Abstract
Type 2 diabetes is an emerging global health epidemic. Foundations for new therapies are arising from understanding interactions between body systems. Bone-derived factors that reduce RANKL (receptor activator of NF-kappa B ligand) signaling in the liver may prevent insulin resistance and the onset of type 2 diabetes. Here we demonstrate that deletion of the epigenetic regulator, Hdac3, in Osx1-expressing osteoprogenitors prevents insulin resistance induced by high fat diet by increasing serum and skeletal gene expression levels of osteoprotegerin (Opg), a natural inhibitor of RANKL signaling. Removal of one Opg allele in mice lacking Hdac3 in Osx1+ osteoprogenitors increases the insulin resistance of the Hdac3-deficient mice on a high fat diet. Thus, Hdac3-depletion in osteoblasts increases expression of Opg, subsequently preserving insulin sensitivity. The Hdac inhibitor vorinostat also increased Opg transcription and histone acetylation of the Opg locus. These results define a new mechanism by which bone regulates systemic insulin sensitivity.
Keywords: osteoblast, energy metabolism, insulin sensitivity, osteoprotegerin, type 2 diabetes mellitus, histone deacetylase 3
Introduction
Type 2 diabetes mellitus causes significant morbidity and mortality around the world. Globally, the incidence of diabetes rose nearly 3-fold (108 million to 422 million) between 1980 and 2014 (World Health Organization, Geneva, 2016), with type 2 diabetes comprising the vast majority of these cases (World Health Organization, Geneva, 1999). Prediabetes, which is associated with a high rate of progression to type 2 diabetes, is also rapidly increasing in prevalence (1). One of the earliest identifiable abnormalities in prediabetes and hepatic insulin resistance is higher fasting insulin concentrations with unchanged endogenous glucose production (2, 3). Current interventions used to treat prediabetes cannot alter the natural history of the disease (4), and therefore new therapeutic approaches are needed.
Bone and osteoblast-derived factors are potential targets for new diabetic therapies because the skeleton, which is insulin sensitive (5, 6), produces endocrine factors that regulate systemic glucose levels and energy metabolism to promote metabolic homeostasis (7–10). For example, osteoblast suppression is linked to metabolic dysfunction in mice (7), and ablation of osteoblasts in adult mice causes insulin insensitivity (11). Hepatic inflammation contributes to some of the earliest changes in the development of insulin resistance during progression to type 2 diabetes (12), and importantly, bone-derived factors that can reduce inflammatory signaling in the liver improve hepatic insulin resistance and prevent type 2 diabetes in mice and possibly in humans (13). The molecular mechanisms behind this phenomenon, however, are not yet understood.
Histone deacetylases (Hdacs) are epigenetic modulators of systemic metabolism and skeletal development. We previously showed that Hdac3 is needed in osteo/chondro-progenitor cells to regulate osteoblast and chondrocyte transcriptomes and endochondral and intramembranous ossification (14–16). Hdac3 deletion in Osx1+ skeletal progenitor cells reduces bone density and increases marrow adiposity, but produces generally leaner mice (16, 17). In contrast, deletion of Hdac3 in committed osteoblasts does not affect marrow adiposity or body size despite reducing bone quality (15). Similarly, broad acting Hdac inhibitors negatively impact pre-osteoblast function and skeletal development but have few negative effects on committed osteoblasts (18). Hdac inhibitors also reduce body weight, glucose, and insulin levels in diabetic models by enhancing oxidative metabolism (19), and Hdac3 was implicated as a likely mediator of these phenotypes (20–24). The role of Hdac3 in regulating energy homeostasis from bone is not yet fully understood, but our previous studies revealed that deletion of Hdac3 in an osteoprogenitor cell population (with Osx1-Cre) causes lean body mass and low fasting glucose levels (17). Moreover, these Hdac3-deficient mice maintained insulin sensitivity and prevented the onset of hepatic steatosis on a long-term (6 month) high fat diet (17). The goal of the study was to identify circulating factors present in Hdac3-deficient osteoprogenitor cells that affect systemic metabolism.
2. Methods
2.1 Animal model and diets
All mice were maintained on a C57BL/6 background and genotyped as previously described (15, 16). For initial experiments with the Hdac3 mouse line, Hdac3fl/fl mice were bred with mice expressing Cre recombinase under control of the osterix (Osx1) promoter, eventually yielding three groups of mice that were studied: Hdac3-conditional knockout (CKO) animals (Hdac3fl/fl; Osx1-Cre+), and Cre-negative control littermates (Hdac3+/+ or Hdac3fl/fl)(25). In a set of experiments designed to assess the role of osteoprotegerin (Opg) in the Hdac3 CKO mouse phenotype, the mice above were crossed with commercially available Opg−/− mice (Tnfrsf11btm1Eac, strain #010672, The Jackson Laboratory), eventually yielding three additional groups of double-mutant mice that were studied alongside the single-mutants described above: A) Opg+/−; Hdac3fl/fl; Osx1-Cre+, B) Opg+/−; Osx1-Cre+, and C) Opg+/− or Opg+/−; Hdac3fl/fl. We also investigated the metabolic biology caused by Hdac3 deficiency by crossing Hdac3fl/fl mice with transgenic mice expressing Cre recombinase under control of a segment (approximately 4 kb) of the human OCN promoter to create Hdac3fl/fl; OCN-Cre+ conditional knockout mice and Cre-negative or Cre-positive control littermates as previously described (15).
