Abstract
Here we describe a conditional doxycycline-dependent mouse model of RET/PTC3 (NCOA4-RET) oncogene-induced thyroid tumorigenesis. In these mice, after 10 days of doxycycline (dox) administration, RET/PTC3 expression induced mitogen activated protein kinase (MAPK) stimulation and a proliferative response which resulted in the formation of hyperplastic thyroid lesions. This was followed, after 2 months, by growth arrest accompanied by typical features of oncogene-induced senescence (OIS), including upregulation of p16INK4A and p21CIP, positivity at the Sudan black B, activation of the DNA damage response (DDR) markers γH2AX and pChk2 T68, and induction of p53 and p19ARF. After 5 months, about half of thyroid lesions escaped OIS and formed tumors that remained dependent on RET/ PTC3 expression. This progression was accompanied by activation of AKT-FOXO1/3a pathway and increased serum TSH levels.
Keywords: Thyroid cancer, RET oncogene, Oncogene-induced senescence, AKT
1. Introduction
More than 80% of follicular cell-derived thyroid carcinomas are represented by papillary thyroid carcinomas (PTC) (Nikiforov and Nikiforova, 2011). Oncogenic conversion of components of the mitogen activated protein kinase (MAPK) pathway is a major driver for PTC formation (Nikiforov and Nikiforova, 2011). Accordingly, in the Cancer Genome Atlas Research Network study, PTC featured genetic lesions targeting BRAF (>60% of the cases), RAS (about 13% of the cases) and gene rearrangements (about 10% of the cases) affecting tyrosine kinase receptors (RTK), such as RET, ALK, NTRK1, NTRK3, MET, FGFR2 and LTK, or few other genes (PPARG and THADA) (Cancer Genome Atlas Research Network, 2014). Gene fusion events are particularly common in PTCs occurred in patients with previous radiation exposure (Ricarte-Filho et al., 2013).
RET fusions (RET/PTC) involve in-frame recombination of the RET TK domain with various gene partners (Santoro and Carlomagno, 2013). RET/PTC3, in particular, is a frequent isoform identified in sporadic and radiation associated PTCs. It results from a paracentric inversion of the long arm of chromosome 10 that causes RET recombination with the NCOA4 (Nuclear receptor coactivator 4) gene. NCOA4, initially identified as a coactivator for nuclear receptors (Yeh and Chang, 1996), has been recently involved in additional biological processes, such as ferritinophagy and DNA replication control (Mancias et al., 2014; Bellelli et al., 2014, 2016).
Multiple evidences suggest that also the phosphatidyl-inositol-3-kinase/AKT(PKB) (PI3K/AKT) pathway plays a primary role in thyroid cancer. In KRAS conditional mouse models, PTEN loss induces thyroid hyperplasia and PTC formation (Yeager et al., 2007; Miller et al., 2009). Genetic ablation of AKT1 blocks thyroid cancer progression in the TRβ(PV/PV) mouse model (Saji et al., 2011). Finally, mutations in components of the PI3K/AKT pathway, such as PTEN, PI3KCA and AKT1, have been reported in thyroid cancer, particularly in radioiodine-refractory PTCs and anaplastic thyroid carcinomas (ATC), suggesting they are involved in progression to aggressive histotypes (García-Rostán et al., 2005; Ricarte-Filho et al., 2009; Xing, 2013; Landa et al., 2016). Noteworthy, PI3K/AKT pathway stimulation cooperates with the physiological thyroid growth factor, thyroid stimulating hormone (TSH), acting through cAMP, to induce DNA synthesis of thyrocytes (Roger et al., 1987; Deleu et al., 1999; Cass et al., 1999; Coulonval et al., 2000; Roger et al., 2010; Zaballos and Santisteban, 2013).
Oncogene activation in primary cells elicits a permanent cell cycle arrest known as oncogene-induced senescence (OIS) (Serrano et al., 1997). OIS is characterized by the upregulation of the p16INK4A (hereafter referred to as p16) and p21CIP1 (hereafter referred to as p21) cell cycle inhibitors (Lin et al., 1998). Mechanistically, OIS may be initiated by aberrant MAPK-mediated replication stress and activation of the DNA damage response (DDR) (Di Micco et al., 2006; Bartkova et al., 2006). OIS has been demonstrated in human precancerous lesions and it is considered a major barrier against tumorigenesis (Collado and Serrano, 2010; Negrini et al., 2010). In mouse models, senescence leads to tumor regression suggesting possible pro-senescence therapeutic approaches (Nardella et al., 2011).
Several evidences have suggested a role for OIS in restraining proliferation of oncogene-bearing thyroid neoplastic cells. Papillary thyroid microcarcinomas show p21 and p16 upregulation, which is lost upon progression to full-blown cancers (Vizioli et al., 2011). Furthermore, OIS induced in vitro by HRAS/G12V is p16-dependent (Vizioli et al., 2014). Recently, Zou and colleagues showed OIS in tumor xenografts from TPO-BRAF V600E transgenic mice (Zou et al., 2016).
It has been previously reported that constitutive expression of RET/PTC oncogenes in mouse thyroid, under the control of follicular cell-specific bovine thyroglobulin (Tg) promoter, is able to initiate thyroid tumorigenesis (Ledent et al., 1991; Jhiang et al., 1996; Santoro et al., 1996; Powell et al., 1998; Burniat et al., 2008). Here, to explore initiation of thyroid lesions and their addiction to oncogene expression, we developed Tg-rtTA/tetO-RET/PTC3 mice whereby expression of the RET/PTC3 oncogene in adult thyroid was conditionally controlled by administration of doxycycline (Chakravarty et al., 2011), an experimental system that is particularly suited to explore early events upon oncogene induction.
2. Materials and methods
2.1. Generation of tetO-RET/PTC3 mice
The Tg-rtTA mouse line (in the FVB background), expressing the reverse tetracycline transactivator (rtTA) in thyroid follicular cells under the control of thyroid-specific bovine thyroglobulin (Tg) promoter, has been described in Chakravarty et al. (2011). To obtain the tetO-RET/PTC3 plasmid, expressing the RET/PTC3 oncogene under the control of doxycycline (dox)-responsive element, the human RET/PTC3 cDNA (expressing the short -RET9- alternatively spliced RET C-ter) was cloned downstream from the TRE element, containing a multimerized tetO operator upstream from the minimal CMV promoter, in the pTRE2pur vector (Clontech Laboratories, Mountain View, CA, USA) (Supplemental informations, Fig. S1). A beta-globin poly-A cassette was cloned downstream from the RET/ PTC3 sequence. The obtained construct was checked by DNA sequencing. To verify its expression, it was transiently transfected in the HeLa Tet-ON cells (Clontech Laboratories), expressing rtTA transactivator, by using the Fugene reagent (Promega, Fitchburg, WI, USA). Twenty-four hours after transfection, RET/PTC3 expression was induced by dox treatment (1 mg/ml) for 24 h and 48 h and expression was confirmed by immunoblot (data not shown). TetO-RET/PTC3 mice (in the FVB background) were generated through fertilized egg microinjection by the Turku Center for Disease Modelling, TCDM, Mouse Biology and Genetics facility (Turku, Finland). Tg-rtTA/tetO-RET/PTC3 double heterozygotes were generated by intercrossing the two corresponding parental lines. Mouse genotyping was performed on tail DNA by PCR using the following amplimers:
Tg-rtTA-f: 5′-CACCCGCCCAACAGAGAAACAGTA-3';
Tg-rtTA-r: 5′-TCAAGAGCGTCAGCAGGCAGCATA-3';
TRE-PTC3 f: 5′-GCAAACCTGCCAGTGGTTAT-3';
TRE-PTC3 r: 5′-GGAGTCAGATGGAGTGGACG-3'.
