Abstract
Background
Although 3D echocardiography (3DE) allows accurate and reproducible quantification of cardiac chambers, it has not been integrated into clinical practice because it relies on manual input, which interferes with workflow. A recently developed automated adaptive analytics algorithm for simultaneous quantification of left ventricular and atrial (LV, LA) volumes was found to be accurate and reproducible in patients with good images. We sought to prospectively test its feasibility and accuracy in consecutive patients in relationship with image quality and reader experience.
Methods
Three hundred consecutive patients underwent 3DE. Image quality was graded as poor, adequate, or good. Images were analyzed by an expert echocardiographer to obtain LV volumes and ejection fraction (EF) and LA volume using the automated analysis (HeartModel, Philips, Andover, MA) with and without editing the endocardial boundaries and using conventional manual tracing (QLAB, Philips, Andover, MA) blinded to the automated measurements as a reference. In a subgroup of 100 patients, automated analysis was repeated by two readers without 3DE experience.
Results
Automated analysis failed in 31/300 patients (10%). Patients with poor image quality (n = 72, 24%) showed suboptimal agreement with the reference technique, especially for LVEF. Importantly, patients with adequate (n = 89, 30%) and good (n = 108, 36%) images showed small biases and excellent correlations without border corrections, which were further improved with editing. In contrast, border corrections by inexperienced readers did not improve the agreement with reference values.
Conclusions
Automated 3DE analysis allows accurate quantification of left-heart size and function in 66% of consecutive patients, while in the remaining patients, its performance is limited/unreliable due to image quality. Border corrections require 3DE experience to improve the accuracy of the automated measurements. In patients with sufficient image quality, this automated approach has the potential to overcome the workflow limitations of the 3D analysis in clinical practice.
Keywords: 3D echocardiography, Cardiac chamber quantification, Automation
Three-dimensional (3D) echocardiography (3DE) has been shown to have advantages over two-dimensional (2D) imaging in multiple areas and thus has been gradually incorporated into clinical routine in many echocardiography laboratories throughout the world. Improved accuracy and reproducibility of the quantification of cardiac chamber size and function is one of the major advantages of 3DE over 2D echocardiography (2DE). This is because the volumetric 3DE approach, which directly counts pixels inside the endocardial surface, does not rely on geometrical assumptions and thus avoids the risk of underestimating chamber volumes due to the use of foreshortened views,1–3 which are common with 2DE. The equipment and analysis software of 3DE is now widely available, and the rising numbers of publications have placed this technology as an evolving new standard for chamber quantification. The higher accuracy and reproducibility translate into improved clinical prognostic significance, which is the reason why 3DE is the recommended technique by the recently published guidelines for quantification of left-heart chambers.4 Nevertheless, currently available analysis techniques rely on extensive user input, which requires expertise and adversely affects the workflow and thus impedes the implementation in busy clinical laboratories.1,5,6 As a result, most clinical laboratories still use traditional, frequently qualitative, 2DE assessment of cardiac function.
To overcome these limitations, we recently tested a new automated approach for left-heart chamber quantification based on an adaptive analytics algorithm. In a single-center study, we reported good accuracy and reproducibility, and improved speed of analysis, compared with the conventional 3DE methodology and cardiac magnetic resonance.7 In a more recent multicenter study, we showed that it is an accurate and robust alternative to conventional manual methodology, which yields almost the same values across laboratories and is more reproducible.8 However, these studies included only patients with good-quality images.
Furthermore, current 3DE acquisition is based on combining multiple beats (usually 4 to 6) to generate a single full-volume data set, which is needed to obtain a high enough frame rate for accurate analysis of cardiac function. This multibeat acquisition is associated with “stich artifacts,” which are particularly common in patients with arrhythmias and those who cannot hold their breath, precluding accurate analysis. To circumvent this limitation, the new automated analysis utilizes a different, high frame rate, single-beat 3D acquisition mode. However, the impact of this new acquisition mode on the accuracy of chamber size and function measurements is unknown.
