Abstract
PURPOSE
To investigate the effect of reducing tooth preparation and ceramic thickness on fracture resistance of lithium disilicate crowns.
MATERIALS AND METHODS
Specimen preparation included a standard complete crown preparation of a typodont mandibular left first molar with an occlusal reduction of 2 mm, proximal/axial wall reduction of 1.5 mm, and 1.0 mm deep chamfer (Group A). Another typodont mandibular first molar was prepared with less tooth reduction: 1 mm occlusal and proximal/axial wall reduction and 0.8 mm chamfer (Group B). Twenty crowns were milled from each preparation corresponding to control group (n=5) and conditioned group of simultaneous thermal and mechanical loading in aqueous environment (n=15). All crowns were then loaded until fracture to determine the fracture load.
RESULTS
The mean (SD) fracture load values (in Newton) for Group A were 2340 (83) and 2149 (649), and for Group B, 1752 (134) and 1054 (249) without and with fatigue, respectively. Reducing tooth preparation thickness significantly decreased fracture load of the crowns at baseline and after fatigue application. After fatigue, the mean fracture load statistically significantly decreased (P<.001) in Group B; however, it was not affected (P>.05) in Group A.
CONCLUSION
Reducing the amount of tooth preparation by 0.5 mm on the occlusal and proximal/axial wall with a 0.8 mm chamfer significantly reduced fracture load of the restoration. Tooth reduction required for lithium disilicate crowns is a crucial factor for a long-term successful application of this all-ceramic system.
Keywords: Fatigue, Lithium disilicate, Tooth preparation, Thermocycling, Fracture load
INTRODUCTION
Tooth preparation for a complete crown restoration requires considerable tooth reduction1 and varies depending on the ceramic system to be used. The preparation guidelines for all-ceramic crowns as recommended by many manufacturers require a minimal ceramic thickness of 1.5 mm on axial/occlusal and 1.0 mm at the cervical region. These invasive preparations lead to the loss of up to 75% of the coronal part of a tooth,1 which raises the question of whether the tooth preparation required by the manufacturers is imperative for the successful application of all-ceramic restorations.
All-ceramic restorations thickness requirements were recommended based on the material's mechanical properties2 and traditional laboratory testing to optimize the restoration strength as a stand-alone item with less concentration on the crown-tooth complex.3 A high ceramic thickness is needed to achieve high aesthetic standards and to avoid restoration fracture during service. However, the restoration fracture is a multifactorial issue influenced by a combination of variables including tooth preparation and restoration geometry, restoration mechanical properties, cementation material, and progressive damage caused by occlusal function.4,5
Clinical studies of restoration fracture are generally expensive, time-consuming, difficult to standardize, and they involve ethical constraints.6,7 Nevertheless, all-ceramic restorations typically fail after many years in service, which indicates fatigue failure rather than acute overload.8 Therefore, in vitro testing which involves cyclic loading in simulated oral environment can provide scientifically based data to assess the failure risks of a restoration in vivo.9 It offers clinically relevant results when compared to the traditional testing methodologies such as static loading of standard test specimens in the form of a bar, a disk, or a restoration.10
Reducing the amount of tooth preparation required for all-ceramic crown necessitates the development of strong restoration which can successfully function and survive in the oral cavity at lower thicknesses. The unique features of lithium disilicate (LD) such as high strength, superior aesthetics, and promising in vivo and in vitro results2,11,12,13,14,15,16,17,18,19,20 make it an attractive material for research and development. The current study aims to evaluate fracture load of LD crowns with suggested minimal tooth preparation by using anatomically correct crown specimens and applying mechanical loading and thermocycling. We hypothesize that fracture load of LD crowns with reduced tooth preparation and subsequent crown thickness will not be significantly different from that recommended by the manufacturer.
MATERIALS AND METHODS
Specimens' preparation included a standard complete crown preparation of a typodont mandibular left first molar (Nissin Dental Products Inc., Kyoto, Japan) with an occlusal reduction of 2 mm, a proximal/axial wall reduction of 1.5 mm, and 1.0 mm deep chamfer (Group A). Another typodont mandibular first molar (Nissin Dental Products Inc., Kyoto, Japan) was prepared with less tooth reduction; 1 mm occlusal and proximal/axial wall reduction and 0.8 mm chamfer (Group B). The preparation was carried out by an experienced prosthodontist using a silicone index of an unprepared tooth to achieve the required tooth reduction. The prepared tooth was then scanned (3Shape A/S, Copenhagen, Denmark). Fully anatomically shaped molar crown was virtually designed according to each preparation. The thickness of the crowns at different surfaces was corresponding to the tooth reduction.
