Abstract
Objective
Pro-inflammatory cytokines are associated with bipolar disorder (BD), but less is known about how cytokines function during the interepisode period. This study examined cytokines, mood symptoms, and sleep in individuals with interepisode BD with complaints of insomnia. We also investigated the effects of a BD-specific modification of cognitive behavior therapy for insomnia (CBTI-BP) on cytokine levels.
Methods
The 22 adults with interepisode BD Type I and insomnia were drawn from a subset of an NIMH-funded study. Participants were randomly allocated to CBTI-BP (n = 11) or Psychoeducation (PE; n = 11). Participants completed a sleep diary, rated self-report measures of mania and depression, and provided samples assayed for interleukin (IL)-6 and tumor necrosis factor soluble receptor 2 (sTNF-R2).
Results
IL-6 was associated with mania symptoms (rs = 0.44, p = .041) and total sleep time (rs = −0.49, p = .026). IL-6 was related to depression symptoms at the trend level (rs = 0.43, p = .052). sTNF-R2 was not significantly related to mood or sleep measures. From pretreatment to posttreatment, CBTI-BP compared to PE was associated with a non-significant, large effect size decrease in IL-6 (z = −1.61, p = .13, d = −0.78) and a non-significant, small-medium effect size decrease in sTNF-R2 (z = −0.79, p = .44, d = −0.38).
Conclusions
These findings provide preliminary evidence that IL-6 is related to mania symptoms and shorter TST in interepisode BD. A treatment that targets sleep in BD could potentially decrease IL-6 although replication is warranted.
Keywords: bipolar disorder, sleep, mood, inflammation, pro-inflammatory cytokines
Bipolar disorder (BD) affects nearly 4% of the population and is among the top ten leading causes of disability (1,2). Mania symptoms, depression symptoms, and sleep disturbance are hallmark features of BD (3–5). While extant research has identified a range of cognitive, behavioral, social, and clinical predictors of core aspects of BD, the biological correlates are not well defined.
There is evidence implicating inflammation as one putative biological mechanism across psychiatric conditions. Peripheral levels of pro-inflammatory cytokines—proteins that coordinate adaptive and innate immune response—are a common measure of systemic inflammation. Altered pro-inflammatory cytokines have been observed in major depressive disorder (6–8), schizophrenia (9), and anxiety disorders (10,11). Additionally, pro-inflammatory cytokines have been linked to poor physical health outcomes (6), neuropsychological impairment (12), and chronic pain (13); outcomes that occur at elevated rates in psychiatric conditions.
Goldstein, Kemp, Soczynska, and McIntyre (14) have proposed that inflammation may also be an important biological mechanism in BD. Their model first describes bidirectional relationships between mania or depression and inflammation. Next, these paths are mediated by sleep disturbance or chronic pain. The resulting inflammation then leads to negative mental and physical health consequences. Episodes of mania and bipolar depression have generally been associated with increased pro-inflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-related molecules (e.g., TNF-α, soluble TNF receptor 1 [sTNF-R1], and soluble TNF receptor 2 [sTNF-R2] (15–21). Previous research has also suggested that the increase in pro-inflammatory cytokines associated with mania and depression may be resolved during the interepisode period (15,22–24). However, more work is needed to determine whether subsyndromal symptoms of mania and depression may be associated with elevated pro-inflammatory cytokines during the interepisode period.
Subsyndromal symptoms during the interepisode period are common and associated with relapse to episodes of mania and depression (3,25,26). Examining the relationship between subsyndromal mood symptoms and pro-inflammatory cytokines may be useful for understanding impairment during the interepisode period. Evidence for the relationship between pro-inflammatory cytokines and symptom measures of mania and depression have been inconsistent or non-significant during acute mood episodes (15,17,18,27–31). However, the relationship between mood symptoms and pro-inflammatory cytokines has not been considered during the interepisode period. Nor has there been work examining the pathway between sleep disturbance and pro-inflammatory cytokines in BD.
Sleep disturbance such as short sleep duration and insomnia have been strongly implicated in BD (3,25,32,33). Shorter sleep duration has been connected to increased IL-6 activity in other clinical samples (34,35), adolescents (36), and older men (37). However, a meta-analysis suggests that these effects may be small (38). Further, the effect of sleep deprivation on TNF-α, sTNF-R1, and sTNF-R2 activity is mixed (39–43). Chronic insomnia is related to increased IL-6 as well as a shift from nighttime to daytime IL-6 and TNF-α secretion (34,44). Total wake time (TWT) during the night, which reflects the sleep disturbance characteristic of insomnia (45), is also positively associated with IL-6 in chronic insomnia (34). Furthermore, cytokines may have a reciprocal influence on sleep disturbance, which is supported by a study that reported that higher TNF levels prior to sleep were associated with greater sleep continuity whereas lower sleep continuity was related to higher TNF production in the morning among adults with rheumatoid arthritis (46). While these studies suggest that pro-inflammatory markers may be related to sleep disturbance in the general population and other disorders, to the best of our knowledge, no studies have examined this relationship in BD. Notably, however, it has been hypothesized that IL-6 is related to sleep disturbance in BD (14,47). Similarly, TNF-related molecules such as TNF-α, sTNF-R1, and sTNF-R2 may also be implicated given evidence for elevated activity during episodes of mania and depression as well as some evidence that sleep disturbance may be related to TNF activity (16–21,35,38,39,41–43).