Single heterozygous Tg-rtTA and tetO-RET/PTC3 mice were bred separately; the mendelian transmission of the transgene in both the strains suggested integration in a single genetic locus. Double heterozygous Tg-rtTA x tetO-RET/PTC3 mice were derived from the breeding of single heterozygous Tg-rtTA with single heterozygous tetO-RET/PTC3 mice. Tg-rtTA/tetO-RET/PTC3 mice were fed with dox chow (on) and then, when required, with regular chow (off) for the indicated time points. Dox was administered via dox-impregnated food pellets (2500 ppm; Harlan-Teklad, Indianapolis, IN, USA) starting at 6—8 weeks of age (P0) for the indicated times. Thyroid lobes were surgically removed upon general anesthesia and either snap-frozen for RNA extraction or fixed in formaldehyde 4% for histological and immunohistochemical analysis. Mice were maintained under specific pathogen-free conditions in the Italian Ministry approved (D.M. 78/213-A, 25.3.2013) Animal Facility of the Università ‘Federico II’, Naples, Italy. This study was conducted in accordance with Italian regulations for animal experimentation and it was approved by the local Committee of the Università ‘Federico II’ as well as the Italian Ministry of Health.
2.2. RNA extraction and RT-PCR
RNA was isolated from snap-frozen thyroids by using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and purified with the RNeasy purification kit (Qiagen, Hilden, Germany). RNA (500 ng) was reverse-transcribed with the Quantitec reverse transcription kit (Qiagen). Quantitative RT-PCR reactions were done in triplicate and fold changes were calculated with the formula: 2 −(sample 1 ΔCt − sample 2 ΔCt), where ΔCt is the difference between the amplification fluorescent threshold of the mRNA of interest and the mRNA of the β-actin used as an internal reference. Primers used to detect RET/PTC3 mRNA are described in Nikiforov et al. (2009).
2.3. Mice studies
Formalin-fixed paraffin embedded (FFPE) thyroid sections (5 µm thickness) were stained with Hematoxylin & Eosin (H&E) by conventional methods or deparaffinized and rehydrated by passages through xylene and alcohol series for immunohistochemical (IHC) staining. Architectural (loss of follicular pattern, solid areas with mitotic figures in absence of colloid and presence of multiple papillary-like structures) and nuclear (enlargement, overlapping, chromatin clearing, ground glass appearance, and pseudo-inclusions) features, or combination thereof, were diagnostic for PTC-like thyroid neoplasia (Baloch et al., 2016). Endogenous peroxidase activity was inactivated by treatment with 3% hydrogen peroxide (30 min at R.T.). Antigen retrieval was performed by incubation for 15 min in boiling citrate buffer (1 mM, pH 6.0). Slides were blocked in 1% BSA for 1 h at R.T. followed by O.N. incubation with primary antibodies and for 30 min at R.T. with biotinylated goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA). The stain was visualized with 3,3′-Diaminobenzidine (DAB) (SIGMA-Aldrich, St. Louis, MO, USA). Primary antibodies were: anti-(pan)AKT (4685), -PTEN (9188), -AKT-pSer473 (4060), -phospho-FOXO1(Thr24)/FOXO3a(Thr32) (9464), - pMEK1/2 (Ser217/Ser221) (9121) and -cleaved Caspase 3 (9661) from Cell signaling Technologies (Beverly, MA, USA); anti-p21 (sc-397), -p19ARF (sc-22784), and -p16 (sc-1207) from Santa Cruz Biotechnology (Dallas, TX, USA); anti Ki-67 (VP-K451) from Vector Laboratories (Peterborough, UK); anti-phospho-histone H2A.X (Ser139, e.g. γH2AX) from Millipore (Temecula, CA, USA); anti-Chk2-pT68 (NB100-92502) from Novus Biologicals (Littleton, CO, USA); anti-p53 (CM5p) from Novocastra (Leica Biosystem, Milano, Italy). Anti-RET is a home-made affinity-purified polyclonal rabbit antibody raised against the tyrosine-kinase portion of human RET. Sudan Black B staining (SBB) was performed as described in Georgakopoulou et al. (2013). The TUNEL (Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling) assay was performed according to manufacturer's instructions (Roche, Indianapolis, IN, USA). Serum TSH and T4 levels were measured as previously described (Ferrara et al., 2013).
2.4. Statistical analysis
Statistical analysis of mean difference for Ki67, p16 and p21 staining was performed as follows: when samples displayed Gaussian distribution and different standard deviation we used t-student, Welch corrected; when samples did not display Gaussian distribution a non parametric Mann-Whitney U test was applied. All P values were two sided and differences were significant when P < 0.05. All statistical analysis were carried out using the GraphPad Instat software program (version 3.06.3).
3. Results
3.1. Induction of RET/PTC3 expression in Tg-rtTA/tetO-RET/PTC3 mice results in thyroid hyperplasia which progresses to neoplasia
