Abstract
Objectives
This study sought to observe the relationship between left atrial (LA) strain and left ventricular diastolic function and determine whether LA strain could be used to detect diastolic dysfunction (DD) and classify its degree when present.
Background
The assessment of diastolic function is complex and multiparametric because most conventional parameters do not follow the progression of DD. Strain imaging is an emerging index of LA function, with recent data demonstrating that LA strain is diminished in diastolic heart failure. However, LA strain is not part of the standard assessment of diastolic function. We hypothesized that LA strain decreases with worsening DD in a stepwise fashion and could thus be useful in evaluating DD.
Methods
We performed a retrospective derivation and validation cohort study to derive and test LA strain thresholds for DD grades (0 to 3) in patients with preserved left ventricular ejection fraction (N = 229). Two-dimensional speckle tracking was used to measure peak LA strain, which was applied as a single parameter to classify DD. American Society of Echocardiography guidelines were used as the reference standard.
Results
In the derivation cohort (n = 90), peak LA strain was significantly different between DD groups, with gradual decreases seen with worsening DD. Receiver-operating characteristic analysis resulted in 3 distinct LA strain thresholds for categorization of DD grades, with good to excellent diagnostic utility (area under the curve: 0.86 to 0.91). In an independent validation group (n = 139) with a spectrum of diastolic function, 11 patients (8%) had indeterminate DD grades using standard criteria, whereas LA strain was measured in all patients and its cutoffs resulted in diagnostic accuracy up to 95%.
Conclusions
LA strain measurements are feasible and allow accurate categorization of DD, because unlike the traditional parameters, it changes progressively with severity of DD. LA strain may become a useful tool for diastolic assessment in future clinical practice.
Keywords: diastolic function, left atrium, left atrial strain, myocardial strain
The principal role of the left atrium (LA) is to modulate left ventricular (LV) filling via its reservoir, conduit, and booster functions. The LA manifests adaptive changes in its structure and mechanics, and these changes are well described in the setting of abnormal patterns of LV filling, known as diastolic dysfunction (DD). The use of Doppler echocardiography is a cornerstone for the diagnosis and categorization of diastolic function. Although there are already well-established algorithms available for DD staging that are widely used in the clinical setting, the criteria required for diagnosis incorporate multiple echocardiography-based parameters and can be cumbersome to acquire and interpret. Furthermore, individual patients may demonstrate a spectrum of diastolic indices that do not clearly meet the strict definition of a particular DD type, thereby making interpretation challenging at times (1,2).
Strain imaging using 2-dimensional speckle tracking of the LA has been used for the assessment of left atrial function (3,4). LA strain is angle-independent, and thus less susceptible to the limitations of Doppler echocardiographic assessment of strain (5). Alterations in LA strain have been described in patients with hypertension, atrial fibrillation and diastolic heart failure (HF) (6–8). However, there is limited data describing the changes in LA strain across DD groups, nor are there available thresholds proposed for use in the clinical evaluation of diastolic function. We hypothesized that LA strain measurements may hold promise as a simple noninvasive tool to aid in the determination of DD severity. The aim of this study was to determine whether LA strain could be used as an accurate diagnostic criterion for the presence and degree of DD, when compared with the current standard guidelines.
Methods
Study Design
We performed a retrospective derivation and validation of DD categories defined by peak LA strain, using the official guidelines for the assessment of diastolic function as a reference. Three cutoffs were obtained for LA strain from the initial derivation cohort to distinguish normal, grade 1 (mild), grade 2 (pseudonormal), and grade 3 (severe/ restrictive) DD. These cutoffs were then applied to an independent group of patients (validation cohort) and compared with the diagnosis conferred by guideline-based DD assessment.
Patients
All patients (both the derivation and the validation cohorts) were identified from our echo-cardiography database of patients who underwent complete 2-dimensional (2D) transthoracic echocar-diography to evaluate diastolic function between January 2010 and May 2014. Inclusion criteria required the patient to have LV ejection fraction (EF) ≥50%, normal sinus rhythm, and no significant valvular heart disease (defined as greater than mild regurgitation or stenosis) or a prosthetic valve. These inclusion criteria were selected to reduce the chance of confounding results by conditions known to alter LA strain based on previous studies (6–8). Patients were excluded if images were of poor quality, or if image loops did not depict all LA segments, did not allow speckle tracking of atrial boundaries (<15% of the patients), or lacked a discernible R-R interval, which might preclude accurate strain measurements.
The derivation cohort of 90 subjects included 15 patients with normal diastolic function, and 3 subgroups of 25 subjects with grades 1, 2, and 3 of DD, each, using classification criteria described in the 2009 American Society of Echocardiography (ASE) guidelines (1). The validation cohort included an additional 139 patients, representing a spectrum of diastolic function from normal to grade 3 DD.
For all patients, heart rate and noninvasive blood pressure was recorded at the time of echocardiogram. Basic demographic information, and any adverse clinical event, defined as death or HF–related hospitalization, was obtained by chart review. An institutional review board approved the study protocol.
