Abstract
Background
The present study optimized ultrasound-assisted extraction conditions to maximize extraction yields of glycyrrhizic acid from licorice.
Methods
The optimal extraction temperature (X1), extraction time (X2), and methanol concentration (X3) were identified using response surface methodology (RSM). A central composite design (CCD) was used for experimental design and analysis of the results to obtain the optimal processing parameters.
Results
Statistical analyses revealed that three variables and the quadratic of X1, X2, and X3 had significant effects on the yields and were followed by significant interaction effects between the variables of X2 and X3 (p < 0.01). A 3D response surface plot and contour plots derived from the mathematical models were applied to determine the optimal conditions. The optimum ultrasound-assisted extraction conditions were as follows: extraction temperature, 69 °C; extraction time, 34 min; and methanol concentration, 57%. Under these conditions, the experimental yield of glycyrrhizic acid was 3.414%, which agreed closely with the predicted value (3.406%).
Conclusion
The experimental values agreed with those predicted by RSM models, thus indicating the suitability of the model employed and the success of RSM in optimizing the extraction conditions.
Keywords: licorice, glycyrrhizic acid, ultrasound-assisted extraction, optimization, response surface methodology
1. Introduction
Licorice is a well-known Chinese herb, which has been used in food and medicinal remedies for thousands of years.1 Licorice has been the most important ingredient of Chinese traditional medicine since ancient times, and is prescribed in 60% of all cases.2 It beneficial due to its antioxidant, antidotal, anti-allergic, anti-inflammatory, antiulcer, antiviral, gastroprotective, and immunomodulatory properties.3, 4
Phytochemical studies have revealed that licorice contains two major types of secondary metabolites: triterpene saponins and flavonoids.5 Triterpenes and flavonoids contain glycyrrhizic acid and glycyrrhetinic acid of triterpene, liquiritinapioside, liquiritin, isoliquiritinapioside and isoliquiritin of flavonoids and so on.6 Glycyrrhizic acid (GA) is the principal saponin in licorice and is an indicator of licorice quality.5, 7 GA is widely used as a major therapeutic agent to treat chronic viral hepatitis and allergic dermatitis.8 It is also known to have anti-inflammatory, anti-carcinogenic, anti-allergic, anti-arthritic, anti-asthmatic, antibacterial, analgesic amphiestrogenic, and hepato-protective properties.9, 10
In recent years, the ultrasound-assisted extraction method has been used to effectively extract chemical constituents from plant materials.11, 12 Ultrasound enhances the extraction efficiency of organic compounds possibly through cavitation, which occurs in the solvent by the passage of ultrasonic waves.13 This facilitates better penetration of the solvent into the sample, increasing the release of compounds from the matrix into the solvent.14 Optimization of the experimental conditions is a critical step in developing a successful ultrasound-assisted extraction process since several process variables, such as, ultrasound power, process temperature, and sonication time affect the extraction efficiency.15
Response surface methodology (RSM) is a collection of statistical and mathematical techniques used for developing, improving, and optimizing processes in which a response of interest is influenced by several variables, with the eventual objective of optimizing this response.16 RSM has a major advantage over the one-factor-at-a time approach in that it allows for the evaluation of the effect of multiple variables and their interactions on the output variables with a lesser number of trials.17 The optimization of the extraction process using RSM would not only serve as a visual aid to have a clearer picture about the effects of various factors on extraction but also help us to locate the region where the extraction is optimized.18 In order to extract the bioactive compounds of the natural products, the RSM has been widely used in many studies.19, 20, 21, 22, 23 For modeling, we used the central composite design (CCD) method for experimental design, and the results were fitted with a polynomial equation in the vicinity of the optimal condition.24
Therefore, the aim of the present study was to optimize variables of the ultrasound-assisted extraction method such as temperature, time, and concentration of methanol using response surface methodology, by employing central composite design to maximize the extraction of GA from licorice.