We generated a doxycycline (dox)-inducible mouse model (Tg-rtTA/tetO-RET/PTC3) to study in vivo effects of transient thyroid-specific expression of the RET/PTC3 oncogene (Supplemental informations, Fig. S1). Preliminarily, RET/PTC3 was found to be expressed upon 10 days of dox chow administration and it was switched-off upon 30 days dox withdrawal by RT-PCR and immunohistochemistry (IHC) (Supplemental informations, Figs. S2A and S2B).
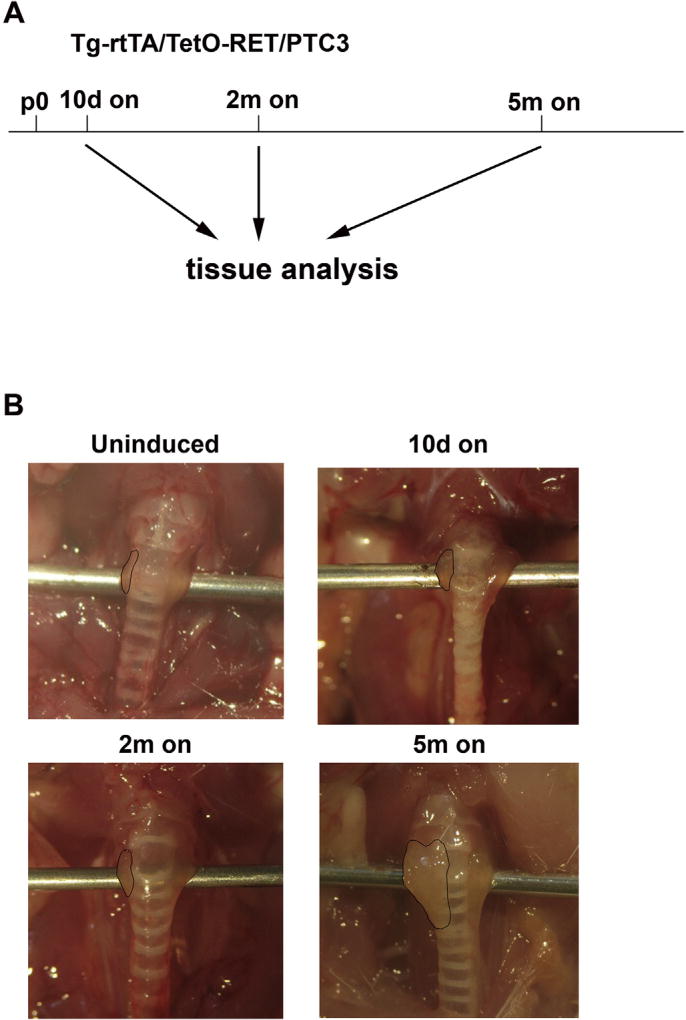
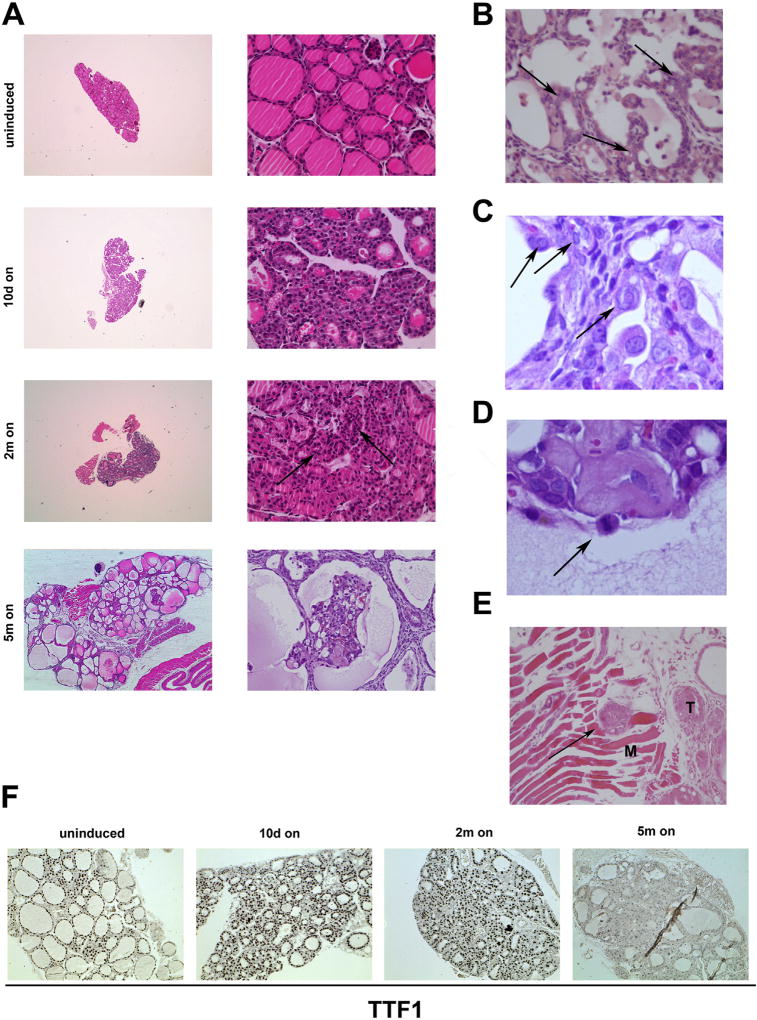
We induced RET/PTC3 expression for 10 days, or 2 or 5 months and analysed thyroid morphology. Macroscopically, after 10 days or 2 months of induction, no significant change of thyroid lobes appearance (size and shape) was noted; upon 5 months of RET/PTC3 induction, a gross enlargement of thyroid lobes was noted (Fig. 1 and Table 1). Microscopically, at 10 days induction, Tg-rtTA/tetO-RET/PTC3 thyroids developed a benign hyperplastic phenotype with follicular cell hypertrophy and crowding (Fig. 2A). After 2 months induction, thyroids showed signs of atypical hyperplasia with nuclear irregularities and solid areas lacking colloid (see arrows in Fig. 2A); however, we could not detect any of the classical nuclear features of PTC, nor a prominent change of thyroid architecture at this time point. Finally, upon 5 months induction, thyroids (7 out of 12 examined) showed histological features of PTC-like neoplasia, including loss of follicular architecture, absence of colloid and presence of multiple papillary-like structures (Fig. 2A and Table 1). Moreover, cytologically, at the 5 months time point, thyroid cells developed nuclear features of PTC, including nuclear enlargement, clearing, overlapping, ground glass appearance, and pseudo-inclusions, as well as mitotic figures (see arrows in Fig. 2B – D). Two out of 12 examined 5 months dox-treated mice showed also extrathyroidal extension of the tumor with skeletal muscle invasion (Fig. 2E). Of note, thyroids from 5 months treated mice also showed reduced staining for the thyroid differentiation marker TTF1 (NKX2-1), consistent with impaired differentiation (Fig. 2F). As a control, single heterozygous Tg-rtTA or tetO-RET/PTC3 mice did not show detectable thyroid morphology abnormalities upon a 2 months dox diet (Supplemental informations, Fig. S2C).
Fig. 1. Thyroid effects of RET/PTC3 expression.
A) RET/PTC3 expression schedule. Tg-rtTA/tetO-RET/PTC3 mice were fed with dox chow (on) for the indicated time points (10 days: 10d on; 2 months: 2m on; 5 months: 5m on). Twelve animals were studied at each time point (Table 1). Age-matched regular chow fed Tg-rtTA/tetO-RET/PTC3 mice were used as controls (uninduced). B) Representative images of thyroids from Tg-rtTA/tetO-RET/PTC3 mice uninduced or fed with dox chow for the indicated times. Thyroid boundaries are marked by dashed lines.
Table 1.
Summary of histological features of RET/PTC3-induced thyroid lesions.
| Experimental groupa | Histopathology
|
|||
|---|---|---|---|---|
| Normal | Hyperplasia | Atypical hyperplasia | PTC-like neoplasiab | |
| Uninduced (3 males and 3 females) | 6/6 | – | – | – |
| 10 days on (6 males and 6 females) | – | 10/12 | 2/12 | – |
| 2 months on (6 males and 6 females) | – | – | 11/12 | 1/12 |
| 5 months on (6 males and 6 females) | – | – | 5/12 | 7/12 |
On = Dox chow fed mice.
See criteria listed in Material and Methods.
Fig. 2. Histological features of RET/PTC3-induced thyroid lesions.
A) Representative H&E staining of thyroids from Tg-rtTA/tetO-RET/PTC3 mice uninduced or fed with dox chow (on) for the indicated time points. Arrows indicate areas of cell crowding with absent colloid. Twelve animals were studied at each time point (Table 1). B–E) Microscopic features of malignancy in Tg-rtTA/TetO-RET/PTC3 mice fed with dox chow for 5 months: ground glass nuclei (arrows) (B); multiple nuclear inclusions (arrows) (C); mitotic figure (arrow) (D); skeletal muscle invasion (M: muscle; T: thyroid) (arrow) (E). (F) Representative staining of thyroids from Tg-rtTA/tetO-RET/PTC3 mice fed with dox chow (on) for the indicated time points with TTF1 antibody. Normally fed Tg-rtTA/tetO-RET/PTC3 mice were used as controls (uninduced) (magnification 40x).
3.2. Thyroid lesions in Tg-rtTA/tetORET/PTC3 mice are addicted to RET/PTC3
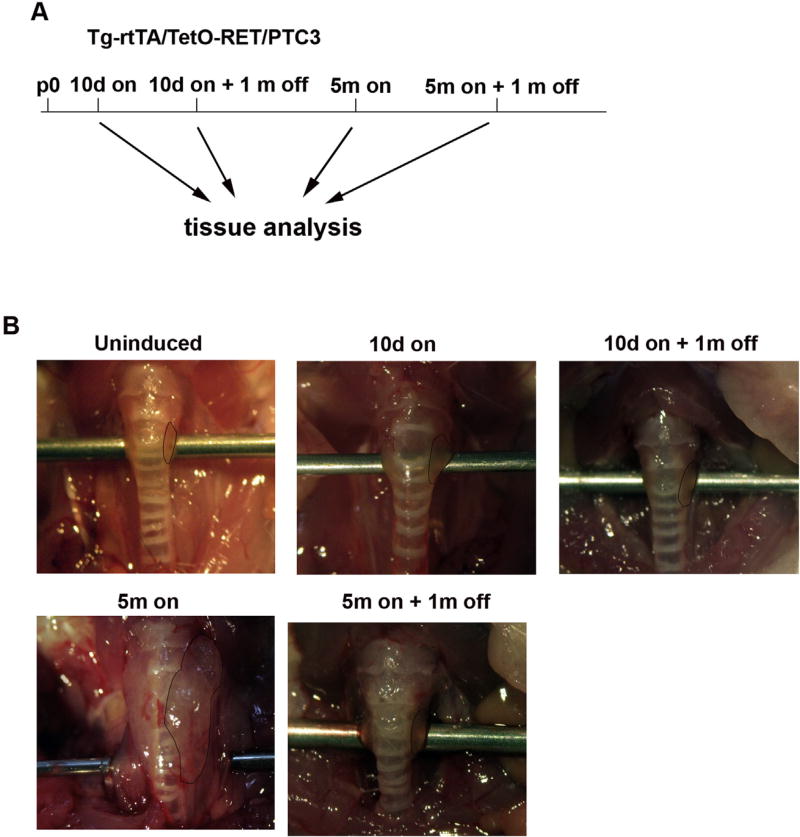
We evaluated effects of 30 days dox withdrawal in Tg-rtTA/tetO-RET/PTC3 mice that were previously induced with dox for 10 days or 5 months (Fig. 3). Macroscopically, upon dox wash-out, both sets of dox-fed mice (5 out of 5 mice examined for each time point) showed a regression of thyroid phenotype (Fig. 3). Microscopically, upon dox wash-out, signs of follicle disruption, with loss of colloid, extensive follicle collapse and only mild residual hyperplastic features were seen in both cases (Fig. 4A). Then, upon a prolonged (2 months) dox deprivation period, thyroid histology returned virtually normal (data not shown).