Accordingly, the main goal of this study was to assess the feasibility of this automated technique in consecutive nonselected patients and evaluate the effects of image quality on its accuracy. The additional goals were to evaluate the effects of reader experience with 3DE and the high frame rate, single-beat acquisition mode on the accuracy of the automated analysis.
METHODS
Population and Study Design
We prospectively studied 300 consecutive nonselected patients (age, 63 ±17; female, 54%; body surface area, 1.9 ± 0.2 m2) referred for clinically indicated transthoracic echocardiograms for a wide range of suspected cardiovascular conditions (Table 1) who underwent in addition 3DE imaging. Noncooperative patients or those who refused to participate were excluded; no other exclusion criteria were applied. The protocol was approved by the Institutional Review Board, and informed consent was obtained from each patient.
Table 1.
Clinical characteristics of the 300 study patients
| Hypertension (0 = no, 1 = yes) |
Coronary artery disease (0 = no, 1 = yes) |
Cardiomyopathy (0 = no, 1 = ischemic, 2 = idiopathic) |
Valvular heart disease (0 = no, 1 = yes) |
Congenital heart disease (0 = no, 1 = yes) |
Congenital heart disease (0 = no, 1 = yes) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| 0 | 93 | 31 | 215 | 72 | 179 | 60 | 261 | 87 | 297 | 99 | 237 | 79 |
| 1 | 207 | 69 | 85 | 28 | 52 | 17 | 39 | 13 | 3 | 1 | 63 | 21 |
| 2 | 69 | 23 | ||||||||||
Images were analyzed by an experienced echocardiographer, who used the automated 3DE software to measure left heart chamber size and function indices, with and without endocardial boundary corrections. To generate a reference standard, the same reader used the conventional approach based on 3D-guided biplane measurements, while blinded to the results of the automated analysis. These comparisons were used to determine the accuracy of the automated analysis when images were classified by quality. In addition, to evaluate the effects of reader experience on the ability to effectively edit endocardial borders and thus potentially improve the accuracy of the automated analysis, measurements were repeated in a subset of randomly selected 100 patients by two readers without 3DE experience (third-year general cardiology fellows) and compared against the same reference standard.
To assess the effects of the high frame rate, single-beat acquisition on the accuracy of the 3DE measurements, 30 patients with good-quality images were imaged in addition using the conventional 4-beat full-volume mode. These 4-beat data sets were analyzed using conventional semiautomated volumetric analysis and used as the reference for comparisons.
Echocardiographic Imaging
Imaging was performed using the EPIQ system (version 7C, Philips Medical Systems, Andover, MA) and an X5-1 phased-array transducer with the patient in the left lateral decubitus position. Before each acquisition, images were optimized for endocardial visualization by modifying the gain, compress, and time-gain compensation controls. Image acquisition included wide-angled, single-beat, high frame rate 3DE data sets (HM ACQ key on the EPIQ system) from the apical position during a single breath hold. Care was taken to include the entire left ventricular (LV) and left atrial (LA) cavity within the 3DE images. Imaging depth and sector width were optimized to obtain the highest possible frame rate. In addition, in a subset of 30 patients, a conventional 4-beat full-volume acquisition was performed in the same setting using the same equipment.
Three-Dimensional Echocardiography Image Analysis
Images were reviewed and analyzed by an expert echocardiographer with extensive training in 3DE. First, the image quality of the 3DE images was graded by reviewing two-, three-, and four-chamber views extracted from the 3D data set as poor (more than two of six contiguous segments not visualized in any view or two of six contiguous segments in at least two different views), adequate (not more than two of six not well visualized contiguous segments in one view and one or fewer in the other views), and good (better than adequate).