2. Methods
2.1. Plant material and reagents
The licorice used in this study was grown in China and purchased from an Oriental pharmacy in Geumsan, South Korea. It was authenticated by Dr. Goya Choi at the Korea Institute of Oriental Medicine (KIOM). Prior to extraction, the sample was pulverized using a disintegrator and then passed through a 600 μm sieve. A voucher specimen was deposited in the herbarium of the Herbal Medicine Resources Group at KIOM. The high performance liquid chromatography grade acetonitrile, methanol, ethanol, and distilled water were obtained from Burdick & Jackson (Muskegon, MI, USA). The analytical grade acetic acid was obtained from J. T. Baker Inc. (Phillipsburg, NJ, USA). The glycyrrhizic acid (GA) standard was purchased from Wako Pure Chemical Industries (Osaka, Japan).
2.2. Sample preparation
Samples (0.5 g) were placed into an extraction vessel with 12 mL of the extraction solvent and sonicated (ultrasonic cleaner 8510; 250 W, 44 kHz; Branson Co., Danbury, CT, USA)3 for various experimental durations and temperatures. The extracted liquid fraction and the residue were then collected separately, and the residue was re-extracted. After extraction, the sample volumes were made up to 25 mL in volumetric flasks and filtered through a 0.2 μm membrane filter prior to HPLC analysis.
2.3. HPLC analysis
The HPLC system consisted of a Waters e2695 liquid chromatography system (Waters, USA), equipped with a Waters 2998 photodiode array detector. Data processing was carried out with the Empower software (Waters, USA). An XBridge C18 column (4.6 mm × 250 mm, 5.0 μm, Waters, USA) was employed. The mobile phase was 7% acetic acid and acetonitrile (60:40, v/v) with a flow-rate of 0.8 mL/min. The injection volume for all the samples was 20 μL. A wavelength of 254 nm was used for the detection of glycyrrhizic acid (GA). The GA peak was identified by comparing its retention time with those of standards, and the concentration was calculated from the calibration curves. Results are expressed as the mean values of assays for each experiment, which were performed in triplicate.
2.4. Experimental design
To further study the interaction between the factors, we optimized the operating conditions by RSM and used the central composite design (CCD) method. The range and center point values of three independent variables presented in Table 1 were based on the results of preliminary experiments. This generated 20 treatments with six replications at the central points to estimate the repeatability of the method (Table 2). Extraction temperature (X1), extraction time (X2), and methanol concentration (X3) were chosen as the independent variables. This design was applied to investigate the optimal working conditions for the extraction of GA from licorice.
Table 1.
Independent variables and codified values employed for optimization of the extraction procedure.
| Independent variables | Code units | Coded levels |
||||
|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | ||
| Temperature (°C) | X1 | 43 | 50 | 60 | 70 | 77 |
| Time (min) | X2 | 28 | 35 | 45 | 55 | 62 |
| Methanol concentration (%) | X3 | 53 | 60 | 70 | 80 | 87 |
Table 2.
Central composite design of three variables with their observed responses.
| No. | X1 (°C) | X2 (min) | X3 (%) | Glycyrrhizic acid (%) |
|---|---|---|---|---|
| 1 | 50 (−1) | 35 (−1) | 60 (−1) | 3.307 |
| 2 | 70 (1) | 35 (−1) | 60 (−1) | 3.414 |
| 3 | 50 (−1) | 55 (1) | 60 (−1) | 3.183 |
| 4 | 70 (1) | 55 (1) | 60 (−1) | 3.272 |
| 5 | 50 (−1) | 35 (−1) | 80 (1) | 3.160 |