Mice were weaned onto a high fat diet (HFD: 60% fat, 20% protein, 20% carbohydrate; Research Diets D12492) by 4 weeks of age. All analyses were conducted with male mice, because wildtype male mice develop HFD-induced metabolic defects more rapidly than female mice (26, 27) and are better for studying effects of a short term HFD regimen. Mice were sacrificed between 9 and 10 weeks of age by carbon dioxide asphyxiation. Blood was collected via cardiac puncture and serum was stored at −80° C. For molecular analyses, tissues were immediately flash frozen in liquid nitrogen and stored at −80° C.
2.2 Body composition measurements
Body composition of individual mice was quantified via dual-energy X-ray absorptiometry (DXA) scanning (PIXImus, GE Healthcare) at a resolution of 0.18 × 0.18 mm pixels, permitting determination of lean mass, fat mass, and bone mineral density in a compartment-specific manner. Scans were performed between 8 and 9 weeks of age. Mice were anesthetized by isoflurane inhalation during scanning. X-ray absorptiometry data from the posterior body (defined as a region of interest extending from the posterior aspect of the ribs to the feet, including the lumbar spine, pelvis, and hindquarters) were processed with manufacturer-supplied software. Body fat percentage for each mouse was normalized to the average of Cre-littermate controls to control for inter-litter variability.
2.3 Fasting glucose, glucose and insulin tolerance tests
Insulin sensitivity was determined at 8 weeks of age (i.e., after 4 weeks of HFD administration) following a 4 hour fast by measuring glucose concentrations before (time 0) and 15, 30, 60, 90, and 120 minutes after an intraperitoneal bolus of insulin (0.50 mU/kg). Following a recovery period of at least 2 days, glucose tolerance was assessed in the mice after a 6 hour fast by measuring blood glucose concentrations before (time 0) and 15, 30, 60, 90, and 120 minutes after administration of an intraperitoneal bolus of glucose (1g/kg), as previously described (17).
2.4 Opg expression in bone and serum
Sera and calvarial bone tissue were collected as previously reported (15, 16). Circulating levels of Opg levels were determined using a Milliplex Luminex xMAP system (Millipore Mouse Bone Panel 1A). Opg mRNA levels were determined by semi-quantitative, real-time PCR as documented (15).
2.5 Serum bone remodeling markers
Circulating levels of procollagen type 1 amino-terminal propeptide (P1NP; bone formation marker), tartrate-resistant acid phosphatase 5b (TRAcP5b; bone resorption marker) and collagen type 1 cross-linked C-telopeptide (CTX; bone resorption marker) were measured with colorimetric assays (Rat/Mouse P1NP EIA #AC-33F1, Immunodiagnostic Systems, Fountain Hills AZ; MouseTRAP ELISA #SB-TR103, Immunodiagnostic Systems, Fountain Hills AZ; RatLaps (CTX-I) EIA #AC-06F1, Immunodiagnostic Systems, Fountain Hills AZ). All samples were tested in duplicate within each assay.
2.6 Cell lines and transcription assays
Mesenchymal-lineage C2C12 cells were maintained and routinely passaged as previously described (28, 29). A mouse Opg promoter-luciferase construct, containing 1.6 kb of promoter sequence spanning from −1486 to +133 bp relative to the Opg transcription start site, was obtained as a kind gift from Dr. Malayannan Subramaniam and Dr. John Hawse at the Mayo Clinic (30). For analyses of Opg transcriptional activity, cells were transfected with Lipofectamine (Invitrogen) and a DNA mixture consisting of 300 ng of this mouse Opg promoter construct, promoterless Renilla luciferase (pRL-null) (10 ng), and either pCMV-Hdac3 expression or control plasmids (100 to 500 ng) in 12-well plates. Following an overnight incubation at 37°C, cells were treated with 10 µM SAHA or vehicle (DMSO) for 24 hours. Luciferase activity in 20 µL of cell lysate was measured using the dual luciferase assay system (Promega) and a Glomax 96 microplate luminometer (Promega) or a BioTek Synergy HT plate reader (BioTek, Winooski, VT, USA). All transfections were performed in triplicate, and data were normalized to the activity of Renilla luciferase. We also re-analyzed our previously reported ChIP-sequencing dataset (28) from mesenchymal lineage MC3T3 cells to examine the effects of the Hdac inhibitor vorinostat (10 µM) on acetylation of histone H4.
2.7 Statistics
Statistics were performed with JMP 12 statistical analysis software (SAS Institute Inc., Cary, NC). Data were compared between groups within each experiment with Student’s t-tests or ANOVA as appropriate, where a significance level of p ≤ 0.05 was used for all comparisons. Single outliers within any group were detected with the two-sided Grubbs’ ESD method (31). Two outliers were identified in the serum CTX dataset (one Hdac3Osx CKO and one Opg+/−; Hdac3Osx CKO) and were excluded from the analysis. Three outliers were identified in the serum TRAcP5b dataset (one Opg+/− mouse, one Opg+/−; Osx1-Cre+ mouse, and one Hdac3Osx CKO) and were excluded from analysis. Data are presented as mean ± standard deviation, unless otherwise indicated. Sample sizes for each data set are indicated in each figure and caption.
