Abstract
The ability to monitor adherence to antiretroviral therapy is critical for the interpretation of outcomes from clinical studies of HIV, and for optimizing patient care. The antiretrovirals tenofovir (TFV), emtricitabine (FTC), and lamivudine (3TC) are commonly included in drug regimens for HIV prevention and treatment. The active form of the drugs tenofovir diphosphate (TFVdp), emtricitabine triphosphate (FTCtp), and lamivudine triphosphate (3TCtp) are found intracellularly in erythrocytes and peripheral blood mononuclear cells (PBMCs). The ability to collect and analyze dried blood spot (DBS) samples is an attractive alternative to PBMC sampling in many resource limited settings. We developed and validated an assay to quantify all three intracellular metabolites over the range of 100–25000 fmol/sample. This assay utilizes a simple protein precipitation/liquid-liquid extraction of a single 3-mm DBS punch (from a Whatman 903 Protein Saver card) with isotopically labeled 13C5-TFVdp included as the internal standard. Following extraction, samples are analyzed by anion exchange chromatography on a Thermo Biobasic AX 5micron column with detection by electrospray ionization in the positive mode on a AB Sciex API-5000 triple quadrupole mass spectrometer with a total run time of 8 minutes. The assay was linear over the entire range (R2>0.996). The assay was accurate (inter-assay %bias within ±3.0%) and precise (inter-assay %CV ≤9.8%). The assay was also reproducible from multiple punches within a spot as well as punches from separate blood spots. Stability was established at room temperature for 3 days, and at −80°C for up to 63 days. Clinical samples were analyzed from subjects on Truvada®, Stribild®, Descovy®, and Triumeq® regimens and intracellular metabolites were detected in all samples as expected, indicating the assay performs well for all current formulations of TFV, FTC, and 3TC.
Keywords: Dried blood spot, validation, HIV, adherence monitoring, antiretroviral, intracellular metabolite
1. Introduction
Nucleos(t)ide reverse transcriptase inhibitors (NRTI) are the cornerstone of HIV treatment and prevention regimens, which require high levels of adherence to prevent drug resistance or breakthrough infections (1). Tenofovir (TFV), emtricitabine (FTC), and lamivudine (3TC) are three of the most commonly utilized NRTIs in pre-exposure prophylaxis (PrEP) applications and for HIV treatment (2, 3).
Plasma NRTI concentrations have been used to monitor adherence but, because of their relatively short half-life (6, 10, and 17 hours for 3TC, FTC, and TFV, respectively), they only provide information on dosing over the prior few days (4, 5). In addition, these concentrations may be confounded by “white coat adherence”, where patients only take their medications in anticipation of a monitoring event (6). Mononuclear cells phosphorylate NRTIs intracellularly to their active metabolites, which have 3–6 times longer half-lives and can be used as a surrogate of adherence over a 2–3 week period (2). However, cell isolation is complex and may not be practical in all settings. Recently, these compounds have been found in red blood cells with similar or longer half-lives (7, 8). The presence of these intracellular metabolites in erythrocytes provides an opportunity to utilize dried blood spots as a means of collecting samples for adherence monitoring (9); and concentration thresholds with associations of high (100%), medium (66%) and low (37%) levels of adherence have been previously defined for TFVdp (7, 10). Dried blood spots present an attractive alternative to plasma or cell processing, especially in the resource-limited settings of many HIV clinical trials.
To date only one method has been published for the analysis of NRTI metabolites in dried blood spots. Zheng et al presented an assay for the indirect analysis of TFVdp and FTCtp in DBS (11). The aim of our validation was to develop and validate a simple and rapid liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for the direct detection of TFVdp, FTCtp, and 3TCtp in DBS samples using weak anion exchange chromatography for use in monitoring adherence. Numerous assays have been developed for detection of NRTI metabolites by both direct and indirect detection with one example of TFVdp, FTCtp, and 3TCtp by direct detection in peripheral blood mononuclear cell (PBMC) samples with a combination of weak anion exchange and ion-pair chromatography (12,13). Our inclusion of 3TCtp in a DBS assay for antiretroviral adherence monitoring is important as it increases the number of studies that can benefit from assistance in interpretation of study drug adherence, particularly in resource limited settings where 3TC is more frequently utilized (14,15).