Echocardiography
All echocardiograms were performed at a single cardiac imaging center. Comprehensive 2D, color and tissue Doppler evaluation was performed by expert sonographers using iE33 imaging system (Philips Healthcare, Andover, Massachusetts). Digital loops were stored and analyzed offline (Xcelera, Philips Healthcare). All traditional echocardiographic and LA strain measurements were performed by an operator blinded to the patient's clinical chart-based DD diagnosis.
Volumetric analysis of the LA and LV were performed using standard 2D methodology (9). Method of disks technique was used to measure LA volume, which was divided by body surface area to obtain left atrial volume index (LAVI). Similarly, in the apical 2-and 4-chamber views, LV end-diastolic and end-systolic volumes were measured using the biplane method of disks and EF was calculated. LV mass was calculated from the parasternal long-axis view using the following formula: {LV mass = 0.8 × [1.04 × (LVEDD+ PWTd + SWTd)3 - (LVEDD)3]} + 0.6, where LVEDD = LV end-diastolic diameter, PWTd = end-diastolic posterior wall thickness, and SWTd = end-diastolic septal wall thickness. LV mass was then indexed for body surface area to generate LV mass index.
Echocardiographic assessment for DD grade was performed as follows. From an apical 4-chamber view, transmitral pulsed-wave Doppler was obtained at the mitral leaflet tips, and peak early (E) and late (A) diastolic filling velocities, E/A ratio, and E-wave deceleration time were obtained. Doppler tissue imaging of the mitral annulus was performed at the septal and lateral positions, from which values for the peak early (e′) velocities were obtained and averaged. Pulmonary vein velocities were obtained from the right or left upper pulmonary vein, including peak S-wave inflow velocity during ventricular systole, peak D-wave inflow velocity during the early phase of ventricular diastole, and the corresponding S/D ratio. The maximum tricuspid regurgitation velocity was measured and used to generate the peak tricuspid regurgitation gradient using the modified Bernoulli equation. This was added to the estimated right atrial pressure, estimated from inferior vena cava collapsibility as seen in the subcostal view during inspiration, to estimate pulmonary arterial systolic pressure (9).
Normal diastolic function was identified when LA volume index <34 ml/m2, with medial mitral annular e′ ≥8 m/s, and lateral mitral annular e′ ≥10 ms/s. DD was diagnosed if the following 3 criteria were met: LA volume index ≥34 ml/m2; medial annular e′ <8 m/s; and lateral annular e′ <10 m/s. Further categorization of DD into severity grades 1, 2, or 3 was performed using the mitral E/A ratio, E-wave deceleration time, average E/e′ value, and peak pulmonary systolic pressure. No distinction of normal versus elevated filling pressures was made in this analysis. Subjects were deemed to have “indeterminate” diastolic function if their E/A inflow, deceleration time, or average E/e′ demonstrated overlap between DD grades.
Assessment of La Strain, Lv Strain, and LA Function
Using 2D speckle tracking software (EchoInsight, Epsilon, Ann Arbor, Michigan) the LA endocardial border was traced in the apical 4-chamber view, taking care to exclude the appendage and pulmonary veins from the LA cavity. Then, a composite LA longitudinal strain curve throughout the cardiac cycle was generated. This curve comprised 6 individual atrial segments (Figure 1). If >1 atrial segment had to be excluded from analysis because of suboptimal visualization and tracking, an alternative loop was selected to ensure as complete an analysis as possible for each subject. Peak strain value was derived from the maximal inflection point on the composite LA strain curve. Individual LA strain curve data was then interpolated to include 100 data points per cardiac cycle, irrespective of the heart rate. This interpolation also allowed us to average strain curves for each subject group for intergroup comparisons.
Figure 1. Example of Speckle Tracking Analysis of LA Strain.
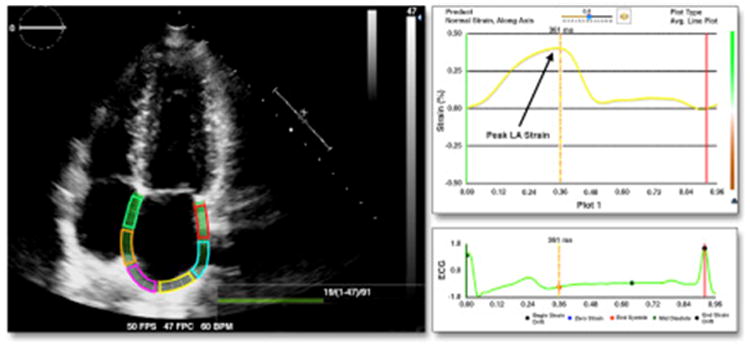
The apical 4-chamber view with the entirety of the left atrium (LA) is pictured, with the endocardium of the LA traced (left). LA strain over time curve and an electrocardiogram signal are shown on the right.