| 6 | 70 (1) | 35 (−1) | 80 (1) | 3.266 |
| 7 | 50 (−1) | 55 (1) | 80 (1) | 3.199 |
| 8 | 70 (1) | 55 (1) | 80 (1) | 3.332 |
| 9 | 43 (−1.68) | 45 (0) | 70 (0) | 3.208 |
| 10 | 77 (1.68) | 45 (0) | 70 (0) | 3.337 |
| 11 | 60 (0) | 28 (−1.68) | 70 (0) | 3.275 |
| 12 | 60 (0) | 62 (1.68) | 70 (0) | 3.260 |
| 13 | 60 (0) | 45 (0) | 53 (−1.68) | 3.332 |
| 14 | 60 (0) | 45 (0) | 87 (1.68) | 3.208 |
| 15 | 60 (0) | 45 (0) | 70 (0) | 3.371 |
| 16 | 60 (0) | 45 (0) | 70 (0) | 3.356 |
| 17 | 60 (0) | 45 (0) | 70 (0) | 3.368 |
| 18 | 60 (0) | 45 (0) | 70 (0) | 3.343 |
| 19 | 60 (0) | 45 (0) | 70 (0) | 3.332 |
| 20 | 60 (0) | 45 (0) | 70 (0) | 3.360 |
2.5. Statistical analysis
All the analyses were carried out in triplicate and the experimental results were expressed as mean values. Statistical analysis was performed using the Minitab 16 (Minitab Inc., State College, PA, USA) software. A response surface analysis and analysis of variance (ANOVA) were employed to determine the regression coefficients and statistical significance of the model terms and to fit the mathematical models of the experimental data that aimed to optimize the overall region for response variable.25 A second order polynomial model was applied to predict the response variables as shown in Eq. (1)
| Y = β0 + β1X1 + β2X2 + β3X3 + β11X12 + β22X22 + β33X32 + β12X1X2 + β13X1X3 + β23X2X3 | (1) |
where Y is the predicted response; β0 is the constant (intercept); β1, β2, and β3 are the regression coefficients for the linear effect terms; β11, β22, and β33 are the quadratic effect terms; and β12, β13, and β23 are the interaction effect terms, respectively.26 The adequacy of the model was determined by evaluating the lack of fit, coefficient of determination (p-value), and the Fisher test value (F-value) obtained from the analysis of variance (ANOVA) that was generated by the software. Statistical significance of the model and model parameters were determined at the 5% probability level.27
3. Results
3.1. HPLC analysis
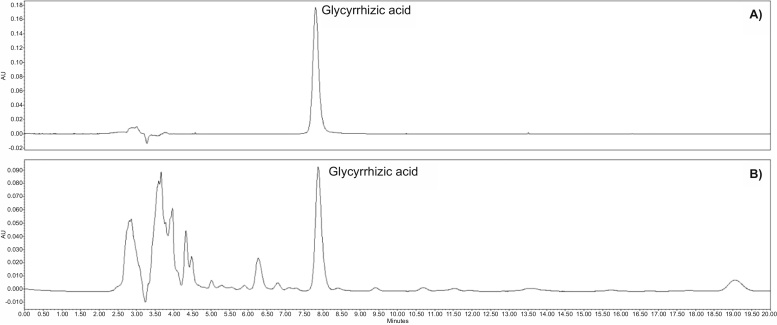
The major compound of licorice, glycyrrhizic acid (GA) was detected at 254 nm and representative HPLC chromatograms are shown in Fig. 1. For GA concentrations from 12.5 to 200 μg/mL, the regression equation was Y = 17842X − 4192.5, which presented good linearity (r2 = 1.0000).
Fig. 1.
HPLC chromatogram of glycyrrhzic acid in standard (a) and sample (b).
3.2. Effect of solvent type on the extraction method
The effect of solvent type on the extraction method was investigated at the beginning of the study. The choice of an appropriate solvent is the most important parameter to be optimized for the extraction of specific constituents.12 The glycyrrhizic acid (GA) molecule has several hydroxyl groups, which renders it easily soluble when extracted by polar solvents.28 In previous studies, different solvents such as methanol, ethanol, and water have been tested.8, 9, 28, 29 To select the appropriate extraction solvent, we measured the yields of GA in different solvents. Table 3 shows significant differences in the extraction yields for the three solvents used (p < 0.001). The yield of GA was highest with methanol (0.845 ± 0.030), and therefore methanol was chosen as the extraction solvent for the design of experiments.
Table 3.
Contents of glycyrrhizic acid for licorice in various solvents.
| Solvent | Content of glycyrrhizic acid (%) |
||
|---|---|---|---|
| Mean | SD | Post-hoca | |
| Methanol | 0.845 | 0.030 | A |
| Ethanol | 0.169 | 0.004 | B |
| Distilled water | NDb | – | C |
Post-hoc by Tukey.