2. Experimental
2.1. Materials
TFVdp (tetraammonium salt) and FTCtp (tetraammonium salt) were synthesized at TriLink Biotechnologies (San Diego, CA, USA) while 3TCtp (triethylammonium salt) was purchased from Toronto Research Chemicals (North York, ON, Canada). The stable, isotopically-labelled internal standard 13C5-TFVdp (tetraammonium salt) was purchased from Moravek Biochemicals Inc. (Brea, CA, USA). Dichloromethane, acetonitrile, and ammonium acetate (all HPLC grade) were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Formic acid (Certified ACS, 88%), ammonium hydroxide (certified ACS plus), and ammonium phosphate dibasic and monobasic (reagent grade) were also purchased from Fisher Scientific. Water was purified by a Hydro Picosystem® UV Plus (Durham, NC, USA). Drug-free human whole blood was used as a control throughout the method and was purchased from Biological Specialty Corporation (Colmar, PA, USA) or collected from healthy volunteers consented on IRB 08-0047, a research specimen collection study approved by the UNC Chapel Hill Biomedical Review Board, in accordance with all industry ethical standards. The filter paper used was Whatman 903 DBS protein saver filter papers (Fisher Scientific).
2.2. LC-MS/MS Instrumentation
A Shimadzu Prominence HPLC system including pumps (LC-20AD), divert valves (FCV-12AH), degasser (DGU-20A), and controller (CBM-20A) are supplied from Shimadzu (Columbia, MD, USA). A Thermo Scientific BioBasic AX (50×2.1mm 5um particle size) column was used under anion exchange conditions with 750mM ammonium acetate (mobile phase A) and 75:25 5mM ammonium acetate:acetonitrile, pH 10.1 (mobile phase B) with a flow rate of 0.400mL/min. The column heater (CTO-20A) was set at 35 °C, and the autosampler (SIL-20AC/HT) was maintained at 10 °C. The injection volume was 15uL. The LC gradient held at 20% B for 0.25 min and increased to 100% B at 1.00 min and held until 3.25 min. Flow was diverted to waste for the first 1.75 min, and allowed to enter the mass spectrometer from 1.75 min to 3.15 min to allow for the detection of analytes and the internal standard at the following retention times: 2.44min (TFVdp, TFVdp-IS), 2.45 min (FTCtp), and 2.46 min (3TCtp). Following the elution of the target compounds the flow was diverted back to waste. The gradient changed to 100% mobile phase A for 4.0 minutes to aid in carryover reduction before reverting back to the starting condition of 20% B for a total run time of 8.0 minutes.
An API-5000 triple quadruple mass spectrometer (SCIEX, Foster City, CA, USA) operated in positive ion TurboIonspray mode was used to acquire data for this method. Selected reaction monitoring was used to detect the analyte [precursor/product] transitions (m/z) as follows: TFVdp [448/270], FTCtp [488/130], 3TCtp [470/112], and 13C5-TFVdp (internal standard) [453/355]. The source temperature was 650 °C and the ion spray voltage was 5500V. The declustering potential (DP) for all compounds was 65V, and the collision energy (CE) was 40V for the method analytes and 22V for the internal standard.
Linear regression of concentration (x) versus peak area ratio of compound to internal standard (y) using a 1/(x2) weighting was used with Sciex Analyst software (version 1.6.2).
2.3. Validation Criteria
TFVdp, FTCtp, and 3TCtp are active metabolites that are intracellularly phosphorylated and subsequently trapped within the phospholipid cellular membrane. These metabolites are highly unstable outside of the cellular environment due to the presence of phosphatase enzyme degradation. Thus, the generation of a true standard or QC at a known concentration in whole blood long enough to spot and dry on a DBS card is not feasible. As a result, certain parameters typically included in method validations are unable to be performed as FDA guidance suggests (16). Many of the traditional evaluations for stability assessments are performed at the low and high QC levels. However, the inability to prepare a sample at those specific concentrations and allow it to dry on a card prevents those tests from being performed in the traditional way. For those evaluations, including stability, spot-to-spot variability, and method reproducibility, clinical samples were substituted. The collection and analysis of clinical samples allowed for a more accurate view of the method performance; but, since these samples are from patients taking antiretroviral therapy, the actual concentration was unknown. Thus, only an assessment of precision and comparison to a theoretical concentration of the sample analyzed soon after initial collection was possible. The precision and accuracy assessments were performed by spiking analyte at target concentrations onto a fresh single dried DBS punch prior to extraction where analyte stability was maintained.
2.4. Preparation of Calibration Standards and Quality Control Samples
Fresh whole blood (drug-free, EDTA anticoagulated) was spotted onto Whatman 903 Protein Saver cards using 40–60uL per spot. These cards were allowed to dry at room temperature for approximately 18–24 hours. Numerous 3mm punches were generated from each spot using a Tomtec Manual DBS Bio-Sampling Punch (3mm). These single punches were placed into separate 1.5mL polypropylene microcentrifuge tubes to serve as the blank matrix for the daily standard and QC preparation.
