Abstract
Objective
Diabetes mellitus (DM) and peripheral arterial disease (PAD) are independently associated with increased risk of amputation. However, the effect of poor glycemic control on adverse limb events has not been studied. We examined the effects of poor glycemic control (high hemoglobin A1c) on the risk of amputation and modified major adverse limb events (mMALE) after lower extremity revascularization.
Methods
Patients undergoing PAD revascularization who had HbA1c levels available within 6 months were identified in the VA database 2003– 2014 (N=26799). The diagnosis of preoperative DM (PreopDM) was defined using DM diagnosis codes and evidence of treatment. Amputation and modified MALE (mMALE) risk was compared for HbA1c levels using Kaplan Meier analysis. Cox proportional hazards models were created to assess the effect of high HbA1c on amputation/mMALE (adjusted for age, gender, race, SES, comorbidities, cholesterol levels, creatinine, supra/infrainguinal procedure, open/endovascular procedure, severity of PAD, year of cohort entry and medications) for all patients and stratified on PreopDM.
Results
High HbA1c levels were present in 33.2% of the cohort while 59.9% had PreopDM. Amputations occurred in 4,359 (16.3%) patients and 10,580 (39.5%) had mMALE. Kaplan Meier curves showed worst outcomes in patient with PreopDM and high HbA1c. In the Cox model, incremental HbA1c levels of 6.1–7.0%, 7.1–8.0% and >8% were associated with 26% (HR 1.26, 95% CI 1.15– 1.39), 53% (HR 1.53, 95%CI 1.37–1.7) and 105% (HR 2.05, 95% CI 1.87–2.26) higher risk of amputation respectively. Similarly, the risk of mMALE also increased by 5% (HR 1.05, 95%CI 0.99–1.11), 21% (HR 1.21, 95%CI 1.13–1.29) and 33% (HR 1.33, 95%CI 1.25–1.42) with worsening HbA1c levels of 6.1–7.0%, 7.1–8.0% and >8% respectively (versus HbA1c ≤6.0%). In stratified analysis by established by PreopDM, the relative risk of amputation/mMALE was much higher with poor glycemic control (HbA1c >7.0%) in patients without PreopDM.
Conclusion
PAD patients with worse perioperative glycemic control have a significantly higher risk of amputation and mMALE. Incremental increases in HbA1c levels are associated with higher hazards of adverse limb outcomes independent of PreopDM status. Poor glycemic control (HbA1c> 7.0%) in patients without a preoperative diagnosis of DM carries twice the relative risk of amputation and mMALE than those with good glycemic control. These results suggest that screening of diabetic status and better management of glycemic control could be a target for improvement of perioperative and long term outcomes in PAD patients.
INTRODUCTION
Peripheral arterial disease (PAD) occurrence in patients with Diabetes Mellitus (DM) is three times greater than those without DM and its prevalence in diabetics above 50 years of age is 29%1,2. Diabetes causes endovascular dysfunction of micro and macrovascular circulation leading to a state of inflammation and accelerated atherosclerosis3,4. Patients with DM have more infra-geniculate arterial involvement and frequently present with tissue loss and life threatening lower extremity infections5,6. Patients with DM and PAD have been shown to have a higher lifetime risk of amputation compared to those with either disease alone3,7,8. However, evidence regarding the mere presence of diabetes as a significant perioperative risk factor for amputation and mortality following peripheral revascularization has been conflicting9–13.
The importance of glycemic control in the perioperative setting of lower extremity revascularization has not been well addressed. In randomized trials of diabetic patients, intensive glycemic control has had mixed results for major cardiovascular events14–16. Data are lacking in regard to the effect of glycemic control prior to surgery on amputation and limb related outcomes, although glycemic control has been described as an important part of limb salvage in diabetics along with control of other risk factors4,17. In a single institution Japanese study, when compared with nondiabetics, diabetic patients with hemoglobin A1c (HbA1c) levels ≥6.8% had a significantly higher risk of major amputation than those with a lower HbA1c18. Another small study of infra-popliteal angioplasty in diabetic patients showed high fasting blood glucose levels were associated with reduced primary patency and possibly amputations at 1 year.19
The objective of our study was to determine the effect of poor glycemic control as defined by elevated perioperative HbA1c on PAD outcomes of amputation and modified major adverse limb events (mMALE) in patients undergoing vascular procedures in a large national cohort. Secondarily, we sought to determine the effect of elevated HbA1c in patients without a pre-operative diabetes diagnosis (PreopDM) but diagnosed after revascularization. We hypothesized that high HbA1c would be associated with worse outcomes in those with or without PreopDM diagnosis and there would be a dose-response effect of worse outcomes with higher HbA1c.
METHODS
Sample and Database
We reviewed national Veterans Health Administration (VHA) data to identify 26,799 vascular patients with at least one PAD revascularization procedure (Supplemental Table I) from 2003–2014 and HbA1c measured within 6 months of the procedure. A comprehensive list of covariates including patient demographics (age at procedure, sex, race), body mass index (BMI), smoking (classified using a validated method20), medications [antiplatelets, antihypertensives, statins, antiglycemics (insulin or oral antiglycemics) and cilostazol], PAD severity (claudication, rest pain, ulcer/gangrene, or unspecified), patient co-morbidities [hypertension, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), atrial fibrillation (AF), carotid disease, depression, and chronic kidney disease or end stage renal disease (CKD/ESRD)], procedure location (infrainguinal vs. suprainguinal reconstruction, or combination), procedure type (open vs. endovascular, or hybrid), and laboratory values [total cholesterol, high density lipoprotein (HDL), low-density lipoprotein (LDL), and creatinine] were abstracted from the Veterans Affairs (VA) Corporate Data Warehouse (CDW) and VA Medical SAS administrative databases. All variables were measured closest to the vascular procedure date.
Study Exposures and Outcome
The exposure was defined as the closest HbA1c measurement to the procedure date (within six months before/after). HbA1c was dichotomized into normal (≤7.0%) vs. abnormal (>7.0%) readings. For our dose-response analysis we further subdivided HbA1c into 4 levels (≤ 6.0%, 6.1–7.0%, 7.1–8.0%, and >8.0%).