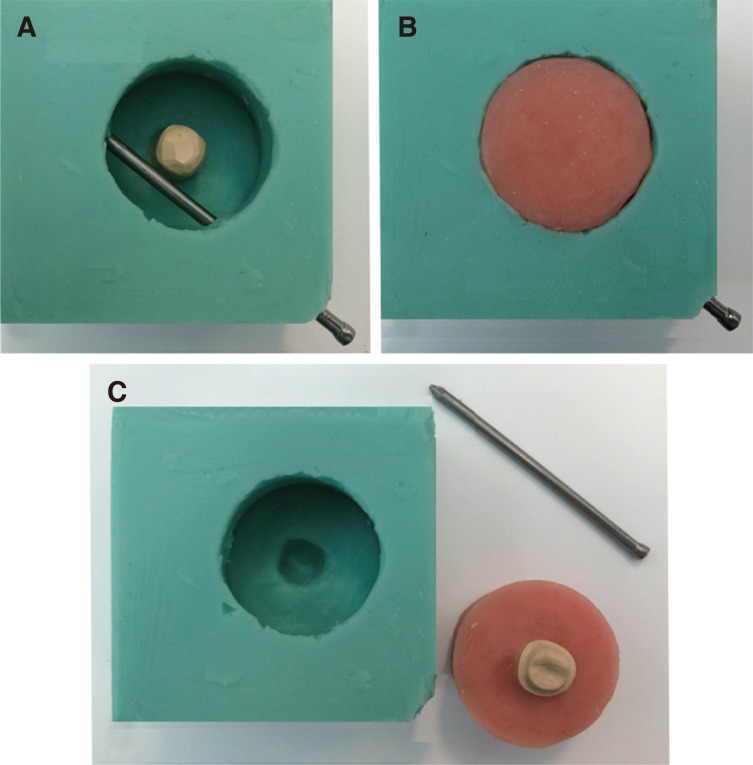
Vinyl polysiloxane impressions (3M ESPE, St. Paul, MN, USA) of the prepared master dies were made to create 20 replicas of each master die representing the manufacturer's recommended tooth preparation (Group A) and the suggested minimal tooth preparation (Group B). All specimens were then designed to suit the specimen cup of the chewing simulator (CS-4.8, SD Mechatronik GmbH, Feldkirchen-Westerham, Germany) and a specially designed jig which was used to hold the specimens during fatigue and a single load to fracture testing. Therefore, a silicone mould replica (Exaktosil N21, Bredent, Germany) of the specimen cup was created, and acrylic resin (Palapress vario, Heraeus Kulzer, Wehrheim, Germany) was mixed and poured to cover the die up to 2.0-mm away from the finish line as shown in Fig. 1.
Fig. 1. Preparation of crown holders according to the specimen cup of the chewing simulator. Epoxy resin die is positioned in its corresponding place in the silicon mould with a hole created using metal pin to fix the specimen during testing (A), the mould is filled with acrylic resin (B), the metal pin and specimen holder are removed from the mould (C).
Twenty specimens were prepared from each of the preparations. Crowns were milled from lithium disilicate e.max CAD blocks (Ivoclar Vivadent, Shaan, Liechtenstein) using 5-axis milling machine (DMG 20/Mori Seiki, Japan). Glazing was combined with the crystallization process using Programat EP 3000 furnace (Ivoclar Vivadent, Shaan, Liechtenstein). Dies were sandblasted with 100 µm AL2O3 at a pressure of 1-bar to enhance their cementation. All crowns were cemented to the epoxy resin dies using the recommended cement (Variolink II, Ivoclar Vivadent, Shaan, Liechtenstein) and according to the manufacturer's instructions. After cementation, the specimens were incubated in water until testing. Crowns were then divided into four subgroups: A1 (n = 5), A2 (n = 15), B1 (n = 5), and B2 (n = 15) without fatigue and with fatigue, respectively.