TFVdp and FTCtp (100mM solutions received from vendor) were diluted to 1mg/mL in 1mM ammonium phosphate (pH 7.4). 3TCtp was weighed and prepared to 1mg/mL in 1mM ammonium phosphate (pH 7.4). These 1mg/mL stocks were then diluted in water to make a set of calibration standard working solutions at 4,000, 8,000, 20,000, 40,000, 200,000, 400,000, 900,000, and 1,000,000 fmol/mL and a quality control (QC) working solution set with concentrations at 4,000, 12,000, 80,000, and 800,000 fmol/mL. TFVdp and FTCtp stocks, as well as the working solutions were stored at −80 °C. The 3TCtp stocks were stored at −20°C.
Calibration standards and QCs were prepared fresh daily by spiking 25uL of the appropriate working solution onto a single 3mm DBS punch from a drug-free whole blood sample in a 1.5mL microcentrifuge tube. The resulting concentrations for the calibration standards were 100, 200, 500, 1,000, 5,000, 10,000, 22,500, and 25,000 fmol/sample with QCs at 100, 300, 2,000 and 20,000 fmol/sample.
The internal standard (13C5-TFVdp) was prepared to 1mg/mL in water and was stored at −80 °C. This solution was diluted with water to the final internal standard working solution of 150ng/mL and was stored at 4 °C.
2.5. Clinical Sample Collection
During the conduct of this validation, clinical samples were collected from patients on multiple fixed-dose combinations (eg Truvada® and Stribild®, TFV/FTC containing regimens, Descovy®, a tenofovir alafenamide/FTC containing regimen, and Triumeq®, a 3TC regimen). All clinical samples used for testing in this validation had a viral load of <40 copies/mL and were adherent to their current regimens for over one consecutive year, as evidenced by consistently undetectable viral loads at clinic visits, and increasing, or maintaining of CD4+ T cell concentrations. Blood samples were collected in EDTA tubes, and small aliquots (40–60uL) were spotted onto the Whatman 903 Protein Saver cards and allowed to dry at room temperature for approximately 18–24 hours. Once dry, multiple 3mm punches were generated from each dried blood spot and placed into separate 1.5mL tubes to be stored at various temperatures until analysis.
2.6. Sample Extraction
The extraction of the analytes from a human dried blood spot was performed using a combination of protein precipitation and a liquid-liquid extraction. In this method, 25uL of the appropriate standard or QC spike was added to a 1.5mL polypropylene tube containing a single dried blood spot punch. A 25uL aliquot of water was added to all clinical samples in place of the analyte spike. Then, 50uL of water containing the internal standard (13C5-TFV-dp) was added to the tube. Samples were sonicated for one minute, with 150uL of 60:40 dichloromethane:acetonitrile subsequently added to the tube. Following vortex and centrifugation steps, the upper (aqueous) layer was removed and transferred to a clean tube. After a second centrifugation step to remove any residual precipitate from the upper layer, the extract was transferred into a 96-well plate for LC-MS/MS analysis.
3. Results and Discussion
3.1. Validation Results
3.1.1 Selectivity
Selectivity was evaluated in six unique lots of human whole blood spotted on Whatman 903 filter paper. No interfering peaks were detected at the retention time of the analytes or internal standard in any of the six lots evaluated. In addition, the clinical samples only produced peaks for the specific analyte(s) in that subject’s regimen: the subjects on TFV/FTC containing regimens only had peaks for TFVdp and FTCtp, and the subjects on 3TC regimens only had peaks present for 3TCtp. Figure 1 illustrates sample chromatograms from a lower limit of quantification (LLOQ) standard (Figure 1A), and clinical DBS samples for subjects taking 3TC (Figure 1B) and TFV/FTC (Figure 1C)-based regimens.
Figure 1.
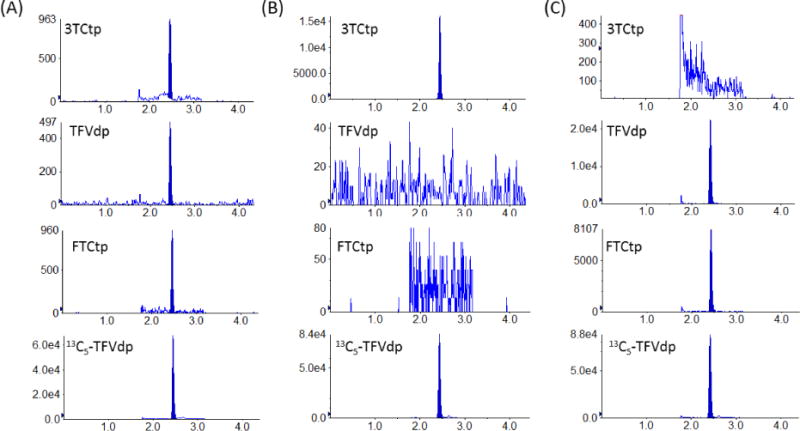
Example chromatograms from a LLOQ standard (Figure 1A) and clinical DBS samples of subjects on 3TC (Figure 1B) and TFV/FTC (Figure 1C) regimens.