One inpatient or two outpatient visit ICD-9 diagnosis code for diabetes or a diabetes medication in the subject’s record prior to the vascular procedure date were used to define PreopDM. Diabetes medications were classified into 3 categories (insulin, oral antiglycemics, or none). The outcomes of interest were incident amputations [mid/hind-foot, below and above knee amputations] and a composite incident amputation/repeat revascularization endpoint [modified Major Adverse Limb Event (mMALE)] during follow-up including any endovascular or open revascularization for supra or infrainguinal disease (Supplemental Table I). The follow-up continued through outcome occurrence, death, or December 31, 2015 (subject was censored). Patients with prior amputations were included in the analysis but incident amputation was defined as the first amputation after the vascular procedure. Long-term survival of the cohort of patients was extracted from the VA vital status file.
Statistical Analysis
Demographic and clinical variables were assessed for the entire cohort and stratified by HbA1c (>7% versus ≤7%). Continuous variables were expressed as means (± standard deviations (SD)) or as medians (± interquartile ranges (IQR)) if they were not normally distributed. Discrete variables were expressed as proportions. Proportions of missing data were also calculated and compared across subjects with any vs. no missing data. Unadjusted associations for HbA1c levels and risk of amputation and mMALE were obtained using Kaplan-Meier curves over entire study period; subjects who did not experience an outcome were censored at death or December 31, 2015. HbA1c was classified as a dichotomous exposure (>7% versus ≤7%) as well as a 4 level exposure (≤6.0%, 6.1–7.0%, 7.1–8.0%, and >8.0%).
Cox proportional hazard regression models were then created to calculate adjusted hazard ratios (HR) and 95% confidence intervals (CI) for HbA1c (4 level exposure) amongst all patients and by stratifying on PreopDM diagnosis. All Cox models adjusted for the covariates described above and procedure year. We excluded smoking due to >30% missing data. All variables were found to meet the proportional hazards assumption via log-log survival curves for amputation and mortality. Wald confidence limits were constructed for all hazard ratios. In a sensitivity analysis we added PreopDM medication use to the Cox models for those with a PreopDM diagnosis. The statistical analysis was done using SAS version 9.4 (SAS Institute, Cary, NC). Two-sided p-values < .05 were considered statistically significant. This study was approved by the Emory University IRB and Atlanta VAMC Research and Development Committee. Informed consent was waived for a retrospective cohort study design with no human patient contact and minimal privacy risks.
RESULTS
Our cohort consisted of 26,799 vascular patients with a median follow up of 3.8 years for amputation and 3.5 years for the mMALE composite endpoint. The majority of the cohort was male (98.3%) with a mean age of 66.3 years (SD 8.9). There were 4,369 (16.3%) patients with amputations and 10,580 (39.4%) patients with mMALE composite endpoint. The demographics and covariates of the cohort are listed in Table I. The mean HbA1c level for the cohort was 6.4% [IQR (5.80–7.50)]. High HbA1c levels (>7%) were present in 33.2% of the cohort while 59.9% had known diagnosis of PreopDM. A vast majority (91.6%) of patients with PreopDM had an abnormal peri-operative HbA1c (Table I). Patients with an abnormal HbA1c (>7%) were more likely to present with ulceration/gangrene (32%) and were more likely to be on insulin therapy for glycemic control (59.8%).
Table I.
Demographics of full cohort and stratification by Hemoglobin A1c (HbA1c) for patients undergoing PAD revascularization from 2003–2014.
| Variable | All | Abnormal HbA1c (>7.0%) | Normal HbA1c (<= 7.0%) |
|---|---|---|---|
| N | 26,799 | 8,901 | 17,898 |
| Age (y), Mean (SD) | 66.3 (8.9) | 65.8 (8.7) | 66.6 (9.0) |
| Sex (% Male) | 98.3 | 98.2 | 98.3 |
| Race, % | |||