Fig. 3. RET/PTC3 withdrawal leads to regression of thyroid lesions.
A) RET/PTC3 expression schedule. Tg-rtTA/tetO-RET/PTC3 mice were fed with dox chow (on) and then with regular chow (off) for the indicated time points. Regular chow fed mice were used as controls (uninduced). B) Representative images of thyroid lobes from Tg-rtTA/tetO-RET/PTC3 mice fed with dox chow for the indicated time points (on) and then regular chow (off) for 1 month. Thyroid boundaries are marked by dashed lines. These images are representative of 5 independent samples for each time point.
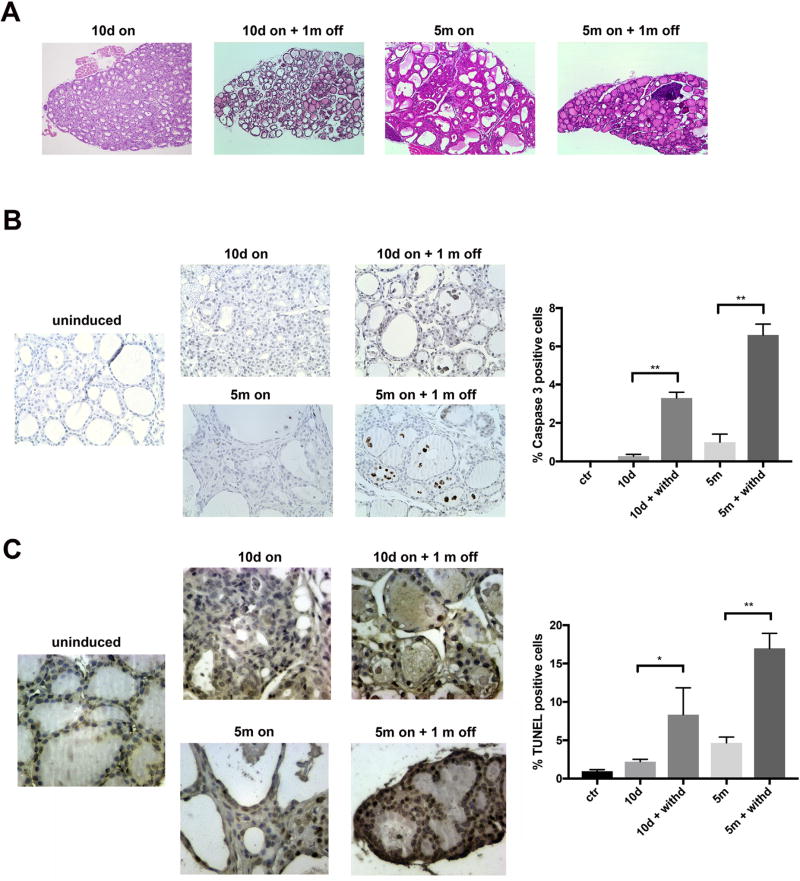
Fig. 4. RET/PTC3 withdrawal leads to apoptotic regression of thyroid lesions.
A) H&E staining of thyroids from Tg-rtTA/tetO-RET/PTC3 mice fed with dox chow for 10 days (10d on) or 5 months (5m on) and then regular chow (off) for 1 month (magnification 20x). B) Left: representative FFPE sections of thyroids from 10 days (10d on) or 5 months (5m on) dox-fed mice, then fed with regular chow (off) for 1 month, stained for cleaved caspase-3. Normally fed Tg-rtTA/tetO-RET/PTC3 mice were used as controls (uninduced) (magnification 40x). Right: bar graphs showing percentage of cleaved caspase-3 positive cells at the indicated time points. Data represent mean ± SD of counting of at least 400 cells from groups of 4–5 independent thyroids. The Mann-Whitney U test was used to determine statistical significance (**p < 0.01). C) Left: representative TUNEL assay stainings performed on FFPE thyroid sections from 10 days (10d on) or 5 months (5m on) dox-fed mice, then fed with regular chow (off) for 1 month. Normally fed Tg-rtTA/tetO-RET/PTC3 mice were used as controls (uninduced) (magnification 40x). Right: bar graphs showing percentage of cleaved caspase-3 positive cells at the indicated time points. Data represent mean ± SD of counting of at least 400 cells from groups of 4–5 independent thyroids. The Mann-Whitney U test was used to determine statistical significance (*<0.05; **p < 0.01).
The reduction of thyroid volume upon RET/PTC3 wash-out, suggested a block of cell proliferation associated with cell death. To verify this hypothesis, we stained thyroid sections from Tg-rtTA/tetO-RET/PTC3 mice with anti-cleaved caspase 3 antibody to identify apoptotic cells. Percentage of cleaved caspase 3 positive cells strongly increased upon 30 days of dox deprivation, particularly in mice previously fed with dox chow for 5 months (Fig. 4B). A TUNEL (Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling) assay which measures DNA fragmentation in vivo confirmed increased apoptosis upon dox deprivation (Fig. 4C). Thus, hyperplastic and neoplastic lesions initiated by RET/PTC3 remained addicted to oncogene expression.
3.3. Thyrocytes of Tg-rtTA/tetO-RET/PTC3 mice undergo oncogene-induced senescence
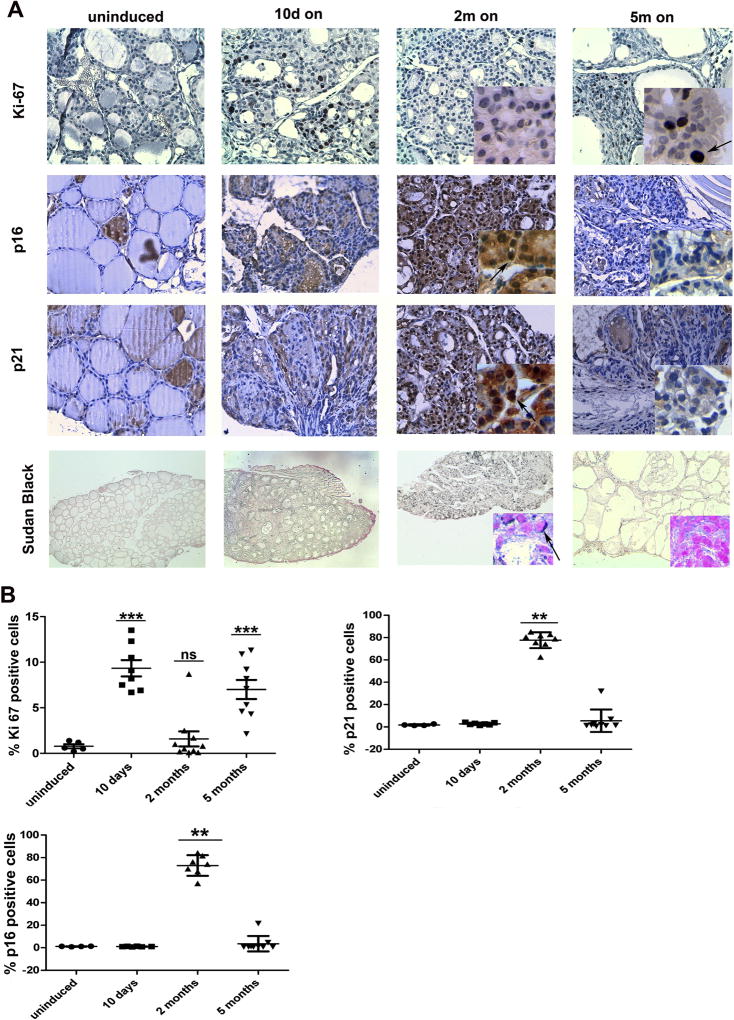
The biphasic nature of thyroid volume increase (e.g. increase at 10 days dox chow followed by stabilization up to 2 months, and finally increase again up to 5 months) prompted us to analyze cell proliferation in Tg-rtTA/tetO-RET/PTC3 mice. As shown in Fig. 5, upon 10 days of RET/PTC3 induction thyroids developed a strong positivity for the Ki-67 proliferation marker, pointing to an initial proliferative burst. Then, upon 2 months dox treatment, only sparse thyroid cells remained positive for Ki-67, pointing to a block of cell proliferation; this was not associated to signs of cell death. Finally, thyroids of 5 months dox-fed mice returned strongly positive for Ki-67 (Fig. 5 and data not shown).