ND, not detected.
3.3. Statistical analysis and model fitting
A total of 20 runs were needed for optimizing the three individual parameters in the current CCD. Table 2 shows the experimental conditions and the GA extraction yield results according to the factorial design. The ANOVA results (Table 4) shows that it is possible to plot the response surface for this experimental design. The correlation measure for testing the goodness-of-fit of the regression equation is the adjusted determination coefficient (R2adj).30 The value of R2adj is 0.902, which is reasonably close to 1 and indicates a high degree of correlation between the observed and predicted values.15 The lack-of-fit (p-value) was calculated as 0.126. The fitness of the model was evaluated through the lack of fit test (p > 0.05), which indicated the adequacy of models to accurately predict the variation.25
Table 4.
Analysis of variance for the fitted quadratic polynomial model of extraction of glycyrrhizic acid.
| Source | DFa | Sum of squares | F-value | p-value |
|---|---|---|---|---|
| Regression | 9 | 30.1335 | 61.57 | 0.000 |
| Linear | 3 | 15.6342 | 95.83 | 0.000 |
| X1 | 1 | 10.8811 | 200.09 | 0.000 |
| X2 | 1 | 0.7373 | 13.56 | 0.001 |
| X3 | 1 | 4.0158 | 73.84 | 0.000 |
| Square | 3 | 9.2582 | 56.75 | 0.000 |
| X1X1 | 1 | 1.5775 | 48.01 | 0.000 |
| X2X2 | 1 | 3.5957 | 80.13 | 0.000 |
| X3X3 | 1 | 4.0850 | 75.12 | 0.000 |
| Interaction | 3 | 5.2411 | 32.12 | 0.000 |
| X1X2 | 1 | 0.0036 | 0.07 | 0.797 |
| X1X3 | 1 | 0.0708 | 1.30 | 0.259 |
| X2X3 | 1 | 5.1667 | 95.01 | 0.000 |
| Residual error | 50 | 2.7191 | – | – |
| Lack of fit | 5 | 0.4597 | 1.83 | 0.126 |
| Pure error | 45 | 2.2594 | – | – |
| Total | 59 | 32.8526 | – | – |
DF, degrees of freedom.
The significance of the F-value depends on the number of degrees of freedom (DF) in the model and is shown in the p-value column (95% confidence level). Thus, the effects lower than 0.05 in this column were considered significant.31 The corresponding variables would be more significant if the F-value becomes greater and the p-value becomes smaller.32 The F-test suggested that the model had a high F-value (F = 61.57) and a low p-value (p < 0.001), indicating that this model was highly significant. In this case, the linear, quadratic, and interaction terms had high model F-values of 95.83, 56.75, and 32.12, respectively, and all p-values (p < 0.001) were low, indicating that this model was highly significant.
3.4. Optimization of GA extraction conditions by RSM
The application of RSM offers, based on parameter estimates, an empirical relationship between the response variable (extraction yield of GA) and the test variables under consideration. By applying multiple regression analysis on the experimental data, the response variable and the test variables are related by the following second-order polynomial equation:
| Y = 33.5559 + 0.5153X1 − 0.1342X2 − 0.3131X3 − 0.2457X12 − 0.3175X22 − 0.3074X32 + 0.0123X1X2 + 0.0543X1X3 + 0.4640X2X3 | (2) |
where Y is the yield of GA (%) and X1, X2, and X3 are the coded variables for extraction temperature, extraction time, and methanol concentration, respectively.
Table 5 shows the regression coefficients for each term in the model and the Student’s t-test statistics and probability values for the significance of the terms. The p-value is used as a tool to check the significance of each coefficient and the interaction strength between each independent variable.33 The corresponding variables will be more significant if the absolute t-value becomes larger and the p-value becomes smaller.34 Thus, the smaller the values of p were, the more significant the corresponding coefficients were. It can be seen from this table that the linear coefficients (X1, X2, X3) were significant at the level of p < 0.01 or p < 0.001. The quadratic terms for all factors X1X1, X2X2, X3X3 and the interaction factors X2X3 were significant, with very small p-value (p < 0.001). The other coefficient terms were considered not significant (p > 0.05).