In a subset of 20 subjects (5 normal subjects, and 5 subjects each with grades 1 to 3 DD), we measured global longitudinal strain of the LV. Using the same software, the LV endocardial border was carefully traced from an apical 4-chamber view to generate a composite LV strain curve over 1 cardiac cycle. Peak longitudinal LV strain was then derived from the minimal inflection point on the LV strain curve. These were interpolated similarly to the LA strain curves to allow for intergroup comparisons.
Calculations of LA functionality to assess conduit, reservoir, and booster function were performed using definitions from previous studies (10,11). LA volumes (maximum, minimum and pre-atrial contraction) were identified by manual review of the LA time-volume curve, with selection of the LA volume at pre-atrial contraction timed to the onset of the p-wave from the surface electrocardiogram. Briefly, reservoir function (or LA expansion index) was calculated with the formula: [(maximal LA volume – minimum LA volume)/minimum LA volume] × 100. Conduit function (passive emptying index) was calculated with the formula: [(maximal LA volume – pre-atrial contraction LA volume)/maximal LA volume] × 100. Booster function (active emptying index) was calculated with the formula: [(pre-atrial contraction LV volume – minimum LA volume)/pre-atrial contraction volume] × 100.
Statistical Analysis
Data are presented as mean ± SD. Significance of differences was tested using analysis of variance without adjusting for multiple comparisons, followed by Student t tests performed between all groups (normal vs. grade 1, normal vs. grade 2, normal vs. grade 3, grade 1 vs. grade 2, grade 2 vs. grade 3) for demographic and echocardiographic data measures. Statistical significance was defined as p < 0.05. Receiver-operating characteristic curves were constructed for peak LA strain, and area under the curve was obtained to assess its diagnostic performance. Then 3 discrete thresholds were selected for peak LA strain, to distinguish normal diastolic function from abnormal DD, grade 0 to 1 DD from grade 2 to 3 DD and grade 3 DD from all other, to maximize the agreement with the ASE guidelines in the derivation cohort (by maximizing the overall accuracy). The optimal thresholds were then applied to LA strain data obtained in the validation cohort to classify DD in these patients. The results of this classification were compared with the gold standard for DD diagnosis based on the 2009 ASE guidelines, and the sensitivity, specificity, positive and negative predictive values, and accuracy were calculated. Linear regression was used to analyze the relationship between LA strain and traditional parameters of diastolic function. Inter-rater agreement for peak LA strain measurements was analyzed using intraclass correlation coefficients.
Results
Derivation Cohort
Within the derivation cohort, patients with normal diastolic function were significantly younger than patients with grades 1 and 2 DD (p < 0.01) (Table 1), but there were no significant differences between groups with regard to sex. Whereas baseline LVEF, body surface area, body mass index, heart rate, and systolic blood pressure were similar between groups, LV mass index demonstrated a progressive increase with worsening DD (p < 0.001). LAVI significantly increased with worsening DD, with an incremental rise in indexed volumes seen with grades 1, 2, and 3 DD. However, difference in LAVI was no longer significant between the more severe DD grades 2 and 3 (Figure 2).
Table 1. Baseline Demographics and Echocardiographic Characteristics of the Derivation Cohort.
| Derivation Cohort (n = 90) | Grade 0 (n = 15) | Grade 1 (n = 25) | Grade 2 (n = 25) | Grade 3 (n = 25) | |
|---|---|---|---|---|---|
| Age, yrs | 64 ± 7 | 54 ± 6 | 70 ± 11 | 65 ± 13 | 61 ± 15 |
| Sex, M/F, % | 50/50 | 53/47 | 44/56 | 48/52 | 40/60 |
| BSA, m2 | 2.1 ± 0.2 | 2.1 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.3 | 2.0 ± 0.3 |
| BMI, kg/m2 | 30 ± 6 | 29 ± 5 | 30 ± 6 | 30 ± 7 | 30 ± 4 |
| SBP, mm Hg | 136 ± 25 | 134 ± 16 | 142 ± 20 | 136 ± 29 | 139 ± 25 |
| HR, beats/min | 69 ± 12 | 69 ± 12 | 68 ± 12 | 68 ± 9 | 70 ± 13 |
| EF, % | 61 ± 6 | 62 ± 12 | 62 ± 5 | 62 ± 6 | 59 ± 7 |
| LVMI, g/m2 | 98 ± 39 | 71 ± 22 | 81 ± 20 | 97 ± 27 | 132 ± 45 |
| LAVI, ml/m2 | 41 ± 17 | 29 ± 13 | 32 ± 9 | 48 ± 14 | 51 ± 18 |
| E, cm/s | 98 ± 28 | 90 ± 13 | 67 ± 15 | 110 ± 22 | 115 ± 26 |
| A, cm/s | 70 ± 24 | 70 ± 14 | 89 ± 24 | 79 ± 20 | 48 ± 12 |
| E/A | 1.6 ± 0.8 | 1.4 ± 0.4 | 0.8 ± 0.2 | 1.5 ± 0.4 | 2.5 ± 0.7 |
| E-wave DT, ms | 206 ± 4 | 220 ± 4 | 234 ± 4 | 201 ± 3 | 170 ± 4 |
| Lateral e′, cm/s | 8 ± 3 | 12 ± 2 | 7 ± 1 | 7 ± 2 | 7 ± 2 |
| Septal e′, cm/s | 6 ± 2 | 9 ± 1 | 5 ± 1 | 6 ± 1 | 5 ± 1 |
| E/e′ | 15 ± 6 | 9 ± 2 | 12 ± 4 | 18 ± 5 | 21 ± 5 |
| S-wave, cm/s | 51 ± 16 | 58 ± 14 | 52 ± 12 | 54 ± 17 | 37 ± 12 |
| D-wave, cm/s | 57 ± 17 | 50 ± 10 | 41 ± 11 | 58 ± 14 | 64 ± 20 |
| S/D | 1.0 ± 0.4 | 1.2 ± 0.3 | 1.4 ± 0.4 | 1.0 ± 0.3 | 0.6 ± 0.2 |
| PASP, mm Hg | 35 ± 14 | 27 ± 12 | 31 ± 8 | 39 ± 14 | 42 ± 17 |
Values are mean ± SD or %.