Fig. 5. Oncogene-induced senescence upon RET/PTC3 expression.
A) Immunohistochemical detection of Ki-67, p16 and p21, and staining with Sudan Black B of thyroids from Tg-rtTA/TetO-RET/PTC3 mice fed with dox chow (on) for the indicated time points (5–12 thyroids were used for each staining and uninduced mice were used as controls). B) Bar graphs showing percentage of cells positive for Ki-67, p16 or p21. At least 400 nuclei were counted from 5 to 10 independent thyroids; average values ± SD are reported. Statistical significance was calculated using t-student, Welch corrected for Ki-67, and Mann-Whitney U test for p21 and p16 (***p < 0.001; **p < 0.01; ns: not significant).
A proliferative arrest after an initial proliferative burst can be caused by oncogene-induced senescence response (OIS). OIS is characterized by increased expression of the cell cycle inhibitors p16 and p21 (Serrano et al., 1997); in cultured thyrocytes OIS was shown to be p16-dependent (Vizioli et al., 2014). This prompted us to explore whether OIS was engaged in Tg-rtTA/tetO-RET/PTC3 mice and whether its evasion accompanied progression from atypical hyperplasia (2 months on) to neoplastic lesions (5 months on). As shown in Fig. 5, thyroids from Ki-67 negative uninduced mice were negative for p21 and p16, thus excluding any aspecific positivity for these markers in quiescent non-proliferating cells. Thyroids from 10 days dox-fed mice (which are strongly Ki-67 positive) were also negative for p21 and p16. On the contrary, thyroids from 2 months dox-fed mice became strongly positive for both p21 and p16, suggesting engagement of an OIS response. Finally, 5 months dox-fed mice glands (which were strongly Ki-67 positive) were almost completely negative for p21 and p16 (see higher magnification insets and arrows in Fig. 5).
To confirm these findings, we applied the modified Sudan Black B (SBB) staining that has been developed to mark lipofuscin accumulation in senescent cells (Georgakopoulou et al., 2013; Evangelou and Gorgoulis, 2017). As show in Fig. 5, thyroids from 2 months dox-fed mice showed a strong positivity for SBB which turned negative in 5 months dox-fed mice. Importantly, both uninduced and 10 days dox-fed mice were negative at SBB staining.
OIS is dependent upon the activation of a DNA Damage Response (DDR) signaling cascade (Bartkova et al., 2005; Gorgoulis et al., 2005). DDR senses the presence of DNA lesions and promotes a p53-dependent checkpoint response (Jackson and Bartek, 2009). It is believed that the initial trigger of OIS is a replication stress caused by oncogene-stimulated hyper-replication (Di Micco et al., 2006; Bartkova et al., 2006). Main players of the DDR and p53 activation are the apical Ser/Thr kinases ATM (Ataxia Teleangiectasia Mutated) and ATR (ATM and Rad3-related) and their target kinases Chk2 and Chk1, respectively (Ciccia and Elledge, 2010). A downstream target of the DDR is the histone variant H2AX which is phosphorylated at Ser139 (a modification also known as γH2AX) (Jackson and Bartek, 2009; Ciccia and Elledge, 2010). Thus, we stained thyroid sections with antibodies against Chk2 pT68 and γH2AX. As shown in Fig. 6, as early as after 10 days of dox treatment, thyroids from Tg-rtTA/tetO-RET/PTC3 mice showed a strong positivity for pChk2 T68 and γH2AX. These markers remained strongly positive after 2 months and persisted also after 5 months of dox treatment upon OIS escape. This is in agreement with what observed in the majority of human cancers and mouse models (Collado and Serrano, 2010; Negrini et al., 2010).
Fig. 6. DNA damage response upon RET/PTC3 expression.
Detection of γH2AX, pChk2 T68, p53 and p19 in thyroids from Tg-rtTA/tetO-RET/PTC3 uninduced or fed with dox chow (on) for the indicated time points (original magnification 40x). These images are representative of 7–12 independent samples for each time point.
In order to confirm OIS and to look for mechanisms of escape upon prolonged dox dosing, we analysed p53 levels by immunohistochemistry. As shown in Fig. 6, p53 staining was negative in thyroids from mice uninduced or induced with dox for 10 days. Increased overall and nuclear p53 was found upon 2 months dox treatment (see arrow in Fig. 6). Strikingly, escape from OIS and tumor progression in 5 months dox-fed mice, was associated with reduced p53 staining, with the majority of thyroid cells showing a negative or only a weak and diffuse signal (see higher magnification insets in Fig. 6).
Oncogenic insults are associated with increased expression of p19ARF which indirectly promotes p53 stability by antagonizing MDM2 (Pomerantz et al.,1998; Sherr, 2006). As shown in Fig. 6, p19 levels were parallel to p53. They were undetectable in thyroids from uninduced mice and remained negative or weakly citoplasmatic after 10 days of dox-chow. Then, a markedly increased expression and nuclear accumulation of p19 was found after 2 months of dox-chow (see higher magnification insets in Fig. 6). Finally, p19 was reduced, parallel to escape from OIS, in 5 months dox-fed mice, with the great majority of thyroid cells scoring negative or with a diffuse and weak nucleo-citoplasmatic staining. Similar behaviour has been described in other cancer mouse models (Evangelou et al., 2013; Velimezi et al., 2013).
3.4. Escape from RET/PTC3-induced senescence is associated to AKT activation and increased TSH levels
Hyperactivation of the PI3K/AKT pathway promotes escape from OIS in cancer models induced by MAPK drivers. Kennedy and coworkers showed that a constitutively active form of AKT (myr-AKT) is able to block RAS-induced OIS in vitro (Kennedy et al., 2011). In a mouse model of melanoma, PTEN downregulation prompted progression of BRAF bearing nevi to melanoma (Vredeveld et al., 2012). Similarly, in a mouse model of thyroid cancer, loss of PTEN was essential to resume proliferation of KRAS-expressing cells (Miller et al., 2009). Finally, in a thyroid-specific TPO-BRAF V600E mouse model, increased AKT activation correlated with senescence escape (Zou et al., 2016).
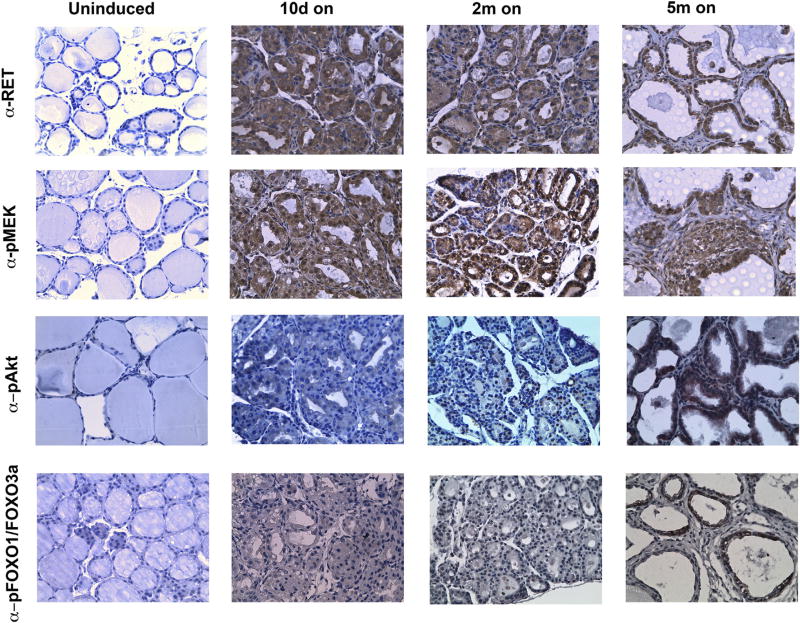
Thus, we analysed activation of the MEK/MAPK and PI3K/AKT pathways in proliferative lesions (10 days on), senescent thyroid lesions (2 months on) and highly proliferative neoplastically advanced lesions (5 months on). As shown in Fig. 7, RET/PTC3 was expressed at similar levels at all the time points tested, ruling out the possibility that proliferative changes were due to fluctuations of oncogene expression. RET/PTC3 expression was paralleled by MEK phosphorylation, that promptly increased in dox-induced thyroids and did not detectably change at the different time points. Of note, at early time points (10 days or 2 months of dox treatment) only a minimal increase of AKT phosphorylation (S473) was detected. Thereafter, parallel to proliferation rescue, glands from 5 months dox-fed mice showed a strong increase in pAKT (Fig. 7). Consistently, a strong phosphorylation of bona fide AKT substrate FOX-O1(Thr24)/FOXO3a (Thr32) was detected in the same samples, suggesting that activation of the AKT-FOXO1/3a pathway was associated to OIS escape. We could not detect any change in PTEN or total AKT levels, excluding that AKT pathway triggering was mediated by their perturbed expression (Supplemental informations, Fig. S3A). Phospho-AKT S473 and p-FOXO1(Thr24)/ FOXO3a (Thr32) IHC staining returned virtually negative in thyroid sections from 5 months-treated mice subjected to 1 month dox-withdrawal; thus, whatever the exact mechanism of AKT/FOXO stimulation, this appeared dependent on sustained RET/PTC3 signaling (Supplemental informations, Fig. S3B).