Table 5.
Regression coefficients result from the data of central composite design (CCD) experiments.
| Term | Coefficient | Standard error | t-value | p-value |
|---|---|---|---|---|
| Intercept | 33.5559 | 0.05491 | 611.086 | 0.000 |
| X1 | 0.5153 | 0.03643 | 14.145 | 0.000 |
| X2 | −0.1342 | 0.03643 | −3.682 | 0.001 |
| X3 | −0.3131 | 0.03643 | −8.593 | 0.000 |
| X1X1 | −0.2457 | 0.03547 | −6.929 | 0.000 |
| X2X2 | −0.3175 | 0.03547 | −8.952 | 0.000 |
| X3X3 | −0.3074 | 0.03547 | −8.667 | 0.000 |
| X1X2 | 0.0123 | 0.04760 | 0.258 | 0.797 |
| X1X3 | 0.0543 | 0.04760 | 1.141 | 0.259 |
| X2X3 | 0.4640 | 0.04760 | 9.747 | 0.000 |
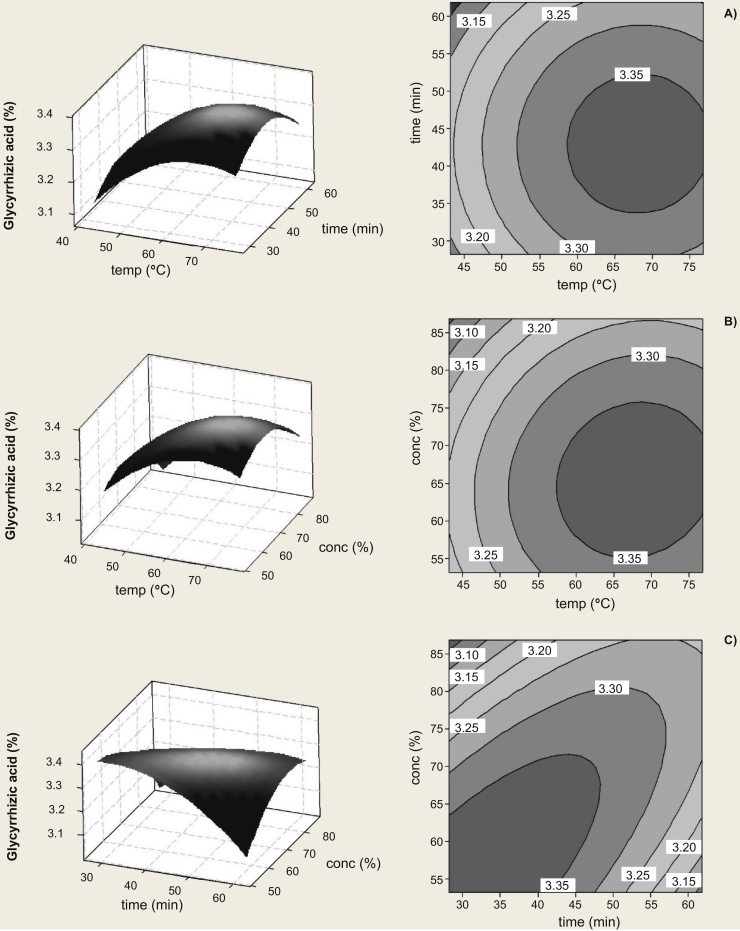
The 3D response surface plot and 2D contour plot are the graphical representations of the regression equation, and the results of the GA extraction yield, as affected by the extraction temperature (X1), extraction time (X2), and methanol concentration (X3), are presented in Fig. 2. In Fig. 2a, the 3D response surface plot and the contour plot were developed for the extraction yield of GA with varying extraction temperature and extraction time at fixed methanol concentration (70%). At a fixed extraction time, the GA yield increased with increasing extraction temperatures ranging from 43 °C to 69 °C. The 3D response surface plot and the contour plot in Fig. 2b show the extraction yield of GA as a function of extraction temperature and methanol concentration at a fixed extraction time (45 min). Higher yields of GA were obtained with higher extraction temperatures and lower methanol concentrations, within the chosen experimental range. The extraction yield of GA affected by different extraction times and methanol concentrations are given in Fig. 2c, when the extraction temperature was fixed at 60 °C. The interaction between the extraction time and the methanol concentration was very significant (p < 0.001). The response curves demonstrate that higher yields are obtained at shorter extraction time and lower methanol concentrations.
