Abstract
Background
Until recently, transplantation from hepatitis C–positive donors was relatively contraindicated as eradication of active hepatitis C previously required an interferon-based regimen that has been associated with rejection in solid organ transplantation. New interferon-free treatment regimens for hepatitis C have fewer adverse events and higher cure rates than interferon-based regimens. Interferon-free regimens have been shown to be safe in the liver transplantation literature, but little is known about the safety and efficacy of treatment in heart transplantation.
Case Description and Discussion
Here we report a case of successful eradication of hepatitis C with a non–interferon-based regimen using ledipasvir-sofosbuvir following combined orthotopic heart and liver transplantation. Based on the prevalence of hepatitis C in the general population, inclusion of hepatitis C–positive donors for heart transplantation can expand this component of the donor pool 3- to 6-fold.
Conclusions
In carefully selected patients and recipients, inclusion of hepatitis C–positive donors may allow for expansion of the donor pool.
Keywords: Donor pool, hepatitis C, OHT/OLT, ledipasvir-sofosbuvir
The wait times for cardiac transplantation and organ allocation are prohibitively long for thousands or patients each year. Currently, the supply-demand mismatch for hearts is substantial, with 4124 Americans awaiting heart transplantation and only 2804 heart transplantations in the year 2015.1 We present a case involving a donor with hepatitis C virus (HCV) that, in the setting of improvements in management and treatment, may serve as a model for safely expanding the donor supply.
HCV infection represents a global epidemic. It is predicted that 130 to 160 million people worldwide and 2.7 to 3.9 million Americans are chronically infected with HCV.2 More specifically, its overall prevalence in the US general population is 1.8% or 1800 per 100,000 people, whereas the prevalence among transplanted heart donors dating back to 1988 is 0.55% or 550 per 100,000 people.3,4 Overall, transplantation of HCV-positive donors had been on the decline since the year 2002, although recent trends suggest that acceptance of donors with HCV may be regaining favor.4 Heart transplantation from a HCV-positive donor has historically been relatively contraindicated. In the past, HCV-positive donors required an interferon-based regimen, which has been associated with rejection in solid organ transplantation.5,6 Furthermore, peg-interferon alpha-2b treatment has been associated with acute allograft failure among heart transplant recipients.7 Recently, new interferon-free treatment regimens for hepatitis C have seen fewer adverse events and higher cure rates than interferon-based regimens.8 Ledipasvir-sofosbuvir is an interferon-free regimen used in isolation or combined with ribavirin for HCV genotypes 1a, 1b, 4, 5, and 6. Among patients with genotype 1, ledipasvir-sofosbuvir is associated with a 99% sustained virologic response rate at 12 weeks.8 Interferon-free regimens have been shown to be safe in the liver transplantation literature, but little is known about the safety and efficacy of treatment in heart transplantation.
With that background, a 62-year-old man with a history of chronic HCV, genotype 1b, complicated by cirrhosis, hepatocellular carcinoma, and dilated cardiomyopathy required combined heart and liver transplantation. The patient’s HCV viral load was more than 10 million copies (greater than 5 million copies is considered a high viral load) and we opted to leave his HCV untreated before transplant but rather consider an expansion of the donor pool by including HCV-positive donors. Shortly thereafter, the patient underwent successful combined heart and liver transplantation with basiliximab induction. The patient developed a mild transaminase elevation that was diagnosed as recurrent acute hepatitis C proven by liver biopsy. The patient was treated with ledipasvir-sofosbuvir and, following a 12-week course, HCV was not detectable by polymerase chain reaction (Fig. 1). At the end of his first year posttransplant, the patient had no episodes of rejection and had normal heart and liver graft function.
Fig. 1.