Then the automated analysis was performed (HeartModel [HM], Philips) to obtain LV end-diastolic (ED) and end-systolic (ES) volumes (EDV, ESV) and LA volume (LAV) measurements, and LV ejection fraction (EF) was calculated. Analysis methodology was described in detail in our recent publications.7,8 Briefly, the software simultaneously detects LV and LA endocardial surfaces using an adaptive analytics algorithm, which uses knowledge-based identification to orient and locate cardiac chambers and patient-specific adaptation of endocardial borders. The algorithm automatically identifies the ED and ES phases of the cardiac cycle, and creates ED and ES 3D casts of the LV cavity and an ES cast of the LA cavity, from which LV and LA volumes are derived directly without geometrical assumptions. Manual corrections of the LV and LA endocardial surfaces are possible, when the operator judges the automatically detected surface as suboptimal. This is achieved by displaying the LA and LV contours on four-, three-, and two-chamber cut planes extracted from the 3DE data sets and allowing the user to edit the contours to optimize the match between the detected and the perceived endocardial boundaries (Figure 1).
Figure 1.
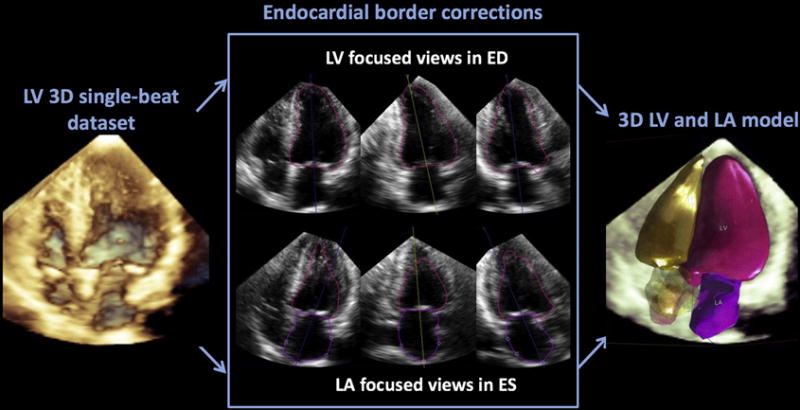
Automated technique for left-heart 3D chamber quantification. Following initial fully automated detection of LV and LA endocardial surfaces from a high-frame rate single-beat 3DE data set (left), the software allows the user to perform manual corrections of the endocardial boundaries when needed (center), resulting in 3D casts of the cardiac chambers (right). The optional corrections are performed in anatomically correct nonforeshortened 2D planes showing focused long-axis views of the left ventricle (top) and left atrium (bottom), both automatically extracted from the 3D data set. (Note that the program displays right ventricular and atrial casts but no volume values are provided because they have not been validated.)
These measurements were compared to LV EDV, ESV, EF, and LAV values obtained with conventional 3DE software using the 3D-guided biplane approach (3DQ, QLAB, Philips) based on manual initialization of the endocardial boundaries in nonforeshortened views extracted from the 3DE data sets. These anatomically correct LV- and LA-focused apical two- and four-chamber views were identified as those in which the long-axis dimension of the relevant chamber was maximized. The ED and ES frames used for analysis were the same ones chosen by the automated technique. After mitral annular points were marked in each view, and an additional point was placed to mark either the LV apex for LV analysis, or the most distal point on the LA roof for the LA analysis, the endocardial border was automatically identified. After manually editing the borders as deemed necessary, LV EDV, ESV, and LAV were obtained and LVEF was calculated. The reader was blinded to the results of the automated measurements during this conventional analysis.
In addition, in a subset of randomly selected 100 patients, the automated analysis was repeated by two readers without 3DE experience who received minimal training with the HM software and were instructed to edit the automatically detected endocardial boundaries when deemed necessary to optimize border position. Their measurements were compared with the same reference values generated by the expert reader using the above 3D-guided biplane methodology, in order to assess the effects of 3DE experience.
Finally, in the subset of 30 patients with both single-beat and 4-beat images, the latter data sets were analyzed by the expert reader using the volumetric approach, which does not rely on geometrical assumptions (4D LV Analysis software, a module of Research Arena 2.0, TomTec Imaging Systems, Unterschleissheim, Germany). This methodology has been extensively used previously, including publications from our laboratory.2,9–11 Briefly, after the long axis of the relevant chamber is identified, the software creates a 3D cast, which is automatically tracked throughout the cardiac cycle using speckle-tracking. Fine-tuning of the endocardial surface was performed interactively to optimize boundary position as necessary. Finally, the actual chamber volume inside each cast was calculated throughout the cardiac cycle and used to determine EDV, ESV, EF, and LAV.