A = peak late diastolic filling velocity; BMI = body mass index; BSA = body surface area; DT = deceleration time; E = peak early diastolic filling velocity; e′ = peak early velocity; EF = ejection fraction; F = female; HR = heart rate; LVMI = left ventricular mass index; LAVI = left atrial volume index; M = male; PASP = pulmonary artery systolic pressure; SBP = systolic blood pressure.
Figure 2. Composite LA Volume Curves by DD Grade.
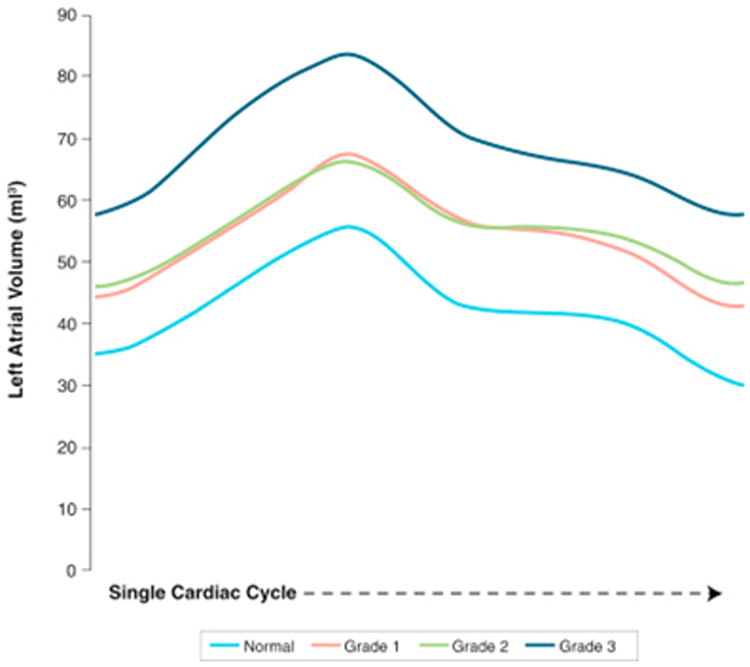
Left atrial (LA) volume curves generated using 2-dimensional speckle tracking software over a single cardiac cycle. With increasing diastolic dysfunction (DD) grade, LA volumes also increase. Note that there is overlap in the volume curves for grades 1 and 2 DD suggesting that it would not be possible to distinguish between these 2 DD grades by LA volumes alone.
Of the conventional diastolic parameters, the mitral inflow E/A ratio, deceleration time, and lateral and medial mitral annular e′ followed the expected trajectory with worsening DD severity. Average E/e′ was the sole conventional parameter that remained significantly different, rising with worsening DD severity, but this trend also lost significance between grades 2 and 3 DD groups.
Evaluation of LA function using volumetric analysis demonstrated impairment in the phases of LA function associated with the presence of DD. Subjects with any degree of DD had higher LA volumes throughout the cardiac cycle, when compared with normal subjects (Figure 2). LA function calculations were feasible in 80% of subjects (72 of 90) (Table 2). All 3 phases of LA function were affected by worsening DD severity. Reservoir function deteriorated with worsening DD, with significant decreases occurring between grades 1 and 2 DD versus normal subjects. Similarly, conduit function was lower in patients with any DD when compared with normal subjects. Interestingly, booster function initially increased with grade 1 DD, but decreased thereafter with worsening DD.
Table 2. LA Phasic Function in Derivation Cohort.
| Grade 0 (n = 15) | Grade 1 (n = 19) | Grade 2 (n = 20) | Grade 3 (n = 18) | |
|---|---|---|---|---|
| Reservoir, LA emptying fraction, % | 131 ±58 | 125 ± 48 | 99 ± 66 | 89 ± 27 |
| Conduit, LA passive emptying fraction, % | 29 ± 15 | 26 ± 18 | 24 ± 12 | 21 ± 8* |
| Booster, LA active emptying fraction, % | 36 ± 9†§ | 39 ± 7 | 28 ± 13‡§ | 15 ± 8* |
Values are mean ± SD.
p < 0.05 for this group versus all other groups.
p < 0.05 for this group versus group 1.
p < 0.05 for this group versus group 2.
p < 0.05 for this group versus group 3.
