Abstract
Background:
Surgical tumour removal remains the preferred treatment for most patients with renal cell carcinoma, and many medical associations have proposed guidelines for the optimal surveillance of patients following surgery. This study evaluated the adherence of Canadian urologists to the follow-up guidelines proposed by the Canadian Urological Association (CUA) in 2009.
Methods:
The study cohort was identified from the Canadian Kidney Cancer Information System, a prospectively populated database from 15 academic institutions in 6 Canadian provinces: British Colombia, Alberta, Manitoba, Ontario, Quebec and Nova Scotia. A total of 1982 patients who underwent radical or partial nephrectomy for stage pT1-3N0M0 renal cancer between January 2011 and June 2016 were included in the cohort. Numbers of abdominal and chest imaging tests performed during the follow-up period were captured and compared with the 2009 CUA guidelines. The level of compliance was measured by means of weighted κ and Pearson correlation statistics. Multivariate logistic regression was used to evaluate factors associated with noncompliance (under- or overtesting) in the postoperative surveillance period.
Results:
Of the 1982 patients, 1380 had stage pT1 disease, 164 had stage pT2 disease, and 438 had stage pT3 disease. There was incongruent adherence to the CUA surveillance guidelines, with a ratio of observed to recommended tests of 0.71 and 2.27 for chest and abdominal imaging, respectively. Overall, moderate correlation between observed and recommended tests was observed, with the highest value found for abdominal imaging in the pT3 group (κ = 0.59 [95% confidence interval 0.52-0.66]). Patients who underwent radical nephrectomy and those who presented with a higher stage of the disease were less likely to receive fewer chest imaging tests than recommended, and those with stage pT2 disease, those with stage pT3 disease, those with conventional clear cell renal cell carcinoma and those with a low-risk histologic type had an increased risk of undertesting.
Interpretation:
In the 6 Canadian provinces, there are large differences between guidelines and clinical practice in imaging surveillance after nephrectomy for renal cell carcinoma. Better adherence to clinical guidelines could improve optimization of health care services.
Surgical resection, via either radical or partial nephrectomy, is the most effective therapeutic option for clinically localized renal cell carcinoma. Although radical nephrectomy has long been considered the gold standard,1 partial nephrectomy, or nephron-sparing surgery, has now replaced it as the preferred treatment for renal masses of up to 7 cm.2 Recurrence rates with the 2 procedures for small tumours are similar, 0%-6%.3-6 Radiologic follow-up after partial or radical nephrectomy aims to identify local recurrence or development of metastatic disease. The most common sites of metastatic recurrence are the lung, liver, bone and brain.7 Although high-level evidence is lacking, it is hypothesized that early diagnosis of recurrence or metastasis could trigger earlier treatment and thus improve patient outcomes.2 Urological associations have proposed different algorithms for follow-up after partial or radical nephrectomy.2,8,9 In 2009, the Canadian Urological Association approved guidelines for the follow-up of patients with localized and locally advanced renal cell carcinoma after radical or partial nephrectomy, with a reprint in 2012.9,10
Despite the publication of these guidelines, recent studies have shown that adoption of and adherence to guidelines by the clinical community remain suboptimal.11,12 Little is known about urologists' compliance with the 2009 Canadian guidelines. We aimed to evaluate the levels of compliance with the guidelines and factors associated with compliance in the real-life Canadian setting by studying a prospective cohort of patients undergoing radical or partial nephrectomy in several academic centres in Canada.
Methods
Setting and data sources
The study cohort was identified from the Canadian Kidney Cancer Information System (CKCis), a multicentre collaboration of 15 academic hospitals in 6 Canadian provinces (British Colombia, Alberta, Manitoba, Ontario, Quebec and Nova Scotia) initiated in January 2011. All patients treated for kidney cancer at urology or medical oncology departments were included. We identified 2 groups of patients, surgical and medical oncology, depending on the treating department. For the current study, we selected patients from the surgical group. Clinical, demographic and pathological data for CKCis are obtained by patient survey and medical record review. Patient characteristics collected include age, sex, body mass index, preoperative renal function (estimated glomerular filtration rate), smoking history, comorbidity status (hypertension, diabetes, cardiovascular disease) and family history of kidney cancer. All patients included in the study underwent preoperative chest imaging and routine blood testing, including complete blood count, extended electrolyte panel, blood urea nitrogen and serum creatinine levels, and liver function tests.13 Tumour characteristics included stage, size and number of renal tumours. Treatment characteristics included year of surgery, type of surgery (radical or partial nephrectomy) and surgical approach (open, laparoscopic or robotic-assisted). The choice of surgical type and approach was dependent on patient and surgeon preferences. These preference factors generally include history of abdominal surgery, tumour complexity and medical comorbidities.13 Cancer staging was based on the American Joint Committee of Cancer staging manual, seventh edition.14
The central CKCis database is updated every 6 months, but each centre records data continuously. The database was recently used in other studies.6,15 Three statisticians and 1 project manager are continually working on data validation and inquiries for missing values.