3.2. Linearity
Three accuracy and precision runs were extracted during this method validation. All calibration curves employed linear regression with 1/x2 weighting and correlation coefficients exceeded 0.99. Calibration standards were included at the beginning and the end of each analytical run to bracket all injected QC and clinical samples. Peak area ratios of the calibration standard to internal standard were used to construct the calibration curves. Acceptance criteria for calibration standards was accuracy ≤ ±15% (≤ ±20% at the LLOQ).
3.3. Accuracy and Precision
Accuracy and precision were evaluated by analysis of six replicates of human blood DBS QC samples prepared at the LLOQ (100 fmol/sample) and at 3 additional concentrations (300, 2,000, and 20,000 fmol/sample) over three runs. Intra-assay statistics were determined from QCs (n=6) within the same analytical run. Inter-assay statistics were determined from replicate analysis of QC samples (n=6) in each of the 3 separate analytical runs (n=18). Precision was calculated as coefficient of variation (%CV) while accuracy was calculated as % bias from the nominal concentration. Both had acceptance criteria of ±15% (±20% at the LLOQ). Accuracy and precision results are shown in Table 1. All results were well within acceptance criteria for method validation.
Table 1.
Precision and Accuracy Results for TFVdp, FTCtp, and 3TCtp in DBS Quality Control Samples
| Analyte | Nominal conc. (fmol/sample) |
Run 1 Accuracy (%Bias) |
Run 1 Precision (%CV) |
Run 2 Accuracy (%Bias) |
Run 2 Precision (%CV) |
Run 3 Accuracy (%Bias) |
Run 3 Precision (%CV) |
Inter-assay Accuracy (%Bias) |
Inter-assay Precision (%CV) |
|---|---|---|---|---|---|---|---|---|---|
| TFVdp | 100 | −5.2 | 5.2 | 2.1 | 4.1 | 3.1 | 8.6 | 0.0 | 7.0 |
| 300 | −4.0 | 5.5 | 0.3 | 2.6 | −0.3 | 4.4 | −1.3 | 4.5 | |
| 2000 | −1.5 | 2.5 | −0.5 | 3.2 | 0.4 | 2.4 | −0.5 | 2.7 | |
| 20000 | −1.6 | 2.3 | −1.2 | 1.5 | 0.3 | 1.6 | −0.8 | 1.9 | |
| FTCtp | 100 | −3.0 | 14.0 | −0.2 | 4.4 | 2.9 | 6.7 | −0.1 | 9.0 |
| 300 | 0.2 | 6.3 | −1.8 | 3.7 | −0.9 | 5.0 | −0.9 | 4.9 | |
| 2000 | 1.8 | 1.6 | −1.0 | 1.5 | −0.3 | 3.6 | 0.1 | 2.6 | |
| 20000 | −0.8 | 2.1 | −3.4 | 1.0 | 0.2 | 2.4 | −1.3 | 2.4 | |
| 3TCtp | 100 | 0.7 | 9.4 | −9.6 | 8.0 | 1.8 | 8.4 | −2.4 | 9.8 |
| 300 | −2.1 | 5.8 | −2.8 | 4.5 | −3.9 | 5.2 | −3.0 | 5.0 | |
| 2000 | 0.1 | 1.5 | −1.5 | 1.6 | 0.8 | 2.6 | −0.2 | 2.1 | |
| 20000 | −0.5 | 1.2 | −3.1 | 0.9 | 0.9 | 2.6 | −0.9 | 2.4 |
3.4. Carryover/Crosstalk
No carryover was observed for any analyte or internal standard following the injection of a blank sample immediately following the highest calibration standard. Cross talk was also evaluated by extracting a high standard without internal standard being added. No peak was seen in the internal standard channel showing no positive bias in the internal standard response at higher analyte concentrations.
3.5. Recovery, Matrix Effects
Three experiments were performed to evaluate recovery and matrix effects. Peak area responses of the analytes and the internal standard extracted from DBS samples at the low, mid, and high QC levels (pre-extracted) were compared with blank extracts spiked with analyte and internal standard corresponding to 100% recovered levels (post-extracted) and neat solution samples (unextracted). Recovery was calculated as the ratio of the mean peak area response of the compound in extracted QCs to the mean peak area response in post-extracted QCs. Matrix effects were calculated as the ratio of the mean peak area response of the compound in post-extracted QCs to mean peak area response of the compound in unextracted solvent QCs. The range of recoveries associated with this assay was 60.4%–65.3% (TFVdp), 61.8%–66.1% (FTCtp), and 60.4%–65.7% (3TCtp) for the three QC levels evaluated. Recovery of the TFVdp-IS was 59.2%–62.3%. The matrix effects ranged from 64.8%–67.4%, 71.7%–72.1%, and 71.7%–79.7% for TFVdp, FTCtp, and 3TCtp, respectively. Matrix effects for the TFVdp-IS was 67.8%–69.4% across all three QC levels. The matrix effect calculation suggests a small amount of signal suppression. However, recovery and matrix effects were consistent between the analytes and internal standard throughout the concentration range evaluated.