| White | 80.1 | 79.1 | 80.6 |
| Black | 18.4 | 19.4 | 18.0 |
| Other | 1.5 | 1.6 | 1.5 |
| Smoking, % | |||
| Current | 43.4 | 38.4 | 45.9 |
| Former | 43.8 | 46.4 | 42.5 |
| Never | 12.8 | 15.2 | 11.5 |
| BMI (kg/m2), Mean (SD) | 27.3 (5.7) | 28.6 (5.7) | 26.7 (5.6) |
| Laboratory | |||
| Total Cholesterol, mg/dL, Mean (SD) | 157.0 (45.1) | 157.8 (49.4) | 156.6 (42.8) |
| LDL, mg/dL, Mean (SD) | 87.5 (34.2) | 86.1 (34.8) | 88.1 (33.9) |
| HDL, mg/dL, Mean (SD) | 39.0 (13.1) | 36.4 (11.4) | 40.2 (13.7) |
| Peri-Surgical HbA1c (w/in 6 months), %, Median (IQR) | 6.4 (5.8–7.5) | 8.1 (7.5–9.2) | 6.0 (5.6–6.4) |
| Creatinine, mg/dL, Median (IQR) | 1.0 (0.9–1.4) | 1.1 (0.9–1.4) | 1.0 (0.8–1.3) |
| Comorbidities, % | |||
| Hypertension | 91.0 | 93.4 | 89.9 |
| CAD | 59.5 | 64.7 | 56.9 |
| CHF | 22.6 | 26.2 | 20.8 |
| COPD | 8.3 | 7.2 | 8.9 |
| AF | 14.5 | 14.2 | 14.7 |
| Carotid Disease | 81.1 | 83.2 | 80.1 |
| Depression | 20.3 | 20.8 | 20.1 |
| CKD or ESRD | 11.2 | 10.8 | 11.5 |
| Procedure Location, % | |||
| Infra-inguinal | 67.5 | 74.6 | 64.0 |
| Supra-inguinal | 19.2 | 13.7 | 22.0 |
| Combination | 13.2 | 11.7 | 14.0 |
| Procedure Type, % | |||
| Open | 33.4 | 30.4 | 34.9 |
| Endovascular | 61.4 | 65.5 | 59.4 |
| Hybrid | 5.2 | 4.1 | 5.7 |
| Statins (% on Any Statin) | 73.1 | 75.9 | 71.7 |
| Antiplatelets (% on Any Antiplatelet) | 87.2 | 88.1 | 86.7 |
| Antihypertensives (% on Any Antihypertensive) | 88.9 | 91.7 | 87.5 |
| Cilostazol (% taking cilostazol) | 10.7 | 10.1 | 11.0 |
| Pre-procedure diabetes diagnosis (%) | 59.9 | 91.6 | 44.1 |
| Abnormal HbA1c (>7.0%) | 33.2 | - | - |
| HbA1c (% in each category) | |||
| ≤6.0 | 37.0 | 0 | 55.4 |
| 6.0–6.9 | 29.8 | 0 | 44.6 |
| 7.0–7.9 | 15.6 | 46.9 | 0 |
| >8.0 | 17.6 | 53.1 | 0 |
| PAD Severity (% in each category) | |||
| Unspecified | 38.9 | 35.9 | 40.4 |
| Claudication | 26.6 | 23.6 | 28.1 |
| Rest Pain | 10.7 | 9.0 | 11.5 |
| Ulceration/Gangrene | 23.8 | 31.5 | 20.0 |
| Pre-Operative Diabetes Medications (% in each category) | |||
| None | 49.3 | 16.1 | 65.9 |
| Oral | 21.3 | 24.2 | 19.8 |
| Insulin | 29.4 | 59.8 | 14.3 |
Unadjusted associations of HbA1c, pre-operative diabetes, and mortality
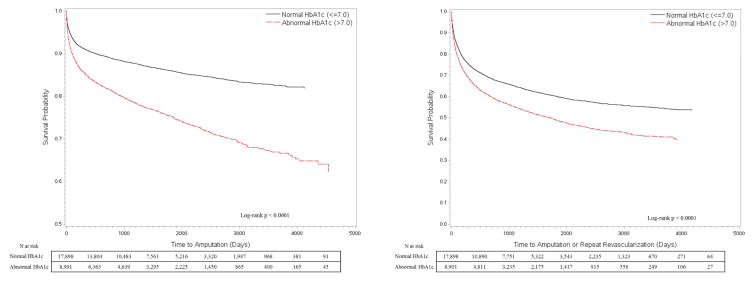
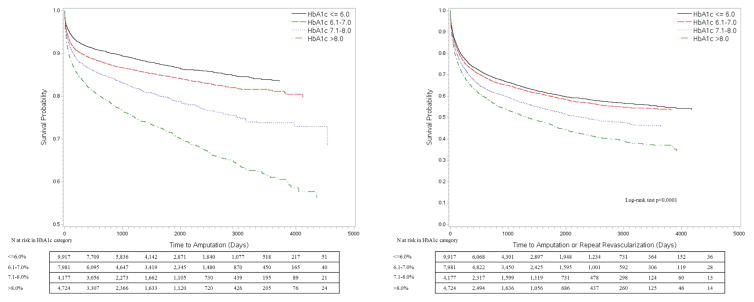
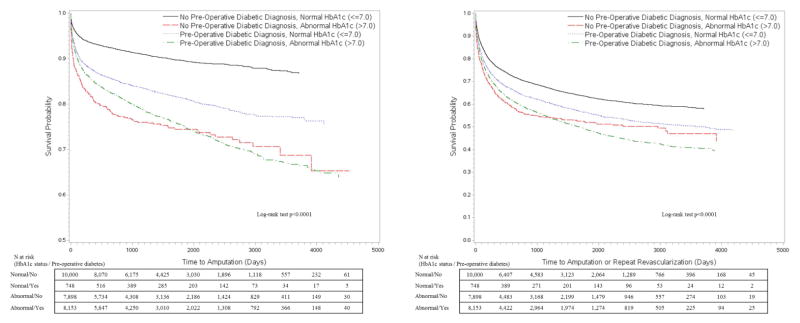
The Kaplan-Meier (KM) curves in Figure 1 depict amputation-free survival (AFS) and mMALE-free survival (MFS) stratified by HbA1c status as a dichotomous variable. Poor glycemic control as defined by a conventional HbA1c level >7% shows a clear disadvantage with regards to amputation and mMALE free survival over time. The AFS at 30-days, 1-yr, 3-yr and 5-yrs was 96.1%, 90.7%, 87.9% and 86% for the normal HbA1c group while it was 94.1%, 84.7%, 79 and 75.2% for the abnormal HbA1c group, respectively. The MFS at 30-days, 1-yr, 3-yr and 5-yrs was 91.2%, 73.6%, 65.1% and 60.2% for the normal HbA1c group while it was 88.5%, 66.4%, 55.3 and 48.9% for the abnormal HbA1c group, respectively. When we divided HbA1c further into 4 levels (=6.0%, 6.1–6.9%, 7.1–8.0% and >8%) as shown in Figure 2, we found more strength in the association with a clear dose response relationship of amputation and mMALE with rising HbA1c levels. We then stratified patients by knowledge of PreopDM and predictably the curves show the best results for patients with a normal HbA1c and known PreopDM (Figure 3). Interestingly, patients with abnormal HbA1c that were not identified preoperatively as having diabetes seemed to do the worst initially, but their amputation free survival and mMALE-free survival improved over the patients with poorly controlled PreopDM as time progressed possibly due to appropriate therapy. The AFS and MFS life table estimates of the interaction of HbA1c and PreopDM diagnosis as well as the 4 level analysis of HbA1c are presented in detail in Supplemental Table II (Appendix).
Figure 1.
Kaplan Meier unadjusted analysis of time to amputation or modified major adverse limb event (amputation or repeat revascularization) by HbA1c levels (≤7 or >7.0%)
Figure 2.