Fig. 2.
Response surface plots and contour plots showing the effects of variables on the response Y (Glycyrrhizic acid yield).
4. Discussion
Response surface methodology plays a key role in identifying the optimum values of the independent variables efficiently, optimum values under which dependent variables could achieve a maximum response.27 The response surface plot and contour plot provide a method to visualize the relationship between responses and experimental levels of each variable and the type of interactions between two test variables.30
In this experiment, at a fixed extraction time the GA yield increased with increasing extraction temperatures. The same result was obtained by Charpe et al.,4 and so it can be considered that the solubility of GA increases with an increase in temperature, and hence the amount of extracted GA also increases. Moreover, it was found out that the GA yield increased with an increase in extraction time followed by a decline with further increases of the extraction time. The extraction yield decreased, which may be due to bioactive degradation upon extended ultrasound activity.12 Mason et al.35 reported that ultrasound can induce acoustic cavitation and rupture of plant cells. When extraction time is extended, plant cells are completely cracked by the effects of acoustic cavitation, and extraction yields increase. However, when plant cells rupture, insoluble substances and cytosol get suspended in the extraction liquid, thus resulting in the lower permeability of the solvent.36 Moreover, specific constituents get re-adsorbed on the ruptured plant particles due to their relatively large specific surface areas, and this decreases the yields of the recovered compounds.11 Therefore, it is counterproductive to extend extraction duration once the maximum extraction yield has been achieved.12
A numerical optimization was performed through the desirability function method to determine the optimum level of process variables to obtain maximum GA extract yield. A methanol concentration of 57%, extraction temperature of 69 °C, and a 34 min extraction time were determined to be the optimal conditions for extraction. The maximum response was found as 3.406% under these operating conditions. The experiment was repeated to recheck the procedure, and this was performed in triplicate, at the optimal conditions, to compare the predicted result with the practical value. A mean value of 3.414%, was obtained from the actual extraction procedure, and this demonstrates the validity of the RSM model, indicating that the model is adequate for this extraction process.
In the present work, response surface methodology with a central composite design (CCD) was applied to investigate the ultrasound-assisted extraction of glycyrrhizic acid from licorice. The experimental results showed that all three process variables, that is, the extraction temperature, extraction time, and methanol concentration, contributed to the extraction of GA. The optimal extraction conditions of GA were determined as follows: extraction temperature 69 °C, extraction time 34 min, and methanol concentration 57%. Under these conditions, the experimental yield of GA was 3.414%, which agreed closely with the predicted yield value (3.406%). The experimental values agreed with those predicted by RSM models, thus indicating the suitability of the model employed and the success of RSM in optimizing the extraction conditions.
Acknowledgement
This research was supported by Project (K17091, K14142) of the Korea Institute of Oriental Medicine.