Results
Hdac3 deficiency in skeletal progenitor cells but not in committed osteoblasts prevents weight gain on a short-term high fat diet
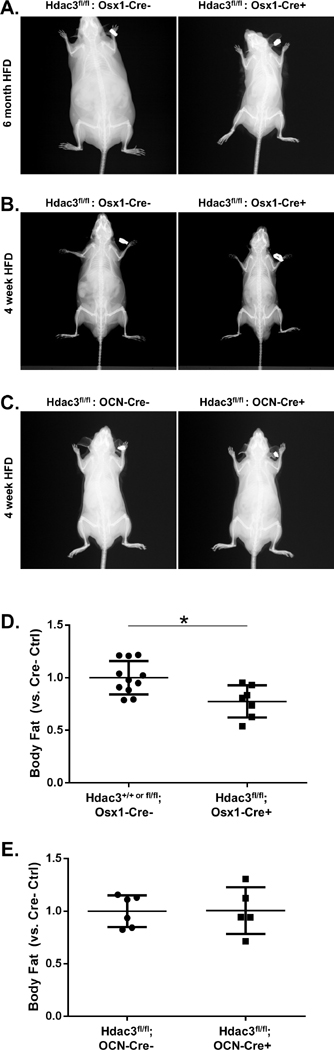
We recently reported that Hdac3fl/fl;Osx1-Cre+ mice (hereafter referred to as Hdac3Osx CKO) maintain a leaner body mass, maintain higher insulin sensitivity, and do not develop hepatic steatosis on a prolonged (6 month long) HFD regimen (17) (Figure 1A). These phenotypes are also observed after just 4 weeks of a HFD (Figure 1B, 1D). Thus Hdac3Osx CKO mice have lower body fat as compared to Cre- control mice. Body fat was not different between Hdac3OCN CKO (which deletes Hdac3 in the mature osteoblasts, rather than osteoprogenitor cells) and Hdac3fl/fl mice (Figure 1C, 1E), indicating that the metabolic effects of loss of Hdac3 are confined to an osteoprogenitor cell population.
Figure 1.
A) X-rays of littermate male Hdac3fl/fl:Osx1-Cre− and Hdac3fl/fl:Osx1-Cre+ mice at 26 weeks of age, after having been fed a high-fat diet between 4 weeks to 26 weeks of age. Leaner body mass and lack of hepatic hypertrophy are notable the Hdac3fl/fl:Osx1-Cre+ animals. B) X-rays of littermate male control (Hdac3fl/fl:Osx1-Cre−) and Hdac3Osx CKO (Hdac3fl/fl:Osx1-Cre+) mice at 8 weeks of age, after having been fed a high-fat diet between 4 and 8 weeks of age. Leaner body mass is notable. C) X-rays of littermate male control (Hdac3fl/fl:OCN-Cre−) and Hdac3OCN CKO (Hdac3fl/fl:OCN-Cre+) mice at 8 weeks of age, after having been fed a high-fat diet between 4 and 8 weeks of age. Body mass is comparable between genotypes. D) Body composition in Hdac3Osx CKO as compared to control littermates; *p<0.05 vs. control. E) Body composition is not different between Hdac3OCN CKO and control littermates.
Circulating Opg levels are elevated in Hdac3Osx CKO mice but not Hdac3OCN CKO mice
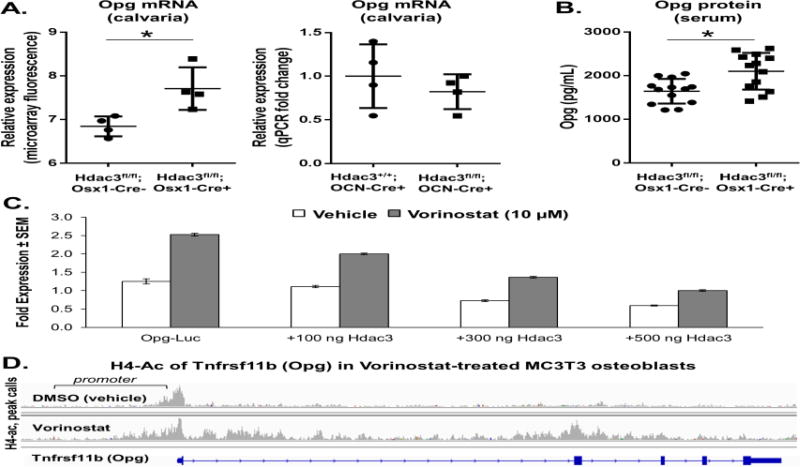
A previous transcriptome analysis identified a number of transcripts for soluble factors that are differentially regulated in calvarial osteoblasts from Hdac3Osx CKO mice compared to controls (16). Among these candidate factors, Opg/Tnfrsf11b was considered as a possible mediator of the metabolic phenotype because it rescued glycemic control and insulin resistance during a HFD regimen (32). Several tissues including bone produce Opg. To validate that Opg is more highly expressed in Hdac3Osx CKO, mice, bone-derived mRNA and circulating levels of Opg were measured by qPCR and ELISA, respectively. Both bone mRNA and serum levels of Opg were significantly increased in the Hdac3Osx CKO mice (Figure 2A). Hdac3 repressed transcription from the Opg promoter in a concentration-dependent manner, while the Hdac inhibitor SAHA increased Opg promoter activity and blunted Hdac3-dependent repression (Figure 2B). SAHA also increased acetylation of the Opg gene in MC3T3 pre-osteoblasts, particularly in the promoter region (Figure 2C). These data demonstrate that Hdac3 directly represses Opg transcription in skeletal progenitor cells. Notably, Opg transcription was not increased in Hdac3OCN CKO mouse bone as compared to control littermates (Figure 2D), suggesting that Hdac3’s regulation of Opg is unique to the osteoprogenitor lineage.
