Abstract
Objective
Total dose of oxytocin received during labor is an important variable in studies of human labor but is difficult to calculate. We sought to identify a surrogate measure for total dose of oxytocin received.
Study Design
For each subject receiving oxytocin during labor, the oxytocin total dose received in labor was calculated as the area under the curve. Maximal oxytocin infusion rate, total duration of oxytocin infusion, and the product of both, defined as the oxytocin product, were then each correlated with the total dose of oxytocin received using the Pearson’s correlation coefficient.
Results
Oxytocin dosing data were available from 402 women at Duke and 6,907 women from Pithagore6. The two variables alone, or combined as the oxytocin product, demonstrated a high correlation with the oxytocin total dose (r > 0.7), with the oxytocin product demonstrating the highest (r > 0.9). This was true whether labor was induced or augmented and whether delivery was vaginal or cesarean.
Conclusion
The oxytocin product, composed of two easily obtained variables, demonstrated a very high correlation with total oxytocin dose received in labor and represents a simple and accurate surrogate for total dose of oxytocin received during labor. The oxytocin product can be used in clinical studies in which oxytocin dose is an important variable.
Keywords: area under the curve, augmentation of labor, labor, labor induction, oxytocin, pregnancy
Oxytocin is an endogenous hormone that is released in large amounts during spontaneous labor.1 In addition, synthetic oxytocin is used clinically for induction and augmentation of labor.2 Due to its short serum half-life and narrow therapeutic range, oxytocin infusions typically begin at low rates and then are incrementally increased until the rate that produces an appropriate uterine contractile response is achieved. The dose at which this occurs varies for individual women and is difficult to predict.1,3,4
The optimal oxytocin dosing regimen for induction or augmentation of labor is not known. Prolonged infusions of oxytocin are associated with decreased uterine contractility.5–8 Clinically, this may present as dysfunctional labor patterns during labor, which increase the risk for cesarean delivery, or as uterine atony following delivery, which is an important risk factor for postpartum hemorrhage.9–11 The mechanism by which this occurs is thought to be due to oxytocin-mediated desensitization of the oxytocin receptor, but it is not known if prolonged infusions directly cause these adverse events or if some other predictor that correlates with the need for long infusions drives the association.5,12,13 Regardless, oxytocin exposure in labor is an important predictor of labor outcomes.
The total dose of oxytocin that a woman receives during labor is difficult to calculate as the infusion rate of oxytocin does not remain constant for each woman, but rather varies during the course of labor. The area under the curve (AUC) of oxytocin infusion rate versus duration of oxytocin infusion gives the total dose of oxytocin received in labor but is difficult to calculate. Calculating the AUC involves determining the rate of administration, as well as the duration of time at each rate, for the entire labor. As total oxytocin exposure during labor is an important clinical variable that is associated with various obstetric outcomes, the objective of this study was to determine if other more readily accessible measures of oxytocin dosing could be utilized as a surrogate for total dose of oxytocin received in labor.
Materials and Methods
The study was approved by the Institutional Review Board of Duke University and by the Sud Est III Institutional Review Board and the French Data Protection Agency (CNIL) for the Pithagore6 Investigators. We conducted a retrospective cohort study to determine if the two variables related to oxytocin dosing and utilization during labor that are readily available from the medical record, namely, maximal rate of infusion and duration of infusion, could be used as a surrogate for total dose of oxytocin received during labor.
Two independent cohorts of women for whom oxytocin dosing data were available were utilized for this study. The first cohort of subjects came from a retrospective cohort study that was conducted at the Duke University Hospital between 2009 and 2010. The study included consecutive women at 36 weeks or more laboring at the Duke University Hospital with a live, nonanomalous fetus who received oxytocin for induction or augmentation of labor. The Duke study was originally designed as a quality initiative to determine oxytocin utilization on the Duke University Hospital labor and delivery unit. Data were abstracted from the medical records by two of the authors (C.A.G. and L.L.L.). The second cohort of subjects came from women who enrolled in the Pithagore6 trial, a cluster-randomized controlled trial conducted at 106 units in France between 2004 and 2006 to evaluate an educational intervention to reduce the rate of severe postpartum hemorrhage.14 The Pithagore6 trial has been previously described in detail.14 The total amount of oxytocin received during labor was available for 402 women from the Duke cohort and for 6,907 women who had enrolled in the Pithagore6 study. For each subject in both cohorts, total dose of oxytocin in labor in mU was calculated by plotting oxytocin infusion rate versus time (Fig. 1) and then computing the AUC using GraphPad Prism version 6.0 (GraphPad Software, Inc, La Jolla, CA) for the Duke cohort and Microsoft Excel (Microsoft Corporation, Redmond, WA) for the Pithagore6 cohort.