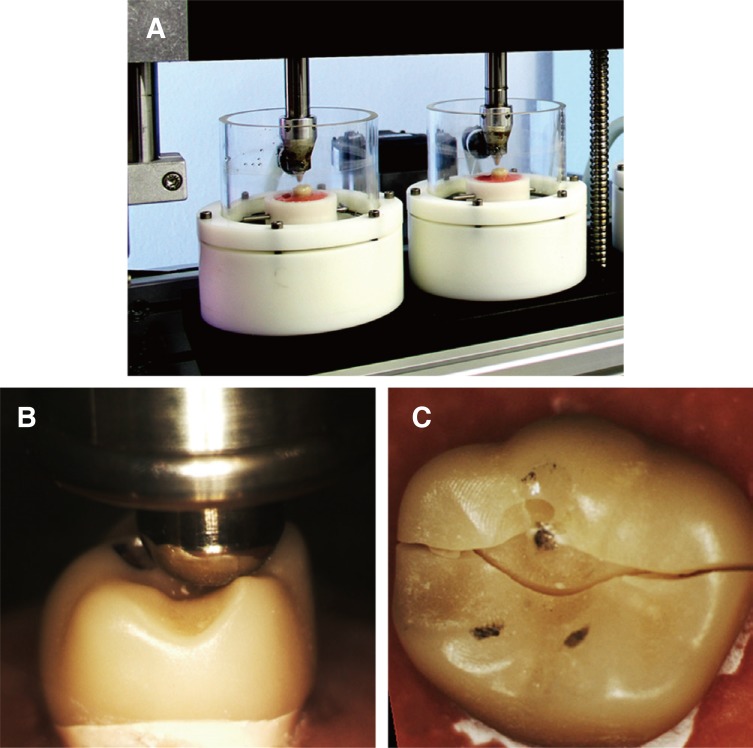
Fifteen specimens from each group (A2 and B2) were subjected to Thermal mechanical loading in the chewing simulator (CS-4.8, SD Mechatronik GmbH, Feldkirchen-Westerham, Germany) as seen in Fig. 2. A number of cycles of 250000 has been frequently reported in the literature to represent one year in service.2,15,17 Thus, specimens in this study were subjected to 1.5 million stress cycles to represent 6 years of clinical service. The loading protocol consisted of three phases. In the first loading phase, the force applied was 50 N for 500,000 cycles. In the second phase, crowns were loaded with 100 N for 500,000 cycles, and in the last phase the load was increased to 150 N for 500,000 cycles. Loading frequency all through testing was set at 1.2 Hz. A stainless steel indenter in a round cone shape with 3.18 mm diameter was used to load the specimens during chewing simulation. The exact initial position of the indenter at the distobuccal cusp was assured using an articulating paper. During each cycle, the indenter contacts the crowns at this point, applies the load, and slides 0.5 mm toward the central fossa. Mouth opening distance was set at 6 mm to simulate aspects of natural occlusion. Concurrently, thermocycling with a temperature extremes of 5℃ and 55℃ in distilled water (dwell time: 30 seconds, pause time: 13 seconds) was performed in the computerized thermocycling unit (SD Mechatronik GmbH, Feldkirchen-Westerham, Germany). The specimens were dried and inspected for cracks, chipping or fracture after each loading phase.
Fig. 2. Specimens undergoing thermal mechanical testing in the chewing simulator (A), tripod occlusal contact of the spherical indenter during single load to fracture test (B & C).
Then, all crowns (fatigued and unfatigued) were loaded in the universal testing machine (Loyds Instrument Model LRX, Fareham, England) until failure. The specimens were fixed in the same reproducible position in the specially designed jig to attain tripod contact configuration between the indenter and the crown by touching the distobuccal cusp and the palatal cusps (Fig. 2). A 6-mm diameter stainless steel spherical indenter was used to apply the load vertically until failure with a crosshead speed of 1 mm/min. Fracture load for each crown was recorded in Newton.
Results were analysed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA). Normal distribution of data was confirmed before statistical assessment used Kolmogorov-Smirnov test. Descriptive statistical analysis was performed and t-test was used to analyse the differences between the groups (P < .05). Including the crown cracked during aging in the fracture test will more likely reduce the mean fracture load of the group compared to the other groups because of the pre-existing damage. Therefore, a fracture force is equivalent to 90% of the lowermost fracture force recorded in the group where the fractured crown belong was assigned to the crown that failed during chewing simulation to obtain a normal distribution of data.
RESULTS
All crowns in Group A survived fatigue testing; no failure was observed during any of the three loading phases resulting in 100% survival rate. A crack was noticed under the indenter contact point of only one specimen in subgroup B2 at the end of the last loading phase (150 N), which corresponds to a failure frequency of 6.7%. All fatigued specimens in both groups suffered excessive wear at the indenter contact point.
Table 1 shows the mean and standard deviation of single load to the fracture test for each group. Independent t-test revealed that tooth preparation and subsequent crown thickness had a significant influence on the fracture load (P < .05). Within each tooth preparation category, the fatigue effect on crown's fracture load appears to depend on the amount of tooth preparation performed. While it statistically significantly decreased by 40% (from 1752 to 1054 N, P < .001) for crowns with suggested minimal tooth preparation, it was not affected (P > .05) in crowns with the recommended tooth preparation where fracture load decreased by only 8.2%.
Table 1. Descriptive statistics (mean and standard deviation) of fracture load in Newtons.
| Group | Fracture load in Newton (SD) | |
|---|---|---|
| Control (n = 5) | After 1.5 m cycle (n = 15) | |
| A | 2340 (83)A, a | 2149 (649)A, a |
| B | 1752 (134)B, a | 1054 (249)B, b |
Within each column; different capital superscripts indicate heterogeneous subsets (P < .05).