An additional evaluation of matrix effects was performed using the experiments previously described by Matuszewski (17). Six different lots of blank whole blood were spotted onto the Whatman 903 Protein Saver filter paper. Multiple 3mm punches from each of these lots were used as blank DBS for this evaluation. These punches were spiked at the low, mid, and high QC levels and extracted in triplicate with the average peak area ratios from the analyses being plotted against QC concentrations for each of the six lots evaluated. The slope values from these six curves were compared. The %CV from the six slope values for TFVdp, FTCtp, and 3TCtp were <5% indicating the lack of matrix effect related to different lots of whole blood used in the DBS extraction. Blank DBS punches from the six selectivity lots were also spiked at the LLOQ level (n=3) to determine if assay performance was consistent at the LLOQ in different lots of whole blood. Precision (%CV) and accuracy (%Bias) were within the ±20% acceptance criteria. These data are presented in Table 2.
Table 2.
TFVdp, FTCtp, and 3TCtp Slope Comparison and LLOQ Precision and Accuracy from DBS Samples Prepared in 6 Lots of Human Whole Blood
| Analyte | Parameter | Lot 1 | Lot 2 | Lot 3 | Lot 4 | Lot 5 | Lot 6 | %CV (Slope) |
|---|---|---|---|---|---|---|---|---|
| TFVdp | Slope | 7.92E-05 | 8.09E-05 | 7.74E-05 | 7.75E-05 | 7.70E-05 | 7.74E-05 | 1.9% |
| LLOQ %CV | 7.8 | 5.9 | 3.2 | 15.5 | 2.9 | 3.9 | ||
| LLOQ %Bias | −1.6 | −2.8 | −4.9 | 0.1 | −8.7 | −2.5 | ||
| FTCtp | Slope | 1.40E-04 | 1.43E-04 | 1.41E-04 | 1.41E-04 | 1.40E-04 | 1.41E-04 | 0.8% |
| LLOQ %CV | 5.5 | 6.7 | 1.4 | 7.3 | 6.8 | 3.3 | ||
| LLOQ %Bias | −5.0 | −3.3 | −0.3 | −3.2 | −8.2 | −0.7 | ||
| 3TCtp | Slope | 1.53E-04 | 1.58E-04 | 1.55E-04 | 1.52E-04 | 1.53E-04 | 1.54E-04 | 1.4% |
| LLOQ %CV | 13.9 | 3.3 | 15.5 | 11.7 | 11.4 | 11.9 | ||
| LLOQ %Bias | −11.2 | −2.6 | −10.2 | −3.1 | −6.8 | −11.8 |
3.6. Method Assessments with Clinical Samples
Since samples cannot be prepared in whole blood (specifically with analytes existing inside cells) with known concentrations prior to spiking on the card, the exact recovery of the analytes from the DBS card cannot be evaluated. It also may be different from the recovery of the calibration standards and QC samples. The inability to evaluate this initial step may introduce some bias into the accuracy of the assay of clinical samples. Knowing this limitation, additional tests were performed with clinical dried blood spot samples to provide further information regarding the ruggedness of the assay. All long-term stability evaluations (room temp and other storage conditions) were performed using clinical samples where an initial analysis determined the theoretical concentration (t=0).
3.7. Reproducibility within Spots
Precision of the assay was assessed using clinical samples from subjects on regimens containing TFV/FTC or 3TC. Three subjects on a TFV/FTC regimen and three subjects on a 3TC regimen had blood spotted on Whatman Protein Saver DBS cards. Three separate 3mm punches were extracted from the same spot to determine the intra-spot precision. An additional evaluation compared responses from three separate 3mm punches from three different spots (inter-spot). Although accuracy could not be assessed, precision [%CV range] of TFVdp [2.8–7.9 intra-spot, 1.7 – 8.1 inter-spot], FTCtp [0.2 – 10.1 intra-spot, 6.4 – 12.7 inter-spot], and 3TCtp [2.9 – 9.8 intra-spot, 2.2 – 9.3 inter-spot] fmol/sample concentrations were all within an acceptable range of 15%. The range of clinical sample concentrations per spot were as follows: 1200–2900 fmol/sample (TFVdp), 200 – 600 fmol/sample (FTCtp), and 100–1000 fmol/sample (3TCtp). These results demonstrate that reproducibility of the assay is acceptable for the analysis of TFVdp, FTCtp, and 3TCtp from clinical DBS samples.