Study cohort
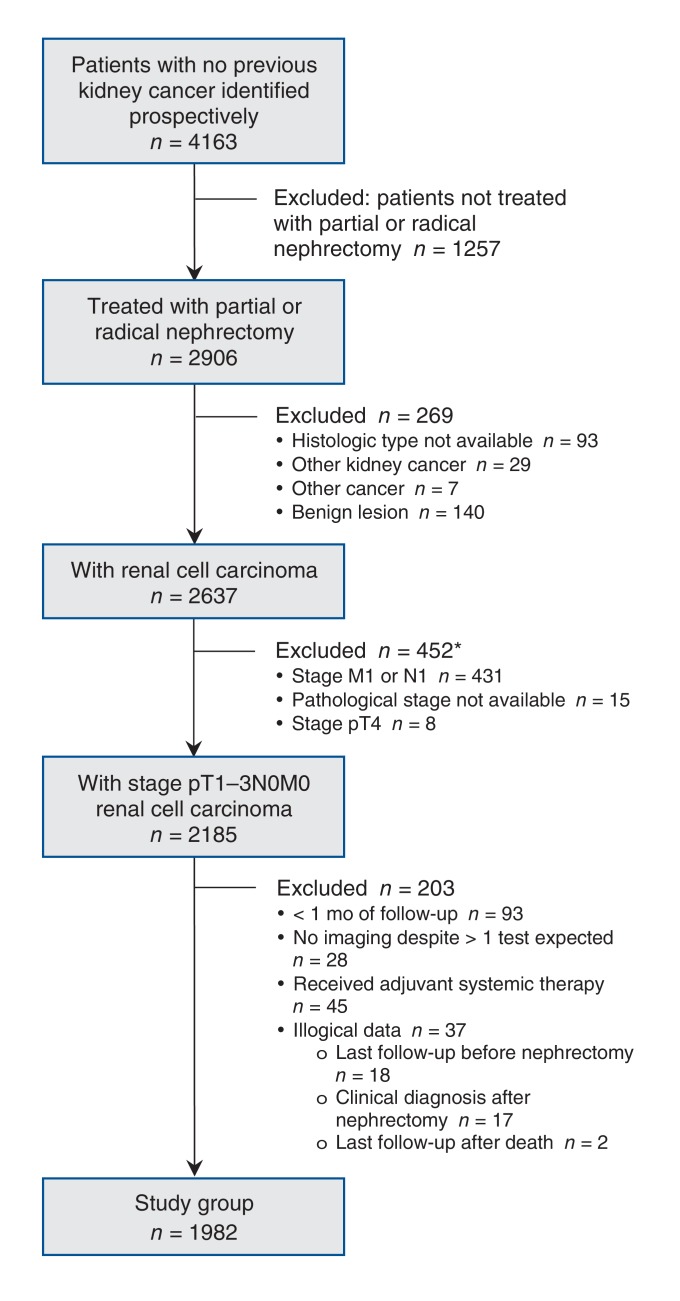
We included patients in whom renal cell carcinoma had been diagnosed between January 2011 and June 2016. To evaluate compliance with the 2009 Canadian Urological Association guidelines, we included only patients whose data were collected prospectively. Patients with no previous history of kidney cancer who were undergoing treatment after January 2011 in 1 of the participating centres across Canada were identified. Several exclusion criteria were applied (Figure 1). We stratified patients by pathological tumour stage into 3 stage groups based on postoperative pathological findings using the 2009 TedfNM staging system.14 All patients in our cohort had stage N0 and M0 status; therefore, from here forward, stages pT1N0M0, pT2N0M0 and pT3N0M0 are referred as pT1, pT2 and pT3, respectively. We defined the surveillance (follow-up) period as the date of nephrectomy until the end of follow-up, which corresponded to the date of disease recurrence, December 2016 or the date of last follow-up (i.e., last patient visit to the treating physician). Recurrence was defined as detection of metastasis in the chest or abdomen as evidenced by imaging (computed tomography, ultrasonography or radiography).
Figure 1.
Flow chart showing patient selection. *Some patients were excluded for more than 1 reason.
Chest and abdominal imaging for surveillance after nephrectomy
The number of chest and abdominal imaging tests performed for each patient was captured in CKCis during the follow-up period. We used the Canadian Urological Association guidelines to estimate the recommended number of chest and abdominal imaging tests for each patient during the specific follow-up period (Table 1). We excluded from this calculation any imaging tests performed during the first 28 days postoperatively as well as repeated tests. A test was considered to be a repetition if the same test was identified at the same location within the previous 30 days.