The aim of this study was to examine inflammation in interepisode BD by empirically evaluating components of the Goldstein et al. (14) model of inflammation in BD. This study also tested if targeting a component of this model—sleep disturbance—can result in changes in measures of inflammation. The first aim was to test the relationship between mood symptoms and pro-inflammatory cytokines in participants with interepisode bipolar I disorder and insomnia. It was hypothesized that mania and depression symptoms would be positively associated with IL-6 and sTNF-R2. The second aim was to test the relationship between sleep disturbance and pro-inflammatory cytokines in BD. It was hypothesized that total sleep time would be negatively associated with IL-6 and sTNF-R2 and total wake time would be positively associated with IL-6 and sTNF-R2. The third aim was to test if a BD-specific modification of cognitive behavior therapy for insomnia (CBTI-BP) can affect pro-inflammatory cytokines in patients with interepisode bipolar I disorder and insomnia. It was hypothesized that CBTI-BP compared to Psychoeducation (PE) would be associated with decreased IL-6 and sTNF-R2 from pretreatment to posttreatment.
Method
Participants
The participants for the current study were drawn from a subset of those enrolled in an NIMH pilot randomized controlled trial (RCT) to determine if CBTI-BP can improve mood state, sleep, and functioning (48). Data were collected from April 2011 to March 2012. OMT collection was added to collect pilot data on inflammatory markers after 29 of the total 58 participants were enrolled, providing a sample of 29 participants for OMT collection. Two participants did not consent to OMT collection and five participants had samples that were unable to be assayed, providing a total sample of 22 participants with OMT for analysis. There were no significant differences on demographic or clinical characteristics between participants with OMT data (n = 22) compared to participants without OMT data (n = 36). Participants that provided OMT samples had significantly longer total wake time, t(54) = 2.30, p = .026. There were no other significant differences for any sleep variables between participants that did or did not provide OMT samples. Twenty-two adults with interepisode bipolar I disorder and insomnia received CBTI-BP (n = 11) or PE (n = 11). Among the randomized participants in the present sample, 3 CBTI-BP participants and 2 PE participants dropped out during treatment. Attrition rates were not significantly different between treatment groups in the present sample, χ2(1, N = 22) = 0.26, p = .61. Demographic characteristics of the present sample are presented in Table 1. Detailed information on study design and inclusion/exclusion criteria can be found elsewhere (see (48)). All study procedures were approved by the University of California, Berkeley Institutional Review Board. Informed consent was obtained for all participants.
Table 1.
Pretreatment demographic and clinical characteristics for CBTI-BP and PE.
| CBTI-BP (n = 11) | PE (n = 11) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| M or N | % or SD | M or N | % or SD | t or χ2 | p | ||
| Age | 37.4 | 14.3 | 35.4 | 9.1 | 0.39 | .70 | |
| Female | 7 | 63.6% | Female | 5 | 45.5% | .39 | |
| Ethnicity (1 declined to answer) | 3.20 | .07 | |||||
| Hispanic or Latino | 2 | 18.2% | 0 | 0.0% | |||
| Not Hispanic or Latino | 8 | 72.7% | 11 | 100.0% | |||
| Race | 6.52 | .48 | |||||
| American Indian/Alaska Native | 0 | 0.0% | 0 | 0.0% | |||
| Asian | 1 | 9.1% | 4 | 36.4% | |||
| African American | 1 | 9.1% | 2 | 18.2% | |||
| White | 6 | 54.6% | 5 | 45.5% | |||
| Bi-racial/Multi-racial | 2 | 18.2% | 0 | 0.0% | |||
| Marital Status (1 declined to answer) | 3.45 | .63 | |||||
| Single | 7 | 63.6% | 7 | 63.6% | |||
| Married/Partnered | 2 | 18.2% | 2 | 18.2% | |||
| Divorced/Separated/Widow | 2 | 18.2% | 2 | 18.2% | |||
| Employed (1 declined to answer) | 2.79 | .42 | |||||
| Full-time | 2 | 18.2% | 0 | 0.0% | |||
| Part-time | 2 | 18.2% | 2 | 18.2% | |||
| Unemployed | 7 | 63.6% | 8 | 72.7% | |||
| Income (2 declined to answer) | 6.49 | .26 | |||||
| <$20,000 | 4 | 36.4% | 6 | 54.6% | |||
| $20,000–$35,000 | 0 | 0.0% | 1 | 9.1% | |||
| $35,000–$50,000 | 2 | 18.2% | 3 | 27.3% | |||
| $50,000–$60,000 | 2 | 18.2% | 1 | 9.1% | |||
| >$60,000 | 1 | 9.1% | 0 | 0.0% | |||
| Bipolar disorder onset | 22.6 | 11.5 | 25.6 | 11.5 | 0.61 | .55 | |
| Bipolar disorder duration | 14.8 | 13.6 | 9.8 | 5.9 | −1.11 | .28 | |
| Mood medication | 11 | 100.0% | 10 | 91.1% | 1.05 | .31 | |
| Sleep medication | 7 | 63.6% | 5 | 45.5% | 0.73 | .39 | |
Treatments
CBTI-BP
Cognitive behavioral therapy for insomnia (CBT-I) was modified to increased safety and tolerability related to stimulus control and sleep restriction (48). To minimize the risk of hypomania or mania relapse resulting from sleep deprivation, time in bed was restricted to no less than 6.5 hours. CBT-I was also modified to target features of sleep in BD by incorporating components of interpersonal and social rhythm therapy, chronotherapy, and motivational interviewing. The first session focused on case formulation, goal setting, motivational interviewing, and sleep/circadian education. Subsequent sessions included behavioral modules (e.g., stimulus control, sleep restriction, and regularizing sleep-wake times) and cognitive modules (e.g., correcting unhelpful sleep-related beliefs and reducing sleep-related worry or vigilance).