3.8. Stability
3.8.1. Extract/Reinjection Reproducibility
The stability of the analytes in DBS extracts was demonstrated two different ways. Extracts from a validation run were stored at 10°C for 5 days. On the day of preparation, these extracts were assayed for a validation run. After the storage period, three replicates of QC1 (300 fmol/sample) and QC3 (20,000 fmol/sample) were reinjected and quantified against the original calibration curve. Comparisons of the resulting concentrations back to the nominal levels for QC1 and QC3 respectively were 4.1% and 9.0% (TFVdp), 4.3% and 1.5% (FTCtp), and 6.1% and 10.5% (3TCtp) deviation. In the second demonstration of extract stability three replicates of QC1 and QC3 from the original run were reinjected and analyzed with the freshly extracted standards and QCs. Comparisons of these concentrations to nominal levels for QC1 and QC3 respectively resulted in −10.3% and 0.2% (TFVdp), −8.3% and −4.2% (FTCtp), and −1.0% and −1.8% (3TCtp).
The stability of the analytes in DBS extracts was also evaluated with regard to reinjecting a plate following prolonged storage. Extracts were stored at 10°C for 5 days. On the day of preparation, these extracts were assayed for a validation run. After the storage period, the calibration curve (n=2) and the QC1, QC2, and QC3 levels (n=3) were reinjected. All reinjected standards and QCs met acceptance criteria of ±15%, demonstrating stability.
3.8.2. Stock/Working Solution Stability
Long-term stability of stock solutions was tested by comparing previous prepared solutions that were stored at −80°C (TFVdp, FTCtp) and −20°C (3TCtp) for various lengths of time, to solutions that were freshly prepared. The percent difference from the stored stock was −2.9% (TFVdp), −7.2% (FTCtp), and 2.6% (3TCtp) showing that TFVdp and FTCtp are stable for up to 894 days when stored at −80°C and up to 191 days for 3TCtp when stored at −20°C. Stability of stock solutions was also evaluated at room temperature. Following initial stock solution preparation, an aliquot of each of the stock solutions was transferred into a tube and stored at room temperature for 17 (TFVdp, FTCtp) and 20 (3TCtp) hours. A second aliquot was transferred to a separate tube and placed back at −80°C (TFVdp, FTCtp) and −20°C (3TCtp). After 17 hours for TFVdp, FTCtp and 20 hours for 3TCtp, the frozen aliquots were removed from the freezer and analyzed. The % difference was 1.0% (TFVdp), −0.3% (FTCtp), and −4.5% (3TCtp) demonstrating all stock solutions were stable at these conditions.
In addition, we evaluated the stability of the spiking solutions prepared in water. Fresh working solutions prepared at the QC 1 and 3 levels were compared to solutions that were stored at −80°C for 162 days. The percent differences between these solutions were −5.2% (TFVdp), −1.4% (FTCtp), −3.0% (3TCtp) for the QC 1 level and −2.5% (TFVdp), −3.5% (FTCtp), and 2.2% (3TCtp) for the QC 3 level demonstrating stability in water.
3.8.3. Storage Stability of DBS Samples
Long-term storage stability was established using clinical dried blood spot samples stored at a variety of temperatures. These DBS samples were extracted at multiple time points to establish matrix stability. The theoretical concentration (t=0) assigned to each sample was the average of three replicate samples from each of the three different subjects on either TFV/FTC or 3TC regimens generated during the initial analysis. These samples were extracted within one day of sample collection to establish the t=0 concentration. The t=0 concentration ranges were as follows: 1200–2900 fmol/sample (TFVdp), 200–600 fmol/sample (FTCtp), and 100–1600 fmol/sample (3TCtp).
Short term room temperature stability was established for three days for all three analytes. The % difference [range] from the t=0 samples was as follows: 0.4% [−0.5 – 1.3%] for TFVdp, −1.0% [−1.0 – −0.9%] for FTCtp, and −2.0% [−6.1 – 2.1%] for 3TCtp.
Longer term stability assessments were performed following 34 and 63 days of room temperature and −80°C storage. Evaluations of 34 and 63 days of room temperature storage demonstrated instability. The stored samples at room temperature showed a mean % difference [range] of −12.9% [−14.5 – −11.5%] TFVdp, −15.6% [−24.8–−10.7%] FTCtp, and −12.5% [−19.4 – −5.4%] 3TCtp following 34 days and −20.6% [−21.9 – −18.4%] TFVdp, −28.5% [−32.8 – −24.3%] FTCtp, and −16.1% [−25.9 – 1.4%] 3TCtp after 63 days, not meeting the acceptance criteria of ±15%. Although these results show failed accuracy, the precision associated with the replicate DBS spots was acceptable. The mean precision [range] for all three analytes at room temperature was 5.9% [1.5% – 10.5%] at 34 days and 9.1% [4.1% – 14.5%] at 63 days.