Within each row; similar small superscripts indicate homogeneous subsets (P > .05).
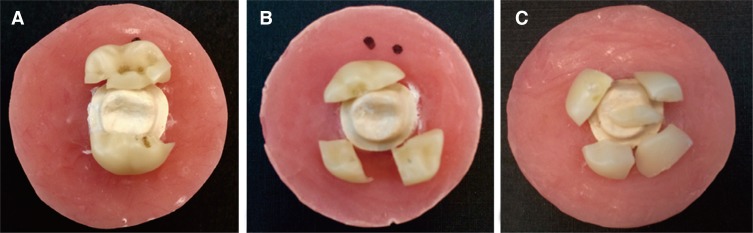
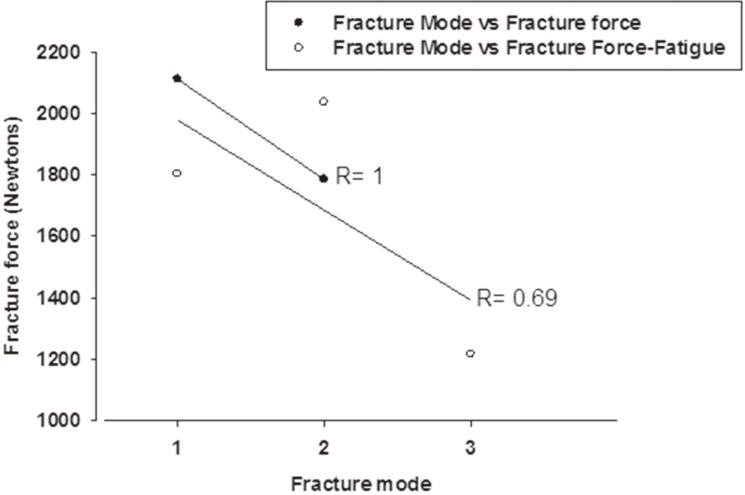
Bulk fracture was the predominant failure observed. Three fracture modes with different number of main fractured pieces were seen among the specimens tested (Fig. 3). Only one specimen failed pre-maturely during the chewing simulation. The remaining 39 specimens exhibited modes of fractures as shown in Table 2. These modes varied along with the corresponding fracture load values. Almost half the specimens exhibited Mode I fracture (49%), followed by Mode III (28%) and lastly Mode II (23%). Fractures forces were above 1900 N for modes I & II and dropped significantly to 1200 N for mode III. The drop in force and increase in number of shattered pieces had linear correlation (negative) and R values were 1 and 0.69 for nonfatigued and fatigued specimens respectively (Fig. 4). Tooth reduction and the subsequent crown thickness had significant effect on the fracture modes. While control groups (A1 and B1) exhibited majorly mode I fracture, fatigued groups significantly differed. It was Mode I for group A2 (60%) and Mode III for group B2 (67%).
Fig. 3. Fracture modes as presented after fracture test. Fracture runs mesiodistally through the central fossa (A), Fracture runs mesiodistally and through the lingual groove (B), Fracture runs mesiodistally, through the mesiobuccal and lingual grooves (C).
Table 2. Fracture modes presented by groups.
| Fracture mode | No of specimens (%) | Load (SD) in Newton | Fracture modes by group (No of specimens) (%) | |||
|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | |||
| I: Fracture along the mesiodistal plane running through the central fossa | 19 (49) | 1933 (583) | 5 (100) | 9 (60) | 3 (60) | 2 (13) |
| II: Fracture runs mesiodistally and along the lingual groove | 9 (23) | 1980 (871) | - | 5 (33) | 2 (40) | 2 (13) |
| III: Fracture runs mesiodistally, along the mesiobuccal and lingual grooves | 11 (28) | 1216 (210) | - | 1 (7) | - | 10 (67) |
| Crack under the indenter contact point during fatigue | n/a | - | n/a | 1 (7) | ||
Fig. 4. Graph showing linear correlation between fracture mode (number of fractured pieces) and fracture force.
DISCUSSION
In this study we hypothesized that fracture load of LD crowns with minimal tooth preparation and subsequent crown thickness will not be significantly different from that prepared as recommended by the manufacturer. However, the results show that crowns with recommended tooth preparation had a significantly higher fracture load than crowns with suggested minimal tooth preparation regardless of fatigue application. We also found that fatigue significantly influenced the fracture load of crowns with suggested minimal tooth preparation but failed to affect crowns with recommended tooth preparation. Therefore, the hypothesis of the study was rejected.