Fig. 1.
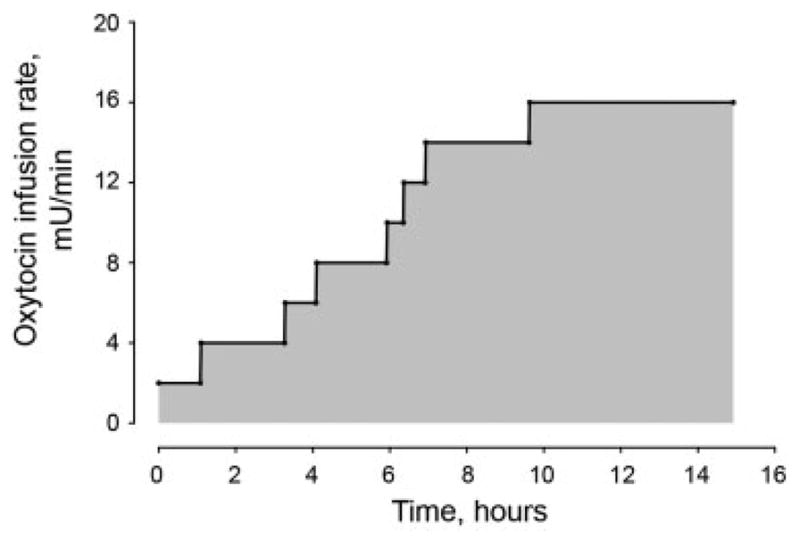
Graphical representative example of total oxytocin dose received in labor from a single subject. To calculate the total dose of oxytocin received during labor, for each woman, a plot must be constructed of oxytocin infusion rate with time. The shaded region, or the area under the curve, represents the total dose of oxytocin received during labor.
For each of the two study populations, maximal rate of oxytocin infusion (in mU/min), the duration of oxytocin infusion (in hours), and the oxytocin product (product of maximal oxytocin infusion rate with total duration of infusion, mU/min × hours) were each correlated with total oxytocin dose received in labor (mU) using a Pearson’s correlation coefficient. Maximal rate of infusion was defined as the highest infusion rate of oxytocin that a subject received at any time during her labor. Duration of infusion was defined as the total time a subject received oxytocin, accounting for periods in which the infusion may have been discontinued during labor. Given the large sample size, differences in continuous variables between each cohort were compared using an unpaired t-test with Welch’s correction, which assumes that the standard deviations for each variable are not similar between the two cohorts. All statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software, Inc) and JMP 11 (Version 11.0, SAS Institute, Inc, Cary, NC). Normally distributed continuous variables were reported as mean and standard deviations, while nonnormally distributed continuous variables were reported as median and interquartile range (IQR). A p-value of < 0.05 was considered to be significant.
Results
There were 402 women from the Duke cohort with complete oxytocin data available, which included the maximal rate of infusion, the duration of infusion, and the total dose received. Of the 6,907 women from the Pithagore6 cohort with total oxytocin dosing available, 6,842 women had oxytocin product data available. Table 1 summarizes basic subject characteristics from each cohort. Women from the Duke cohort were significantly younger, had greater body mass index at delivery, and delivered infants with smaller birth weights compared with women in the Pithagore6 cohort. In addition, women at Duke were more likely to have been induced and the Duke cohort had a higher cesarean delivery rate compared with women from France (Table 1).
Table 1.