PE
PE provided information about sleep, stress, diet, health, exercise, and mood in BD. PE did not facilitate or plan for behavior change.
Materials and Procedure
Structured Clinical Interview for DSM-IV (SCID)
The SCID is a semi-structured interview used to assess Axis I diagnostic criteria for the DSM-IV-TR (45). The SCID has demonstrated good reliability (49). The SCID was used to assess diagnostic criteria for BD Type I.
Duke Structured Interview for Sleep Disorders (DSISD)
The DSISD is a semi-structured interview that assesses sleep disorders defined by DSM-IV and International Classification of Sleep Disorders-2 criteria (50). The DSISD was used to assess diagnostic criteria for primary insomnia.
Young Mania Rating Scale (YMRS)
The YMRS is an 11-item measure rated on a five-point scale used to assess mania symptoms. It has been shown to have good reliability and validity (51). The YMRS was also examined with the sleep items (item #1) removed.
Quick Inventory of Depressive Symptomatology (QIDS)
The QIDS is a 16-item measure rated on a four-point scale used to assess depression symptoms (52). The measure has demonstrated good reliability and validity (52). The QIDS was also examined with the sleep items (items #1–4) removed.
Sleep diary
The sleep diary was based on the Expanded Consensus Sleep Diary for Morning, a validated self-report measure of sleep patterns (53,54). Sleep diary was recorded each morning for one week preceding the laboratory assessment. In the present study, total sleep time (TST) and total wake time (TWT) were the primary measures of sleep disturbance. TST was selected because shortened TST is linked to increased mania symptoms (33), depression symptoms (32), and pro-inflammatory cytokines (34,55,56). TWT was selected because it reflects the core insomnia diagnostic criteria (45), is associated with negative mood in BD (25), and is related to pro-inflammatory cytokines in insomnia (34). TWT was calculated as a composite of sleep onset latency (SOL), wake after sleep onset (WASO), and early morning awakening (EMA; (25)).
Collection and measurement of cytokines
Oral Mucosal Transudate (OMT) samples were collected with OraSure devices (OraSure Technologies, Bethlehem, PA). OMT has been validated for assessing pro-inflammatory cytokines and is correlated with levels found in plasma (57). Samples were collected one week before and after the eight week treatment. All samples were collected between 3:00 and 7:00 P.M. (58). The OraSure device contains a cotton fiber pad treated with a hypertonic salt solution that enhances the transport of OMT across the gingival crevice and oral mucosa (59,60). The cotton pad is held between the right lower cheek and gum for 3 minutes. The cotton pad is then inserted into a tube containing a buffer solution to preserve the sample. Following collection, oral fluids were centrifuged at 800 g for 15 minutes and then stored at −80°C until processed. All biochemical analyses were conducted by ProNovus Bioscience, LLC (Mountain View, CA).
All biochemical assays were conducted with IL-6 and sTNF-R2 Quantikine ELISA kits (R&D Systems, Minneapolis, MN). sTNF-R2 is the soluble receptor for TNF, which reflects TNF activity and is more stable than measuring TNF directly (61,62). Assay sensitivities were 0.11 pg/mL and 2.3 pg/mL for IL-6 and sTNF-RII, respectively. Minimum detectable dose was 0.01 pg/mL and 1.4 pg/mL for IL-6 and sTNF-RII, respectively. Intra- and inter-assay coefficients of variation (CV) for the IL-6 ELISA were 3.6% and 5.2%, respectively. Intra- and inter-assay CV for the sTNF-RII ELISA were 0.6% and 1.02%, respectively. Sensitivity and reliability values were provided by ProNovus Bioscience, LLC (Mountain View, CA). All assay kits were validated and all samples were assayed on the same lot. Each sample was tested in duplicate. The Shapiro-Wilk normality test indicated that unadjusted IL-6 (W = .64, p < .001) and sTNF-R2 (W = .73, p < .001) values were not normally distributed. Hence, values were log transformed, which resulted in values that fit a normal distribution for IL-6 (W = .95, p = .270) and sTNF-R2 (W = .95, p = .312). Histograms for unadjusted and log transformed values for IL-6 and sTNF-R2 are displayed in Figure S1, Supplemental Digital Content 1.
Medications
A pharmacotherapy tracking log was completed at pretreatment and posttreatment. Dose, time of day taken, frequency of use, missed doses, and side effects were assessed. 21 participants (95.5%) were prescribed mood stabilizers and 12 participants (54.5%) were prescribed sleep medication.
Data analysis
The first two aims were addressed with non-parametric Spearman correlations. The third aim used hierarchical linear models (HLM) with restricted maximum likelihood estimation. This statistical method can account for the relationships between repeated measurements and does not have the same missing data restrictions of traditional regression analyses. The fixed part of the model included an indicator for treatment condition (CBTI-BP and PE), time (pretreatment and posttreatment), and the interaction between treatment condition and time. The random part of the model included a random intercept for participant.
This study was a pilot study, and thus was not powered to obtain statistically significant effects at the .05 level (63,64). Reporting and interpreting results will emphasize effect sizes in addition to statistical significance (65). The correlation coefficient is a measure of effect size, and will be interpreted as 0.10 = small effect size, 0.30 = medium effect size, and 0.50 = large effect size (66,67). The treatment effect on the change in pro-inflammatory cytokines from pretreatment to posttreatment was expressed as Cohen’s d, and will be interpreted as 0.20 = small effect size, 0.50 = medium effect size, and 0.80 = large effect size (66). Given that this was a pilot study with a small sample size, covariates were not included to reduce the possibility of overfitting models (68). All statistical analyses were conducted with R (69).