Figure 2.
A) Opg mRNA expression is increased in calvarial bone tissue of Hdac3Osx CKO mice, but is not increased in Hdac3OCN CKO mice. B) Opg expression is increased in the serum (protein) of Hdac3Osx CKO mice. *p<0.05 vs. Cre- control mice. C) Hdac3 dose-dependently represses Opg transcriptional activity, whereas inhibition of Hdac activity by the pan-Hdac inhibitor vorinostat increased Opg transcriptional activity and prevented repression by Hdac3. Means ± standard error are presented. D) ChIP-seq performed on osteoblastic MC3T3 cells treated with DMSO or vorinostat with antibodies recognizing tetra-acetylated H4. H4-Ac peak calls near the Opg gene were made with MACS analysis.
Serum remodeling markers are minimally affected by loss of one Opg allele
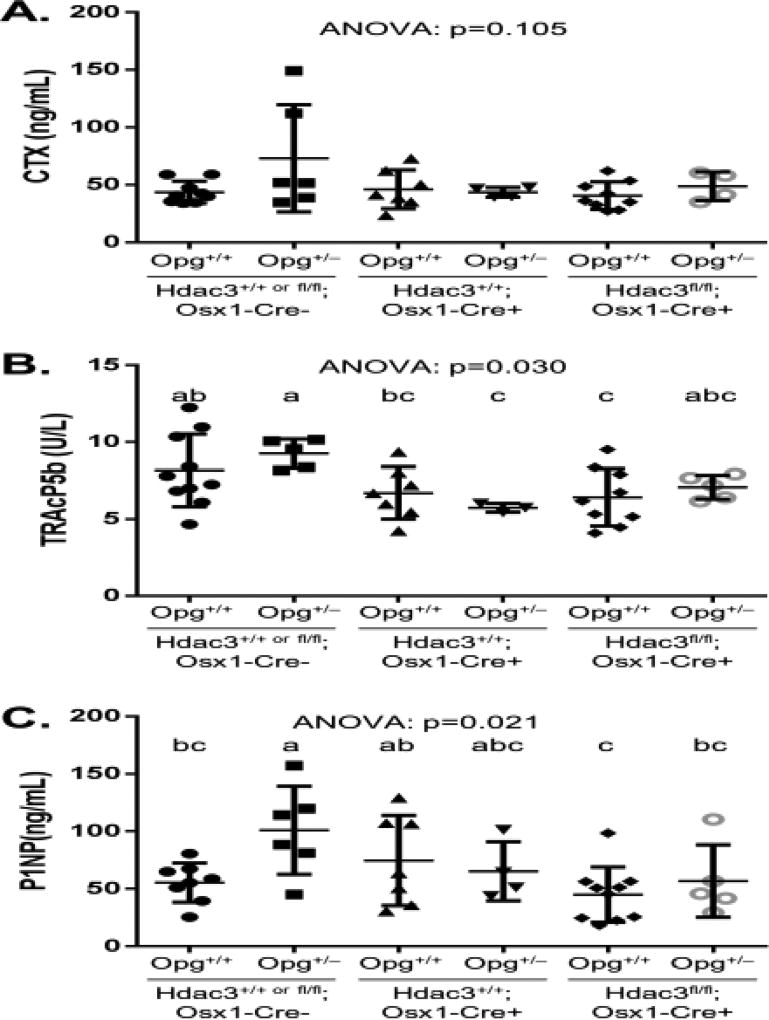
To determine if Opg is the bone-derived factor that provides a protective metabolic phenotype to Hdac3Osx CKO mice on a HFD, we crossed Hdac3fl/fl;Osx1-Cre+ animals with Opg+/− animals to generate double mutant Opg+/−; Hdac3fl/fl; Osx1-Cre+ mice, single gene mutants, and controls. These animals were placed onto a HFD at 4 weeks of age. Serum remodeling markers were quantified to determine whether altered bone remodeling activity may have influenced the observed metabolic phenotype in the Hdac3Osx CKO mice, and whether Opg would be involved in this process. We did not detect significant differences (ANOVA: p=0.105) between groups with regards to serum CTX levels (Figure 3). While serum TRAcP5b levels did slightly and significantly (ANOVA: p=0.030) vary between groups, we noted no differences between Opg+/+ and Opg+/− mice of the same Hdac3 genotype. Combined with the serum CTX data, these results suggest that loss of one allele of Opg did not substantially impact bone resorption in either Hdac3Osx CKO or control mice. With regards to bone formation, serum P1NP levels did significantly (ANOVA: p=0.021) vary between groups, but were only elevated in the Opg+/− control group as compared to other genotypes (Figure 3).
Figure 3.
Serum remodeling markers including A) collagen type 1 cross-linked C-telopeptide (CTX), a marker of osteoclast resorption activity, B) Tartrate-resistant acid phosphatase 5b (TRAcP5b), a marker of osteoclast number, and C) type 1 procollagen N-terminal fragment (P1NP), a marker of osteoblastic bone formation activity. ANOVA p-values are indicated. Post-hoc analyses (when appropriate) are indicated by superscript letters above each group, where groups with similar superscript letters are not significantly different from one another.