Kaplan Meier unadjusted analysis of time to amputation or modified major adverse limb event (amputation or repeat revascularization) by incremental HbA1c levels (≤6.0%, 6.1–7.0%, 7.1–8.0% or >8.0%)
Figure 3.
Kaplan Meier unadjusted analysis of time to amputation or modified major adverse limb event (amputation or repeat revascularization) by HbA1c levels (≤7 or >7.0%) and preoperative knowledge of diabetes diagnosis.
Adjusted associations of HbA1c with amputation and revascularization: all patients
In Cox proportional hazards regression, the effect of poor glycemic control persisted on increased risk of amputation and mMALE after adjusting for covariates described in methods section (Table II). Given the strong dose-response association between HbA1c level and amputation risk in the KM analysis, the Cox regression models were run with a granular HbA1c 4 level classification. Amongst all patients, abnormal HbA1c levels of 6.1–7.0%, 7.1–8.0% and >8% were associated with 26% (HR 1.26, 95% CI 1.15– 1.39), 53% (HR 1.53, 95%CI 1.37–1.7) and 105% (HR 2.05, 95% CI 1.87–2.26) higher risk of amputation, respectively, compared to those with an HbA1c level ≤6.0%. Similarly, the risk of mMALE also increased by 5% (HR 1.05, 95%CI 0.99–1.11), 21% (HR 1.21, 95%CI 1.13–1.29) and 33% (HR 1.33, 95%CI 1.25–1.42) with worsening HbA1c levels of 6.1–7.0%, 7.1–8.0% and >8%, respectively, as compared to those with HbA1c ≤6.0% (Table II, Model 1). Black race and CKD/ESRD were independently associated with an increased risk of amputation and mMALE, while rising HDL was protective from adverse limb events. Endovascular procedures had a lower risk of amputations (HR 0.81) but a higher risk of mMALE (HR 1.26), possibly needing future revascularizations. Severity of PAD in terms of Critical Limb Ischemia (CLI) i.e. rest pain/tissue loss versus claudication, had a higher risk of adverse limb events [HR 1.93 for rest pain and HR 7.52 for ulcer/gangrene]. A large portion of the cohort (38.9%) had unspecified PAD presentation, but the HR was 1.79 for this group suggesting they were likely a combination of claudicants and CLI patients. When PreopDM diagnosis was added to the Cox model the effect of HbA1c on amputation and mMALE risk was attenuated but still stayed significant and maintained the dose response relationship with increasing HbA1c (Table II, Model 2). Abnormal HbA1c levels of 6.1–7.0%, 7.1–8.0% and >8% were associated with 13% (HR 1.13, 95% CI 1.02–1.24), 28% (HR 1.28, 95%CI 1.14–1.44) and 72% (HR 1.72, 95% CI 1.54–1.91) higher risk of amputation respectively as compared to those with HbA1c ≤6.0% with the addition of PreopDM in the model. PreopDM was also associated with a higher risk of amputation and mMALE contrary to the KM analysis, suggesting that PreopDM diagnosis may be an effect modifier in this model. Therefore, we stratified the analysis based on PreopDM diagnosis to further delineate the effect of increased HbA1c on adverse limb outcomes in these subgroups.
Table II.
Cox proportional hazard regression model results for effect of elevated Hemoglobin A1c on amputation and modified major adverse limb events (mMALE) for all patients.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
|
| ||||
| Variable | Major Amputation | mMALE | Major Amputation | mMALE |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| HbA1c (vs ≤6.0) | ||||
| 6.0–6.9 | 1.26 (1.15 , 1.39) | 1.05 (0.99 , 1.11) | 1.13 (1.02 , 1.24) | 1.01 (0.95 , 1.08) |
| 7.0–7.9 | 1.53 (1.37 , 1.70) | 1.21 (1.13 , 1.29) | 1.28 (1.14 , 1.44) | 1.15 (1.06 , 1.24) |
| >8.0 | 2.05 (1.87 , 2.26) | 1.33 (1.25 , 1.42) | 1.72 (1.54 , 1.91) | 1.26 (1.17 , 1.35) |
| Female Sex (vs male) | 0.65 (0.47 , 0.88) | 0.95 (0.80 , 1.12) | 0.64 (0.47 , 0.88) | 0.95 (0.80 , 1.12) |
| Black race (vs white race) | 1.41 (1.30 , 1.53) | 1.10 (1.04 , 1.16) | 1.41 (1.30 , 1.53) | 1.10 (1.04 , 1.16) |
| HDL (10 mg/dL increase) | 0.92 (0.89 , 0.94) | 0.94 (0.93 , 0.96) | 0.92 (0.89 , 0.95) | 0.95 (0.93 , 0.96) |
| CKD or ESRD | 1.76 (1.55 , 1.99) | 1.24 (1.13 , 1.36) | 1.70 (1.51 , 1.93) | 1.23 (1.12 , 1.35) |
| Endovascular versus Open | 0.81 (0.75 , 0.87) | 1.26 (1.20 , 1.32) | 0.80 (0.75 , 0.86) | 1.25 (1.20 , 1.31) |
| PAD Severity (vs Claudication) | ||||
| Rest Pain | 1.93 (1.64 , 2.28) | 1.32 (1.22 , 1.43) | 1.94 (1.64 , 2.28) | 1.32 (1.22 , 1.43) |
| Ulcer/Gangrene | 7.52 (6.64 , 8.51) | 2.30 (2.16 , 2.44) | 7.33 (6.48 , 8.30) | 2.28 (2.15 , 2.43) |
| Unspecified | 1.79 (1.57 , 2.05) | 0.81 (0.76 , 0.86) | 1.80 (1.57 , 2.05) | 0.81 (0.76 , 0.86) |
| Pre-Operative DM Diagnosis | NA | NA | 1.40 (1.27 , 1.55) | 1.10 (1.03 , 1.16) |
HR: Hazard ratio; CI: confidence interval; HDL: High density lipoprotein; CKD: Chronic kidney disease; ESRD: end stage renal disease; PAD: peripheral arterial disease; DM: Diabetes mellitus
Model 1 and 2 both adjusted for age, body mass index, low density lipoprotein, creatinine, comorbidities (hypertension, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, atrial fibrillation, carotid artery stenosis, depression), supra-inguinal vs infra-inguinal procedure, medications (anti-platelets, statin and anti-hypertensives) and procedure year. Model 2 further adjusted for pre-operative diabetes diagnosis.