Results
Means, standard deviations, and intercorrelations for study variables are presented in Tables 1–3. Results indicated that mania symptoms were associated with IL-6 with a medium-large effect size (rs = 0.44, p = .041) and with sTNF-R2 with a small, non-significant effect size, (rs = −0.10, p = .68). Depression symptoms were associated with IL-6 with a trend-level, medium-large effect size (rs = 0.43, p = .052) and sTNF-R2 with a small, non-significant effect size (rs = 0.22, p = .37). Mania symptoms without the sleep items were associated with IL-6 with a medium effect size (rs = 0.45, p = .035) and with sTNF-R2 with a small, non-significant effect size (rs = −0.19, p = .43). Depression symptoms without the sleep items were associated with IL-6 with a medium effect size (rs = 0.43, p = .044) and sTNF-R2 with a small, non-significant effect size (rs = 0.18, p = .46).
Table 3.
Spearman correlations between pro-inflammatory cytokines, mood, and sleep at pretreatment.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. IL-6 | - | |||||||
| 2. sTNF-R2 | 0.08 | - | ||||||
| 3. YMRS | 0.44* | −0.10 | - | |||||
| 4. YMRSa | 0.45† | 0.22 | 0.16 | - | ||||
| 5. QIDS | 0.45* | −0.19 | 0.89*** | 0.32 | - | |||
| 6. QIDSa | 0.43* | 0.18 | 0.22 | 1.00*** | 0.39† | - | ||
| 7. TST | −0.49* | 0.05 | −0.35 | −0.21 | −0.53* | −0.25 | - | |
| 8. TWT | −0.14 | −0.17 | −0.05 | −0.02 | 0.07 | 0.05 | 0.01 | - |
Note.
Sleep items removed.
p < .001;
p < .05;
p < .10.
Average TST was associated with IL-6 with a medium effect size (rs = −0.49, p = .026) and sTNF-R2 with a small, non-significant effect size (rs = 0.05, p = .84). Average TWT was associated with IL-6 (rs = −0.14, p = .54) and sTNF-R2 with non-significant, small effect sizes (rs = −0.17, p = .48).
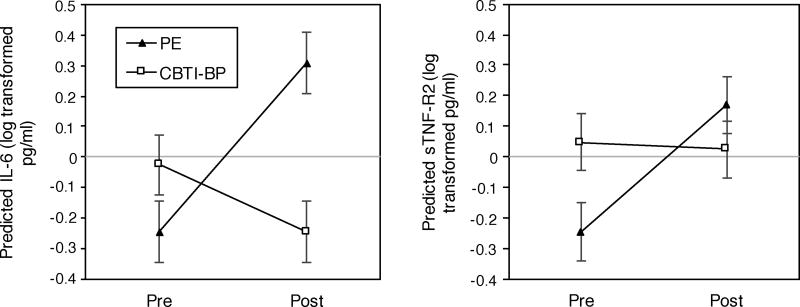
At pretreatment, there was a non-significant, small-medium effect size difference in IL-6 for CBTI-BP compared to PE (B = 0.22, SE = 0.43, z = 0.51, p = .61, d = 0.24). Change in IL-6 from pretreatment to posttreatment for CBTI-BP compared to PE was not significant (B = −0.78, SE = 0.48, z = −1.61, p = .13), but was associated with a large effect size (d = −0.78; Figure 1A). The difference in IL-6 at posttreatment for CBTI-BP compared to PE was associated with a non-significant, large effect size decrease (B = −0.55, SE = 0.48, z = −1.17, p = .24, d = −0.88). IL-6 increased from pretreatment to posttreatment for PE at the trend level (B = 0.55, SE = 0.33, z = 1.67, p = .095, d = 0.88). Change in IL-6 from pretreatment to posttreatment for CBTI-BP was associated with a non-significant, small-medium effect size (B = −0.22, SE = 0.35, z = −0.63, p = .53, d = −0.35).
Figure 1.
Graphs of fitted values derived from hierarchical linear models of pretreatment to posttreatment change in log transformed IL-6 and sTNF-R2 for CBTI-BP compared to PE. A) Predicted IL-6 by time period and treatment group. B) Predicted sTNF-R2 by time period and treatment group.
Note. CBTI-BP sample size: pretreatment (n = 11) and posttreatment (n = 8). PE sample size: pretreatment (n = 11) and posttreatment (n = 9). Error bars refer to the standard error of the predicted means.
There was a non-significant, small-medium effect size difference in sTNF-R2 at pretreatment for CBTI-BP compared to PE (B = 0.29, SE = 0.45, z = 0.64, p = .52, d = 0.31). Change in sTNF-R2 from pretreatment to posttreatment for CBTI-BP compared to PE was associated with a non-significant, small-medium effect size decrease (B = −0.34, SE = 0.43, z = −0.79, p = .44, d = −0.38; Figure 1B). There was a non-significant, small-medium effect size difference in sTNF-R2 at posttreatment for CBTI-BP compared to PE (B = −0.15, SE = 0.52, z = −0.28, p = .78, d = −0.34). Change in sTNF-R2 from pretreatment to posttreatment for PE was not significant (B = 0.42, SE = 0.38, z = 1.09, p = .28), but was associated with a large effect size increase (d = 0.96). Change in sTNF-R2 from pretreatment to posttreatment for CBTI-BP was associated with a non-significant, small effect size (B = −0.02, SE = 0.40, z = −0.06, p = .95, d = −.06).
Discussion
The present study aimed to provide a preliminary test for sleep-related predictions derived from the Goldstein et al. (14) conceptual model of inflammation in BD. At the outset we recognize that the present study was underpowered. As such, interpretation of results will include a focus on effect sizes in addition to statistical significance at the .05 level (65).