Table 1: Canadian Urological Association guidelines for imaging during surveillance after radical or partial nephrectomy for localized and locally advanced renal cell carcinoma (adapted with permission of the Canadian Urological Association9)*.
| Cancer stage; examination | No. of months postoperatively | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 12 | 18 | 24 | 30 | 36 | 48 | 60 | 72 | |
| pT1 | ||||||||||
| History and physical examination | x | x | x | x | x | x | ||||
| Blood tests† | x | x | x | x | x | x | ||||
| Chest radiography‡ | x | x | x | x | x | x | ||||
| Computed tomography or abdominal ultrasonography§ | x | x | ||||||||
| pT2 | ||||||||||
| History and physical examination | x | x | x | x | x | x | x | x | x | |
| Blood tests† | x | x | x | x | x | x | x | x | x | |
| Chest radiography‡ | x | x | x | x | x | x | x | x | x | |
| Computed tomography or abdominal ultrasonography§ | x | x | x | |||||||
| pT3 | ||||||||||
| History and physical examination | x | x | x | x | x | x | x | x | x | |
| Blood tests† | x | x | x | x | x | x | x | x | x | |
| Chest radiography‡ | x | x | x | x | x | x | x | x | x | |
| Computed tomography or abdominal ultrasonography§ | x | x | x | x | x | x | ||||
| pTxN+ | ||||||||||
| History and physical examination | x | x | x | x | x | x | x | x | x | x |
| Blood tests† | x | x | x | x | x | x | x | x | x | x |
| Chest radiography‡ | x | x | x | x | x | x | x | x | x | x |
| Computed tomography or abdominal ultrasonography§ | x | x | x | x | x | x | x | x | x | x |
*If patient is symptomatic or if blood test gives abnormal result, earlier radiologic investigations may be indicated.
†Include complete blood count, measurement of serum chemistry and liver function tests.
‡Can be alternated with chest computed tomography.
§Can be alternated with abdominal ultrasonography in patients with stage pT1-2N0 disease.
Statistical analysis
Patient characteristics are presented as proportions, means (and 95% confidence intervals [CIs]) or medians (and interquartile range), as appropriate.
We defined 3 levels of compliance with the guidelines: 1) compliant testing (number of tests observed = number recommended), 2) undertesting (number observed < number recommended) and 3) overtesting (number observed > number recommended).
To measure the level of compliance, we applied weighted κ statistics and the Pearson correlation statistic, overall and by stage group. We used the Pearson correlation statistic to test whether the numbers of chest and abdominal imaging tests observed were in agreement with the numbers of tests recommended, and the κ statistic to assess the consistency between compliance levels in chest surveillance and abdominal surveillance. We measured levels of compliance and numbers of tests performed over the entire follow-up period and at different time points (6, 12, 18 and 24 mo).
We used multivariate logistic regression to evaluate factors associated with noncompliance (over- or undertesting) with chest and abdominal imaging during the surveillance period.
We performed all analyses using SAS software, version 9 (SAS Institute Inc.). All tests were 2-sided, with a significance threshold of 5%.
We considered several covariates, such as age (> 75 yr v. ≤ 75 yr), family history of kidney cancer, smoking status at diagnosis and type of surgery (radical v. partial), as potential confounders or predictors of noncompliance. Tumour characteristics obtained from the pathology report associated with nephrectomy include tumour stage (pT2 or pT3 v. pT1), tumour grade (high v. low), surgical margin status (positive v. negative) and histologic type. High grade was defined as Fuhrman grade 3 or 4, and low grade as Fuhrman grade 1 or 2. The lower-risk histologic type category included papillary and chromophobe renal cell carcinoma. We also evaluated several comorbidities, including other cancer, diabetes, hypertension, obesity, hypothyroidism, heart disease, kidney stone, renal disease, osteoarthritis, gout, gastroesophageal reflux disease, depression and chronic pulmonary disease.
Ethics approval
Patients had provided consent for data entry into the CKCis database prospectively, and all participating centres have research ethics board approval for projects using the CKCis database.
Results
Patient characteristics
The study cohort included 1982 patients with stage pT1-3 renal cell carcinoma treated with radical or partial nephrectomy. Patient characteristics are presented in Table 2. Of the 1982 patients, 1380 (69.6%) had stage pT1 disease, 164 (8.3%) had stage pT2 disease, and 438 (22.1%) had stage pT3 disease. The mean age of the patients was 60.3 years, and 1314 (66.3%) were men. Nearly half (846 [42.7%]) of the nephrectomy procedures were radical, and most patients (1413 [71.3%]) had clear cell renal cell carcinoma. A total of 208 patients (10.5%) were smokers, 291 (14.7%) had complications related to the nephrectomy procedure, and 111 (5.6%) had a family history of renal tumours. The most common comorbidities were hypertension (949 patients [47.9%]), dyslipidemia (379 [19.1%]), heart disease (377 [19.0%]) and diabetes (349 [17.6%]). No major differences were observed in comorbidity distribution between stage groups (data not shown).