Statistics
For each parameter, the comparisons included linear regression with Pearson correlation coefficients and Bland-Altman analyses to assess the bias and limits of agreement (defined as 2 SD around the mean). Values of P < .05 by t tests were considered statistically significant.
RESULTS
The average frame rate for the single-beat data sets was 19 ± 3 Hz. Semiautomated 3DE-derived maximal LV EDV ranged between 62 and 555 mL (median, 153 mL), ESV between 22 and 468 mL (median, 80 mL), EF between 5% and 79% (median, 47%), and LAV between 15 and 242 mL (median, 72 mL).
The automated software failed in 31 (10%) out of the 300 consecutive nonselected patients because of substandard image quality or complex congenital heart disease. Figure 2 shows an example of a failed analysis, both for the LV and LA (top and bottom panels, respectively). These 31 patients were not included in the evaluation of the accuracy of the automated analysis. The comparisons in the remaining 269 patients showed overall excellent agreement between the automated 3DE and 3D-guided biplane volume measurements, with minimal biases and correlation coefficients above 0.90 for volumes, but a lower value of 0.79 for LVEF (Figure 3). However, there was a considerable number of patients in whom the agreement between the two techniques was suboptimal, as evidenced by the outlying data points (Figure 3, top) especially obvious for LVEF, and relatively wide limits of agreement (Figure 3, bottom). Figure 4 shows the same data without the 72 patients with poor-quality images (total of 197 patients) and depicts a considerably tighter distribution of data points, resulting in even better correlations across all measured parameters, including LVEF with r = 0.94 and narrower limits of agreement. Interestingly, the levels of intertechnique agreement were similar between patients with good and adequate images (Table 2), indicating that the automated measurements were accurate in 197/300 (66%) of consecutive patients. On the other hand, as expected, the intertechnique agreement was worse in patients with poor image quality, with wide limits of agreement for all parameters and a correlation of only 0.49 for LVEF, despite higher correlations for volumes.
Figure 2.
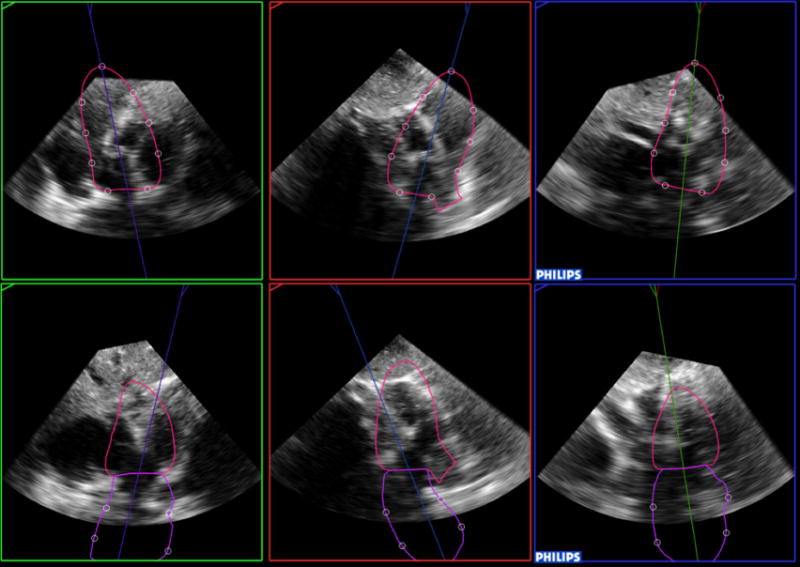
Example of a failed analysis, both for the left ventricle and left atrium (top and bottom, respectively). The software was not able to correctly identify cardiac chambers and extracted from the 3D data set incorrect apical views, one of which (top left) coincidentally looks similar to a subcostal view.