Adjusted associations of HbA1c with amputation and revascularization: pre-operative diabetes diagnosis
In patients with PreopDM, the increased risk of amputation and mMALE persisted for increasing levels of HbA1c in Cox regression analysis (Table III). Patients with HbA1c 6.1–7.0% and 7.1–8.0% had significantly higher risk of adverse limb events (HR 1.18 and 1.29 for amputation; HR 1.03 and 1.15 for mMALE, respectively) versus those with ≤6.0% HbA1c. Those with a perioperative HbA1c >8.0% were at the highest risk with a 73% higher hazard of amputation (HR 1.73, 95% CI 1.52–1.97) and a 27% higher hazard of mMALE (HR 1.27, 95% CI 1.16–1.38) as compared to those with HbA1c ≤6.0%. Similar effects of other covariates were found in this model as the primary model with all patients.
Table III.
Cox proportional hazard model results for effect of elevated Hemoglobin A1c (HbA1c) on amputation and modified major adverse limb events (mMALE) stratified by an established preoperative diabetes mellitus (DM) diagnosis.
| Preoperative DM diagnosis | No Preoperative DM Diagnosis | |||
|---|---|---|---|---|
|
| ||||
| Variable | Major Amputation | mMALE | Major Amputation | mMALE |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| HbA1c (vs <6.0) | ||||
| 6.0–6.9 | 1.18 (1.04 , 1.35) | 1.03 (0.95 , 1.12) | 0.97 (0.82 , 1.15) | 0.98 (0.90 , 1.08) |
| 7.0–7.9 | 1.29 (1.12 , 1.47) | 1.15 (1.05 , 1.26) | 1.49 (1.06 , 2.11) | 1.19 (0.95 , 1.48) |
| >=8.0 | 1.73 (1.52 , 1.97) | 1.27 (1.16 , 1.38) | 1.98 (1.51 , 2.59) | 1.52 (1.25 , 1.84) |
| Female Sex (vs Male) | 0.64 (0.44 , 0.92) | 1.01 (0.82 , 1.25) | 0.66 (0.36 , 1.19) | 0.86 (0.65 , 1.14) |
| Black race (vs white race) | 1.31 (1.19 , 1.44) | 1.10 (1.02 , 1.17) | 1.85 (1.57 , 2.16) | 1.11 (1.00 , 1.22) |
| HDL (10 mg/dL increase) | 0.91 (0.88 , 0.94) | 0.93 (0.91 , 0.95) | 0.94 (0.89 , 0.99) | 0.96 (0.94 , 0.99) |
| CKD or ESRD | 1.77 (1.55 , 2.02) | 1.25 (1.13 , 1.39) | 1.43 (1.01 , 2.02) | 1.16 (0.93 , 1.44) |
| Endovascular versus Open | 0.82 (0.76 , 0.89) | 1.20 (1.13 , 1.27) | 0.74 (0.64 , 0.86) | 1.39 (1.28 , 1.51) |
| PAD Severity (vs Claudication) | ||||
| Rest Pain | 1.64 (1.33 , 2.02) | 1.29 (1.16 , 1.43) | 2.58 (1.95 , 3.42) | 1.37 (1.22 , 1.54) |
| Ulcer/Gangrene | 6.63 (5.74 , 7.65) | 2.18 (2.03 , 2.35) | 9.56 (7.51 , 12.17) | 2.66 (2.39 , 2.96) |
| Unspecified | 1.86 (1.60 , 2.17) | 0.87 (0.80 , 0.94) | 1.61 (1.24 , 2.08) | 0.72 (0.65 , 0.79) |
HR: Hazard ratio; CI: confidence interval; HDL: High density lipoprotein; CKD: Chronic kidney disease; ESRD: end stage renal disease; PAD: peripheral arterial disease.
Models also adjusted for age, body mass index, low density lipoprotein, creatinine, comorbidities (hypertension, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, atrial fibrillation, carotid artery stenosis, depression), supra-inguinal vs infra-inguinal procedure, medications (anti-platelets, statin and anti-hypertensives) and procedure year.
A majority (82.8%) of the patients with PreopDM were on medications: 35.0% on oral antiglycemic and 47.9% on insulin. We performed a sensitivity analysis by adding use of diabetic medications to the Cox model. The effect of HbA1c was mildly attenuated for each outcome but remained significant in its association with amputations for all levels of HbA1c [HbA1c 6.1–7.0%: HR 1.14 (1.0–1.3); HbA1c 7.1–8.0%: HR 1.18 (1.02–1.35); HbA1c >8%: HR 1.51 (1.32–1.73)]. Major adverse limb composite endpoint was not significantly higher for the 6.1–7.0% HbA1c group as compared to those with ≤6.0% HbA1c but mMALE remained 11% and 19% higher for the 7.1–8.0% and >8% HbA1c groups. This suggests that despite being on medical treatment for DM, if patients had a high HbA1c perioperatively, their long term limb outcomes were not good. Patients on insulin therapy had a worse risk of amputation [HR 1.29 (1.13–1.46)] and mMALE [1.13 (1.03, 1.23)] as compared to those not on any medications or on oral hypoglycemic, likely due to severity of DM.