The results of this study agree with Rekow et al.21 who indicated that crown material and crown thickness were the most significant factors that influence fracture probability. Similarly, Guess et al.22 linked the high reliability of LD crowns to two main reasons: the thickness of their specimens especially at the occlusal surface that reached 2 mm and the capability of the CAD/CAM technology to produce homogenous blanks with minimal flaws and microstructural defects. They cited that the load required to produce bulk fracture in CAD/CAM LD can be expected to drop rapidly as the thickness is reduced.22 This claim is also supported by a recent clinical study,23 which included 41 lithium disilicate CAD/CAM posterior crown. The authors reported that the thickness of the only crown failed by fracture during 4 year observation period was not kept at a minimum thickness of 1.5 mm in the fissure line which led to catastrophic failure.23
On the other hand, a similar study by Seydler et al.2 suggested that the wall thickness of posterior LD crowns can be decreased to 1 mm which is not in harmony with the current study. However, such disagreement can be related to experimental variabilities as Seydler et al.2 applied 1.2 million cycles with a maximum load of 108 N before conducting the fracture test. Furthermore, their thermal and mechanical loading were conducted separately, and the thermocycling protocol was different. Lastly, Seydler et al.2 used uniform crown thicknesses and applied the post-fatigue fracture test by loading only one cusp perpendicular to its surface.
Current study showed that fatigue loading for 1.5 million cycles did not significantly affect fracture load of LD crowns in Group A (recommended tooth preparation). On the contrary, a previous study15 reported that cyclic loading significantly reduced the fracture load of monolithic LD crowns. LD crowns have demonstrated good clinical performance with low prevalence of mechanical failure along different follow up periods.20,23,24,25,26,27 The material showed excellent in vitro results through fatigue testing.12,13,28,29 LD also revealed significantly higher reliability than porcelain layered Y-TZP crowns22 and more fracture resistance than zirconia/fluorapatite press over crown.28 Therefore, our findings confirm those of previous in vivo and in vitro studies where tooth preparation and crown thickness were maintained as recommended by the manufacturer.12,13,23,24,25,26,27,28,29
On the other hand, crowns with suggested minimal tooth preparation showed significantly reduced fracture load and catastrophic fracture mode. This can be caused by cyclic loading in the chewing simulator which made these crowns (B2) weaker and significantly decreased the mean fracture load and resulted in different fracture mode.
Regardless, our results indicate that strength of monolithic LD crown is adequate for posterior crown restoration even at reduced thickness as all unfatigued specimens showed initial fracture load higher than the range (1200-1300 N) recommended by Chitmongkolsuk et al.16 for new all-ceramic restorations. The modes of fractures observed in this study varied along with the corresponding fracture load values. Fractures forces were above 1900 N for modes I & II and dropped significantly to 1200 N for mode III. A negative linear correlation was found between the force to fracture and facture mode observed. Fatigue significantly changed the fracture mode of the crowns in both groups. This can be logically related to the effect of fatigue in developing internal stresses within the ceramic, weakening the structure and causing failure at low compression forces. Similar fracture paths were observed in previous study where single load to fracture was applied using 6 mm diameter ceramic ball at a stable position on the occlusal surface of maxillary molar crowns.15
During laboratory simulation, the forces applied on a restoration should be determined carefully to be clinically representative. Clinically, the location of a crown in the dental arch governs the load it might be subject to during function.30,31 This load is greatly variable amongst subjects.32,33 However, an occlusal force between 10 and 120 N has been frequently accepted as sufficient values to represent the occlusal load during chewing or swallowing.34,35,36,37 Thus, specimens in this study were loaded in three phases with 50 N, 100 N, and 150 N for 500,000 cycles each aiming to simulate the variable forces a restoration can be exposed to during function. Several recent studies applied 1.2 million stress cycles to represent 5 years of in vivo service considering that 250000 cycles represent one clinical year.2,15,17 Therefore, 1.5 million cycles were applied in this study to simulate 6 clinical years. Mechanical loading was combined with a 0.5 mm sliding lateral movement in order to mimic the lateral movement of the jaw during mastication and its evident deteriorating influence on the restoration.38,39 Wet conditions and thermocycling were also considered to imitate the chemical effect of aqueous environment and temperature fluctuation on ceramic.
Literature shows that the need for simulating the periodontal ligament in fatigue testing is questionable. Heintze et al.40 suggested that it is not necessary when testing crown specimens; the idea which seems to be agreed on by many authors.2,14,15,22,28,29,41,42 Heintze et al.40 argue that an artificial periodontium which is mostly represented by a thin silicone layer would reduce the axial force when the die moves and the subsequent movement will be unstandardized. It is important when testing FDPs specimens because it can increase the tensile forces at the gingival side of the connector area which might be more clinically representative40 and hence appear in many studies testing FDPs.16,17,18,19,43 In the current study, the researchers did not use artificial periodontium as it moved occlusally leaving a gap between the acrylic resin base and epoxy resin die for the trail specimens exposed to chewing simulator. Using epoxy resin dies instead of natural teeth as substrate might be considered as a limitation of this study because the modulus of elasticity of the supporting material can influence stress distribution21,44 and fracture resistance of ceramic restoration.45,46,47,48 Yucel et al.48 found a similar stress distribution in restorations when the substrates were dentine and epoxy resin dies. However, the stress distribution on the restorations attached to steel or brass dies were different.