References
- 1.Renjie L. Optimization of extraction process of Glycyrrhiza glabra polysaccharides by response surface methodology. Carbohydr Polym. 2008;74:858–861. [Google Scholar]
- 2.Wang Y.C., Yang Y.S. Simultaneous quantification of flavonoids and triterpenoids in licorice using HPLC. J Chromatogr B. 2007;850:392–399. doi: 10.1016/j.jchromb.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 3.Yang L., Li L.L., Liu T.T., Zu Y.G., Yang F.J., Zhao C.J. Development of sample preparation method for isoliquiritigenin, liquiritin, and glycyrrhizic acid analysis in licorice by ionic liquids-ultrasound based extraction and high-performance liquid chromatography detection. Food Chem. 2013;138:173–179. doi: 10.1016/j.foodchem.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 4.Charpe T.W., Rathod V.K. Extraction of glycyrrhizic acid from licorice root using ultrasound: process intensification studies. Chem Eng Process. 2012;54:37–41. [Google Scholar]
- 5.Tao W., Duan J., Zhao R., Li X., Yan H., Li J. Comparison of three officinal Chinese pharmacopoeia species of Glycyrrhiza based on separation and quantification of triterpenesaponins and chemometrics analysis. Food Chem. 2013;141:1681–1689. doi: 10.1016/j.foodchem.2013.05.073. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y.P., Meng X.S., Bao Y.R., Wang S., Kang T.G. Simultaneous quantitative determination of nine active chemical compositions in traditional Chinese medicine Glycyrrhiza by RP-HPLC with full-time five-wavelength fusion method. Am J Chin Med. 2013;41:211–219. doi: 10.1142/S0192415X13500158. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q.E., Ma S., Fu B., Lee F.S., Wang X. Development of multi-stage countercurrent extraction technology for the extraction of glycyrrhizic acid (GA) from licorice (Glycyrrhiza uralensis Fisch) Biochem Eng J. 2004;21:285–292. [Google Scholar]
- 8.Tanahashi T., Mune T., Morita H., Tanahashi H., Isomura Y., Suwa T. Glycyrrhizic acid suppresses type 2 11 beta-hydroxysteroid dehydrogenase expression in vivo. J Steroid Biochem Mol Biol. 2002;80:441–447. doi: 10.1016/s0960-0760(02)00033-x. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S., Sharma R., Pandotra P., Jaglan S., Gupta A.P. Chromolithic method development, validation and system suitability analysis of ultra-sound assisted extraction of glycyrrhizic acid and glycyrrhetinic acid from Glycyrrhiza glabra. Nat Prod Commun. 2012;7:991–994. [PubMed] [Google Scholar]
- 10.Tian M., Yan H., Row K.H. Extraction of glycyrrhizic acid and glabridin from licorice. Int J Mol Sci. 2008;9:571–577. doi: 10.3390/ijms9040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong J., Liu Y., Liang Z., Wang W. Investigation on ultrasound-assisted extraction of salvianolic acid B from Salvia miltiorrhiza root. Ultrason Sonochem. 2010;17:61–65. doi: 10.1016/j.ultsonch.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang X.S., Wu Y.F., Dai S.L., Chen R., Shao Y. Ultrasound-assisted extraction of geniposide from Gardenia jasminoides. Ultrason Sonochem. 2012;19:1155–1159. doi: 10.1016/j.ultsonch.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Rostagno M.A., Palma M., Barroso C.G. Ultrasound-assisted extraction of soy isoflavones. J Chromatogr A. 2003;1012:119–128. doi: 10.1016/s0021-9673(03)01184-1. [DOI] [PubMed] [Google Scholar]
- 14.Morelli L.L., Prado M.A. Extraction optimization for antioxidant phenolic compounds in red grape jam using ultrasound with a response surface methodology. Ultrason Sonochem. 2012;19:1144–1149. doi: 10.1016/j.ultsonch.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Bimakr M., Rahman R.A., Taip F.S., Adzahan N.M., Sarker M.Z., Ganjloo A. Optimization of ultrasound-assisted extraction of crude oil from winter melon (Benincasahispida) seed using response surface methodology and evaluation of its antioxidant activity, total phenolic content and fatty acid composition. Molecules. 2012;17:11748–11762. doi: 10.3390/molecules171011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baş D., Boyacı İ.H. Modeling and optimization I: Usability of response surface methodology. J Food Eng. 2007;78:836–845. [Google Scholar]
- 17.Liyana-Pathirana C., Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93:47–56. [Google Scholar]
