed] [Google Scholar]
- 48.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 49.McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–62. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucivero V, Prontera M, Mezzapesa DM, Petruzzellis M, Sancilio M, Tinelli A, Di Noia D, Ruggieri M, Federico F. Different roles of matrix metalloproteinases-2 and −9 after human ischaemic stroke. Neurol Sci. 2007;28:165–70. doi: 10.1007/s10072-007-0814-0. [DOI] [PubMed] [Google Scholar]
- 51.Chang DI, Hosomi N, Lucero J, Heo JH, Abumiya T, Mazar AP, del Zoppo GJ. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:1408–19. doi: 10.1097/01.WCB.0000091765.61714.30. [DOI] [PubMed] [Google Scholar]
- 52.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–7. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suofu Y, Clark JF, Broderick JP, Kurosawa Y, Wagner KR, Lu A. Matrix metalloproteinase-2 or −9 deletions protect against hemorrhagic transformation during early stage of cerebral ischemia and reperfusion. Neuroscience. 2012;212:180–9. doi: 10.1016/j.neuroscience.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos AR, Corredor RG, Obeso BA, Trakhtenberg EF, Wang Y, Ponmattam J, Dvoriantchikova G, Ivanov D, Shestopalov VI, Goldberg JL, Fini ME, Bajenaru ML. beta1 integrin-focal adhesion kinase (FAK) signaling modulates retinal ganglion cell (RGC) survival. PLoS One. 2012;7:e48332. doi: 10.1371/journal.pone.0048332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tezel G, Chauhan BC, LeBlanc RP, Wax MB. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3025–33. doi: 10.1167/iovs.02-1136. [DOI] [PubMed] [Google Scholar]
- 56.Akiyama H, Nakazawa T, Shimura M, Tomita H, Tamai M. Presence of mitogen-activated protein kinase in retinal Muller cells and its neuroprotective effect ischemia-reperfusion injury. Neuroreport. 2002;13:2103–7. doi: 10.1097/00001756-200211150-00022. [DOI] [PubMed] [Google Scholar]
- 57.Luo JM, Cen LP, Zhang XM, Chiang SW, Huang Y, Lin D, Fan YM, van Rooijen N, Lam DS, Pang CP, Cui Q. PI3K/akt, JAK/STAT and MEK/ERK pathway inhibition protects retinal ganglion cells via different mechanisms after optic nerve injury. Eur J Neurosci. 2007;26:828–42. doi: 10.1111/j.1460-9568.2007.05718.x. [DOI] [PubMed] [Google Scholar]
- 58.Ishizuka F, Shimazawa M, Umigai N, Ogishima H, Nakamura S, Tsuruma K, Hara H. Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. Eur J Pharmacol. 2013;703:1–10. doi: 10.1016/j.ejphar.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Gao S, Andreeva K, Cooper NG. Ischemia-reperfusion injury of the retina is linked to necroptosis via the ERK1/2–RIP3 pathway. Mol Vis. 2014;20:1374–87. [PMC free article] [PubMed] [Google Scholar]
- 60.Galan A, Dergham P, Escoll P, de-la-Hera A, D’Onofrio PM, Magharious MM, Koeberle PD, Frade JM, Saragovi HU. Neuronal injury external to the retina rapidly activates retinal glia, followed by elevation of markers for cell cycle re-entry and death in retinal ganglion cells. PLoS One. 2014;9:e101349. doi: 10.1371/journal.pone.0101349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chintala SK, Zhang X, Austin JS, Fini ME. Deficiency in matrix metalloproteinase gelatinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J Biol Chem. 2002;277:47461–8. doi: 10.1074/jbc.M204824200. [DOI] [PubMed] [Google Scholar]
- 62.Manabe S, Gu Z, Lipton SA. Activation of matrix metalloproteinase-9 via neuronal nitric oxide synthase contributes to NMDA-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2005;46:4747–53. doi: 10.1167/iovs.05-0128. [DOI] [PubMed] [Google Scholar]
- 63.Chen YD, Xu X, Xia X, Wu H, Liu K, Zheng Z, Zhu D. MMP9 is involved in glycation end-products induced increase of retinal vascular permeability in rats and the therapeutic effect of minocycline. Curr Eye Res. 2008;33:977–83. doi: 10.1080/02713680802450984. [DOI] [PubMed] [Google Scholar]
- 64.Mathalone N, Lahat N, Rahat MA, Bahar-Shany K, Oron Y, Geyer O. The involvement of matrix metalloproteinases 2 and 9 in rat retinal ischemia. Graefes Arch Clin Exp Ophthalmol. 2007;245:725–32. doi: 10.1007/s00417-006-0362-y. [DOI] [PubMed] [Google Scholar]
- 65.Markiewicz L, Pytel D, Mucha B, Szymanek K, Szaflik J, Szaflik JP, Majsterek I. Altered Expression Levels of MMP1, MMP9, MMP12, TIMP1, and IL-1beta as a Risk Factor for the Elevated IOP and Optic Nerve Head Damage in the Primary Open-Angle Glaucoma Patients. BioMed Res Int. 2015;2015:812503. doi: 10.1155/2015/812503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang B, Bailey WM, Kopper TJ, Orr MB, Feola DJ, Gensel JC. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflammation. 2015;12:218. doi: 10.1186/s12974-015-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geudens N, Timmermans L, Vanhooren H, Vanaudenaerde BM, Vos R, Van De Wauwer C, Verleden GM, Verbeken E, Lerut T, Van Raemdonck DE. Azithromycin reduces airway inflammation in a murine model of lung ischaemia reperfusion injury. Transpl Int. 2008;21:688–95. doi: 10.1111/j.1432-2277.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 68.Verleden SE, Vandooren J, Vos R, Willems S, Dupont LJ, Verleden GM, Van Raemdonck DE, Opdenakker G, Vanaudenaerde BM. Azithromycin decreases MMP-9 expression in the airways of lung transplant recipients. Transpl Immunol. 2011;25:159–62. doi: 10.1016/j.trim.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 69.Cory TJ, Birket SE, Murphy BS, Hayes D, Jr, Anstead MI, Kanga JF, Kuhn RJ, Bush HM, Feola DJ. Impact of azithromycin treatment on macrophage gene expression in subjects with cystic fibrosis. J Cyst Fibros. 2014;13:164–71. doi: 10.1016/j.jcf.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li DQ, Zhou N, Zhang L, Ma P, Pflugfelder SC. Suppressive effects of azithromycin on zymosan-induced production of proinflammatory mediators by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:5623–9. doi: 10.1167/iovs.09-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sadrai Z, Hajrasouliha AR, Chauhan S, Saban DR, Dastjerdi MH, Dana R. Effect of topical azithromycin on corneal innate immune responses. Invest Ophthalmol Vis Sci. 2011;52:2525–31. doi: 10.1167/iovs.10-5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wacker K, Denker S, Hildebrand A, Eberwein P, Reinhard T, Schwartzkopff J. Short-term azithromycin treatment promotes cornea allograft survival in the rat. PLoS One. 2013;8:e82687. doi: 10.1371/journal.pone.0082687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang L, Su Z, Zhang Z, Lin J, Li DQ, Pflugfelder SC. Effects of azithromycin on gene expression profiles of proinflammatory and anti-inflammatory mediators in the eyelid margin and conjunctiva of patients with meibomian gland disease. JAMA Ophthalmol. 2015;133:1117–23. doi: 10.1001/jamaophthalmol.2015.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]