Figure 3.
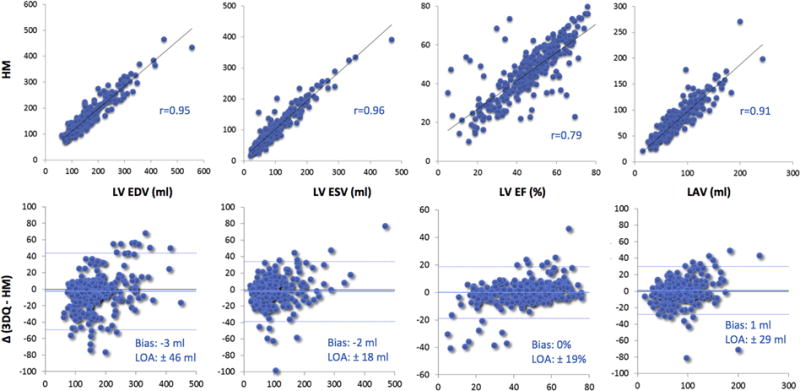
Intertechnique comparisons for left heart chamber quantification in the entire study group (n = 269). Correlation and Bland-Altman analysis for the automated measurements with contour correction against the conventional manual technique. LOA, Limits of agreement.
Figure 4.
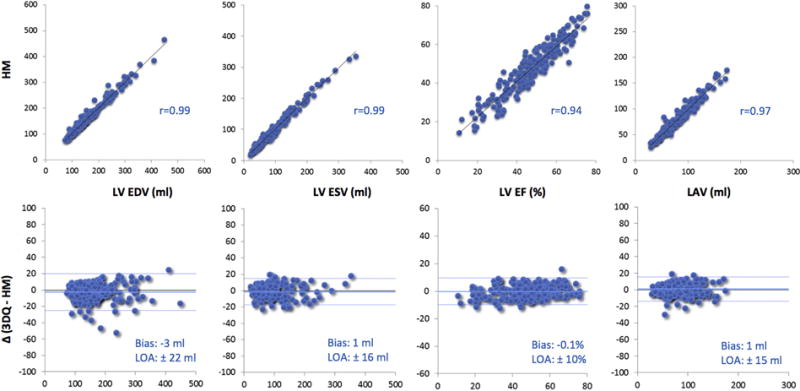
Intertechnique comparisons for left heart chamber quantification in patients with adequate or better image quality (n = 197). Data are presented in the same format as in Figure 3. LOA, Limits of agreement.
Table 2.
Impact of image quality on the accuracy of the automated chamber quantification
| Quality | EDV (mL) | ESV (mL) | EF (%) | LAV (mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Bias ± SD | r value | Bias ± SD | r value | Bias ± SD | r value | Bias ± SD | r value | |
| Good (n = 108) | −0.5 ± 9.0 | 0.99 | −0.6 ± 7.0 | 0.99 | 0.1 ± 4.4 | 0.94 | 1.3 ± 6.5 | 0.97 |
| Adequate (n = 89) | −5.1 ± 13 | 0.98 | −2.0 ± 9.2 | 0.99 | −0.4 ± 5.5 | 0.93 | 0.4 ± 8.2 | 0.97 |
| Poor (n = 72) | −2.7 ± 41 | 0.89 | −4.8 ± 32 | 0.89 | 0.3 ± 16 | 0.49 | 1.8 ± 26 | 0.81 |
Comparisons between the automated analysis and 3D-guided biplane measurements in the three image quality groups (n = 269).
The effects of experience were tested in 71/100 patients, after excluding 19 patients with poor-quality images and 10 in whom the automated algorithm failed. As expected with the deterministic nature of the fully automated analysis, measurements made without endocardial border corrections were identical irrespective of reader experience. However, unlike the effects of border corrections made by the expert, which improved the accuracy of the measurements, when borders were edited by readers without 3DE experience, no clear improvement was noted (Table 3).