Our first hypothesis was partially supported. IL-6 was positively correlated with mania and depression symptoms, both with and without the sleep items included. However, sTNF-R2 was associated with symptom measures of mood with small effect sizes. These results are consistent with previous research that has demonstrated that IL-6 is elevated during mania and depressive episodes (15,16,18). The present study provides preliminary evidence that even during the interepisode period, IL-6 may be related to subsyndromal mania and depression symptoms with medium-large effect sizes. This is noteworthy as previous research has suggested that the increase in pro-inflammatory cytokines associated with mania and depression may be resolved during the interepisode period (22–24).
In partial support of the second hypothesis, a negative relationship was observed between IL-6 and TST, which suggests that as TST decreased, IL-6 levels increased. However, only small effect sizes were observed between sTNF-R2 and TST. Results for IL-6 are consistent with Goldstein et al. (14), as well as empirical research that demonstrates a link between short TST and inflammation (34,55,56). This study also provides preliminary support for a theoretical paper that hypothesized that IL-6 is related to sleep disturbance in BD (47). To the best of our knowledge, this is the first study to provide evidence for this hypothesized relationship.
Small effect sizes were observed between TWT and pro-inflammatory cytokines. This finding is surprising given that other studies suggest that sleep disturbance is positively correlated with an inflammatory response (34,38,44). Given the small sample size, small effect sizes, and the cross-sectional nature of the data, it is not possible to make definite conclusions about these results. One possibility that will need to be tested in future, fully-powered studies is if the relationship between pro-inflammatory cytokines and sleep disturbance in BD is better captured by a measure of sleep duration (e.g., TST) or a measure of nocturnal wakefulness (e.g., TWT).
The third aim was to test if CBTI-BP can affect pro-inflammatory cytokines in patients with interepisode bipolar I disorder and insomnia. These findings provide preliminary evidence that CBTI-BP may reduce IL-6 in patients with interepisode bipolar I disorder and insomnia. The change in IL-6 from pretreatment to posttreatment for CBTI-BP compared to PE was associated with a large effect size decrease. An inspection of the mean values indicated that patients who received CBTI-BP had lower IL-6 from pretreatment to posttreatment compared with patients who received PE. The group difference effect size was large, although the difference did not reach statistical significance. Results for sTNF-R2 were in the same direction as IL-6, but with small effect sizes. Taken together, a nonpharmacological intervention that improves sleep (e.g., CBTI-BP) may be a promising intervention to target pro-inflammatory cytokines in BD.
The within group change in pro-inflammatory cytokines is also notable. The small-medium effect size decrease in IL-6 in the CBTI-BP condition should be evaluated in comparison to the PE group, which was associated with a large effect size increase in IL-6. Similar results were observed with sTNF-R2, but with smaller effect sizes. It is possible that for participants in the PE condition, sleep disturbance, as well as other factors related to inflammation such as return to mood episode, were not addressed to the same degree as CBTI-BP. Indeed, CBTI-BP compared to PE was associated with reduced risk of mood episode relapse and improved sleep (48). In a fully powered study it will be important to determine what factors mediate changes in pro-inflammatory cytokines as a result of treatment.
Several limitations are important to consider. First, the interpretability and generalizability of these findings are limited by the small sample size. Second, this study included adults with both insomnia and BD. While insomnia occurs in 50% of interepisode BD patients (3), findings from this sample may not generalize to all patients with BD. Third, due to recommendations for small sample sizes and risk of overfitting statistical models (68), we did not control for variables known to influence pro-inflammatory cytokines such as age, sex, socioeconomic status, race and ethnicity, or body mass index (70). In a fully powered study, it may be informative to evaluate the influence of these factors. Fourth, 95.5% of participants were prescribed mood stabilizers. Although this may bias the results, we suggest that the present study is notable because effects were observed despite most participants taking medication known to reduce inflammation (71). Last, sTNF-R2 was related to study variables with small effect sizes. Although there is evidence that sTNF-R2 is related to BD (16), it is possible that this elevation is characteristic of acute illness phase rather than the interepisode period. Future studies will be necessary to further define the role of sTNF-R2 with respect to sleep and mood during the interepisode period.
In sum, this study provides preliminary evidence that IL-6 may be related to mood symptoms and sleep duration in participants with interepisode bipolar I disorder and insomnia. Furthermore, targeting sleep disturbance with CBTI-BP may result in changes in IL-6. Although the results of this study are encouraging, replication and further investigation in larger samples is warranted.
Supplementary Material
Table 2.
Pro-inflammatory cytokines, mood, and sleep at pretreatment and posttreatment for CBTI-BP and PE.