Genetically deleting one allele of Opg impairs the enhanced insulin sensitivity of Hdac3Osx CKO mice on a HFD regimen
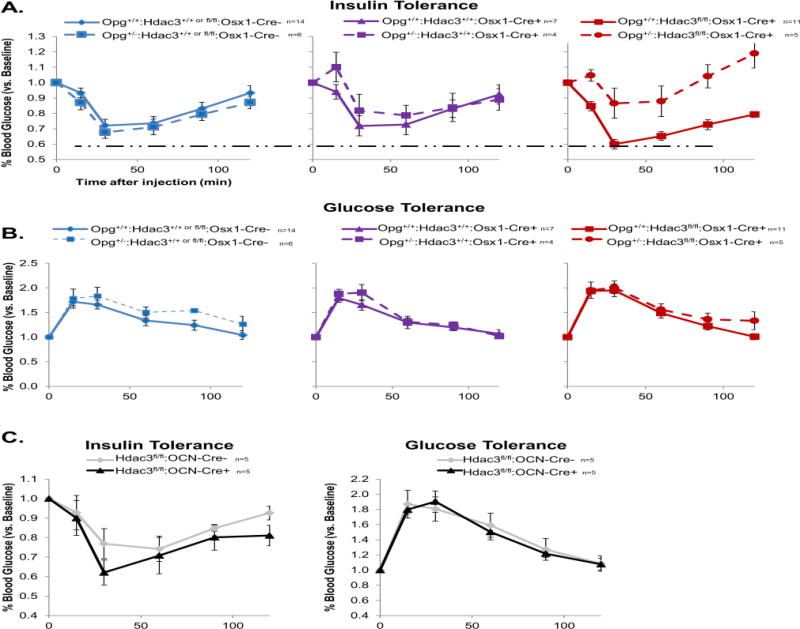
Insulin tolerance was assessed at 8 weeks of age, after 4 weeks on a HFD regimen. Consistent with our earlier studies (17), Hdac3Osx CKO animals maintained a greater response to insulin as compared to either Cre-control or Cre+ control groups during a HFD regimen (Figure 4A), despite the comparable body fat between Hdac3Osx CKO and Osx1-Cre+ control mice (Figure 1). No differences were seen in serum glucose responses to a glucose challenge between groups (Figure 4B). We confirmed that these effects are unique to the osteoprogenitor lineage, as Hdac3OCN CKO mice did not show improved insulin sensitivity or altered glucose handling on a HFD as compared to Hdac3fl/fl mice (Figure 4C). The inactivation of one Opg allele had no effect on insulin sensitivity or glucose tolerance (Figure 4A–B). However, double mutant Opg+/−; Hdac3Osx CKO animals showed a striking loss of the high insulin sensitivity that was observed in the Hdac3Osx CKO animals (Figure 4A). These data demonstrate that Opg is a major contributor to the metabolic phenotype of Hdac3Osx CKO mice.
Figure 4.
Double mutant Opg+/−: Hdac3Osx CKO mice lose insulin sensitivity. A) After 4 weeks on a HFD, Opg+/+: Hdac3Osx CKO (solid red line) are more sensitive to insulin than either Cre-negative (blue) or Cre-expressing (purple) controls (note dashed horizontal line indicating a greater maximal response in Hdac3Osx CKO mice as compared to either control). Opg+/−: Hdac3Osx CKO (dashed red line), however, lose this high insulin sensitivity. B) Glucose metabolism, as assessed by response to an intraperitoneal injection of glucose in a glucose tolerance test, did not vary between groups. C) Neither insulin sensitivity, as assessed by an intraperitoneal insulin tolerance test, nor glucose tolerance are affected in Hdac3OCN CKO mice. Means ± standard error are presented. Sample sizes for each group are indicated next to each figure legend.
Discussion
Recent reports showing that the skeleton can impact energy metabolism raise the intriguing possibility that bone-derived proteins might be able to help treat or prevent type 2 diabetes, and that bone health and metabolic health are inextricably linked. This latter idea is supported by the finding that fracture risk is increased and bone quality is compromised in type 2 diabetic patients (33–35). The current study confirmed Hdac3 as an important factor in osteoblastic regulation of systemic metabolism as its conditional deletion in the osteoprogenitor cell population protected systemic metabolism against the challenge of a high-fat diet regimen. Hdac3Osx CKO animals maintained lower body fat and fasting glucose levels, and importantly, prevented insulin resistance on a HFD. These data provide new insights into the mechanistic link between the skeleton, namely osteoblasts, and whole body metabolism.
A fully human monoclonal anti-RANKL antibody, denosumab, was recently approved for clinical use as a treatment for osteoporosis. This anti-resorptive skeletal therapy, in some ways, mimics the biological effects of Opg, as Opg acts as a natural decoy receptor for Rankl, and in doing so, prevents osteoclast formation. Whether therapies like denosumab can affect systemic glucose regulation in humans is controversial, and much of the debate focuses on the potential role of osteocalcin/Bglap (36, 37), which our studies suggest does not play a role in the metabolic biology of Hdac3-insufficent mice (38). A retrospective analysis of literature documenting the clinical anti-osteoporosis efficacy of these drugs failed to find an effect of anti-resorptive skeletal therapies including denosumab on fasting glucose levels (39), although the original studies were not designed to address diabetic biology. However, at least one recent report provides evidence that denosumab can modestly but significantly lower fasting glucose levels in diabetic patients prior to administration of anti-diabetic medications (40). Similarly, a recent prospective study reported that a single 60 mg dose of denosumab improved metrics of hepatic insulin resistance index and HbA1c levels in postmenopausal osteoporotic women (41). Thus, prospective clinical data support the idea that targeting RANKL/NF-κB can affect systemic glucose homeostasis in humans.