Table 3.
Impact of experience on the accuracy of the automated chamber quantification
| HM experience | EDV (mL) | ESV (mL) | EF (%) | LAV (mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Bias ± SD | r value | Bias ± SD | r value | Bias ± SD | r value | Bias ± SD | r value | |
| No editing | −12 ± 29 | 0.97 | −2.2 ± 30 | 0.96 | −3.4 ± 17 | 0.69 | −6.7 ± 19 | 0.95 |
| Experienced with editing | 4.9 ± 26 | 0.98 | 1.8 ± 17 | 0.99 | 0.3 ± 10 | 0.90 | −1.2 ± 14 | 0.98 |
| Inexperienced with editing | −17 ± 39 | 0.95 | −20 ± 33 | 0.96 | 7.4 ± 17 | 0.71 | −8.2 ± 19 | 0.95 |
Comparisons between 3D-guided biplane and the automated measurements with and without endocardial boundary corrections, by experienced and inexperienced readers, in patients with adequate or good-quality images (n = 71).
The effects of the high frame rate single-beat acquisition mode were tested in 30 patients with good-quality images. Frame rates were similar for the single-beat and the 4-beat full-volume acquisition modes: 19 ± 2 and 20 ± 2 (P = .43). Without contour corrections, intertechnique comparisons showed strong correlations (Table 4), with the single-beat images slightly underestimating LV volumes, EF, and LAV. Contour corrections improved the measurements only modestly, indicating that with good-quality images, border corrections are not essential.
Table 4.
Evaluation of the effects of single-beat acquisition
| Single beat acquisition effects | EDV (mL) | ESV (mL) | EF (%) | LAV (mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Bias ± SD | r value | Bias ± SD | r value | Bias ± SD | r value | Bias ± SD | r value | |
| No editing | −12 ± 16 | 0.96 | −1.2 ± 18 | 0.95 | −4.8 ± 6.7 | 0.92 | −5.6 ± 10 | 0.96 |
| With editing | 2.5 ± 17 | 0.96 | 5.2 ± 14 | 0.97 | −3.0 ± 5.2 | 0.95 | 5.4 ± 8.1 | 0.97 |
Comparisons between the automated analysis of high frame rate single-beat data sets and 3D-guided biplane measurements from 4-beat full-volume data sets in patients with good-quality images (n = 30).
DISCUSSION
Left-heart chamber quantification is critical for both clinical management and clinical trials. The recent chamber quantification guidelines emphasize that 3DE measurements are preferred over 2DE because of their superior accuracy and reproducibility.4 However, 3DE has not been widely incorporated into routine clinical practice because of the workflow limitations. We recently tested this automated technique and found it to be fast, accurate, and reproducible, while providing almost the same values across laboratories.7,8 These previous studies were performed in patients with good image quality by strict inclusion criteria similar to those used in the present study and analyzed by 3DE expert readers. The purpose of the current study was to assess additional clinically important questions, including (1) the feasibility of this new automated technique in consecutive, nonselected patients in a busy echocardiography laboratory, (2) the importance of reader 3DE experience, and (3) the implications of the new single-beat acquisition mode.
We found that the new automated technique failed in about 10% of the patients. This rate of failure of the automated algorithm is not surprising and is comparable to the percentage of patients with substandard images not suitable for echocardiographic analysis. When compared with 3D-guided biplane measurements performed by an expert reader, this algorithm provided unreliable results in 24% of the patients because of poor image quality. In this subgroup, we considered the measurements to be unreliable, despite the high correlations with the reference technique for volumes, because of the wide limits of intertechnique agreement, indicating inaccuracy in individual patients and also because of the moderate at best correlation for LVEF, probably as a result of compounding of errors in EDV and ESV measurements.