Adjusted associations of HbA1c with amputation and revascularization: no pre-operative diabetes diagnosis
In patients without PreopDM, the effect of mildly elevated HbA1c (6.1–7.0%) was not significant for risk of amputation or mMALE. Of note these patients were not on any diabetic medication preoperatively (Table III). Therefore, they were likely recognized postoperatively or had early diabetes in the perioperative period. However, patient with HbA1c in the 7.1–8.0% range had a significantly higher risk of amputation [HR 1.49, 95% CI 1.06–2.11] and a trend towards higher mMALE [HR 1.19, 95% CI 0.95–1.48]. Those with the poorest glycemic control (HbA1c >8.0%) had the worst risk of amputation [HR 1.98, 95% CI 1.51–2.59] and mMALE [HR 1.52, 95% CI 1.25–1.84], even when compared to those with PreopDM and an HbA1c >8.0%. This group of patients likely had unrecognized severe diabetes that went untreated before their revascularization.
DISCUSSION
Our study shows that elevated perioperative hemoglobin A1c is associated with a higher incidence of long-term amputation and mMALE for PAD patients. The association is further strengthened upon delineating HbA1c levels (≤6.0%, 6.1–7.0%, 7.1–8.0& and >8.0%) to show a dose-response relationship between degree of glycemic control and adverse limb outcomes. Patients with the poorest glycemic control (HbA1c >8.0%) are at twice the long-term risk of amputation and 33% higher risk for mMALE as compared to those with a normal HbA1c. This association is attenuated but still significant in patients with PreopDM diagnosis and those on treatment for their diabetes, suggesting a possible role for better glycemic control perioperatively to improve long-term outcomes. Patients without recognized DM in the preoperative setting and elevated HbA1c (>7.0%) are associated with the worst risk of amputation and mMALE in the cohort, suggesting the importance of preoperative recognition of diabetes before revascularization for PAD.
The impact of diabetes as a comorbid condition in patients with PAD is significant, both clinically and economically21. Most studies to date have only explored the presence of diabetes as a risk factor for lower extremity outcomes but not glycemic control. Malmstedt et al used a Swedish vascular registry to show that DM was associated with a lower amputation-free survival after leg bypass for critical limb ischemia after adjusting for demographics, comorbidities and other risk factors for amputation12. In contrast, another European study showed no difference in limb salvage at 1 year for patients with and without diabetes undergoing femoro-distal reconstruction for CLI13. Hicks et al and Brothers et al recently used the Vascular Quality initiative data in separate analyses to show that infra-geniculate bypasses or endovascular interventions did not have any difference in amputation or MALE risk at 1 year in patients with and without diabetes in multivariable models10, 22. Takahara et al conducted a study on all balloon angioplasty procedures for CLI done at their Japanese institution and found similar findings as us that diabetes and poor glycemic control as defined by abnormal HbA1c was significantly associated with limb loss18. In a separate single center study, high fasting blood glucose as a marker of glycemic control in patients undergoing infra-popliteal angioplasty was found to be associated with significantly decreased primary patency19. Since our main goal was to study the effect of glycemic control on lower extremity outcomes, we included all PAD revascularization procedures and not only endovascular procedures in our analysis. We adjusted for level of intervention (supra/infra-inguinal), severity of PAD as well as endovascular/open nature of intervention. Despite these adjustments we found significant effect of glycemic control (in an incremental fashion) on long term adverse limb events after revascularization in diabetic patients. Our study confirmed that presence of DM does impact major amputation and adverse limb events but the level of glycemic control had a stronger association with these outcomes. Amongst patients with high HbA1c (>7%), 8.4% did not have known PreopDM diagnosis prior to surgery. Furthermore, we found a significantly higher risk of limb loss and need for revascularization in patients without PreopDM but had evidence of poor glycemic control. This may suggest the need for screening for diabetes prior to a major revascularization procedure and perhaps using HbA1c as a screening tool.
Hemoglobin A1c (HbA1c) concentration reflects mean blood glycemic levels over a 3–6 month period and has been used as a marker for glycemic control in a number of large clinical trials14–16,23. Hyperglycemia augments the atherosclerotic process by several mechanisms including activation of the protein kinase C pathway and by increasing atherogenic lipid deposition, platelet activation and inducing a hypercoagulable state. These pathways lead to a pro-inflammatory state that augments smooth muscle migration and proliferation, and ultimately increase endothelial dysfunction and oxidative stress24. Higher concentrations of HbA1c may promote arterial stiffness and accelerated atherosclerosis in proportion to the HbA1c level, even at a level below the diagnostic threshold for diabetes25. According to a report from the Atherosclerosis Risk in Communities (ARIC) study, graded HbA1c rise was associated with greater PAD incidence in diabetic adults suggesting a role for advanced glycation products and severity of hyperglycemia in accelerated atherosclerosis in the lower limbs26. The mechanism of association between poor glycemic control and adverse limb outcomes may also involve disparate pathways than atherosclerosis and inflammation. Poor glycemic control is associated with impaired wound healing, decreased immunity and peripheral neuropathy through tissue accumulation and crosslinking in extracellular matrix, thus impairing healing of diabetic foot ulcers (DFU) patients with PAD27,28.