Subject demographics
| Variable | Duke N = 402 |
Pithagore6 N = 6,907 |
p-Value |
|---|---|---|---|
| Age, ya | 27.0 ± 6.6 | 29.9 ± 5.2 | <0.0001 |
| Race/ethnicity, n (%) | Not available | ||
| White | 129 (32.1) | ||
| Black | 149 (37.1) | ||
| Hispanic | 95 (23.6) | ||
| Asian/Pacific Islander | 22 (5.5) | ||
| Other | 2 (0.5) | ||
| Missing | 5 (1.2) | ||
| BMI, kg/m2a,b | 33.0 ± 7.2 | 27.9 ± 4.4 | <0.0001 |
| Gestational age at delivery, wka | 38.8 ± 2.4 | 39.8 ± 1.6 | <0.0001 |
| Induced labor, n (%) | 306 (76.1) | 2,115 (30.6) | <0.0001 |
| Mode of delivery, n (%) | |||
| Vaginal | 288 (71.6) | 6062 (87.8) | <0.0001 |
| Cesarean | 114 (28.4) | 845 (12.2) | |
| Birthweight, ga | 3,196 ± 627 | 3,389 ± 374 | <0.0001 |
Abbreviation: BMI, body mass index.
Mean ± standard deviation.
BMI at delivery.
Women in the Duke cohort received higher median maximal oxytocin infusion rates and longer duration of infusions, resulting in a significantly greater oxytocin product than women in the Pithagore6 cohort (Table 2). Similarly, women in the Duke cohort received significantly greater total dose of oxytocin (median [IQR]) than women in the Pithagore6 cohort (3,535 [1,194, 9,822] vs. 1,370 [540, 2,940] mU, p < 0.0001) (Table 2).
Table 2.
Oxytocin dosing variables
| Variable | Dukea N = 402 |
Pithagore6b N = 6,907 |
p-Value |
|---|---|---|---|
| Maximal oxytocin infusion rate, mU/min | 9.0 (5.0, 16.0) | 7.5 (5.0, 12.5) | <0.0001 |
| Total duration of oxytocin infusion, h | 11.6 (6.4, 20.4) | 4.1 (2.2, 6.3) | <0.0001 |
| Oxytocin product, mU/min × h | 173 (46, 880) | 32.5 (11.8, 72.6) | <0.0001 |
| Oxytocin total dose (AUC), mU | 3,525 (1,194, 9,822) | 1,370 (540, 2,940) | <0.0001 |
Abbreviation: AUC, area under the curve.
The Duke data are from 402 women.
The Pithagore6 data are as follows: 6,854 women with maximal infusion rate, 6,895 women with total duration of oxytocin infusion, 6,842 women with oxytocin product, and 6,907 with oxytocin total dose (AUC).
Note: Data presented are median (interquartile range).
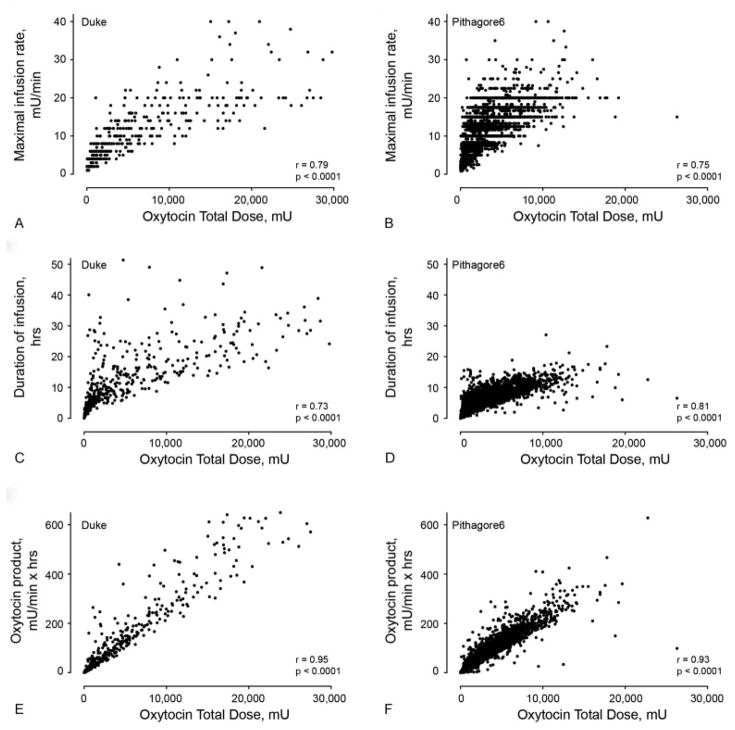
Maximal oxytocin infusion rate correlated well with oxytocin total dose in both the Duke and Pithagore6 cohorts (r = 0.79 [95% confidence interval [CI]: 0.75, 0.82] and r = 0.75 [95% CI: 0.74, 0.76], respectively, Table 3, Fig. 2). Similarly, the duration of oxytocin infusion correlated well with oxytocin total dose in both groups (r = 0.73 [95% CI: 0.68, 0.77] and r = 0.81 [95% CI: 0.80, 0.82], respectively). The oxytocin product correlated best with oxytocin total dose in both cohorts compared with either maximal rate of oxytocin infusion or duration of infusion (r = 0.95 [95% CI: 0.93, 0.96] and r = 0.93 [95% CI: 0.93, 0.93], for the Duke and Pithagore6 cohorts, respectively, Table 3, Fig. 2).