Opg’s role in systemic metabolic biology is still uncertain. Lacombe et al reported that young Opg-deficient mice are hypoglycemic, more glucose tolerant, and have less white adipose tissue (epididymal fat) as compared to control littermates (37). These phenotypic changes were linked to increased levels of circulating undercarboxylated osteocalcin/Bglap in the Opg-deficient mice (37). Meanwhile, Kondegowda et al showed that recombinant Opg improved glucose homeostasis in young, aged, and STZ-induced diabetic models by increasing in β-cell proliferation and β-cell mass (42). Similarly, administration of recombinant Opg to diabetic (leptin-deficient ob/ob) mice reduced fasting glucose, circulating insulin, and HOMA-IR, but the beneficial effects of Opg were attributed to the prevention of hepatic inflammation (32). Other murine studies reported harmful effects of Opg on β-cells leading to hyperglycemia and hypoinsulinemia (43), or no effect of Opg overexpression on glucose homeostasis (44, 45). In recent clinical studies, serum Opg levels were reported to be elevated in men with impaired glucose tolerance or type 2 diabetes (46) and significantly associated with higher risk for impaired glucose regulation (47) or unrelated to the risk of developing type 2 diabetes (32). Thus, whether Opg has a beneficial or harmful effect on systemic metabolic biology, including glucose homeostasis and insulin sensitivity, remains a controversial topic. In our own studies, Opg levels were increased in Hdac3Osx CKO mice, and deleting one allele of Opg negated the enhanced insulin sensitivity of these animals. Notably, deleting one allele of Opg had few effects on markers of bone turnover, suggesting that the mechanism of metabolic rescue in these mice, while currently unknown, is likely not linked to changes in bone resorption activity or decarboxylation of osteocalcin/Bglap. This is consistent with our observations that both total and undercarboxylated osteocalcin/Bglap levels are lower (not higher) in Hdac3Osx CKO mice (17) and that osteoblastic bone formation activity is substantially suppressed in Hdac3Osx CKO mice (16). It is possible that increased circulating Opg in Hdac3Osx CKO mice targets and inhibits RANK-related inflammatory signaling in the liver to preserve insulin sensitivity, as previously reported in other animal models (32); this concept will be explored in future studies.
Studies defining the skeleton’s role as an endocrine organ affecting energy metabolism are important, as they may help identify new therapeutic targets for millions of people suffering from obesity and metabolic disorders worldwide. In addition, these studies improve our understanding of how epigenetic drugs affect skeletal health. Several small molecule pan-inhibitors of Hdacs are already FDA-approved for cancer treatment and mood disorders (48–50). Preclinical studies indicate that these drugs are also effective at treating many other diseases, including neurological disorders, inflammation, and obesity, due to their ability to reprogram diseased cells (19, 51–53). Inhibitors specific for individual Hdacs are now being designed and tested. Several Hdac3-selective inhibitors, RGFP966 and BG45, have been tested in preclinical models for Huntington’s disease (54, 55), and multiple myeloma (56) and type I diabetes (57). As clinical use of Hdac inhibitors and other forms of epigenetic therapies expands, a firm understanding of how specific Hdacs contribute to bone and metabolic homeostasis will provide insight into their potential benefits to patients.
Acknowledgments
The American Diabetes Association (1-16-JDF-062), NIH (T32 AR056950, F32 AR60140), the Minnesota Obesity Center (Subaward H412621701, P30DK050456-18) and the Mayo Clinic Center for Regenerative Medicine supported this work. The authors thank Dr. David Razidlo, Mr. Xiaodong Li, and Ms. Bridget Stensgard for technical assistance and animal husbandry.
Footnotes
Author Contributions: Experimental design: MEML, JJW. Data collection and analysis: MEML, JLP, KY, NEC, EWB. Drafting manuscript: MEML and JJW. All authors have read and approved the final submitted manuscript.