The role of intensive glucose control in the prevention of cardiovascular outcomes has been controversial. The Action to Control Cardiovascular Risk in Diabetes Study Group (ACCORD) trial targeted treating patients aggressively to hemoglobin A1c values below 6.0% versus using standard therapy to a value between 7.0% and 7.9%, and found higher cardiovascular mortality in the intensively treated group at 3.5 years with no significant reduction in the number of cardiovascular events including nonfatal myocardial infarction and stroke14. The United Kingdom Prospective Diabetes Study (UKPDS), Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), and the Veterans Affairs Diabetes Trial (VADT) studies did not show harm of an intensive glycemic control regimen but at the same time failed to show a statistically significant risk reduction in the development of macrovascular complications (non-fatal myocardial infarction, non-fatal stroke, and death from cardiovascular causes) with intensive glycemic control15,16,29. We lack PAD trials that focus on limb related and diabetic foot endpoints related to glycemic control. A recent meta-analysis by the Society for Vascular Surgery (SVS) reported results for 19,234 patients in nine diabetes trials that included amputation as secondary endpoints in their analysis for glycemic control. It found that intensive control was associated with decreased risk of amputation, better sensory nerve function, and potentially overall incidence of DFU and infection30. A few recent observational studies have shown that there is considerable regional variation and racial disparity in the intensity of diabetic care as measured by annual serum cholesterol and hemoglobin A1c testing across the United States31,32. Compared with the lowest quartile of diabetic testing, diabetic patients in regions with the highest quartile of diabetic testing had significantly improved amputation free survival [HR= 0.94] and MALE free survival [HR= 0.92] persisting up to 2 years after lower extremity revascularization. Nondiabetic patients with CLI, in comparison, did not benefit to the same extent from undergoing revascularization in regions with high-quality outpatient diabetic care31. Another study in the VA system observed that patients with improved process measures like glucose control were less likely to undergo lower extremity bypass surgery or amputation33. The recent clinical practice guidelines from the SVS in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine recommend adequate glycemic control (HbA1c < 7% with strategies to minimize hypoglycemia) to reduce the incidence of DFUs and infections, with subsequent risk of amputation (Grade 2B)4. Our study findings support the role of glycemic control with a target of HbA1c <7.0% to decrease amputation and adverse limb events in patients with PAD with PreopDM before revascularization. Our results also suggest possible usefulness of screening for diabetes using HbA1c in PAD patients scheduled for revascularization. However, further prospective investigation will be required to verify whether glycemic interventions to target a modest HbA1c goal of 7%, offer risk reduction in PAD revascularization patients with known diabetes or those at risk for diabetes and whether surgery should be delayed in an elective setting to ensure a better perioperative glycemic control.
Our study has several limitations. Data on laterality of vascular procedures and amputations was unavailable, possibly overestimating the number and timing of procedure-relevant outcomes. We only assessed the impact of perioperative HbA1c level closest to the procedure. Patients could have been on better glycemic control later on. Our study is observational using administrative data, and the analysis may be susceptible to residual confounding. The nature of the database precludes access to technical details such as runoff vessels or patency as well as lack of ankle brachial index data. The presence of absence of infection as well as extent of tissue loss is not well captured, therefore we couldn’t assign patients to some of the newer classifications being used for CLI such as Wound, Ischemia, and foot Infection (WIfI). Only each subject’s first vascular procedure during the study timeframe (2003–2014) was included in the analysis. We adjusted for year of entry into cohort but our approach may have led to selection bias and has implications for the generalizability of the study. Our study is based on VHA data and it is overwhelmingly comprised of male patients. Results may differ in a non-VA population.
In conclusion, poor glycemic control (high HbA1c) is incrementally associated with increased risk of amputation and mMALE in PAD patients undergoing revascularization. Presence of diabetes preoperatively is also an independent risk factor of adverse limb events. Patients with no known diabetes but impaired glycemic control are at worse risk of adverse limb related outcomes with HbA1c levels above 7.0% as compared to those with PreopDM. Our results suggest a possible role of tighter glycemic control in reduction of amputation and need for further revascularization in diabetics as well as a possible utility of screening for diabetes using HbA1c levels prior to revascularization for PAD rather than a pre-existing diagnosis of DM.
Supplementary Material
Take Home Message.
60% of 26,799 patients who underwent revascularization for PAD had DM, many of whom were undiagnosed, and those who had DM with poor control had worse outcomes, including amputation and major adverse limb event (MALE) when their HgA1C showed poor glycemic control(>7.0)
Acknowledgments
Supported by the following grant(s):
Arya: American Heart Association Mentored Clinical and Population Research Award (15MCPRP25580005); NIH-NIA 1R03AG050930; American Geriatric Society/Society for Vascular Surgery (SVS) Foundation Jahnigen Career Development Award.
Brewster: NIH- NHLBI, 1KO8HL119592; SVS Foundation/American College of Surgeons Mentored Clinical Scientist Research Career Development Award; Department of Defense, CDMRP/OPORP; OP140015
Wilson: Veteran Affairs Merit Grant I01-CX001025
This material is the result of work supported with resources and the use of facilities at the Atlanta VA Medical Center, Decatur GA.
The funding organizations did not participate directly in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Note to editor: Abstract presented at the Southern Association for Vascular Surgery (2017) plenary session and awarded the best paper prize.
DISCLOSURES The authors report no conflicts or relations with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004 Aug 10;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch A, Criqui M, Treat-Jacobson D, Regensteiner J, Creager M, Olin J, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317– 1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 3.Jude EB, Oyibo SO, Chalmers N, Boulton AJM. Peripheral arterial disease in diabetic and nondiabetic patients: A comparison of severity and outcome. Diabetes Care. 2001;24(8):1433–1437. doi: 10.2337/diacare.24.8.1433. [DOI] [PubMed] [Google Scholar]