LA = left atrial.
In contrast, peak LA strain values demonstrated a steady decrease with worsening DD severity, maintaining significance between all DD grades (Table 3, Figure 3). There was excellent inter-rater agreement in peak LA strain measurement (r = 0.94). There were no strong correlations between LA strain and peak tricuspid regurgitation velocity, LAVI, or septal or lateral e′ or E/e′ (r values between 0.17 and 0.41). In a subset of patients for whom we measured LV strain, there was a significant difference between normal subjects and grade 3 DD subjects (global longitudinal strain: -19 ± 1% vs. -13 ± 4%; p < 0.05), but there was not a consistent reduction in LV strain with graded DD severity (Figure 4).
Table 3. LA Strain Results in Derivation Cohort.
| Grade 0 (n = 15) | Grade 1 (n = 25) | Grade 2 (n = 25) | Grade 3 (n = 25) | |
|---|---|---|---|---|
| Peak LA strain, % | 37 ± 13 | 29 ± 8 | 22 ± 9 | 13 ± 6 |
Values are mean ± SD. p < 0.05 among all groups.
LA = Left atrial.
Figure 3. Composite LA Strain Curves for Individual DD Grades.
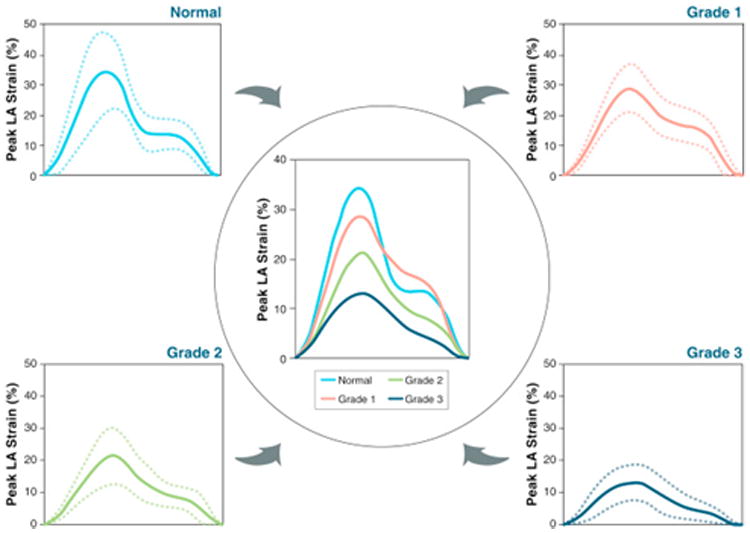
At the 4 corners, composite LA strain curves are depicted as mean of each subgroup (solid lines) with standard deviation (dotted lines). Center panel shows all 4 LA strain curves in a single plot to facilitate comparisons. Abbreviations as in Figures 1 and 2.
Figure 4. Composite LV Strain Curves for Individual DD Grades.
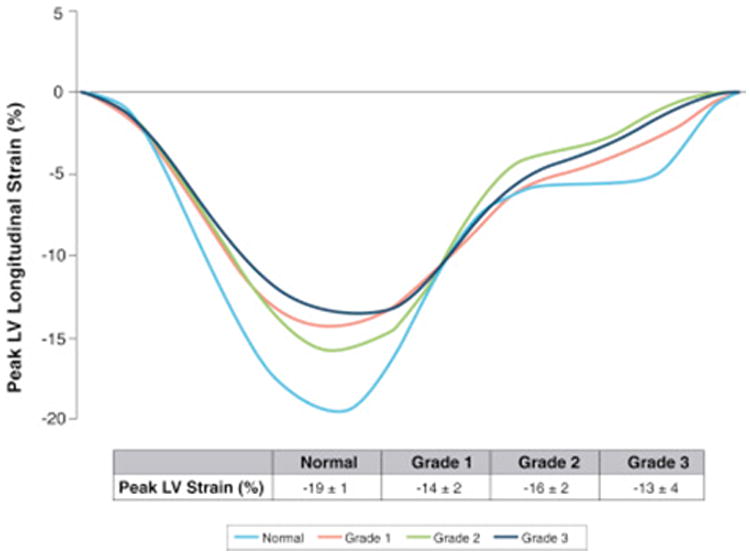
Subset of patients with longitudinal left ventricular (LV) strain curves shown by diastolic dysfunction (DD) grade, demonstrating that with grade 3 DD dysfunction there is a significant decrease in peak longitudinal strain of the LV, despite preserved ejection fraction. However, there is overlap of LV strain curves observed between grades 1 and 2 DD.