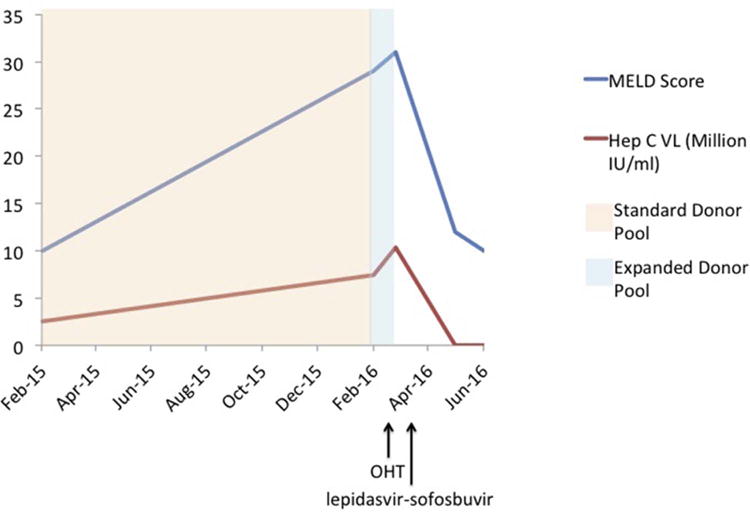
Hepatitis C viral load (hep C VL) and Model for End-Stage Liver Disease (MELD) score trends in relationship to donor pool size, transplantation date, and treatment with ledipasvir-sofosbuvir. Suitable organs were found shortly after donor pool expansion to include hepatitis C–positive donors. A sustained virologic response was rapidly achieved after ledipasvir-sofosbuvir treatment. OHT, orthotopic heart transplant.
We believe that in carefully selected patients and recipients, inclusion of HCV-positive donors may allow for expansion of the donor pool. The prevalence of HCV in the general population is 1800 per 100,000 and the prevalence of HCV among deceased liver donors is 3100 per 100,000.3,4 Given that the prevalence of HCV among current heart donors is 550 per 100,000, acceptance of HCV-positive donors has the potential to expand the HCV-positive patient contribution to the donor pool 3- (1800/550) to 6- (3100/550) fold, which would be a major step forward in bridging the donor supply-demand mismatch.3,4
Before such a strategy can be widely implemented, prospective studies including longer patient follow-up are necessary to better document the duration of sustained virologic response and rates of graft loss, rejection, vasculopathy, and death on a larger scale. Nonetheless, given the improved treatment strategies for combating HCV together with the implications for the donor, the strategy of inclusion of hepatitis C–positive donors in the donor pool warrants additional attention.
Acknowledgments
All authors have no relevant disclosures.
Footnotes
Disclosures
All authors have no relevant disclosures.
References
- 1.United Network for Organ Sharing based on OPTN data as of July 29, 2016. Available at: https://www.unos.org/data/transplant-trends/. Accessed August 8, 2016.
- 2.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–15. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–62. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 4.United Network for Organ Sharing based on OPTN data as of April 28, 2017. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Accessed May 1, 2017.
- 5.Aljumah AA, Saeed MA, Al Flaiw AI, Traif I, Al Alwan AM, Al Qurashi SH, et al. Efficacy and safety of treatment of hepatitis C virus infection in renal transplant recipients. World J Gastroenterol. 2012;18:55–63. doi: 10.3748/wjg.v18.i1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanai FM, Mousa D, Al-Mdani A, Al-Shoail G, Al-Ashgar H, Al Meshari K, et al. Safety and efficacy of peginterferon-α2a plus ribavirin treatment in renal transplant recipients with chronic hepatitis C. J Hepatol. 2013;58:1096–103. doi: 10.1016/j.jhep.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Wang BY, Chang HH, Chen IM, Shih CC, Yang AH. Peginterferon alpha-2b and acute allograft failure in a heart transplant recipient. Ann Thorac Surg. 2010;89:1645–7. doi: 10.1016/j.athoracsur.2009.09.084. [DOI] [PubMed] [Google Scholar]
- 8.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]