References
- 1.Dinneen SF, Maldonado D, 3rd, Leibson CL, Klee GG, Li H, Melton LJ, 3rd, et al. Effects of changing diagnostic criteria on the risk of developing diabetes. Diabetes care. 1998;21(9):1408–13. doi: 10.2337/diacare.21.9.1408. [DOI] [PubMed] [Google Scholar]
- 2.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nature medicine. 2005;11(2):183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55(12):3536–49. doi: 10.2337/db06-0319. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54(4):1150–6. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(4):677–80. doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- 6.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan-Speranza TC, Henneicke H, Gasparini SJ, Blankenstein KI, Heinevetter U, Cogger VC, et al. Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. The Journal of clinical investigation. 2012;122(11):4172–89. doi: 10.1172/JCI63377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(13):5266–70. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kode A, Mosialou I, Silva BC, Joshi S, Ferron M, Rached MT, et al. FoxO1 protein cooperates with ATF4 protein in osteoblasts to control glucose homeostasis. The Journal of biological chemistry. 2012;287(12):8757–68. doi: 10.1074/jbc.M111.282897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshikawa Y, Kode A, Xu L, Mosialou I, Silva BC, Ferron M, et al. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. J Bone Miner Res. 2011;26(9):2012–25. doi: 10.1002/jbmr.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes Mellitus and Inflammation. Current diabetes reports. 2013 doi: 10.1007/s11892-013-0375-y. [DOI] [PubMed] [Google Scholar]
- 13.Kiechl S, Wittmann J, Giaccari A, Knoflach M, Willeit P, Bozec A, et al. Blockade of receptor activator of nuclear factor-kappaB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med. 2013 doi: 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- 14.Carpio LR, Bradley EW, McGee-Lawrence ME, Weivoda MM, Poston DD, Dudakovic A, et al. Histone deacetylase 3 supports endochondral bone formation by controlling cytokine signaling and matrix remodeling. Sci Signal. 2016;9(440):ra79. doi: 10.1126/scisignal.aaf3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGee-Lawrence ME, Bradley EW, Dudakovic A, Carlson SW, Ryan ZC, Kumar R, et al. Histone deacetylase 3 is required for maintenance of bone mass during aging. Bone. 2013;52(1):296–307. doi: 10.1016/j.bone.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razidlo DF, Whitney TJ, Casper ME, McGee-Lawrence ME, Stensgard BA, Li X, et al. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PloS one. 2010;5(7):e11492. doi: 10.1371/journal.pone.0011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGee-Lawrence ME, White TA, LeBrasseur NK, Westendorf JJ. Conditional deletion of Hdac3 in osteoprogenitor cells attenuates diet-induced systemic metabolic dysfunction. Molecular and cellular endocrinology. 2015;410:42–51. doi: 10.1016/j.mce.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGee-Lawrence ME, McCleary-Wheeler AL, Secreto FJ, Razidlo DF, Zhang M, Stensgard BA, et al. Suberoylanilide hydroxamic acid (SAHA; vorinostat) causes bone loss by inhibiting immature osteoblasts. Bone. 2011;48(5):1117–26. doi: 10.1016/j.bone.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galmozzi A, Mitro N, Ferrari A, Gers E, Gilardi F, Godio C, et al. Inhibition of Class I Histone Deacetylases Unveils a Mitochondrial Signature and Enhances Oxidative Metabolism in Skeletal Muscle and Adipose Tissue. Diabetes. 2012 doi: 10.2337/db12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alenghat T, Meyers K, Mullican SE, Leitner K, Adeniji-Adele A, Avila J, et al. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature. 2008;456(7224):997–1000. doi: 10.1038/nature07541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutson SK, Chyla BJ, Amann JM, Bhaskara S, Huppert SS, Hiebert SW. Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. The EMBO journal. 2008;27(7):1017–28. doi: 10.1038/emboj.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montgomery RL, Potthoff MJ, Haberland M, Qi X, Matsuzaki S, Humphries KM, et al. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. The Journal of clinical investigation. 2008;118(11):3588–97. doi: 10.1172/JCI35847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331(6022):1315–9. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Z, Miller RA, Patel RT, Chen J, Dhir R, Wang H, et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nature medicine. 2012;18(6):934–42. doi: 10.1038/nm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Mishina Y, Liu F. Osterix-Cre transgene causes craniofacial bone development defect. Calcified tissue international. 2015;96(2):129–37. doi: 10.1007/s00223-014-9945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PloS one. 2012;7(9):e46057. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medrikova D, Jilkova ZM, Bardova K, Janovska P, Rossmeisl M, Kopecky J. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes (Lond) 2012;36(2):262–72. doi: 10.1038/ijo.2011.87. [DOI] [PubMed] [Google Scholar]
- 28.Dudakovic A, Evans JM, Li Y, Middha S, McGee-Lawrence ME, van Wijnen AJ, et al. Histone deacetylase inhibition promotes osteoblast maturation by altering the histone H4 epigenome and reduces Akt phosphorylation. The Journal of biological chemistry. 2013;288(40):28783–91. doi: 10.1074/jbc.M113.489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGee-Lawrence ME, Li X, Bledsoe KL, Wu H, Hawse JR, Subramaniam M, et al. Runx2 protein represses Axin2 expression in osteoblasts and is required for craniosynostosis in Axin2-deficient mice. The Journal of biological chemistry. 2013;288(8):5291–302. doi: 10.1074/jbc.M112.414995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramaniam M, Hawse JR, Bruinsma ES, Grygo SB, Cicek M, Oursler MJ, et al. TGFbeta inducible early gene-1 directly binds to, and represses, the OPG promoter in osteoblasts. Biochemical and biophysical research communications. 2010;392(1):72–6. doi: 10.1016/j.bbrc.2009.12.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11(1):1–21. [Google Scholar]