| CBTI-BP | PE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Pretreatment (n=11) |
Posttreatment (n=8) |
Pretreatment (n=11) |
Posttreatment (n=9) |
||||||||
| M or N | % or SD | M or N | % or SD | M or N | % or SD | M or N | % or SD | t | p | ||
| Cytokines | |||||||||||
| IL-6a (pg/ml) | 0.85 | 0.73 | 0.69 | 0.46 | 0.77 | 1.27 | 2.48 | 5.25 | −1.46 | .16 | |
| IL-6b (pg/ml) | −0.58 | 1.03 | −0.59 | 0.75 | −0.81 | 0.89 | −0.24 | 1.37 | −1.61 | .13 | |
| sTNF-R2a (pg/ml) | 67.40 | 70.66 | 60.97 | 53.12 | 42.99 | 38.02 | 55.57 | 45.36 | −0.81 | .43 | |
| sTNF-R2b (pg/ml) | 3.72 | 1.04 | 3.79 | 0.87 | 3.54 | 0.65 | 3.82 | 0.62 | −0.79 | .44 | |
| Mood | |||||||||||
| YMRS | 3.14 | 2.72 | 3.50 | 3.38 | 3.38 | 3.95 | 4.50 | 3.46 | 0.53 | .60 | |
| YMRSc | 3.39 | 2.41 | 3.25 | 2.96 | 3.14 | 2.84 | 4.00 | 3.15 | −0.01 | .99 | |
| QIDS | 9.91 | 6.39 | 8.25 | 7.21 | 9.50 | 4.58 | 5.67 | 3.39 | 0.59 | .56 | |
| QIDSc | 7.09 | 6.32 | 6.25 | 6.43 | 7.18 | 4.49 | 3.67 | 3.46 | 0.82 | .42 | |
| Sleep | |||||||||||
| Time in Bed | 503.93 | 86.51 | 480.89 | 78.51 | 574.82 | 86.47 | 588.57 | 127.04 | −1.31 | .21 | |
| Total Sleep Time | 396.01 | 82.86 | 409.14 | 100.72 | 441.53 | 87.33 | 491.01 | 94.96 | −1.04 | .31 | |
| Total Wake Time | 104.97 | 34.89 | 73.06 | 28.63 | 135.94 | 41.51 | 97.56 | 73.10 | 0.18 | .86 | |
| Sleep Onset Latency | 36.85 | 21.22 | 23.82 | 9.88 | 54.25 | 38.11 | 54.46 | 86.25 | −0.75 | .46 | |
| Wake After Sleep Onset | 26.98 | 18.48 | 29.18 | 25.87 | 19.45 | 9.24 | 6.83 | 7.03 | 1.49 | .16 | |
| Early Morning Awakening | 28.98 | 34.13 | 22.46 | 20.92 | 25.55 | 25.42 | 17.68 | 37.25 | −0.19 | .86 | |
| Sleep Efficiency | 79.71 | 6.64 | 83.60 | 8.35 | 75.89 | 7.18 | 84.13 | 8.32 | −0.93 | .37 | |
Note.
Log transformed.
Unadjusted.
Sleep items removed. The t statistic and p value refer to the interaction between condition (CBTI-BP and PE) and time (pretreatment and posttreatment).
Acknowledgments
Source of Funding: Support for this study was provided by National Institute of Mental Health grants T32MH020006 (MRD), K01MH111953 (AMS), R34MH080958 (AGH).
Acronyms
- BD
Bipolar disorder
- IL-6
interleukin-6
- sTNF-R2
tumor necrosis factor soluble receptor 2
- OMT
oral mucosal transudate
- TWT
total wake time
- TST
total sleep time
- SOL
sleep onset latency
- WASO
wake after sleep onset
- EMA
early morning awakening
- RCT
randomized controlled trial
- CBTI-BP
cognitive behavior therapy for insomnia modified for bipolar disorder
- PE
psychoeducation
- SCID
structured clinical interview for DSM-IV
- YMRS
Young Mania Rating Scale
- QIDS
Quick Inventory of Depressive Symptomatology
Footnotes
Conflicts of Interest: The authors declare no financial conflicts of interest.
References
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray C, Lopez A. The Global Burden of Disease. World Health Organization. Cambridge: Harvard University Press; 1996. [Google Scholar]
- 3.Harvey AG, Schmidt DA, Scarnà A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005 Jan;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Rubinow DR, Holmes C, Abelson JM, Zhao S. The epidemiology of DSM-III-R bipolar I disorder in a general population survey. Psychol Med. 1997;27:1079–89. doi: 10.1017/s0033291797005333. [DOI] [PubMed] [Google Scholar]
- 5.Giglio LMF, Andreazza AC, Andersen M, Ceresér KM, Walz JC, Sterz L, Kapczinski F. Sleep in bipolar patients. Sleep Breath. 2009 May;13:169–73. doi: 10.1007/s11325-008-0215-5. [DOI] [PubMed] [Google Scholar]
- 6.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008 Jan;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnadas R, Harrison NA. Depression Phenotype, Inflammation, and the Brain: Implications for Future Research. Psychosom Med. 2016 May;78:384–88. doi: 10.1097/PSY.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 8.Byrne ML, Whittle S, Allen NB. The Role of Brain Structure and Function in the Association Between Inflammation and Depressive Symptoms: A Systematic Review. Psychosom Med. 2016 May;78:389–400. doi: 10.1097/PSY.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 9.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogelzangs N, Beekman aTF, de Jonge P, Penninx BWJH. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belem da Silva CT, Costa M de A, Bortoluzzi A, Pfaffenseller B, Vedana F, Kapczinski F, Manfro GG. Cytokine Levels in Panic Disorder: Evidence for a Dose-Response Relationship. Psychosom Med. 2016 Aug;79:1. doi: 10.1097/PSY.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 12.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005 Aug;104:788–93. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 13.Backonja M Misha, Coe CL, Muller DA, Schell K. Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol. 2008;195:157–63. doi: 10.1016/j.jneuroim.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009 Aug;70:1078–90. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 15.Munkholm K, Vinberg M, Vedel Kessing L. Cytokines in bipolar disorder: A systematic review and meta-analysis. J Affect Disord. 2013 Jan;144:16–27. doi: 10.1016/j.jad.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Munkholm K, Braüner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013 Sep;47:1119–33. doi: 10.1016/j.jpsychires.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Barbosa IG, Huguet RB, Mendonça VA, Sousa LP, Neves FS, Bauer ME, Teixeira AL. Increased plasma levels of soluble TNF receptor I in patients with bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2011 Mar;261:139–43. doi: 10.1007/s00406-010-0116-z. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. J Affect Disord. 2006 Feb;90:263–67. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Kapczinski F, Dal-Pizzol F, Teixeira AL, Magalhaes PVSS, Kauer-Sant’Anna M, Klamt F, Moreira JCF, de Bittencourt Pasquali MA, Fries GR, Quevedo J, Gama CS, Post R, Kauer-Sant’Anna M, Klamt F, Moreira JCF, Augusto de Bittencourt Pasquali M, Fries GR, Quevedo J, Gama CS, Post R. Peripheral biomarkers and illness activity in bipolar disorder. J Psychiatr Res. 2011 Feb;45:156–61. doi: 10.1016/j.jpsychires.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Brietzke E, Teixeira AL. Similar immune profile in bipolar disorder and schizophrenia: selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disord. 2010 Jun;12:453–54. doi: 10.1111/j.1399-5618.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 21.Doganavsargil-Baysal O, Cinemre B, Aksoy UM, Akbas H, Metin O, Fettahoglu C, Gokmen Z, Davran F. Levels of TNF-α, soluble TNF receptors (sTNFR1, sTNFR2), and cognition in bipolar disorder. Hum Psychopharmacol Clin Exp. 2013 Mar;28:160–67. doi: 10.1002/hup.2301. [DOI] [PubMed] [Google Scholar]
- 22.Brietzke E, Stertz L, Fernandes BS, Kauer-Sant’anna M, Mascarenhas M, Escosteguy Vargas A, Chies JA, Kapczinski F. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord. 2009 Aug;116:214–17. doi: 10.1016/j.jad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Guloksuz S, Aktas Cetin E, Cetin T, Deniz G, Oral ET, Nutt DJ. Cytokine levels in euthymic bipolar patients. J Affect Disord. 2010;126:458–62. doi: 10.1016/j.jad.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Rapaport MH. Immune parameters in euthymic bipolar patients and normal volunteers. J Affect Disord. 1994 Nov;32:149–56. doi: 10.1016/0165-0327(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 25.Talbot LS, Stone S, Gruber J, Hairston IS, Eidelman P, Harvey AG. A test of the bidirectional association between sleep and mood in bipolar disorder and insomnia. J Abnorm Psychol. 2012 Feb;121:39–50. doi: 10.1037/a0024946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marangell LB. The Importance of Subsyndromal Symptoms in Bipolar Disorder. J Clin Psychiatry. 2004;65:24–27. [PubMed] [Google Scholar]
- 27.Hope S, Dieset I, Agartz I, Steen NE, Ueland T, Melle I, Aukrust P, Andreassen OA. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res. 2011 Dec;45:1608–16. doi: 10.1016/j.jpsychires.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Tsai S-YM, Yang Y-Y, Kuo C-J, Chen C-C, Leu S-JC. Effects of symptomatic severity on elevation of plasma soluble interleukin-2 receptor in bipolar mania. J Affect Disord. 2001 May;64:185–93. doi: 10.1016/s0165-0327(00)00252-4. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y-K, Suh I-B, Kim H, Han C-S, Lim C-S, Choi S-H, Licinio J. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: effects of psychotropic drugs. Mol Psychiatry. 2002 Jan;7:1107–14. doi: 10.1038/sj.mp.4001084. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y-K, Myint A-M, Lee B-H, Han C-S, Lee S-W, Leonard BE, Steinbusch HWM. T-helper types, 1, 2, and 3 cytokine interactions in symptomatic manic patients. Psychiatry Res. 2004 Dec;129:267–72. doi: 10.1016/j.psychres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Maes M, Bosmans E, Calabrese J, Smith R, Meltzer HY. Interleukin-2 and interleukin-6 in schizophrenia and mania: Effects of neuroleptics and mood stabilizers. J Psychiatr Res. 1995 Mar;29:141–52. doi: 10.1016/0022-3956(94)00049-w. [DOI] [PubMed] [Google Scholar]
- 32.Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar Disord. 2006 Jun;8:271–74. doi: 10.1111/j.1399-5618.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- 33.Leibenluft E, Albert PS, Rosenthal NE, Wehr TA. Relationship between sleep and mood in patients with rapid-cycling bipolar disorder. Psychiatry Res. 1996 Jul;63:161–68. doi: 10.1016/0165-1781(96)02854-5. [DOI] [PubMed] [Google Scholar]
- 34.Burgos I, Richter L, Klein T, Fiebich B, Feige B, Lieb K, Voderholzer U, Riemann D. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: A pilot study. Brain Behav Immun. 2006 May;20:246–53. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997 May;82:1313–16. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 36.Park H, Tsai KM, Dahl RE, Irwin MR, McCreath H, Seeman TE, Fuligni AJ. Sleep and Inflammation During Adolescence. Psychosom Med. 2016;78:677–85. doi: 10.1097/PSY.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smagula SF, Stone KL, Redline S, Ancoli-Israel S, Barrett-Connor E, Lane NE, Orwoll ES, Cauley JA Osteoporotic Fractures in Men (MrOS) Research Group. Actigraphy- and Polysomnography-Measured Sleep Disturbances, Inflammation, and Mortality Among Older Men. Psychosom Med. 2016;78:686–96. doi: 10.1097/PSY.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, And Inflammation: A Systematic Review And Meta-Analysis Of Cohort Studies And Experimental Sleep Deprivation. Biol Psychiatry. 2015 Jun;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, Gourby B, Bourrilhon C, Florence G, Gomez-Merino D. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine. 2011 Nov;56:318–24. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, Basta M, Fang J, Sarrigiannidis A, Chrousos GP. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol - Endocrinol Metab. 2007;292 doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- 41.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee B-N, Smith EO, Szuba MP, Van Dongen HPA, Dinges DF. Soluble TNF-α receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001 Jan;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 42.Chennaoui M, Drogou C, Sauvet F, Gomez-Merino D, Scofield DE, Nindl BC. Effect of acute sleep deprivation and recovery on Insulin-like Growth Factor-I responses and inflammatory gene expression in healthy men. Eur Cytokine Netw. 2014;25:52–57. doi: 10.1684/ecn.2014.0356. [DOI] [PubMed] [Google Scholar]