Table 3.
Pearson’s correlations of oxytocin parameters with oxytocin AUC, all subjects
| Variable | Pearson’s correlations (95% CI) for each listed parameter with oxytocin AUCa | |
|---|---|---|
| Duke N = 402 |
Pithagore6 N = 6,907 |
|
| Maximal oxytocin infusion rate | 0.79 (0.75, 0.82) | 0.75 (0.74, 0.76) |
| Duration of infusion | 0.73 (0.68, 0.77) | 0.81 (0.80, 0.82) |
| Oxytocin product | 0.95 (0.93, 0.96) | 0.93 (0.93, 0.93) |
Abbreviation: AUC, area under the curve.
All Pearson’s correlations listed had p-values of <0.0001.
Fig. 2.
Correlations of oxytocin maximal infusion rate, duration of oxytocin infusion, and the oxytocin product each with total oxytocin dose (calculated by the AUC) received in labor among women from Duke and Pithagore6. (A and B) Correlation of maximal oxytocin infusion rate with oxytocin total dose from Duke (A, r = 0.79, p < 0.0001) and Pithagore6 (B, r = 0.75, p < 0.0001). (C and D) Correlation of duration of oxytocin infusion with oxytocin total dose from Duke (C, r = 0.73, p < 0.0001) and Pithagore6 (D, r = 0.81, p < 0.0001). (E and F) Correlation of the oxytocin product (maximal infusion rate × duration of infusion) with oxytocin total dose from Duke (E, r = 0.95, p < 0.0001) and Pithagore6 (F, r = 0.93, p < 0.0001). AUC, area under the curve.
Duration of infusion was defined as the total time that a subject received oxytocin, which accounted for periods in which the infusion was discontinued either during labor or prior to delivery. For example, among subjects for whom their oxytocin infusion was temporarily discontinued (i.e., for fetal intolerance of labor or to provide the laboring mother a break from oxytocin), the duration of infusion variable did not include the time that oxytocin was not being given. For the Duke cohort, the time from the initiation of oxytocin to delivery was also available for analysis. This variable was the period of time from the start of oxytocin until delivery. Similar to duration of infusion, the period of time from initiation of oxytocin to delivery also correlated well with oxytocin total dose (r = 0.74 [95% CI: 0.68, 0.78]) in the Duke cohort. In addition, when the time from initiation of oxytocin to delivery was included in the oxytocin product (maximal oxytocin infusion rate × time from initiation of oxytocin to delivery), this also correlated extremely well with oxytocin total dose (r = 0.95 [95% CI: 0.92, 0.96]).
Both cohorts included women who received oxytocin for labor induction as well as those who labored spontaneously but received oxytocin for augmentation of labor. Table 4 provides the correlations from both cohorts for the three studied variables, maximal rate of infusion, duration of infusion, and the oxytocin product, each compared with oxytocin total dose, stratified by whether oxytocin was utilized for induction or augmentation of labor (Table 4). Within the Pithagore6 cohort, the relationships persisted demonstrating that the oxytocin product correlated best with the total oxytocin dose received in labor (Table 4). Within the Duke cohort, which was much smaller, the 95% CIs for the Pearson’s correlations for the oxytocin product with oxytocin total dose overlapped with some of the other measures of oxytocin exposure within the subgroup analysis (Table 4). Results from each cohort were also stratified by mode of delivery, which also demonstrated similar findings (Table 4).
Table 4.