Importantly, however, the automated analysis of left-heart chambers was highly accurate in 66% of the patients with images of adequate or good quality, and the accuracy was similar in these two subgroups, indicating that this algorithm is suitable for routine use in two-thirds of nonselected consecutive patients seen in a busy clinical laboratory. The accuracy of the algorithm was further improved by endocardial border corrections when deemed necessary and performed by an experienced reader, but not by readers without 3DE training. However, given the high accuracy of the fully automated measurements without border corrections in patients with adequate or better image quality, one may argue that corrections are not essential in this patient population. Therefore, images that fulfill the simple quality criteria defined in this study can be analyzed with relative accuracy by less experienced readers who do not have sufficient 3DE training or lack confidence to edit endocardial borders in 3DE images.
Finally, we found that the automated analysis of left-heart chamber size and function from high frame rate single-beat 3DE images, with or even without corrections, is accurate when compared with conventional volumetric analysis of 4-beat full-volume data sets. This new acquisition mode has the advantage of avoiding one of the most frequent problems of multibeat 3DE imaging, commonly referred to as “stich” artifacts. The consequence of this incremental improvement is that it allows clinical use of 3DE imaging in a considerably higher number of patients in whom 3D analysis has been challenging, such as patients with frequent arrhythmias (21% in our cohort) and/or those unable to hold their breath.
Another advantage of the approach tested in this study is that in addition to the LV quantification, it simultaneously measures LAV from the same 3DE data set. As recommended by the guidelines,4 LAV measurements should be performed in LA focused views to avoid foreshortening and thus underestimating volumes. The new software automatically detects the LA boundaries in 3D space, measures the actual LAV without any geometrical assumptions, and is thus more accurate than 2DE methodology, which requires dedicated LA focused views for accurate biplane measurements.12 Similar to LV endocardial contour editing, the new software allows correction of LA borders. These corrections are also performed on three anatomically correct nonforeshortened LA-focused views, which are also automatically extracted from the 3DE data set and displayed similar to that used for LV border editing (Figure 1, center bottom). With this approach, accurate automated LAV measurements were obtained in this study.
Limitations
This study has several limitations. First, the percentage of patients with adequate or better image quality may vary between echocardiography laboratories and is likely to be higher in laboratories with 3DE experience, such as ours, as well as certain subpopulations of patients. Also, the image quality criteria defined in this study are arbitrary and may be defined differently in other studies, which is likely to affect the percentage of patients suitable for analysis.
Additionally, the inexperienced readers who participated in our study had no prior experience with analysis of 3DE images, with the exception of brief training with the HM software. Therefore, our conclusion that border corrections by nonexperts do not improve the accuracy of chamber quantification should be interpreted with the understanding that this limitation is likely to alleviated with training and experience with 3DE.
Another limitation is the potential bias in the part of the study designed to evaluate the effects of reader experience. This is because the subgroup of 100 studies was analyzed by both an expert and nonexperts using the automated technique, while both were compared to the reference standard generated by the same expert.
One might wonder about our choice of the reference technique for the evaluation of the effects of the single-beat acquisition mode. For this part of the study we used the volumetric analysis over the 3D-guided biplane approach used in the main protocol, in order to maximize the accuracy of the reference technique and thus to improve our ability to detect differences in a smaller sample.
CONCLUSION
In summary, the new, automated 3DE analysis allows accurate measurements of LV and LA volumes in two of three consecutive nonselected patients with adequate or better image quality. While 3DE experience is needed to maximize the accuracy of this approach by endocardial border corrections, its fully automated default version is reasonably accurate and can be performed by less experienced readers in this patient population. This automated approach has the potential to overcome the workflow limitations of 3DE and may thus facilitate further integration of 3DE quantification in routine clinical practice.
Acknowledgments
We thank Mary Kay Bianchi, David Prater, Jane Vogel, and Alexandra Goncalves from Philips Healthcare for their help and support.
The study was supported by a research grant from Philips Healthcare.
Abbreviations
- 2D
Two-dimensional
- 2DE
Two-dimensional echocardiography
- 3D
Three-dimensional
- 3DE
Three-dimensional echocardiography
- ED
End-diastolic
- EDV
End-diastolic volume
- EF
Ejection fraction
- ES
End-systolic
- ESV
End-systolic volume
- HM
HeartModel
- LA
Left atrial
- LAV
Left atrial volume
- LV
Left ventricular
Footnotes
Thomas Ryan, MD, FASE, served as guest editor for this report.