The % difference [range] from the t=0 samples to the −80°C replicates were 2.2% [−2.3 – 7.2%] TFVdp, −1.0% [−4.1 – 1.1%] FTCtp, and 2.5% [−3.5 – 13.0%] 3TCtp following 34 days of storage and 0.3% [−4.5 – 3.5%] TFVdp, −1.7% [−8.3 – 5.8%] FTCtp, and 0.1% [−6.7 – 4.9%] 3TCtp showing that samples stored at −80°C are stable for up to 63 days. Storage stability results can be seen in Figure 2.
Figure 2.
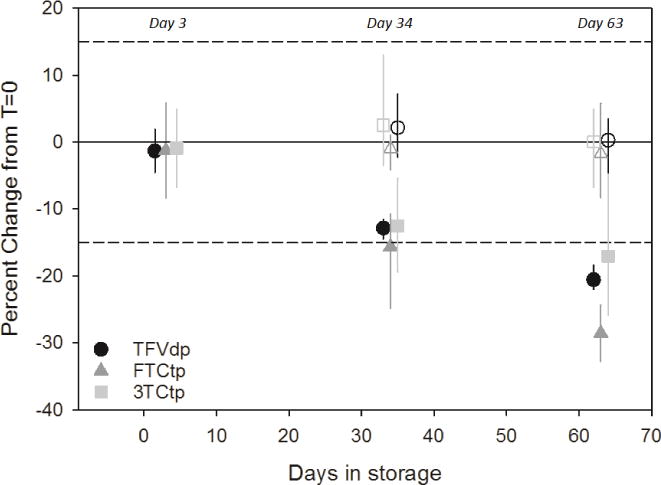
Stability Assessment for Clinical DBS Samples Stored at Room Temperature (closed symbols) and −80°C (open symbols)
Although our testing has only established stability for up to 63 days when stored at −80°C, our results are consistent with the stability profiles reported for TFVdp and FTCtp indicating these phosphorylated metabolites are stable when stored at −80°C but have limited stability at ambient temperatures (11).
3.8.4. Limitations
To evaluate method performance, clinical samples are required. Because the true intracellular concentrations in collected samples cannot be replicated, this limits the ability to easily determine the impact of selective biological variables in method precision and accuracy. Therefore, any evaluations of variables including hematocrit effects and inter-subject recovery cannot be completely assessed. While metabolites could be spiked into tubes containing DBS punches from samples with different hematocrits or different blood lots, these results can never provide a true assessment of the assay performance. The results presented for this validation indicate the assay is precise in regard to variables including punch location and for samples collected from multiple subjects. Although these subjects are considered to be adherent, no direct observation of the last dose was observed, causing the limitation that the clinical samples evaluated may not be indicative of complete adherence. Also the lack of isotopically labeled internal standards for FTCtp and 3TCtp is a limitation for monitoring the possibility of matrix effects that could cause ion suppression or enhancement. However the retention times of these two analytes were <0.02 minutes from the internal standard used and the assay was reproducible even without the stable-labeled internal standards.
4. Conclusions
An LC-MS/MS assay has been validated for the direct analysis of TFVdp, FTCtp, and 3TCtp in dried blood spots. This is, to the best of our knowledge, the first assay in DBS to quantify the intracellular metabolites of TFV, FTC, and 3TC in a single assay. The extraction allows for the direct analysis of the phosphorylated metabolites following a liquid-liquid extraction and direct injection, while avoiding the time-consuming steps used in assays for indirect analysis. The assay had good linearity (R2>0.996), accuracy (inter-assay bias within ±3.0%), and precision (inter-assay %CV ≤9.8%). Clinical samples collected and analyzed during the validation demonstrate that the calibration range selected is appropriate for the detection of these metabolites. Stability of all three metabolites was established for at least 3 days at room temperature and up to 63 days at −80°C. Our assay will enrich the model developed by Anderson et al (7, 10) with our inclusion of FTCtp and 3TCtp as it allows for adherence monitoring in people on alternative antiretroviral regimens. A directly observed therapy clinical trial is underway in our laboratory to define these concentrations and to evaluate relationships between DBS and PBMC concentrations. Thus, the ability to simultaneously analyze dried blood samples for these three commonly prescribed nucleoside/tide reverse transcriptase inhibitors provides a simple way to collect and monitor the adherence of subjects using multiple antiretroviral regimens.
Highlights.
First assay to simultaneously quantify TFVdp, FTCtp, and 3TCtp in DBS.
Assay precision and accuracy within ±15%.
Calibration range utilized clinically relevant concentrations.
Measure of adherence for MOST HIV treatment and prevention regimens.
Acknowledgments
This research was supported in part by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program P30 AI50410 as well as the NIH funded program, R01 Al22319. The authors would like to acknowledge the contributions of the sample donors, the UNC Clinical and Translational Research Center, and the UNC Center for AIDS research.