Table 2: Characteristics of patients with renal cell carcinoma treated with radical or partial nephrectomy.
| Characteristic | Cancer stage; no. (%) of patients* | |||
|---|---|---|---|---|
| All patients n = 1982 |
pT1n = 1380 | pT2n = 164 | pT3 n = 438 |
|
| Age at procedure, yr | ||||
| Mean (95% CI) | 60.3 (59.7-60.8) | 59.5 (58.9-60.1) | 58.4 (58.9-60.4) | 63.2 (62.1-64.2) |
| Median (IQR) | 60.7 (52.5-68.4) | 60.2 (52.0-68.0) | 59.4 (49.5-67.5) | 63.1 (55.8-71.0) |
| Age > 75 yr at procedure | 198 (10.0) | 116 (8.4) | 17 (10.4) | 66 (15.1) |
| Male sex | 1314 (66.3) | 883 (64.0) | 112 (68.3) | 319 (72.8) |
| High grade (n = 1830)† | 866 (43.7) | 458 (33.2) | 85 (51.8) | 318 (72.6) |
| Radical nephrectomy | 846 (42.7) | 356 (25.8) | 140 (85.4) | 351 (80.1) |
| Positive surgical margin (n = 1935) | 129 (6.5) | 65 (4.7) | 1 (0.6) | 64 (14.6) |
| Histologic type‡ | ||||
| Clear cell renal cell carcinoma | 1413 (71.3) | 949 (68.8) | 97 (59.1) | 368 (84.0) |
| Papillary renal cell carcinoma | 325 (16.4) | 254 (18.4) | 35 (21.3) | 35 (8.0) |
| Chromophobe renal cell carcinoma | 155 (7.8) | 115 (8.3) | 24 (14.6) | 16 (3.6) |
| Renal cell carcinoma, unspecified | 89 (4.5) | 62 (4.5) | 8 (4.9) | 19 (4.3) |
| Computed tomography§ | 1048 (52.9) | 640 (46.4) | 95 (57.9) | 314 (71.7) |
| Intra- or postoperative medical complication(s) | 291 (14.7) | 195 (14.1) | 25 (15.2) | 72 (16.4) |
| Family history of renal cell carcinoma | 111 (5.6) | 79 (5.7) | 7 (4.3) | 25 (5.7) |
| Status (n = 1639)¶ | ||||
| Alive with disease | 161 (8.1) | 68 (4.9) | 20 (12.2) | 73 (16.7) |
| No evidence of disease | 1655 (83.5) | 1227 (88.9) | 128 (78.0) | 300 (68.5) |
| Overall death | 81 (4.1) | 22 (1.6) | 13 (7.9) | 46 (10.5) |
| Lost to follow-up | 81 (4.1) | 61 (4.4) | 3 (1.8) | 17 (3.9) |
| Unknown | 4 (0.2) | 2 (0.1) | 0 (0.0) | 2 (0.4) |
Note: CI = confidence interval, IQR = interquartile range.
*Except where noted otherwise.
†Fuhrman grade 3 (moderately to poorly differentiated tumour cells) or 4 (poorly differentiated tumour cells).
‡Primary histologic type from pathology report.
§Patient received at least 1 computed tomography examination of the chest or abdomen during surveillance after nephrectomy.
¶At last follow-up visit for kidney cancer.
The mean length of postoperative surveillance was 18.6 (range 1-63) months, and the median was 15 (interquartile range 7-28) months. The rate of recurrence in the pT1, pT2 and pT3 groups was 1.9%, 14.6% and 28.6%, respectively, at 1 year, and 3.7%, 24.1% and 39.3%, respectively, at 2 years (data not shown).
Compliance with imaging guidelines
Over the follow-up period, 1948 chest and 2986 abdominal imaging tests were performed (Table 3). The corresponding estimated recommended numbers of tests were 2754 and 1317. Overall, the ratio of observed to recommended tests was 0.71 for chest imaging and 2.27 for abdominal imaging. For chest imaging, the highest disparity was observed among patients with stage pT2 disease (0.54), and for abdominal testing, among those with stage pT1 disease (4.55). This corresponds to a compliance level of 42.9% for chest imaging and of 35.5% for abdominal imaging. A total of 739 patients (37.3%) received fewer chest imaging tests than recommended, and 1096 (55.3%) received more abdominal imaging tests than recommended. Ninety-four patients (57.3%) with stage pT2 disease and 187 (42.7%) of those with stage pT3 disease received fewer chest imaging tests than recommended, and 878 patients (63.6%) with stage pT1 disease and 96 (58.5%) of those with stage pT2 disease received more abdominal imaging tests than recommended. Overall, whereas 67.6% of all recommended tests were chest examinations, only 39.5% of observed tests were chest studies. Furthermore, 56.1% of chest examinations performed were radiography tests, and 43.0% of abdominal examinations performed were ultrasound studies.