References
- 1.Mor-Avi V, Jenkins C, Kuhl HP, Nesser HJ, Marwick T, Franke A, et al. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovasc Imaging. 2008;1:413–23. doi: 10.1016/j.jcmg.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Mor-Avi V, Yodwut C, Jenkins C, Kuhl H, Nesser HJ, Marwick TH, et al. Real-time 3D echocardiographic quantification of left atrial volume: multicenter study for validation with CMR. JACC Cardiovasc Imaging. 2012;5:769–77. doi: 10.1016/j.jcmg.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, et al. Quantitative assessment of left ventricular size and function: side-by-side comparison of real-time three-dimensional echocardiography and computed tomography with magnetic resonance reference. Circulation. 2006;114:654–61. doi: 10.1161/CIRCULATIONAHA.106.626143. [DOI] [PubMed] [Google Scholar]
- 4.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Muraru D, Badano LP, Ermacora D, Piccoli G, Iliceto S. Sources of variation and bias in assessing left ventricular volumes and dyssynchrony using three-dimensional echocardiography. Int J Cardiovasc Imaging. 2012;28:1357–68. doi: 10.1007/s10554-011-9985-0. [DOI] [PubMed] [Google Scholar]
- 6.Tsang W, Kenny C, Adhya S, Kapetanakis S, Weinert L, Lang RM, et al. Interinstitutional measurements of left ventricular volumes, speckle-tracking strain, and dyssynchrony using three-dimensional echocardiography. J Am Soc Echocardiogr. 2013;26:1253–7. doi: 10.1016/j.echo.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Tsang W, Salgo IS, Medvedofsky D, Takeuchi M, Prater D, Weinert L, et al. Transthoracic 3D echocardiographic left heart chamber quantification using an automated adaptive analytics algorithm. JACC Cardiovasc Imaging. 2016;9:769–82. doi: 10.1016/j.jcmg.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Medvedofsky D, Mor-Avi V, Amzulescu M, Fernandez-Golfin C, Hinojar R, Monaghan MJ, et al. Three-dimensional echocardiographic quantification of the left-heart chambers using an automated adaptive analytics algorithm: multicentre validation study. Eur Heart J Cardiovasc Imaging. 2017 doi: 10.1093/ehjci/jew328. (in press) [DOI] [PubMed] [Google Scholar]
- 9.Yodwut C, Weinert L, Klas B, Lang RM, Mor-Avi V. Effects of frame rate on three-dimensional speckle-tracking-based measurements of myocardial deformation. J Am Soc Echocardiogr. 2012;25:978–85. doi: 10.1016/j.echo.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Addetia K, Bhave NM, Tabit CE, Gomberg-Maitland M, Freed BH, Dill KE, et al. Sample size and cost analysis for pulmonary arterial hypertension drug trials using various imaging modalities to assess right ventricular size and function end points. Circ Cardiovasc Imaging. 2014;7:115–24. doi: 10.1161/CIRCIMAGING.113.000932. [DOI] [PubMed] [Google Scholar]
- 11.Maffessanti F, Patel AR, Patel MB, Walter JJ, Mediratta A, Medvedofsky D, et al. Non-invasive assessment of the haemodynamic significance of coronary stenosis using fusion of cardiac computed tomography and 3D echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18:670–80. doi: 10.1093/ehjci/jew147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kebed K, Kruse E, Addetia K, Ciszek B, Thykattil M, Guile B, et al. Atrial-focused views improve the accuracy of two-dimensional echocardiographic measurements of the left and right atrial volumes: a contribution to the increase in normal values in the guidelines update. Int J Cardiovasc Imaging. 2017;33:209–18. doi: 10.1007/s10554-016-0988-8. [DOI] [PubMed] [Google Scholar]