Pearson’s correlations of oxytocin parameters with oxytocin AUC among listed clinical subgroups
| Variable | Pearson’s correlations (95% CI) for each listed parameter with oxytocin AUCa | |
|---|---|---|
| Duke | Pithagore6 | |
| Induced labor | N = 306 | N = 2,115 |
| Maximal oxytocin infusion rate | 0.78 (0.64, 0.87) | 0.76 (0.74, 0.77) |
| Duration of infusion | 0.84 (0.74, 0.91) | 0.79 (0.78, 0.80) |
| Oxytocin product | 0.91 (0.84, 0.94) | 0.92 (0.92, 0.93) |
| Spontaneous augmented labor | N = 96 | N = 4,792 |
| Maximal oxytocin infusion rate | 0.85 (0.75, 0.91) | 0.72 (0.69, 0.74) |
| Duration of infusion | 0.79 (0.65, 0.87) | 0.80 (0.78, 0.81) |
| Oxytocin product | 0.92 (0.87, 0.96) | 0.92 (0.91, 0.93) |
| Vaginal delivery | N = 288 | N = 6,062 |
| Maximal oxytocin infusion rate | 0.78 (0.65, 0.87) | 0.75 (0.74, 0.76) |
| Duration of infusion | 0.87 (0.78, 0.92) | 0.81 (0.80, 0.82) |
| Oxytocin product | 0.96 (0.93, 0.98) | 0.93 (0.92, 0.93) |
| Cesarean delivery | N = 114 | N = 845 |
| Maximal oxytocin infusion rate | 0.81 (0.68, 0.89) | 0.77 (0.74, 0.80) |
| Duration of infusion | 0.87 (0.77, 0.92) | 0.82 (0.80, 0.84) |
| Oxytocin product | 0.93 (0.87, 0.96) | 0.93 (0.92, 0.93) |
Abbreviation: AUC, area under the curve.
All Pearson’s correlations listed had p-values of <0.0001.
Comment
Oxytocin is an important clinical exposure that affects labor outcomes. Calculating the total dose of oxytocin that a woman receives during labor is difficult and time-consuming as the infusion rate is typically not constant and varies throughout her labor. Here, we demonstrate that the oxytocin product, the product of total duration of oxytocin infusion during labor with the maximal infusion rate, correlates highly with the total dose received suggesting that the oxytocin product is a valid surrogate for total dose received. Importantly, the oxytocin product is composed of two variables, total duration received and maximal infusion rate, both of which are variables typically easily abstracted from the medical record.
Our data are generated from two relatively large, yet distinct patient populations. Oxytocin utilization, including maximal infusion rates and duration of infusion, also differed significantly between the two cohorts. Despite this, similar correlation findings were seen with each of the three studied oxytocin variables with total oxytocin dose between the two cohorts. Not only did oxytocin dosing differ between the two cohorts but also the characteristics of women and deliveries suggest that the populations may be different. Together, this adds strength to the association that we found of the oxytocin product with total oxytocin dose and suggests that the correlations would remain strong when applied to other populations. It is unknown if our findings are generalizable to other delivery units whose oxytocin utilization may differ but our study does include data from distinct patient populations whose oxytocin exposure differed. Finally, the oxytocin product does not estimate the actual total dose and thus cannot be used to estimate or predict the total oxytocin dosing during labor, but it can be used as a surrogate for oxytocin exposure as the oxytocin product highly correlates with total dosing.
We found that two variables that are easily identified in the medical record, and commonly collected as part of clinical obstetric research, namely, the maximal oxytocin infusion rate and the duration of oxytocin infusion, when combined as a product, correlate highly with the total amount of oxytocin received in labor. As the total dose of oxytocin received in labor is associated with numerous obstetric clinical outcomes, and is difficult to calculate, the oxytocin product is an easily obtained surrogate for total dose received that can be used in clinical studies.
Acknowledgments
Funding
This work was supported by NIH grant K08-HD070872 to CAG. The Pithagore6 project was funded by the French Ministry of Health under its Clinical Research Hospital Program (contract number 27–35) and the Caisse Nationale d’Assurance Maladie (CNAMTS).
The authors would like to thank the staff of the Pithagore6 maternity units and the members of the Pithagore6 group.