Receiver-operating characteristic curves demonstrated area under the curve values of 0.86 or greater for differentiating between DD groups, reflecting excellent diagnostic performance (Table 4, Figure 5). For peak LA strain, the optimal cutoff values derived in this cohort were as follows: grade 0 from grade 1 to 3 DD using a peak LA strain value of >35%; grade 0 to 1 DD from grade 2 to 3 DD using a peak LA strain value of >24%, and grade 0 to 2 DD from grade 3 DD using a peak LA strain value of >19%.
Table 4. ROC Threshold Analysis for LA Strain in Validation Group Using 2009 DD Guidelines.
| Derivation Group | Validation Group | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| AUC | Optimal Cutoff | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Grade 0 vs. grade 1–3 | 0.86 | 35% | 90 | 59 | 61 | 90 | 72 |
| Grade 0–1 vs. grade 2–3 | 0.89 | 24% | 75 | 92 | 75 | 92 | 88 |
| Grade 0–2 vs. grade 3 | 0.91 | 19% | 90 | 95 | 64 | 99 | 95 |
AUC = area under the curve; DD = diastolic dysfunction; LA = left atrial; NPV = negative predictive value; PPV = positive predictive value; ROC = receiver-operating characteristic.
Figure 5. ROC Curves for LA Strain.
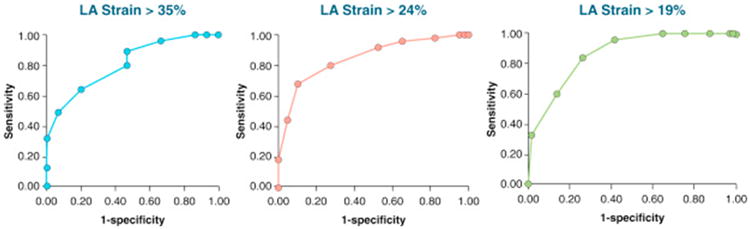
Three distinct curves were obtained to differentiate grade 0 from grades 1 to 3 DD (left), grade 0 to 1 DD from grades 2 to 3 DD (middle), and grades 0 to 2 DD from grade 3 DD (right). ROC = receiver-operating characteristic; other abbreviations as in Figures 1 and 2.
When we analyzed clinical events, there was a trend toward increased rates of death and admission for HF with worsening DD grade. In the normal diastolic cohort, 1 subject (6%) incurred an adverse clinical event. For patients with DD, the lowest event rate was seen in grade 1 with 2 subjects (8%) demonstrating an adverse clinical event, whereas event rates rose considerably thereafter with 7 events in patients with grade 2 DD (28%) and 9 events in patients with grade 3 DD (36%).
Validation Cohort
Of 139 patients, 11 (8%) had indeterminate DD grades using traditional echocar-diographic parameters. The sensitivity, specificity, negative and positive predictive value, and accuracy of LA strain for differentiation of degrees of DD are listed in Table 4. With regard to LA strain, the cutoff value of >35% to identify normal diastolic function was excellent with 90% sensitivity, but specificity and accuracy were modest. The cutoff value of >24% to differentiate grade 1 DD or normal diastolic function from grades 2 and 3 DD demonstrated better accuracy. The LA strain threshold of >19% to separate grade 3 DD performed the best of all LA strain thresholds, with sensitivity, specificity, and accuracy upward of 90%.
Discussion
Our findings demonstrate that the single additional measurement of LA strain using 2D speckle tracking may be a valuable diagnostic tool in the evaluation of DD. While there were a number of conventional echocardiographic parameters analyzed in our derivation cohort, only peak LA strain changed progressively and remained significantly different between all DD grades. The LA strain thresholds defined to categorize patients in our derivation group demonstrated excellent diagnostic accuracy in the independent validation cohort. This was particularly evident in the more advanced stages of DD where an LA strain cutoff of 19%, was 95% accurate in identifying patients defined as having grade 3 DD. These findings suggest a possible role for using peak LA strain in the determination of DD, particularly at the more advanced stages of diastolic disease when distinction between grades can be difficult. Validation of the strain results for our referent healthy normal subjects is evident by our acquired mean LA strain values of 37 ± 13%, which is comparable to the ranges previously reported in the literature (3,12).
With increasing severity of DD, we observed a significant increase in LV mass index. While 2D assessment of LV mass may oversimplify the complexity of remodeling patterns observed in HF with preserved EF, these results support the maladaptive LV remodeling process that accompanies progressive worsening in diastolic function (13). Despite these geometric changes in the LV, systolic LV function was preserved and not significantly different between DD groups, according to our inclusion criteria. However, there was an emergence of abnormal LV strain in severe DD despite preserved EF, which is similar to the findings of the PARAMOUNT (Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion) trial (14).