Fig. 7. Escape from OIS is associated with increased phospho-AKT/FOXO.
Immunohistochemical detection of RET/PTC3, pMEK1/2 (S217/S221), pAKT (S473) and pFOXO1(T24)/FOXO3a(T32) in thyroids from Tg-rtTA/tetO-RET/PTC3 uninduced or fed with dox chow (on) for the indicated time points (original magnification 40x). These images are representative of 7–12 independent samples for each time point.
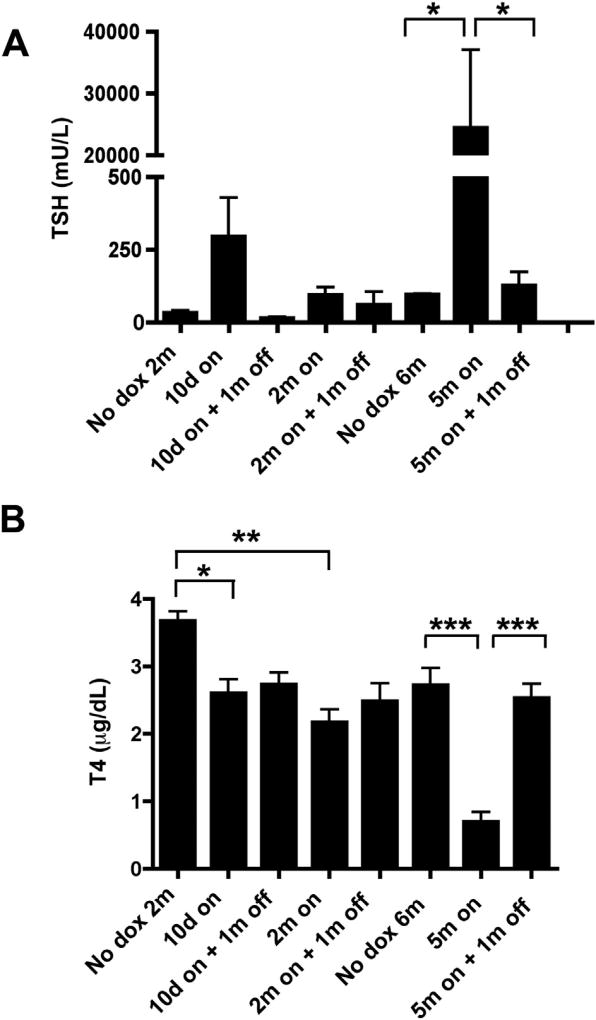
Tumorigenesis and escape from OIS in thyroid cells expressing oncogenic BRAF V6000E was associated to increased TSH levels (Franco et al., 2011; Zou et al., 2016). Thus, we analysed TSH levels in Tg-rtTA/tetO-RET/PTC3 mice subjected or not to a dox chow for 10 days, or 2 or 5 months followed or not by 1 month dox withdrawal. As a control, we used 2 and 6 months old uninduced mice. As shown in Fig. 8, activation of RET/PTC3 for 5 months caused a strong increase of TSH, that upon dox withdrawal moved towards normal values, consistent with the addiction of thyroid lesions to RET/PTC3 expression. Increased TSH levels were likely caused by disruption of thyroid function, as indicated by the parallel decrease of T4 level at 5 months that was rescued upon dox withdrawal. Smaller TSH and T4 changes were noted at 10 days and 2 months of treatment (Fig. 8).
Fig. 8. T4 and TSH levels in Tg-rtTA/tetO-RET/PTC3 mice.
A, B) Serum T4 and TSH levels in Tg-rtTA/tetO-RET/PTC3 mice uninduced (2 or 6 months old) or induced with dox chow for the indicated time points (on) and, then, regular chow (off) for 1 month.
4. Discussion
By characterizing a conditional Tg-rtTA/tetO-RET/PTC3 mouse model, here we show that RET/PTC3 expression is not only sufficient but also necessary for maintenance of thyroid neoplasia. Accordingly, 5 months dox administration prompted formation of thyroid neoplasms with architectural and nuclear features reminiscent of PTC; in 2/12 cases malignancy was demonstrated by soft tissue invasion. Dox withdrawal led to apoptotic regression of RET/ PTC3-initiated lesions. A limitation of this study is that the genetic model employed is not optimal to explore long term effects of chronic oncogene exposure of the thyroid cells; other conditional mouse models, such as those based on the CRE/lox system, would be more appropriate to explore these effects (Franco et al., 2011).
By analysing the time-frame of tumor formation, we observed that RET/PTC3 induced a rapid (10 days) proliferative burst which was followed by engagement of an OIS program, with growth arrest, upregulation of p21 and p16 and positivity at Sudan Black B. This is in agreement with data from Vizioli and co-workers who described OIS upon oncogene expression in primary cultured thyrocytes in vitro and upregulation of p21 and p16 in vivo in papillary thyroid microcarcinomas (Vizioli et al., 2011). Mechanistically, in Tg-rtTA/tetO-RET/PTC3 mice, OIS was linked to activation of DDR, as shown by positivity for pChk2 T68 and γH2AX, and accumulation of p53 and p19. Tumor progression in Tg-rtTA/tetO-RET/PTC3 mice was accompanied by escaped OIS as shown by loss of p21 and p16 expression. Interestingly, loss of p16 and p21 has been also reported in human PTCs (Pickett et al., 2005; Brzeziński et al., 2005; Lam et al., 2007). OIS escape associated p21 downregulation in Tg-rtTA/tetO-RET/PTC3 mice was consistent with reduced p53 and p19 expression. Several mechanisms may account for downregulation of the INK4 (CDKN2A) locus encoding both p16 and p19. For instance, Cdc6 overexpression, which is a downstream effector of the MAPK cascade, was shown to silence the INK4 locus (Gonzalez et al., 2006; Petrakis et al., 2012). Other mechanisms, such as promoter methylation or altered activity of Polycomb repressive complexes (PRC1 and PRC2), which are known to suppress p16 expression, can be also taken into account (LaPak and Burd, 2014).
Recent reports have suggested a role for the PI3K/AKT pathway in promoting escape from OIS (Kennedy et al., 2011; Vredeveld et al., 2012). In aged mice, PI3K/AKT activation, following PTEN loss, has been shown to mediate p16 suppression (Zeng et al., 2013). Accordingly, in 5 months induced Tg-rtTA/tetO-RET/PTC3 mice, thyroids showed increased phosphorylation of AKT and its downstream substrate FOXO1/FOXO3a.