Table 3: Number of imaging tests observed versus number recommended in Canadian guidelines.
| Comparison; variable | Cancer stage | |||
|---|---|---|---|---|
| pT1 | pT2 | pT3 | Total | |
| Aggregate results | ||||
| Chest | ||||
| No. of tests observed | 1194 | 226 | 528 | 1948 |
| No. of tests recommended | 1525 | 418 | 811 | 2754 |
| Ratio of observed to recommended | 0.78 | 0.54 | 0.65 | 0.71 |
| % of tests that were radiography examinations | 67.3 | 51.3 | 32.8 | 56.1 |
| Abdomen | ||||
| No. of tests observed | 2022 | 294 | 670 | 2986 |
| No. of tests recommended | 444 | 122 | 751 | 1317 |
| Ratio of observed to recommended | 4.55 | 2.41 | 0.89 | 2.27 |
| % of tests that were ultrasound examinations | 52.5 | 37.4 | 16.6 | 43.0 |
| Per-patient basis, no. (%) of patients | ||||
| Chest | ||||
| Undertesting | 458 (33.2) | 94 (57.3) | 187 (42.7) | 739 (37.3) |
| Compliant | 635 (46.0) | 52 (31.7) | 63 (37.2) | 850 (42.9) |
| Overtesting | 287 (20.8) | 18 (11.0) | 88 (20.1) | 393 (19.8) |
| Abdomen | ||||
| Undertesting | 32 (2.3) | 10 (6.1) | 140 (32.0) | 182 (9.2) |
| Compliant | 470 (34.1) | 58 (35.4) | 176 (40.2) | 704 (35.5) |
| Overtesting | 878 (63.6) | 96 (58.5) | 122 (27.8) | 1096 (55.3) |
Table 4 presents compliance levels for the first 6, 12, 18 and 24 months postoperatively. Only minor changes over time were observed for both chest and abdominal imaging.
Table 4: Levels of compliance during 6, 12, 18 and 24 months after nephrectomy.
| Cancer stage; time, mo | Chest radiography or computed tomography | Abdominal ultrasonography or computed tomography | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of tests recommended | Undertesting, no. (%) of patients | Compliant, no. (%) of patients | Overtesting, no. (%) of patients | No. of tests recommended | Undertesting, no. (%) of patients | Compliant, no. (%) of patients | Overtesting, no. (%) of patients | |
| pT1 | ||||||||
| 6 (n = 1380) | 0 | 0 (0.0) | 1096 (79.4) | 284 (20.6) | 0 | 0 (0.0) | 838 (60.7) | 542 (39.3) |
| 12 (n = 798) | 1 | 263 (33.0) | 408 (51.1) | 127 (15.9) | 0 | 0 (0.0) | 320 (40.1) | 478 (59.9) |
| 18 (n = 406) | 1 | 114 (28.1) | 203 (50.0) | 89 (21.9) | 0 | 0 (0.0) | 140 (34.5) | 266 (65.5) |
| 24 (n = 168) | 2 | 58 (34.5) | 77 (45.8) | 33 (19.6) | 1 | 7 (4.2) | 62 (36.9) | 99 (58.9) |
| pT2 | ||||||||
| 6 (n = 164) | 1 | 75 (45.7) | 75 (45.7) | 14 (8.5) | 0 | 0 (0.0) | 85 (51.8) | 79 (48.2) |
| 12 (n = 91) | 2 | 48 (52.7) | 32 (35.2) | 11 (12.1) | 1 | 11 (12.1) | 44 (48.4) | 36 (39.6) |
| 18 (n = 50) | 3 | 27 (54.0) | 17 (34.0) | 6 (12.0) | 1 | 4 (8.0) | 22 (44.0) | 24 (48.0) |
| 24 (n = 27) | 4 | 15 (55.6) | 9 (33.3) | 3 (11.1) | 1 | 1 (3.7) | 11 (40.7) | 15 (55.6) |
| pT3 | ||||||||
| 6 (n = 438) | 1 | 169 (38.6) | 187 (42.7) | 82 (18.7) | 1 | 119 (27.2) | 225 (51.4) | 94 (21.5) |
| 12 (n = 177) | 2 | 72 (40.7) | 69 (39.0) | 36 (20.3) | 2 | 54 (30.5) | 76 (42.9) | 47 (26.6) |
| 18 (n = 84) | 3 | 35 (41.7) | 32 (38.1) | 17 (20.2) | 3 | 28 (33.3) | 33 (39.3) | 23 (27.4) |
| 24 (n = 33) | 4 | 14 (42.4) | 12 (36.4) | 7 (21.2) | 4 | 11 (33.3) | 13 (39.4) | 9 (27.3) |
There was low consistency in compliance levels between chest and abdominal imaging (overall κ estimate = 0.30 [95% CI 0.27-0.32]). The highest level of agreement was observed in the pT3 group (κ = 0.59 [95% CI 0.52-0.66]).