In addition, examining the crowns for failure during fatigue testing using Scanning Electronic Microscopy (SEM) could offer a great advantage by detecting micro-cracks and internal defects. Nevertheless, the aim of this study was to test the specimens ultimate fracture load and survival after 6 years of simulated clinical service and to simulate routine clinical procedures in judging the failure.
CONCLUSION
Reducing the amount of tooth preparation required for lithium disilicate crowns by 0.5 mm on the occlusal and proximal/axial wall with a 0.8 mm chamfer significantly reduced the fracture load of the restoration and was likely to be a factor attributing to crown failure during chewing simulation.
Footnotes
This study was partially supported by the Australian Prosthodontic Society (Grant No 4337803/12/3726). Ceramic materials were generously provided by Ivoclar Vivadent, Schaan, Liechtenstein.
References
- 1.Edelhoff D, Sorensen JA. Tooth structure removal associated with various preparation designs for posterior teeth. Int J Periodontics Restorative Dent. 2002;22:241–249. [PubMed] [Google Scholar]
- 2.Seydler B, Rues S, Müller D, Schmitter M. In vitro fracture load of monolithic lithium disilicate ceramic molar crowns with different wall thicknesses. Clin Oral Investig. 2014;18:1165–1171. doi: 10.1007/s00784-013-1062-8. [DOI] [PubMed] [Google Scholar]
- 3.Shahrbaf S, van Noort, R, Mirzakouchaki B, Ghassemieh E, Martin N. Fracture strength of machined ceramic crowns as a function of tooth preparation design and the elastic modulus of the cement. Dent Mater. 2014;30:234–241. doi: 10.1016/j.dental.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Rekow ED, Silva NR, Coelho PG, Zhang Y, Guess P, Thompson VP. Performance of dental ceramics: challenges for improvements. J Dent Res. 2011;90:937–952. doi: 10.1177/0022034510391795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soares CJ, Martins LR, Fonseca RB, Correr-Sobrinho L, Fernandes Neto AJ. Influence of cavity preparation design on fracture resistance of posterior Leucite-reinforced ceramic restorations. J Prosthet Dent. 2006;95:421–429. doi: 10.1016/j.prosdent.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Steiner M, Mitsias ME, Ludwig K, Kern M. In vitro evaluation of a mechanical testing chewing simulator. Dent Mater. 2009;25:494–499. doi: 10.1016/j.dental.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Sibbald B, Roland M. Understanding controlled trials. Why are randomised controlled trials important? BMJ. 1998;316:201. doi: 10.1136/bmj.316.7126.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiskott HW, Nicholls JI, Belser UC. Stress fatigue: basic principles and prosthodontic implications. Int J Prosthodont. 1995;8:105–116. [PubMed] [Google Scholar]
- 9.Naumann M, Metzdorf G, Fokkinga W, Watzke R, Sterzenbach G, Bayne S, Rosentritt M. Influence of test parameters on in vitro fracture resistance of post-endodontic restorations: a structured review. J Oral Rehabil. 2009;36:299–312. doi: 10.1111/j.1365-2842.2009.01940.x. [DOI] [PubMed] [Google Scholar]
- 10.Kelly JR. Clinically relevant approach to failure testing of allceramic restorations. J Prosthet Dent. 1999;81:652–661. doi: 10.1016/s0022-3913(99)70103-4. [DOI] [PubMed] [Google Scholar]
- 11.Attia A, Kern M. Influence of cyclic loading and luting agents on the fracture load of two all-ceramic crown systems. J Prosthet Dent. 2004;92:551–556. doi: 10.1016/j.prosdent.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Clausen JO, Abou Tara M, Kern M. Dynamic fatigue and fracture resistance of non-retentive all-ceramic full-coverage molar restorations. Influence of ceramic material and preparation design. Dent Mater. 2010;26:533–538. doi: 10.1016/j.dental.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht T, Kirsten A, Kappert HF, Fischer H. Fracture load of different crown systems on zirconia implant abutments. Dent Mater. 2011;27:298–303. doi: 10.1016/j.dental.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Mitsias M, Koutayas SO, Wolfart S, Kern M. Influence of zirconia abutment preparation on the fracture strength of single implant lithium disilicate crowns after chewing simulation. Clin Oral Implants Res. 2014;25:675–682. doi: 10.1111/clr.12058. [DOI] [PubMed] [Google Scholar]