- 4.Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016 Feb;63(2 Supplement):3S–21S. doi: 10.1016/j.jvs.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 5.DeRubertis BG, Pierce M, Ryer EJ, Trocciola S, Kent KC, Faries PL. Reduced primary patency rate in diabetic patients after percutaneous intervention results from more frequent presentation with limb-threatening ischemia. J Vasc Surg. 2008;47(1):101–108. doi: 10.1016/j.jvs.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008 May;51(5):747–755. doi: 10.1007/s00125-008-0940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphries MD, Brunson A, Li C-S, Melnikow J, Romano PS. Amputation trends for patients with lower extremity ulcers due to diabetes and peripheral artery disease using statewide data. J Vasc Surg. 2016 Dec;64(6):1747–1755.e1743. doi: 10.1016/j.jvs.2016.06.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. J Am Coll Cardiol. 2012 Nov 20;60(21):2230–2236. doi: 10.1016/j.jacc.2012.08.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biancari F, Salenius JP, Heikkinen M, Luther M, Ylönen K, Lepäntalo M. Risk-scoring method for prediction of 30-day postoperative outcome after infrainguinal surgical revascularization for critical lower-limb ischemia: A finnvasc registry study. World J Surg. 2007;31(1):217–225. doi: 10.1007/s00268-006-0242-y. [DOI] [PubMed] [Google Scholar]
- 10.Hicks CW, Najafian A, Farber A, Menard MT, Malas MB, Black JH, III, et al. Diabetes does not worsen outcomes following infrageniculate bypass or endovascular intervention for patients with critical limb ischemia. J Vasc Surg. 2016 Dec;64(6):1667–1674.e1661. doi: 10.1016/j.jvs.2016.07.107. [DOI] [PubMed] [Google Scholar]
- 11.Mellière D, Berrahal D, Desgranges P, Allaire E, Becquemin JP, Perlemuter L, et al. Influence of diabetes on revascularisation procedures of the aorta and lower limb arteries: Early results. Eur J Vasc Endovasc Surg. 1999;17(5):438–441. doi: 10.1053/ejvs.1998.0806. [DOI] [PubMed] [Google Scholar]
- 12.Malmstedt J, Leander K, Wahlberg E, Karlstrom L, Alfredsson L, Swedenborg J. Outcome after leg bypass surgery for critical limb ischemia is poor in patients with diabetes: a population-based cohort study. Diabetes Care. 2008 May;31(5):887–892. doi: 10.2337/dc07-2424. [DOI] [PubMed] [Google Scholar]
- 13.Wolfle KD, Bruijnen H, Loeprecht H, Rumenapf G, Schweiger H, Grabitz K, et al. Graft patency and clinical outcome of femorodistal arterial reconstruction in diabetic and non-diabetic patients: results of a multicentre comparative analysis. Eur J Vasc Endovasc Surg. 2003 Mar;25(3):229–234. doi: 10.1053/ejvs.2002.1849. [DOI] [PubMed] [Google Scholar]
- 14.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009 Jan 08;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 16.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 17.Fernando ME, Seneviratne RM, Tan YM, Lazzarini PA, Sangla KS, Cunningham M, et al. Intensive versus conventional glycaemic control for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2016 Jan 13;(1):Cd010764. doi: 10.1002/14651858.CD010764.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahara M, Kaneto H, Iida O, Gorogawa S-i, Katakami N, Matsuoka T-a, et al. The Influence of Glycemic Control on the Prognosis of Japanese Patients Undergoing Percutaneous Transluminal Angioplasty for Critical Limb Ischemia. Diabetes Care. 2010;33(12):2538–2542. doi: 10.2337/dc10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S, Armstrong EJ, Sherif W, Alvandi B, Westin GG, Singh GD, et al. Association of elevated fasting glucose with lower patency and increased major adverse limb events among patients with diabetes undergoing infrapopliteal balloon angioplasty. Vasc Med. 2014 Aug;19(4):307–314. doi: 10.1177/1358863X14538330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, et al. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011 Dec;13(12):1233–1239. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malone M, Lau NS, White J, Novak A, Xuan W, Iliopoulos J, et al. The effect of diabetes mellitus on costs and length of stay in patients with peripheral arterial disease undergoing vascular surgery. Eur J Vasc Endovasc Surg. 2014 Oct;48(4):447–451. doi: 10.1016/j.ejvs.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Brothers TE, Zhang J, Mauldin PD, Tonnensen BH, Robison JG, Vallabhaneni R, et al. Predicting outcomes for infrapopliteal limb-threatening ischemia using the Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2016 Jan;63(1):114–124. doi: 10.1016/j.jvs.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 23.Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA. 2006;295(14):1688–1697. doi: 10.1001/jama.295.14.1688. [DOI] [PubMed] [Google Scholar]
- 24.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and Vascular Disease. Pathophysiology, Clinical Consequences, and Medical Therapy: Part I. Circulation. 2003;108(12):1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 25.Lee YH, Shin MH, Choi JS, Rhee JA, Nam HS, Jeong SK, et al. HbA1c is significantly associated with arterial stiffness but not with carotid atherosclerosis in a community-based population without type 2 diabetes: The Dong-gu study. Atherosclerosis. 2016 Apr;247:1–6. doi: 10.1016/j.atherosclerosis.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 26.Selvin E, Wattanakit K, Steffes MW, Coresh J, Sharrett AR. HbA1c and peripheral arterial disease in diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2006 Apr;29(4):877–882. doi: 10.2337/diacare.29.04.06.dc05-2018. [DOI] [PubMed] [Google Scholar]
- 27.Peppa M, Stavroulakis P, Raptis SA. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009 Jul-Aug;17(4):461–472. doi: 10.1111/j.1524-475X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 28.Aubert CE, Michel PL, Gillery P, Jaisson S, Fonfrede M, Morel F, et al. Association of peripheral neuropathy with circulating advanced glycation end products, soluble receptor for advanced glycation end products and other risk factors in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014 Nov;30(8):679–685. doi: 10.1002/dmrr.2529. [DOI] [PubMed] [Google Scholar]
- 29.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 30.Hasan R, Firwana B, Elraiyah T, Domecq JP, Prutsky G, Nabhan M, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016 Feb;63(2 Supplement):22S–28S.e22. doi: 10.1016/j.jvs.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Brooke BS, Kraiss LW, Stone DH, Nolan B, De Martino RR, Reiber GE, et al. Improving outcomes for diabetic patients undergoing revascularization for critical limb ischemia: does the quality of outpatient diabetic care matter? Ann Vasc Surg. 2014 Oct;28(7):1719–1728. doi: 10.1016/j.avsg.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suckow BD, Newhall KA, Bekelis K, Faerber AE, Gottlieb DJ, Skinner JS, et al. Hemoglobin A1c Testing and Amputation Rates in Black, Hispanic, and White Medicare Patients. Ann Vasc Surg. 2016 Oct;36:208–217. doi: 10.1016/j.avsg.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins TC, Beyth RJ, Nelson DB, Petersen NJ, Suarez-Almazor ME, Bush RL, et al. Process of Care and Outcomes in Patients with Peripheral Arterial Disease. J Gen Intern Med. 2007;22(7):942–948. doi: 10.1007/s11606-007-0203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.