The mean and median numbers of recommended and observed tests and the Pearson correlation coefficient between these measurements are presented in Table 5. Again, moderate correlation was observed, with the highest value found for abdominal imaging in the pT3 group (r = 0.69 [95% CI 0.63-0.74]).
Table 5: Level of agreement between observed versus recommended number of imaging tests of chest and abdomen.
| Area; cancer stage | No. of tests recommended | No. of tests observed | Correlation between recommended and observed, Pearson coefficient (95% CI) | ||
|---|---|---|---|---|---|
| Mean (95% CI) | Median (IQR) | Mean (95% CI) | Median (IQR) | ||
| Chest | |||||
| Overall (n = 1655) | 1.34 (1.27-1.41) | 1 (0-2) | 1.00 (0.93-1.06) | 1 (0-2) | 0.55 (0.51-0.58) |
| pT1 (n = 1159) | 1.05 (0.99-1.11) | 1 (0-2) | 0.88 (0.81-0.94) | 0 (0-1) | 0.52 (0.47-0.56) |
| pT2 (n = 141) | 2.53 (2.17-2.87) | 2 (1-4) | 1.32 (1.08-1.56) | 1 (0-2) | 0.59 (0.46-0.68) |
| pT3 (n = 355) | 1.81 (1.62-2.00) | 1 (0-3) | 1.24 (1.09-1.39) | 1 (0-2) | 0.58 (0.51-0.64) |
| Abdomen | |||||
| Overall (n = 1655) | 0.65 (0.60-0.70) | 0 (0-1) | 1.45 (1.38-1.52) | 1 (0-2) | 0.47 (0.43-0.50) |
| pT1 (n = 1159) | 0.32 (0.29-0.34) | 0 (0-1) | 1.39 (1.31-1.47) | 1 (0-2) | 0.53 (0.49-0.57) |
| pT2 (n = 141) | 0.78 (0.66-0.90) | 1 (0-1) | 1.69 (1.40-1.98) | 1 (0-3) | 0.63 (0.52-0.72) |
| pT3 (n = 355) | 1.70 (1.53-1.86) | 1 (0-3) | 1.56 (1.40-1.71) | 1 (0-2) | 0.69 (0.63-0.74) |
Note: CI = confidence interval, IQR = interquartile range.
Factors associated with compliance with guidelines
Multivariate logistic regression revealed several associations (Table 6). For chest imaging, patients who underwent radical nephrectomy and those who presented with a higher grade were less likely to receive fewer tests than recommended, by 39% (odds ratio [OR] 0.61 [95% CI 0.45-0.82]) and 33% (OR 0.67 [95% CI 0.51-0.87]), respectively. Patients with stage pT2 disease (OR 5.36 [95% CI 3.37-8.54]), those with stage pT3 disease (OR 2.64 [95% CI 1.86-3.74]), those with conventional clear cell renal cell carcinoma (OR 2.28 [95% CI 1.12-4.4) and those with a low-risk histologic type (OR 3.14 [95% CI 1.51-6.50]) had an increased risk of undertesting.
Table 6: Factors associated with noncompliance in chest and abdominal imaging on multivariate logistic regression.