The mechanism of AKT activation in Tg-rtTA/tetO-RET/PTC3 remains unknown; it may be secondary to both cell autonomous and non cell autonomous mechanisms. We observed a strong increase of blood TSH level in 5 months dox-fed Tg-rtTA/tetO-RET/PTC3 mice, likely caused by release of the negative feedback on the hypotalamic-pituitary axis due to thyroid function disruption and reduced T4. TSH may cooperate with PI3K/AKT activation to foster growth as it has been shown in many thyroid systems. Alternatively, TSH may autonomously promote signaling to PI3K/AKT cascade as shown in WRT thyroid cells (Cass et al., 1999; reviewed in Roger et al., 2010). Whatever the case, it is feasible that, in Tg-rtTA/tetO-RET/PTC3 mice, TSH elevation impacted OIS escape. Accordingly, Franco et al. (2011) reported that in a transgenic mouse model featuring conditional BRAFV600E expression, genetic knockdown of TSHR or its downstream effector Gsα significantly delayed cancer development. More recently, Zou et al. (2016) showed that OIS is induced in TPO-BRAF V600E tumors when transplanted into TPO-BRAF WT mice but not in TPO-BRAF V600E mice which feature increased TSH levels. Similar to our model, xenografts from TPO-BRAF V600E escaping OIS also showed a strong pAKT S473 signal (Zou et al., 2016). Regardless the exact mechanism, the Tg-rtTA/tetO-RET/PTC3 model here described suggests that besides RET/PTC3-MAPK targeting, also restoring senescence by AKT and TSH pathway blockade might be exploited therapeutically in RET/PTC-initiated thyroid cancers.
Supplementary Material
Acknowledgments
We gratefully acknowledge F. Carlomagno for statistical analysis and critical evaluation of the manuscript. This study was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC)Associazione Italiana per la Ricerca sul Cancro, by the Epigen grant of the Italian National Research Council (CNR), by the MOVIE project of the Regione Campania, by NIH grant DK-15070 from NIH to S. Refetoff and NIH grant CA-50706 to J.A. Fagin.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.mce.2017.06.023.
References
- Baloch ZW, Seethala RR, Faquin WC, Papotti MG, Basolo F, Fadda G, Randolph GW, Hodak SP, Nikiforov YE, Mandel SJ. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a changing paradigm in thyroid surgical pathology and implications for thyroid cytopathology. Cancer Cytopathol. 2016;124:616–620. doi: 10.1002/cncy.21744. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsí Z, Koed K, Krämer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Ørntoft T, Lukas J, Bartek J. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Ørntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Bellelli R, Castellone MD, Guida T, Limongello R, Dathan NA, Merolla F, Cirafici AM, Affuso A, Masai H, Costanzo V, Grieco D, Fusco A, Santoro M, Carlomagno F. NCOA4 transcriptional coactivator inhibits activation of DNA replication origins. Mol. Cell. 2014;55:123–137. doi: 10.1016/j.molcel.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Bellelli R, Federico G, Matte A, Colecchia D, Iolascon A, Chiariello M, Santoro M, De Franceschi L, Carlomagno F. NCOA4 deficiency impairs systemic iron homeostasis. Cell Rep. 2016;14:411–421. doi: 10.1016/j.celrep.2015.12.065. [DOI] [PubMed] [Google Scholar]
- Brzeziński J, Migodziński A, Toczek A, Tazbir J, Dedecjus M. Patterns of cyclin E, retinoblastoma protein, and p21Cip1/WAF1 immunostaining in the oncogenesis of papillary thyroid carcinoma. Clin. Cancer Res. 2005;11:1037–1043. [PubMed] [Google Scholar]
- Burniat A, Jin L, Detours V, Driessens N, Goffard JC, Santoro M, Rothstein J, Dumont JE, Miot F, Corvilain B. Gene expression in RET/PTC3 and E7 transgenic mouse thyroids: RET/PTC3 but not E7 tumors are partial and transient models of human papillary thyroid cancers. Endocrinology. 2008;149:5107–5117. doi: 10.1210/en.2008-0531. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass LA, Summers SA, Prendergast GV, Backer JM, Birnbaum MJ, Meinkoth JL. Protein kinase A-dependent and -independent signaling pathways contribute to cyclic AMP-stimulated proliferation. Mol. Cell Biol. 1999;19:5882–5891. doi: 10.1128/mcb.19.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty D, Santos E, Ryder M, Knauf JA, Liao XH, West BL, Bollag G, Kolesnick R, Thin TH, Rosen N, Zanzonico P, Larson SM, Refetoff S, Ghossein R, Fagin JA. Small-molecule MAPK inhibitors restore radio-iodine incorporation in mouse thyroid cancers with conditional BRAF activation. J. Clin. Invest. 2011;121:4700–4711. doi: 10.1172/JCI46382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol. Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulonval K, Vandeput F, Stein RC, Kozma SC, Lamy F, Dumont JE. Phosphatidylinositol 3-kinase, protein kinase B and ribosomal S6 kinases in the stimulation of thyroid epithelial cell proliferation by cAMP and growth factors in the presence of insulin. Biochem. J. 2000;348:351–358. [PMC free article] [PubMed] [Google Scholar]
- Deleu S, Pirson I, Coulonval K, Drouin A, Taton M, Clermont F, Roger PP, Nakamura T, Dumont JE, Maenhaut C. IGF-1 or insulin, and the TSH cyclic AMP cascade separately control dog and human thyroid cell growth and DNA synthesis, and complement each other in inducing mitogenesis. Mol. Cell. Endocrinol. 1999;149:41–51. doi: 10.1016/s0303-7207(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, Maestro R, Pelicci PG, d'Adda di Fagagna F. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- Evangelou K, Bartkova J, Kotsinas A, Pateras IS, Liontos M, Velimezi G, Kosar M, Liloglou T, Trougakos IP, Dyrskjot L, Andersen CL, Papaioannou M, Drosos Y, Papafotiou G, Hodny Z, Sosa-Pineda B, Wu XR, Klinakis A, Ørntoft T, Lukas J, Bartek J, Gorgoulis VG. The DNA damage checkpoint precedes activation of ARF in response to escalating oncogenic stress during tumorigenesis. Cell Death Differ. 2013;20:1485–1497. doi: 10.1038/cdd.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelou K, Gorgoulis VG. Sudan Black B, the specific histochemical stain for lipofuscin: a novel method to detect senescent cells. Methods Mol. Biol. 2017;1534:111–119. doi: 10.1007/978-1-4939-6670-7_10. [DOI] [PubMed] [Google Scholar]
- Ferrara AM, Liao XH, Gil-Ibáñez P, Marcinkowski T, Bernal J, Weiss RE, Dumitrescu AM, Refetoff S. Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology. 2013;154:2533–2541. doi: 10.1210/en.2012-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AT, Malaguarnera R, Refetoff S, Liao XH, Lundsmith E, Kimura S, Pritchard C, Marais R, Davies TF, Weinstein LS, Chen M, Rosen N, Ghossein R, Knauf JA, Fagin JA. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc. Natl. Acad. Sci. U. S. A. 2011;108:1615–1620. doi: 10.1073/pnas.1015557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rostán G, Costa AM, Pereira-Castro I, Salvatore G, Hernandez R, Hermsem MJ, Herrero A, Fusco A, Cameselle-Teijeiro J, Santoro M. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005;65:10199–10207. doi: 10.1158/0008-5472.CAN-04-4259. [DOI] [PubMed] [Google Scholar]
- Georgakopoulou EA, Tsimaratou K, Evangelou K, Fernandez Marcos PJ, Zoumpourlis V, Trougakos IP, Kletsas D, Bartek J, Serrano M, Gorgoulis VG. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging (Albany NY) 2013;5:37–50. doi: 10.18632/aging.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Klatt P, Delgado S, Conde E, Lopez-Rios F, Sanchez-Cespedes M, Mendez J, Antequera F, Serrano M. Oncogenic activity of Cdc6 through repression of the INK4/ARF locus. Nature. 2006;440:702–706. doi: 10.1038/nature04585. [DOI] [PubMed] [Google Scholar]