LAVI also rose with worsening DD, although there was no significant difference between subjects with grades 2 and 3 DD, suggesting that, at this stage, LA distension appears to plateau. The mean E/e′ did initially rise with worsening DD, but again lost significance between the more severe grades of DD. By comparison, LA strain values were significantly different between all DD groups, suggesting that LA strain is a reliable marker of LA dysfunction that occurs with gradual progression of DD. This discrepancy between LA structural remodeling (i.e., dilation) and LA dysfunction (i.e., stiffness) may be a reflection of the different physiology of HF with preserved EF, as one study showed that systolic HF was associated with greater LA remodeling, whereas diastolic HF was associated with a greater degree of LA stiffness (15). The clinical importance of worsening LA stiffness is evident from the sharp rise in adverse clinical events seen in our derivation cohort population with a near 4 ×-fold increase in incidence for grade 3 compared with patients with grade 1 DD.
Assessment of diastolic function using the noninvasive modality of Doppler echocardiography is the current standard of care, but it is fraught with important technical and practical limitations. Previous studies analyzing simultaneous Doppler echocardiography and invasive hemodynamic assessment have shown rising E/e′ values to correlate with LV end-diastolic pressure. However, increases in the single parameter of E/e′ in patients with preserved EF may not be perfectly accurate (16,17). This has led to the opinion that multiple echocardiographic parameters are required for accurate assessment of DD. However, the acquisition and measurements for the currently used spectrum of diastolic parameters can be time-consuming. Furthermore, there are patients who have discrepant values of DD parameters leading to an “indeterminate” diagnosis. Approximately 8% of patients in the validation cohort had indeterminate diastolic classification using the 2009 guidelines, underscoring the need for alternative parameters that could reduce the number of indeterminate diagnoses. One proposed solution to this conundrum has been the development of a novel grade 1a of DD, but this complicates rather than streamlines the current algorithm (2). In fact, the recent publication of the 2016 guidelines emphasizes the goal of simplifying the echocardiographic approach to diastolic function assessment (24).
With the rising prevalence of hypertension and diastolic HF, the use of LA strain as an adjunctive measure of LA function is an emerging area of interest, as changes in strain have been shown to be independent of LA volume in patients with HF and preserved LVEF (18). Furthermore, peak LA strain was shown to correlate well with LV filling pressures in studies of patients with systolic HF, suggesting peak LA strain to be a potentially good noninvasive marker of elevated filling pressures (19,20). However peak LA strain is susceptible to the effects of age, obesity, valvular disease, such as mitral regurgitation, and atrial fibrillation, which we accounted for in this study by limiting our study population to patients with normal LVEF and no significant valvulopathy (21-23). Thus we feel that our LA strain values may reliably reflect the gradual deterioration of LA function with DD and preserved EF.
The feasibility of strain acquisition is another well-known strength of the speckle tracking technique, as it is angle-independent and less susceptible to artifact. Our study demonstrated that acquisition and measurement of strain was feasible in the majority of patients to define atrial function, whereas phasic indices of LA function derived from LA volume were feasible in 85% of the derivation cohort. We did not include patients with incomplete echocardiographic parameters of diastolic function, a factor known to limit the conventional assessment of DD. In contrast, LA strain can be calculated from a single apical 4-chamber view, which is a fundamental view obtained in all routine echocardiograms.
Study Limitations
This was a single-center, retrospective study with a selected group of patients with no history of atrial fibrillation, valvulopathy, or systolic dysfunction. For reference purposes, we used the 2009 ASE guidelines (1) to establish the presence and category of DD, but there is no other gold standard available to diagnose DD, which confines our ability to compare LA strain's diagnostic performance. Additionally LA strain, although feasible, requires access to and familiarity with 2D speckle tracking software, which is increasingly available for clinical use, but predominantly used in the research arena. Lastly, the very recently released 2016 diastolic ASE guidelines have proposed an alternative algorithm for DD classification, which we did not use in the derivation or validation of our LA strain thresholds (24). These new guidelines will undoubtedly be validated in clinical practice and will offer a new and more streamlined approach to DD assessment. We feel that it is possible that in the future, LA strain could have added value in conjunction with the 2016 guidelines, and that the incorporation of LA strain could help to reduce the frequency of the “indeterminate” classification.
Conclusions
Although our findings do not support supplanting the currently recommended algorithms for DD diagnosis, they do suggest that LA strain is a feasible tool, which can be used to detect and categorize DD. We demonstrated in this study that, when compared with the conventional echocardiographic parameters, only LA strain demonstrated a graded and significant decrease between all stages of DD. Further large-scale, prospective studies are needed to validate LA strain cutoff values, before this methodology could be integrated into the clinical evaluation of DD. Nevertheless, our results support this index as a potential marker of LA dysfunction and an adjunctive measure of the mechanical effects the LA sustains in the setting of DD.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS
Assessment of diastolic LV function relies on a number of echocardiographic parameters for accurate diagnosis and categorization, but the current algorithms are complex and frequently lead to indeterminate classification. We found that LA strain decreases with worsening diastolic dysfunction in a stepwise fashion, allowing accurate classification of its severity. LA strain may become a useful tool for diastolic assessment in future clinical practice.