- 18.Banik R.M., Pandey D.K. Optimizing conditions for oleanolic acid extraction from Lantana camara roots using response surface methodology. Ind Crop Prod. 2008;27:241–248. [Google Scholar]
- 19.Liu Y., Wei S., Liao M. Optimization of ultrasonic extraction of phenolic compounds from Euryale ferox seed shells using response surface methodology. Ind Crop Prod. 2013;49:837–843. [Google Scholar]
- 20.Wang X., Wu Y., Chen G., Yue W., Liang Q., Wu Q. Optimisation of ultrasound assisted extraction of phenolic compounds from Sparganii rhizoma with response surface methodology. Ultrason Sonochem. 2012;20:846–854. doi: 10.1016/j.ultsonch.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Yang B., Liu X., Gao Y. Extraction optimization of bioactive compounds (crocin, geniposide and total phenolic compounds) from Gardenia (Gardenia jasminoides Ellis) fruits with response surface methodology. Innov Food Sci Emerg Technol. 2009;10:610–615. [Google Scholar]
- 22.Xu Q., Shen Y., Wang H., Zhang N., Xu S., Zhang L. Application of response surface methodology to optimise extraction of flavonoids from fructus sophorae. Food Chem. 2013;138:2122–2129. doi: 10.1016/j.foodchem.2012.11.099. [DOI] [PubMed] [Google Scholar]
- 23.Zou T.B., Wang M., Gan R.Y., Ling W.H. Optimization of ultrasound-assisted extraction of anthocyanins from mulberry, using response surface methodology. Int J Mol Sci. 2011;12:3006–3017. doi: 10.3390/ijms12053006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdollahi Y., Zakaria A., Abdullah A.H., FardMasoumi H.R., Jahangirian H., Shameli K. Semi-empirical study of ortho-cresol photo degradation in manganese-doped zinc oxide nanoparticles suspensions. Chem Cent J. 2012;6:88–95. doi: 10.1186/1752-153X-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prasad K.N., Kong K.W., Ramanan R.N., Azlan A., Ismail A. Determination and optimization of flavonoid and extract yield from Brown Mango using response surface methodology. Sep Sci Technol. 2012;47:73–80. [Google Scholar]
- 26.Hossain M.B., Brunton N.P., Patras A., Tiwari B., O'Donnell C.P., Martin-Diana A.B. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanummajorana L.) using response surface methodology. Ultrason Sonochem. 2012;19:582–590. doi: 10.1016/j.ultsonch.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L.L., Xu M., Wang Y.M., Wu D.M., Chen J.H. Optimizing ultrasonic ellagic acid extraction conditions from infructescence of Platycarya strobilacea using response surface methodology. Molecules. 2010;15:7923–7932. doi: 10.3390/molecules15117923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian M., Yan H., Row K.H. Simultaneous extraction and separation of liquiritin, glycyrrhizic acid, and glabridin from licorice root with analytical and preparative chromatography. Biotechnol Bioprocess Eng. 2008;13:671–676. doi: 10.1007/s12257-008-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashemi P., Beyranvand S., Mansur R.S., Ghiasvand A.R. Development of a simple device for dispersive liquid-liquid microextraction with lighter than water organic solvents: isolation and enrichment of glycyrrhizic acid from licorice. Anal Chim Acta. 2009;655:60–65. doi: 10.1016/j.aca.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 30.Zhong K., Wang Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr Polym. 2010;80:19–25. [Google Scholar]
- 31.Cai W., Gu X., Tang J. Extraction, purification, and characterization of the polysaccharides from Opuntia milpa alta. Carbohydr Polym. 2008;71:403–410. [Google Scholar]
- 32.Gan C.Y., Latiff A.A. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2011;124:1277–1283. [Google Scholar]
- 33.Muralidhar R.V., Chirumamilla R.R., Ramachandran V.N., Marchant R., Nigam P. Racemic resolution of RS-baclofen using lipase from Candida cylindracea. Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet. 2001;66:227–232. [PubMed] [Google Scholar]
- 34.Quanhong L., Caili F. Application of response surface methodology for extraction optimization of germinant pumpkin seeds protein. Food Chem. 2005;92:701–706. [Google Scholar]
- 35.Mason T.J., Paniwnyk L., Lorimer J.P. The uses of ultrasound in food technology. Ultrason Sonochem. 1996;3:S253–60. [Google Scholar]
- 36.Zhao S., Kwok K.C., Liang H. Investigation on ultrasound assisted extraction of saikosaponins from Radix Bupleuri. Sep Purif Technol. 2007;55:307–312. doi: 10.1016/j.seppur.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]