- 32.Kiechl S, Wittmann J, Giaccari A, Knoflach M, Willeit P, Bozec A, et al. Blockade of receptor activator of nuclear factor-kappaB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med. 2013;19(3):358–63. doi: 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- 33.Melton LJ, 3rd, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23(8):1334–42. doi: 10.1359/JBMR.080323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farr JN, Drake MT, Amin S, Melton LJ, 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(4):787–95. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farr JN, Khosla S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone. 2016;82:28–34. doi: 10.1016/j.bone.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferron M, Lacombe J. Regulation of energy metabolism by the skeleton: Osteocalcin and beyond. Arch Biochem Biophys. 2014 doi: 10.1016/j.abb.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Lacombe J, Karsenty G, Ferron M. In vivo analysis of the contribution of bone resorption to the control of glucose metabolism in mice. Molecular metabolism. 2013;2(4):498–504. doi: 10.1016/j.molmet.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGee-Lawrence ME, White TA, LeBrasseur NK, Westendorf JJ. Conditional deletion of Hdac3 in osteoprogenitor cells attenuates diet-induced systemic metabolic dysfunction. Molecular and cellular endocrinology. 2015 doi: 10.1016/j.mce.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz AV, Schafer AL, Grey A, Vittinghoff E, Palermo L, Lui LY, et al. Effects of antiresorptive therapies on glucose metabolism: results from the, FIT, HORIZON-PFT, and FREEDOM trials. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28(6):1348–54. doi: 10.1002/jbmr.1865. [DOI] [PubMed] [Google Scholar]
- 40.Napoli N, Vittinghoff E, Pannacciulli N, Crittenden D, Yun J, Wang A, et al. Effect of Denosumab on Fasting Glucose Concentrations in Postmenopausal Women with Osteoporosis: Results From Subjects With Diabetes or Prediabetes From the FREEDOM Trial. In: ASBMR, editor. ASBMR Annual Meeting; Houston, TX. American Society for Bone and Mineral Research; 2014. p. Oral Presentation 1104. [Google Scholar]
- 41.Passeri E, Benedini S, Costa E, Corbetta S. A Single 60 mg Dose of Denosumab Might Improve Hepatic Insulin Sensitivity in Postmenopausal Nondiabetic Severe Osteoporotic Women. International Journal of Endocrinology. 2015;2015 doi: 10.1155/2015/352858. Article ID 352858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kondegowda NG, Fenutria R, Pollack IR, Orthofer M, Garcia-Ocana A, Penninger JM, et al. Osteoprotegerin and Denosumab Stimulate Human Beta Cell Proliferation through Inhibition of the Receptor Activator of NF-kappaB Ligand Pathway. Cell Metab. 2015;22(1):77–85. doi: 10.1016/j.cmet.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toffoli B, Bernardi S, Candido R, Sabato N, Carretta R, Corallini F, et al. Osteoprotegerin induces morphological and functional alterations in mouse pancreatic islets. Molecular and cellular endocrinology. 2011;331(1):136–42. doi: 10.1016/j.mce.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 44.Ominsky MS, Stolina M, Li X, Corbin TJ, Asuncion FJ, Barrero M, et al. One year of transgenic overexpression of osteoprotegerin in rats suppressed bone resorption and increased vertebral bone volume, density, and strength. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24(7):1234–46. doi: 10.1359/jbmr.090215. [DOI] [PubMed] [Google Scholar]
- 45.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 46.O'Sullivan EP, Ashley DT, Davenport C, Penugonda L, Kelleher G, Devlin N, et al. A comparison of osteoprotegerin with adiponectin and high-sensitivity C-reactive protein (hsCRP) as a marker for insulin resistance. Metabolism. 2013;62(1):34–8. doi: 10.1016/j.metabol.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Niu Y, Yang Z, Li X, Zhang W, Lu S, Zhang H, et al. Association of osteoprotegerin with impaired glucose regulation and microalbuminuria: the REACTION study. BMC Endocr Disord. 2015;15:75. doi: 10.1186/s12902-015-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. The Journal of biological chemistry. 2001;276(39):36734–41. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 49.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26(9):1351–6. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 50.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Blanchard F, Chipoy C. Histone deacetylase inhibitors: new drugs for the treatment of inflammatory diseases? Drug Discov Today. 2005;10(3):197–204. doi: 10.1016/S1359-6446(04)03309-4. [DOI] [PubMed] [Google Scholar]
- 52.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiech NL, Fisher JF, Helquist P, Wiest O. Inhibition of histone deacetylases: a pharmacological approach to the treatment of non-cancer disorders. Curr Top Med Chem. 2009;9(3):257–71. doi: 10.2174/156802609788085241. [DOI] [PubMed] [Google Scholar]
- 54.Wells CE, Bhaskara S, Stengel KR, Zhao Y, Sirbu B, Chagot B, et al. Inhibition of histone deacetylase 3 causes replication stress in cutaneous T cell lymphoma. PloS one. 2013;8(7):e68915. doi: 10.1371/journal.pone.0068915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia H, Pallos J, Jacques V, Lau A, Tang B, Cooper A, et al. Histone deacetylase (HDAC) inhibitors targeting HDAC3 and HDAC1 ameliorate polyglutamine-elicited phenotypes in model systems of Huntington's disease. Neurobiology of disease. 2012;46(2):351–61. doi: 10.1016/j.nbd.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minami J, Suzuki R, Mazitschek R, Gorgun G, Ghosh B, Cirstea D, et al. Histone deacetylase 3 as a novel therapeutic target in multiple myeloma. Leukemia. 2014;28(3):680–9. doi: 10.1038/leu.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chou DH, Holson EB, Wagner FF, Tang AJ, Maglathlin RL, Lewis TA, et al. Inhibition of histone deacetylase 3 protects beta cells from cytokine-induced apoptosis. Chem Biol. 2012;19(6):669–73. doi: 10.1016/j.chembiol.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]