TRANSLATIONAL OUTLOOK
LA strain thresholds for DD classification generated from this study need further validation outside of a single-center population. They also should be studied in conjunction with the recently released 2016 ASE diastolic guidelines.
Acknowledgments
Dr. Singh was supported by funding from the National Institutes of Health T32 Training Grant
Abbreviations and Acronyms
- 2D
2-dimensional
- A
peak late diastolic filling velocity
- ASE
American Society of Echocardiography
- DD
diastolic dysfunction
- E
peak early diastolic filling velocity
- e′
peak early velocity
- EF
ejection fraction
- HF
heart failure
- LA
left atrium
- LAVI
left atrial volume index
- LV
left ventricle
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Kuwaki H, Takeuchi M, Chien-Chia Wu V, et al. Redefining diastolic dysfunction grading: combination of E/A ≤ 0.75 and deceleration time >140 ms and E/ε′ ≥10. J Am Coll Cardiol Img. 2014;7:749–58. doi: 10.1016/j.jcmg.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Cameli M, Caputo M, Mondillo S, et al. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound. 2009;7:6. doi: 10.1186/1476-7120-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saraiva RM, Demirkol S, Buakhamsri A, et al. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr. 2010;23:172–80. doi: 10.1016/j.echo.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Vianna-Pinton R, Moreno CA, Baxter CM, Lee KS, Tsang TS, Appleton CP. Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr. 2009;22:299–305. doi: 10.1016/j.echo.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Mondillo S, Cameli M, Caputo ML, et al. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr. 2011;24:898–908. doi: 10.1016/j.echo.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Inaba Y, Yuda S, Kobayashi N, et al. Strain rate imaging for noninvasive functional quantification of the left atrium: comparative studies in controls and patients with atrial fibrillation. J Am Soc Echocardiogr. 2005;18:729–36. doi: 10.1016/j.echo.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–5. doi: 10.1161/CIRCIMAGING.108.813071. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. e14. [DOI] [PubMed] [Google Scholar]
- 10.Okamatsu K, Takeuchi M, Nakai H, et al. Effects of aging on left atrial function assessed by two-dimensional speckle tracking echocardiography. J Am Soc Echocardiogr. 2009;22:70–5. doi: 10.1016/j.echo.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Otani K, Takeuchi M, Kaku K, et al. Impact of diastolic dysfunction grade on left atrial mechanics assessed by two-dimensional speckle tracking echocardiography. J Am Soc Echocardiogr. 2010;23:961–7. doi: 10.1016/j.echo.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Morris DA, Takeuchi M, Krisper M, et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging. 2015;16:364–72. doi: 10.1093/ehjci/jeu219. [DOI] [PubMed] [Google Scholar]
- 13.Katz DH, Beussink L, Sauer AJ, Freed BH, Burke MA, Shah SJ. Prevalence, clinical characteristics, and outcomes associated with eccentric versus concentric left ventricular hypertrophy in heart failure with preserved ejection fraction. Am J Cardiol. 2013;112:1158–64. doi: 10.1016/j.amjcard.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraigher-Krainer E, Shah AM, Gupta DK, et al. for the PARAMOUNT Investigators. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–56. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
- 16.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 17.Dokainish H, Nguyen JS, Sengupta R, et al. Do additional echocardiographic variables increase the accuracy of E/e′ for predicting left ventricular filling pressure in normal ejection fraction? An echocardiographic and invasive hemodynamic study. J Am Soc Echocardiogr. 2010;23:156–61. doi: 10.1016/j.echo.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Santos AB, Kraigher-Krainer E, Gupta DK, et al. for the PARAMOUNT Investigators. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:1096–103. doi: 10.1002/ejhf.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cameli M, Sparla S, Losito M, et al. Correlation of left atrial strain and Doppler measurements with invasive measurement of left ventricular enddiastolic pressure in patients stratified for different values of ejection fraction. Echocardi-ography. 2016;33:398–405. doi: 10.1111/echo.13094. [DOI] [PubMed] [Google Scholar]
- 20.Cameli M, Lisi M, Mondillo S, et al. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound. 2010;8:14. doi: 10.1186/1476-7120-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Wang K, Su D, et al. Noninvasive assessment of left atrial phasic function in patients with hypertension and diabetes using two-dimensional speckle tracking and volumetric parameters. Echocardiography. 2014;31:727–35. doi: 10.1111/echo.12492. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi H, Oishi Y, Mizuguchi Y, et al. Contribution of obesity to left atrial and left ventricular dysfunction in asymptomatic patients with hypertension: a two-dimensional speckle-tracking echocardiographic study. J Am Soc Hypertens. 2014;8:54–63. doi: 10.1016/j.jash.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Ma XX, Boldt LH, Zhang YL, et al. Clinical relevance of left atrial strain to predict recurrence of atrial fibrillation after catheter ablation: a meta-analysis. Echocardiography. 2016;33:724–33. doi: 10.1111/echo.13184. [DOI] [PubMed] [Google Scholar]
- 24.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]