- 43.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007 Sep;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin H-M, Vela-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002 Jul;51:887–92. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 45.First MB, Spitzer RL, Miriam G, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 46.Bjurström MF, Olmstead R, Irwin MR. Reciprocal Relationship Between Sleep Macrostructure and Evening and Morning Cellular Inflammation in Rheumatoid Arthritis. Psychosom Med. 2017 Jan;79:24–33. doi: 10.1097/PSY.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ritter PS, Kretschmer K, Pfennig A, Soltmann B. Disturbed sleep in bipolar disorder is related to an elevation of IL-6 in peripheral monocytes. Med Hypotheses. 2013 Dec;81:1031–33. doi: 10.1016/j.mehy.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 48.Harvey AG, Soehner AM, Kaplan KA, Hein K, Lee J, Kanady J, Li D, Rabe-Hesketh S, Ketter TA, Neylan TC, Buysse DJ. Treating Insomnia Improves Mood State, Sleep, and Functioning in Bipolar Disorder: A Pilot Randomized Controlled Trial. J Consult Clin Psychol. 2015 Jan;83:564–77. doi: 10.1037/a0038655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skre I, Onstad S, Torgersen S, Kringlen E. High interrater reliability for the Structured Clinical Interview for DSM-III-R Axis I (SCID-I) Acta Psychiatr Scand. 1991;84:167–73. doi: 10.1111/j.1600-0447.1991.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 50.Edinger J, Wyatt J, Olsen M, Stechuchak K, Carney CE, Chiang A, Krystal A, Lineberger M, Means M, Radtke R. Reliability and validity of the Duke Structured Interview for Sleep Disorders for insomnia screening. Sleep. 2009;32:A265. [Google Scholar]
- 51.Young RC, Biggs JT, Ziegler VE, Meyer Da. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 52.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996 May;26:477. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 53.Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, Morin CM. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012 Feb;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006 Sep;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 55.von Känel R, Dimsdale JE, Ancoli-Israel S, Mills PJ, Patterson TL, McKibbin CL, Archuleta C, Grant I. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer’s disease. J Am Geriatr Soc. 2006 Mar;54:431–37. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 56.Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005 Jan;67:187–94. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- 57.Nishanian P, Aziz N, Chung J, Detels R, Fahey JL. Oral fluids as an alternative to serum for measurement of markers of immune activation. Clin Diagn Lab Immunol. 1998 Jul;5:507–12. doi: 10.1128/cdli.5.4.507-512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosom Med. 2002 Jan;66:124–31. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- 59.Ferri RS. Oral mucosal transudate testing for HIV-1 antibodies: A clinical update. J Assoc Nurses AIDS Care. 1998;9:68–72. doi: 10.1016/S1055-3290(98)80062-9. [DOI] [PubMed] [Google Scholar]
- 60.Gallo D. Evaluation of a System Using Oral Mucosal Transudate for HIV-1 Antibody Screening and Confirmatory Testing. JAMA J Am Med Assoc. 1997;277:254. [PubMed] [Google Scholar]
- 61.Kreuzer KA, Rockstroh JK, Sauerbruch T, Spengler U. A comparative study of different enzyme immunosorbent assays for human tumor necrosis factor-alpha. J Immunol Methods. 1996 Sep;195:49–54. doi: 10.1016/0022-1759(96)00090-7. [DOI] [PubMed] [Google Scholar]
- 62.Diez-Ruiz a, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 63.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004 May;10:307–12. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 64.Lee EC, Whitehead AL, Jacques RM, Julious SA. The statistical interpretation of pilot trials: should significance thresholds be reconsidered? BMC Med Res Methodol. 2014 Jan;14:41. doi: 10.1186/1471-2288-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cumming G. The new statistics: why and how. Psychol Sci. 2014 Jan;25:7–29. doi: 10.1177/0956797613504966. [DOI] [PubMed] [Google Scholar]
- 66.Cohen J. Statistical power analysis for the behavioral sciences. Vol. 2nd, Statistical Power Analysis for the Behavioral Sciences. 1988 [Google Scholar]
- 67.Kraemer HC, Morgan GA, Leech NL, Gliner JA, Vaske JJ, Harmon RJ. Measures of Clinical Significance. J Am Acad Child Adolesc Psychiatry. 2003;42:1542–1529. doi: 10.1097/00004583-200312000-00022. [DOI] [PubMed] [Google Scholar]
- 68.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004 Jan;66:411–21. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 69.R Development Core Team R. R: A Language and Environment for Statistical Computing. Vol. 1. R Foundation for Statistical Computing; 2016. p. 409. [Google Scholar]
- 70.O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009 Oct;23:887–97. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boufidou F, Nikolaou C, Alevizos B, Liappas IA, Christodoulou GN. Cytokine production in bipolar affective disorder patients under lithium treatment. J Affect Disord. 2004 Oct;82:309–13. doi: 10.1016/j.jad.2004.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.