Footnotes
Note
This work was presented in part at the 35th Annual Meeting of the Society for Maternal-Fetal Medicine, San Diego, CA, February 2015.
Conflict of Interest
None.
References
- 1.Leake RD, Weitzman RE, Glatz TH, Fisher DA. Plasma oxytocin concentrations in men, nonpregnant women, and pregnant women before and during spontaneous labor. J Clin Endocrinol Metab. 1981;53(04):730–733. doi: 10.1210/jcem-53-4-730. [DOI] [PubMed] [Google Scholar]
- 2.ACOG Committee on Practice Bulletins – Obstetrics. ACOG Practice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114(2 Pt 1):386–397. doi: 10.1097/AOG.0b013e3181b48ef5. [DOI] [PubMed] [Google Scholar]
- 3.Leake RD, Weitzman RE, Fisher DA. Oxytocin concentrations during the neonatal period. Biol Neonate. 1981;39(3–4):127–131. doi: 10.1159/000241417. [DOI] [PubMed] [Google Scholar]
- 4.Seitchik J, Amico J, Robinson AG, Castillo M. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics. Am J Obstet Gynecol. 1984;150(03):225–228. doi: 10.1016/s0002-9378(84)90355-7. [DOI] [PubMed] [Google Scholar]
- 5.Grotegut CA, Feng L, Mao L, Heine RP, Murtha AP, Rockman HA. β-Arrestin mediates oxytocin receptor signaling, which regulates uterine contractility and cellular migration. Am J Physiol Endocrinol Metab. 2011;300(03):E468–E477. doi: 10.1152/ajpendo.00390.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crall HD, Mattison DR. Oxytocin pharmacodynamics: effect of long infusions on uterine activity. Gynecol Obstet Invest. 1991;31(01):17–22. doi: 10.1159/000293094. [DOI] [PubMed] [Google Scholar]
- 7.Balki M, Cristian AL, Kingdom J, Carvalho JC. Oxytocin pretreatment of pregnant rat myometrium reduces the efficacy of oxytocin but not of ergonovine maleate or prostaglandin F 2 alpha. Reprod Sci. 2010;17(03):269–277. doi: 10.1177/1933719109351934. [DOI] [PubMed] [Google Scholar]
- 8.Magalhaes JK, Carvalho JC, Parkes RK, Kingdom J, Li Y, Balki M. Oxytocin pretreatment decreases oxytocin-induced myometrial contractions in pregnant rats in a concentration-dependent but not time-dependent manner. Reprod Sci. 2009;16(05):501–508. doi: 10.1177/1933719108329954. [DOI] [PubMed] [Google Scholar]
- 9.Grotegut CA, Paglia MJ, Johnson LN, Thames B, James AH. Oxytocin exposure during labor among women with postpartum hemorrhage secondary to uterine atony. Am J Obstet Gynecol. 2011;204(01):56.e1–56.e6. doi: 10.1016/j.ajog.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belghiti J, Kayem G, Dupont C, Rudigoz RC, Bouvier-Colle MH, Deneux-Tharaux C. Oxytocin during labour and risk of severe postpartum haemorrhage: a population-based, cohort-nested case-control study. BMJ Open. 2011;1(02):e000514. doi: 10.1136/bmjopen-2011-000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjaergaard H, Olsen J, Ottesen B, Dykes AK. Incidence and outcomes of dystocia in the active phase of labor in term nulliparous women with spontaneous labor onset. Acta Obstet Gynecol Scand. 2009;88(04):402–407. doi: 10.1080/00016340902811001. [DOI] [PubMed] [Google Scholar]
- 12.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J Biol Chem. 2001;276(22):19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 13.Grotegut CA, Mao L, Pierce SL, Swamy GK, Heine RP, Murtha AP. Enhanced uterine contractility and stillbirth in mice lacking G protein-coupled receptor kinase 6 (GRK6): implications for oxytocin receptor desensitization. Mol Endocrinol. 2016;30(04):455–468. doi: 10.1210/me.2015-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deneux-Tharaux C, Dupont C, Colin C, et al. Multifaceted intervention to decrease the rate of severe postpartum haemorrhage: the PITHAGORE6 cluster-randomised controlled trial. BJOG. 2010;117(10):1278–1287. doi: 10.1111/j.1471-0528.2010.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]