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr, Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhiang SM, Sagartz JE, Tong Q, Parker-Thornburg J, Capen CC, Cho JY, Xing S, Ledent C. Targeted expression of the ret/PTC1 oncogene induces papillary thyroid carcinomas. Endocrinology. 1996;137:375–378. doi: 10.1210/endo.137.1.8536638. [DOI] [PubMed] [Google Scholar]
- Kennedy AL, Morton JP, Manoharan I, Nelson DM, Jamieson NB, Pawlikowski JS, McBryan T, Doyle B, McKay C, Oien KA, Enders GH, Zhang R, Sansom OJ, Adams PD. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol. Cell. 2011;42:36–49. doi: 10.1016/j.molcel.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AK, Lo CY, Leung P, Lang BH, Chan WF, Luk JM. Clinicopathological roles of alterations of tumor suppressor gene p16 in papillary thyroid carcinoma. Ann. Surg. Oncol. 2007;14:1772–1779. doi: 10.1245/s10434-006-9280-9. [DOI] [PubMed] [Google Scholar]
- Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Invest. 2016;126:1052–1066. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPak KM, Burd CE. The molecular balancing act of p16(INK4a) in cancer and aging. Mol. Cancer Res. 2014;12:167–183. doi: 10.1158/1541-7786.MCR-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Dumont J, Vassart G, Parmentier M. Thyroid adenocarcinomas secondary to tissue-specific expression of simian virus-40 large T-antigen in transgenic mice. Endocrinology. 1991;129:1391–1401. doi: 10.1210/endo-129-3-1391. [DOI] [PubMed] [Google Scholar]
- Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Yeager N, Baker K, Liao XH, Refetoff S, Di Cristofano A. Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res. 2009;69:3689–3694. doi: 10.1158/0008-5472.CAN-09-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardella C, Clohessy JG, Alimonti A, Pandolfi PP. Pro-senescence therapy for cancer treatment. Nat. Rev. Cancer. 2011;11:503–511. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability–an evolving hallmark of cancer. Nat. Rev. Mol. Cell. Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, Fagin JA, Falciglia M, Weber K, Nikiforova MN. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J. Clin. Endocrinol. Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- Petrakis TG, Vougas K, Gorgoulis VG. Cdc6: a multi-functional molecular switch with critical role in carcinogenesis. Transcription. 2012;3:124–129. doi: 10.4161/trns.20301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CA, Agoff SN, Widman TJ, Bronner MP. Altered expression of cyclins and cell cycle inhibitors in papillary thyroid cancer: prognostic implications. Thyroid. 2005;15:461–473. doi: 10.1089/thy.2005.15.461. [DOI] [PubMed] [Google Scholar]
- Pomerantz J, Schreiber-Agus N, Liégeois NJ, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee HW, Cordon-Cardo C, DePinho RA. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- Powell DJ, Jr, Russell J, Nibu K, Li G, Rhee E, Liao M, Goldstein M, Keane WM, Santoro M, Fusco A, Rothstein JL. The RET/PTC3 oncogene: metastatic solid-type papillary carcinomas in murine thyroids. Cancer Res. 1998;58:5523–5528. [PubMed] [Google Scholar]
- Ricarte-Filho JC, Li S, Garcia-Rendueles ME, Montero-Conde C, Voza F, Knauf JA, Heguy A, Viale A, Bogdanova T, Thomas GA, Mason CE, Fagin JA. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J. Clin. Invest. 2013;123:4935–4944. doi: 10.1172/JCI69766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, Janakiraman M, Solit D, Knauf JA, Tuttle RM, Ghossein RA, Fagin JA. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger PP, Servais P, Dumont JE. Induction of DNA synthesis in dog thyrocytes in primary culture: synergistic effects of thyrotropin and cyclic AMP with epidermal growth factor and insulin. J. Cell Physiol. 1987;130:58–67. doi: 10.1002/jcp.1041300110. [DOI] [PubMed] [Google Scholar]
- Roger PP, van Staveren WC, Coulonval K, Dumont JE, Maenhaut C. Signal transduction in the human thyrocyte and its perversion in thyroid tumors. Mol. Cell Endocrinol. 2010;321:3–19. doi: 10.1016/j.mce.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Saji M, Narahara K, McCarty SK, Vasko VV, La Perle KM, Porter K, Jarjoura D, Lu C, Cheng SY, Ringel MD. Akt1 deficiency delays tumor progression, vascular invasion, and distant metastasis in a murine model of thyroid cancer. Oncogene. 2011;30:4307–4315. doi: 10.1038/onc.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M, Carlomagno F. Central role of RET in thyroid cancer. Cold Spring Harb. Perspect. Biol. 2013;5(12):a009233. doi: 10.1101/cshperspect.a009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M, Chiappetta G, Cerrato A, Salvatore D, Zhang L, Manzo G, Picone A, Portella G, Santelli G, Vecchio G, Fusco A. Development of thyroid papillary carcinomas secondary to tissue-specific expression of the RET/PTC1 oncogene in transgenic mice. Oncogene. 1996;12:1821–1826. [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Divorcing ARF and p53: an unsettled case. Nat. Rev. Cancer. 2006;(6):663–673. doi: 10.1038/nrc1954. [DOI] [PubMed] [Google Scholar]
- Velimezi G, Liontos M, Vougas K, Roumeliotis T, Bartkova J, Sideridou M, Dereli-Oz A, Kocylowski M, Pateras IS, Evangelou K, Kotsinas A, Orsolic I, Bursac S, Cokaric-Brdovcak M, Zoumpourlis V, Kletsas D, Papafotiou G, Klinakis A, Volarevic S, Gu W, Bartek J, Halazonetis TD, Gorgoulis VG. Functional interplay between the DNA-damage-response kinase ATM and ARF tumour suppressor protein in human cancer. Nat. Cell. Biol. 2013;15:967–977. doi: 10.1038/ncb2795. [DOI] [PubMed] [Google Scholar]
- Vizioli MG, Santos J, Pilotti S, Mazzoni M, Anania MC, Miranda C, Pagliardini S, Pierotti MA, Gil J, Greco A. Oncogenic RAS-induced senescence in human primary thyrocytes: molecular effectors and inflammatory secretome involved. Oncotarget. 2014;5:8270–8283. doi: 10.18632/oncotarget.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizioli MG, Possik PA, Tarantino E, Meissl K, Borrello MG, Miranda C, Anania MC, Pagliardini S, Seregni E, Pierotti MA, Pilotti S, Peeper DS, Greco A. Evidence of oncogene-induced senescence in thyroid carcinogenesis. Endocr. Relat. Cancer. 2011;18:743–757. doi: 10.1530/ERC-11-0240. [DOI] [PubMed] [Google Scholar]
- Vredeveld LC, Possik PA, Smit MA, Meissl K, Michaloglou C, Horlings HM, Ajouaou A, Kortman PC, Dankort D, McMahon M, Mooi WJ, Peeper DS. Abrogation of BRAFV600E–induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes Dev. 2012;26:1055–1069. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager N, Klein-Szanto A, Kimura S, Di Cristofano A. Pten loss in the mouse thyroid causes goiter and follicular adenomas: insights into thyroid function and Cowden disease pathogenesis. Cancer Res. 2007;67:959–966. doi: 10.1158/0008-5472.CAN-06-3524. [DOI] [PubMed] [Google Scholar]
- Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaballos MA, Santisteban P. FOXO1 controls thyroid cell proliferation in response to TSH and IGF-I and is involved in thyroid tumorigenesis. Mol. Endocrinol. 2013;27:50–62. doi: 10.1210/me.2012-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng N, Yang KT, Bayan JA, He L, Aggarwal R, Stiles JW, Hou X, Medina V, Abad D, Palian BM, Al-Abdullah I, Kandeel F, Johnson DL, Stiles BL. PTEN controls β-cell regeneration in aged mice by regulating cell cycle inhibitor p16ink4a. Aging Cell. 2013;12:1000–1011. doi: 10.1111/acel.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou M, Baitei EY, Al-Rijjal RA, Parhar RS, Al-Mohanna FA, Kimura S, Pritchard C, Binessa HA, Alzahrani AS, Al-Khalaf HH, Hawwari A, Akhtar M, Assiri AM, Meyer BF, Shi I. TSH overcomes Braf (V600E)-induced senescence to promote tumor progression via downregulation of p53 expression in papillary thyroid caner. Oncogene. 2016;35:1909–1918. doi: 10.1038/onc.2015.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.