- 15.Zhao K, Wei YR, Pan Y, Zhang XP, Swain MV, Guess PC. Influence of veneer and cyclic loading on failure behavior of lithium disilicate glass-ceramic molar crowns. Dent Mater. 2014;30:164–171. doi: 10.1016/j.dental.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Chitmongkolsuk S, Heydecke G, Stappert C, Strub JR. Fracture strength of all-ceramic lithium disilicate and porcelain-fused-to-metal bridges for molar replacement after dynamic loading. Eur J Prosthodont Restor Dent. 2002;10:15–22. [PubMed] [Google Scholar]
- 17.Schultheis S, Strub JR, Gerds TA, Guess PC. Monolithic and bi-layer CAD/CAM lithium-disilicate versus metal-ceramic fixed dental prostheses: comparison of fracture loads and failure modes after fatigue. Clin Oral Investig. 2013;17:1407–1413. doi: 10.1007/s00784-012-0830-1. [DOI] [PubMed] [Google Scholar]
- 18.Kheradmandan S, Koutayas SO, Bernhard M, Strub JR. Fracture strength of four different types of anterior 3-unit bridges after thermo-mechanical fatigue in the dual-axis chewing simulator. J Oral Rehabil. 2001;28:361–369. doi: 10.1046/j.1365-2842.2001.00742.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosentritt M, Siavikis G, Behr M, Kolbeck C, Handel G. Approach for valuating the significance of laboratory simulation. J Dent. 2008;36:1048–1053. doi: 10.1016/j.jdent.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Pieger S, Salman A, Bidra AS. Clinical outcomes of lithium disilicate single crowns and partial fixed dental prostheses: a systematic review. J Prosthet Dent. 2014;112:22–30. doi: 10.1016/j.prosdent.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Rekow ED, Harsono M, Janal M, Thompson VP, Zhang G. Factorial analysis of variables influencing stress in all-ceramic crowns. Dent Mater. 2006;22:125–132. doi: 10.1016/j.dental.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Guess PC, Zavanelli RA, Silva NR, Bonfante EA, Coelho PG, Thompson VP. Monolithic CAD/CAM lithium disilicate versus veneered Y-TZP crowns: comparison of failure modes and reliability after fatigue. Int J Prosthodont. 2010;23:434–442. [PubMed] [Google Scholar]
- 23.Reich S, Schierz O. Chair-side generated posterior lithium disilicate crowns after 4 years. Clin Oral Investig. 2013;17:1765–1772. doi: 10.1007/s00784-012-0868-0. [DOI] [PubMed] [Google Scholar]
- 24.Kern M, Sasse M, Wolfart S. Ten-year outcome of three-unit fixed dental prostheses made from monolithic lithium disilicate ceramic. J Am Dent Assoc. 2012;143:234–240. doi: 10.14219/jada.archive.2012.0147. [DOI] [PubMed] [Google Scholar]
- 25.Fasbinder DJ, Dennison JB, Heys D, Neiva G. A clinical evaluation of chairside lithium disilicate CAD/CAM crowns: a two-year report. J Am Dent Assoc. 2010;141:10S–14S. doi: 10.14219/jada.archive.2010.0355. [DOI] [PubMed] [Google Scholar]
- 26.Valenti M, Valenti A. Retrospective survival analysis of 261 lithium disilicate crowns in a private general practice. Quintessence Int. 2009;40:573–579. [PubMed] [Google Scholar]
- 27.Suputtamongkol K, Anusavice KJ, Suchatlampong C, Sithiamnuai P, Tulapornchai C. Clinical performance and wear characteristics of veneered lithia-disilicate-based ceramic crowns. Dent Mater. 2008;24:667–673. doi: 10.1016/j.dental.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altamimi AM, Tripodakis AP, Eliades G, Hirayama H. Comparison of fracture resistance and fracture characterization of bilayered zirconia/fluorapatite and monolithic lithium disilicate all ceramic crowns. Int J Esthet Dent. 2014;9:98–110. [PubMed] [Google Scholar]
- 29.Carvalho AO, Bruzi G, Giannini M, Magne P. Fatigue resistance of CAD/CAM complete crowns with a simplified cementation process. J Prosthet Dent. 2014;111:310–317. doi: 10.1016/j.prosdent.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Ferrario VF, Sforza C, Serrao G, Dellavia C, Tartaglia GM. Single tooth bite forces in healthy young adults. J Oral Rehabil. 2004;31:18–22. doi: 10.1046/j.0305-182x.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 31.Tortopidis D, Lyons MF, Baxendale RH, Gilmour WH. The variability of bite force measurement between sessions, in different positions within the dental arch. J Oral Rehabil. 1998;25:681–686. doi: 10.1046/j.1365-2842.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- 32.Kohyama K, Mioche A, Martin JF. Chewing patterns of various texture foods studied by electromyography in young and elderly populations. J Texture Stud. 2007;33:269–283. [Google Scholar]