Abbreviations
- TFV
tenofovir
- FTC
emtricitabine
- 3TC
lamivudine
- NRTI
nucleos(t)ide reverse transcriptase inhibitor
- TFVdp
tenofovir diphosphate
- FTCtp
emtricitabine triphosphate
- 3TCtp
lamivudine triphosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–F19. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 2.Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother. 2011 Feb;66(2):240–50. doi: 10.1093/jac/dkq447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derdelinckx I, Wainberg MA, Lange JMA, Hill A, Halima Y, Boucher CAB. Criteria for Drugs Used in Pre-Exposure Prophylaxis Trials against HIV Infection. PLoS Med. 2006;3(11):e454. doi: 10.1371/journal.pmed.0030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Truvada [Package Insert] Gilead Sciences. Foster City, CA: 2011. [Google Scholar]
- 5.Epivir [Package Insert] GlaxoSmithKline. Research Triangle Park, NC: 2017. [Google Scholar]
- 6.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. White Coat Compliance” Limits the Reliability of Therapeutic Drug Monitoring in HIV-1—Infected Patients. HIV Clinical Trials. 2008;9(4):238–246. doi: 10.1310/hct0904-238. [DOI] [PubMed] [Google Scholar]
- 7.Castillo-Mancilla J, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, Fernandez C, Langness J, Kiser JJ, Bushman LR, Anderson PL. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013 Feb;29(2):384–390. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo-Mancilla J, Seifert S, Campbell K, Coleman S, McAllister K, Zheng JH, Gardner EM, Liu A, Glidden DV, Grant R, Hosek S, Wilson CM, Bushman LR, MaWhinney S, Anderson PL. Emtrictabine-triphosphate in dried blood spots as a marker of recent dosing. Antimicrob Agents Chemother. 2016 Oct 21;60(11):6692–6697. doi: 10.1128/AAC.01017-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi M, Glidden DV, Liu A, Anderson PL, Horng J, Defechereux P, Guanira JV, Grinsztejn B, Chariyalertsak S, Bekker LG, Grant RM. iPrEx Study Team. Strong Correlation Between Concentrations of Tenofovir (TFV) Emtricitabine (FTC) in Hair and TFV Diphosphate and FTC Triphosphate in Dried Blood Spots in the iPrEx Open Label Extension: Implications for Pre-exposure Prophylaxis Adherence Monitoring. J Infect Dis. 2015 Nov 1;212(9):1402–6. doi: 10.1093/infdis/jiv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson PL, Liu A, Castillo-Mancilla J, Gardner EM, Seifert S, McHugh C, Wagner T, Campbell K, Morrow M, Ibrahim M, Buchbinder S, Bushman LR, Kiser JJ, MaWhinney S. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy:the DOT-DBS Study. Antimicrob Agents Chemother. 2017 Oct 16; doi: 10.1128/AAC.01710-17. pii: AAC.01710-17. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng JH, Rower C, McAllister K, Castillo-Mancilla J, Klein B, Meditz A, Guida LA, Kiser JJ, Bushman LR, Anderson PL. Application of an intracellular assay for determination of Tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using Dried Blood Spots. J Pharm Biomed Anal. 2016 Apr 15;122:16–20. doi: 10.1016/j.jpba.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen RS, Rosing H, Schellens JHM, Beijnen JH. Mass Spectrometry in the Quantitative Analysis of Therapeutic Intracellular Nucleotide Analogs. Mass Spectrometry Reviews. 2011;30:321–343. doi: 10.1002/mas.20280. [DOI] [PubMed] [Google Scholar]
- 13.Kuklenyik Z, Martin A, Pau C, Holder A, Youngpairoj AS, Zheng Q, Cong M, Gerardo Garcia-Lerma J, Heneine W, Pirkle JL, Barr JR. On-line coupling of anion exchange and ion-pair chromatography for measurement of intracellular triphosphate metabolites of reverse transcriptase inhibitors. Journal of Chromatography B Volume. 2009 Nov 1;877(29):3659–3666. doi: 10.1016/j.jchromb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Lesotho Country Operation Plan (COP/ROP) 2017 Pepfar, www.pepfar.gov/documents/organization/272218.
- 15.WHO. March 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection; Recommendations for a public health approach. [PubMed] [Google Scholar]
- 16.Analytical Procedures and Methods Validation for Drugs and Biologics. The Food and Drug Administration. 2015 Jul 27; 80 FR 44357. [Google Scholar]
- 17.Matuszewski B. Standard line slopes as a measure of a relative matrix effect in quantitative HPLC-MS bioanalysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2006 Jan 18;830(2):293–300. doi: 10.1016/j.jchromb.2005.11.009. [DOI] [PubMed] [Google Scholar]