| Variable | Probability of undertesting of chest, OR (95% CI) |
Probability of overtesting of abdomen, OR (95% CI) |
||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Male v. female | 0.95 (0.76-1.17) | 0.90 (0.70-1.17) | 0.90 (0.74-1.11) | 0.97 (0.76-1.25) |
| Age at procedure, yr (> 75 v. < 75) | 1.39 (1.00-1.93) | 1.43 (0.96-2.12) | 0.80 (0.58-1.02) | 1.02 (0.68-1.51) |
| High grade (yes v. no) | 0.84 (0.68-1.03) | 0.67 (0.51-0.87) | 0.67 (0.55-0.82) | 0.97 (0.75-1.24) |
| Positive surgical margin (yes v. no) | 1.16 (0.78-1.75) | 0.84 (0.51-1.38) | 0.89 (0.60-1.32) | 1.61 (0.98-2.64) |
| Radical nephrectomy (yes v. no) | 1.06 (0.86-1.30) | 0.61 (0.45-0.82) | 0.62 (0.51-0.76) | 1.06 (0.80-1.39) |
| Histologic type* | ||||
| Other renal cell carcinoma | Reference | Reference | Reference | Reference |
| Low risk | 1.24 (0.99-1.57) | 3.14 (1.51-6.50) | 0.94 (0.75-1.18) | 0.69 (0.37-1.29) |
| Conventional clear cell | 0.89 (0.71-1.12) | 2.28 (1.12-4.48) | 1.08 (0.87-1.34) | 0.87 (0.48-1.56) |
| Cancer stage | ||||
| pT1 | Reference | Reference | Reference | Reference |
| pT2 | 3.00 (2.11-4.26) | 5.36 (3.37-8.54) | 0.90 (0.63-1.26) | 0.71 (0.46-1.09) |
| pT3 | 1.42 (1.12-1.81) | 2.64 (1.86-3.74) | 0.27 (0.21-0.35) | 0.23 (0.16-0.32) |
| Smoker (yes v. no) | 0.81 (0.60-1.09) | 0.88 (0.64-1.22) | 1.03 (0.78-1.36) | 0.96 (0.71-1.30) |
| Family history of kidney cancer (yes v. no) | 1.39 (0.90-2.07) | 1.48 (0.91-2.40) | 0.92 (0.61-1.39) | 1.04 (0.64-1.69) |
Note: CI = confidence interval, OR = odds ratio.
*Primary histologic type from pathology report; low-risk category includes papillary and chromophobe renal cell carcinoma.
For abdominal imaging, no factors associated with the probability of having received more tests than recommended were found. A trend toward an increase in the number of abdominal tests in patients with positive margins was observed (OR 1.61 [95% CI 0.98-2.64]). However, the probability of abdominal overtesting was 77% lower in the pT3 group than in the pT1 group.
Interpretation
We found a suboptimal level of compliance with the Canadian Urological Association guidelines for postoperative surveillance in patients treated for clinically localized renal cell carcinoma in 15 academic hospitals across 6 Canadian provinces. Overall, the agreement between the number of tests recommended and the number performed was low. Only half of the patients received chest and abdominal imaging consistent with guideline recommendations. We observed undercompliance with chest imaging guidelines of 29.3%, and a concomitant potential overuse of abdominal testing of 226.7%, with the highest discrepancy observed among patients with lower-stage disease (pT1 and pT2). The highest level of noncompliance was observed among patients with stage pT1 disease, for whom the number of abdominal tests was 4 times higher than recommended. For chest imaging, several factors were found to be associated with suboptimal surveillance: patients who underwent radical nephrectomy and those who presented with a higher stage of disease had a decreased risk of being undertested, whereas patients with stage pT2 or pT3 disease and those with conventional clear cell carcinoma or a low-risk histologic type had an increased risk of undertesting. Yet, higher grade of disease was associated with reduced overtesting. This may have been due to the fact that the guidelines recommend a higher frequency of abdominal imaging for patients with higher-grade disease than for lower-grade groups.
For both chest and abdominal imaging, no patterns or preferences of use of imaging modality (i.e., radiography and ultrasonography are less expensive than computed tomography) or changes over time were seen during the surveillance period. In addition, our results showed that clinicians may not be paying attention to the recommended frequency of specific imaging based on stage, and that is why overtesting is more prominent in the low-stage groups. On the other hand, our findings may suggest that there is a discrepancy in recurrence patterns between the guideline recommendations and what urologists actually encounter in their clinical practice. In any case, our study opens this discussion and creates awareness about suboptimal surveillance and the important economic implications that the overtesting may have. Further studies are needed to evaluate the impact of noncompliance with the recommended surveillance guidelines on clinical outcomes and costs.
A recent US study also revealed suboptimal postnephrectomy surveillance of patients treated from 1991 to 2007.11 For patients followed for at least 1 year, initial abdominal and chest imaging were performed in 69% and 78% of patients, respectively. By year 5, annual rates of abdominal and chest imaging had decreased to 28% and 39%, respectively, among patients with high-risk disease (stage T3 or T4) and to 21% and 25%, respectively, among those with low- to moderate-risk disease (stage pT1 or pT2). A second US study among patients treated with nephrectomy between 2000 to 2009 confirmed these results, with rates of chest and abdominal imaging of 65%-80% and 58%-76%, respectively.12 Our results for chest imaging are consistent with those 2 studies, but we found overuse of abdominal imaging. This might be explained by differences in the time period studied. The discrepancy may also be related to differences between the Canadian and US health care systems.