- 33.Kohyama K, Mioche L. Chewing behavior observed at different stages of mastication for six foods, studied by electromyography and jaw kinematics in young and elderly subjects. J Texture Stud. 2004;35:395–414. [Google Scholar]
- 34.Bates JF, Stafford GD, Harrison A. Masticatory function - a review of the literature. III. Masticatory performance and efficiency. J Oral Rehabil. 1976;3:57–67. doi: 10.1111/j.1365-2842.1976.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 35.Kohyama K, Hatakeyama E, Sasaki T, Dan H, Azuma T, Karita K. Effects of sample hardness on human chewing force: a model study using silicone rubber. Arch Oral Biol. 2004;49:805–816. doi: 10.1016/j.archoralbio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Schindler HJ, Stengel E, Spiess WE. Feedback control during mastication of solid food textures-a clinical-experimental study. J Prosthet Dent. 1998;80:330–336. doi: 10.1016/s0022-3913(98)70134-9. [DOI] [PubMed] [Google Scholar]
- 37.De Boever JA, McCall WD, Jr, Holden S, Ash MM., Jr Functional occlusal forces: an investigation by telemetry. J Prosthet Dent. 1978;40:326–333. doi: 10.1016/0022-3913(78)90042-2. [DOI] [PubMed] [Google Scholar]
- 38.Kim B, Zhang Y, Pines M, Thompson VP. Fracture of porcelain-veneered structures in fatigue. J Dent Res. 2007;86:142–146. doi: 10.1177/154405910708600207. [DOI] [PubMed] [Google Scholar]
- 39.Kim JW, Kim JH, Thompson VP, Zhang Y. Sliding contact fatigue damage in layered ceramic structures. J Dent Res. 2007;86:1046–1050. doi: 10.1177/154405910708601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heintze SD, Cavalleri A, Zellweger G, Büchler A, Zappini G. Fracture frequency of all-ceramic crowns during dynamic loading in a chewing simulator using different loading and luting protocols. Dent Mater. 2008;24:1352–1361. doi: 10.1016/j.dental.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Dhima M, Carr AB, Salinas TJ, Lohse C, Berglund L, Nan KA. Evaluation of fracture resistance in aqueous environment under dynamic loading of lithium disilicate restorative systems for posterior applications. Part 2. J Prosthodont. 2014;23:353–357. doi: 10.1111/jopr.12124. [DOI] [PubMed] [Google Scholar]
- 42.Johansson C, Kmet G, Rivera J, Larsson C, Vult Von. Fracture strength of monolithic all-ceramic crowns made of high translucent yttrium oxide-stabilized zirconium dioxide compared to porcelain-veneered crowns and lithium disilicate crowns. Acta Odontol Scand. 2014;72:145–153. doi: 10.3109/00016357.2013.822098. [DOI] [PubMed] [Google Scholar]
- 43.Rosentritt M, Behr M, Gebhard R, Handel G. Influence of stress simulation parameters on the fracture strength of allceramic fixed-partial dentures. Dent Mater. 2006;22:176–182. doi: 10.1016/j.dental.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 44.Wakabayashi N, Anusavice KJ. Crack initiation modes in bilayered alumina/porcelain disks as a function of core/veneer thickness ratio and supporting substrate stiffness. J Dent Res. 2000;79:1398–1404. doi: 10.1177/00220345000790060801. [DOI] [PubMed] [Google Scholar]
- 45.Campbell SD. A comparative strength study of metal ceramic and all-ceramic esthetic materials: modulus of rupture. J Prosthet Dent. 1989;62:476–479. doi: 10.1016/0022-3913(89)90184-4. [DOI] [PubMed] [Google Scholar]
- 46.Mahmood DJ, Linderoth EH, Vult Von. The influence of support properties and complexity on fracture strength and fracture mode of all-ceramic fixed dental prostheses. Acta Odontol Scand. 2011;69:229–237. doi: 10.3109/00016357.2010.549508. [DOI] [PubMed] [Google Scholar]
- 47.Scherrer SS, de Rijk WG. The fracture resistance of all-ceramic crowns on supporting structures with different elastic moduli. Int J Prosthodont. 1993;6:462–467. [PubMed] [Google Scholar]
- 48.Yucel MT, Yondem I, Aykent F, Eraslan O. Influence of the supporting die structures on the fracture strength of all-ceramic materials. Clin Oral Investig. 2012;16:1105–1110. doi: 10.1007/s00784-011-0606-z. [DOI] [PubMed] [Google Scholar]