Limitations
All the patients included in the cohort were followed in academic institutions; therefore, the results may have limited generalizability in nonacademic institutions or in countries with very different surveillance patterns. Second, owing to the absence of information on why the imaging tests were performed, we cannot discount the possibility that overuse of abdominal imaging was due to onset of new symptoms as opposed to routine screening for recurrence. In addition, as the available data did not include patient-reported symptoms or outcomes, this study could not account for patient preferences, fears or concerns. Third, we cannot exclude the possibility that noncompliance may reflect urologists' disagreement with the surveillance guidelines because of a lack of strong evidence. Fourth, our study considered only patient-related factors; no hospital- and/or physician-related factors were considered for adjustment in the analyses. However, neither urologists' disagreement with the guidelines nor factors related to the hospital or the physician would explain the discrepancy between abdominal and chest imaging.
Conclusion
This was a Canadian study evaluating surveillance imaging performed after partial or radical nephrectomy in the contemporary era. It revealed suboptimal follow-up imaging and noncompliance with the recent clinical guidelines. Additional efforts should be made to raise awareness among urologists about appropriate follow-up imaging in this setting.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/5/4/E834/suppl/DC1.
Supplementary Material
Footnotes
Funding: Alice Dragomir obtained salary support from the Coté-Sharp Family Foundation at the McGill University Health Centre. Since June 2016, she has been a Fonds de recherche du Québec - Santé Junior 1 Research Scholar.
References
- 1.Robson CJ, Churchill BM, Anderson W. The results of radical nephrectomy for renal cell carcinoma. J Urol. 1969;101:297–301. doi: 10.1016/s0022-5347(17)62331-0. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Bensalah A, Bex A, et al. Guidelines on renal cell carcinoma 2014. Arnhem (Netherlands): European Association of Urology; 2017. [accessed 2017 Sept. 12]. Available https://uroweb.org/wp-content/uploads/10-Renal-Cell-Carcinoma_LR.pdf.
- 3.Antonelli A, Cozzoli A, Nicolai M, et al. Nephron-sparing surgery versus radical nephrectomy in the treatment of intracapsular renal cell carcinoma up to 7cm. Eur Urol. 2008;53:803–9. doi: 10.1016/j.eururo.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Van Poppel H. Efficacy and safety of nephron-sparing surgery. Int J Urol. 2010;17:314–26. doi: 10.1111/j.1442-2042.2010.02482.x. [DOI] [PubMed] [Google Scholar]
- 5.Lavallée LT, Tanguay S, Jewett MA, et al. Surgical management of stage T1 renal tumours at Canadian academic centres. Can Urol Assoc J. 2015;9:99–106. doi: 10.5489/cuaj.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forbes CM, Rendon RA, Finelli A, et al. Disease progression and kidney function after partial vs. radical nephrectomy for T1 renal cancer. Urol Oncol. 2016;34:486.e17–486.e23. doi: 10.1016/j.urolonc.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 7.Janzen NK, Kim HL, Figlin RA, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–52. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 8.Donat SM, Diaz M, Bishoff JT, et al. Follow-up for clinically localized renal neoplasms: AUA Guideline. J Urol. 2013;190:407–16. doi: 10.1016/j.juro.2013.04.121. [DOI] [PubMed] [Google Scholar]
- 9.Kassouf W, Siemens R, Morash C, et al. Follow-up guidelines after radical or partial nephrectomy for localized and locally advanced renal cell carcinoma. Can Urol Assoc J. 2009;3:73–6. doi: 10.5489/cuaj.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jewett M, Finelli A, Kollmannsberger C, et al. Management of kidney cancer: Canadian kidney cancer forum consensus update 2011. Can Urol Assoc J. 2012;6:16–22. doi: 10.5489/cuaj.11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim EH, Vetter JM, Kuxhausen AN, et al. Limited use of surveillance imaging following nephrectomy for renal cell carcinoma. Urol Oncol. 2016;34:237.e11–8. doi: 10.1016/j.urolonc.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feuerstein MA, Atoria CL, Pinheiro LC, et al. Patterns of surveillance imaging after nephrectomy in the Medicare population. BJU Int. 2016;117:280–6. doi: 10.1111/bju.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel P, Nayak JG, Liu Z, et al. A multicentered, propensity matched analysis comparing laparoscopic and open surgery for pT3a renal cell carcinoma. J Endourol. 2017;31:645–50. doi: 10.1089/end.2016.0787. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 15.Mason R, Kapoor A, Liu Z, et al. The natural history of renal function after surgical management of renal cell carcinoma: results from the Canadian Kidney Cancer Information System. Urol Oncol. 2016;34:486.e1–486.e7. doi: 10.1016/j.urolonc.2016.05.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.