Abstract
Rationale, aims and objectives
Prolongation of the corrected QT (QTc) interval is associated with increased morbidity and mortality. The association between QTc interval-prolonging medications (QTPMs) and risk factors with magnitude of QTc interval lengthening is unknown. We examined the contribution of risk factors alone and in combination with QTPMs to QTc interval lengthening.
Method
The ARIC Study assessed 15,792 participants with a resting, standard 12-lead electrocardiogram and ≥ 1 measure of QTc interval over four examinations at three-year intervals (1987–1998). From 54,638 person-visits, we excluded participants with QRS ≥ 120 ms (n=2,333 person-visits). We corrected the QT interval using the Bazett and Framingham formulas. We examined QTc lengthening using linear regression for 36,602 person-visit observations for 14,160 controlling for age ≥ 65 years, female sex, left ventricular hypertrophy, QTc > 500 ms at the prior visit and CredibleMeds® categorized QTPMs (Known, Possible, or Conditional risk). We corrected standard errors for repeat observations per person.
Results
Eighty percent of person-visits had at least one risk factor for QTc lengthening. Use of QTPMs increased over the four visits from 8% to 17%. Among persons not using QTPMs, history of prolonged QTc interval and female sex were associated with the greatest QTc lengthening, 39 ms and 12 ms, respectively. In the absence of risk factors, Known QTPMs and ≥ 2 QTPMs were associated with modest but greater QTc lengthening than Possible or Conditional QTPMs. In the presence of risk factors, ≥ 2 QTPM further increases QTc lengthening. In combination with risk factors, the association of all QTPM categories with QTc lengthening was greater than QTPMs alone.
Conclusion
Risk factors, particularly female sex and history of prolonged QTc interval, have stronger associations with QTc interval lengthening than any QTPM category alone. All QTPM categories augmented QTc interval lengthening associated with risk factors.
Keywords: QTc interval, QTc prolonging medications, torsade de pointes, risk factors
Introduction
Prolongation of the heart rate corrected QT (QTc) interval on electrocardiogram (ECG) has been linked to adverse outcomes, including torsade de pointes (TdP) and sudden cardiac death [1–6]. Several independent risk factors for QTc interval prolongation have been identified, including numerous medications [7,8]. The association between the magnitude of QTc interval lengthening and QTc interval prolonging medications (QTPMs) and other risk factors, individually and in combination, is currently unknown. Up to 25% of insured outpatients receive at least one potential QTPM and up to 10% may also receive an interacting medications [9–11]. In addition to the increasing number of available medications with the potential to prolong the QTc interval, many medications have recently suffered revised drug labeling or have been withdrawn from the market due to evidence linking these medications with TdP [1,12–20]. Furthermore, healthcare providers frequently prescribe QTPMs in patients who already have multiple risk factors for QTc lengthening [10,21]. This may be further complicated when both cardiovascular and non-cardiovascular QTPMs (e.g., antidepressants, antiinfectives) are prescribed [22–25]. Based upon existing data, a prescriber is unable to estimate the potential magnitude of QTc interval lengthening provoked by a QTPM in association with existing risk factors for QTc lengthening. The objective of this analysis of a longitudinal community-based cohort was to determine the magnitude of QTc interval prolongation associated with (i) categories of QTPMs with varying risk of TdP, (ii) risk factors known to increase the risk of QTc interval prolongation, and (iii) the joint effect of QTPM use with risk factors for QTc interval prolongation.
Methods
The Atherosclerosis Risk in Communities (ARIC) study was designed to investigate the causes of atherosclerosis and its clinical outcomes, and variation in cardiovascular risk factors, medical care, and disease by race, gender, location and date. The ARIC study included a longitudinal cohort of men and women (n=15,792) selected through probability sampling from four communities in the United States: northwest suburbs of Minneapolis, MN; Washington County, MD; Jackson, MS; and Forsyth County, NC [26]. The first examination visit took place between 1987 and 1989; three subsequent examination visits were performed at approximately three-year intervals. At each visit, participants underwent standardized methods to collect data on height, weight, blood pressure, various chronic conditions and related risk factors for heart diseases, a standard resting 12-lead ECG and medication survey [26]. The medication survey is a part of the core data collection and was conducted by trained interviewers; the survey was introduced during the first examination and continued to be administered to all participants through Visit 4. The medication survey and ECG were obtain at this same visit. The QT interval from the digital 12-lead ECG was determined by the NOVACODE program [27]. The NOVACODE program generates an average waveform derived from all 12 simultaneously measured leads. This allows the system to determine the QT from the earliest QRS onset to the latest offset of the T-wave. All ECG waveforms were verified by visual inspection as part of standard ARIC protocol and a globally averaged QT interval was generated from the earliest QRS onset and T-wave offset using all leads [28]. The ARIC study has been approved by the Institutional Review Boards of all participating institutions. Participants provided written informed consent at each ARIC study visit.
The ARIC Study assessed all participants with a resting, standard ECG and one or more measures of QTc interval over four examinations at three-year intervals (15,792 participants, 54,638 person-visits, mean follow-up 7.5 years). We excluded participants with QRS ≥ 120 ms and participants missing medication data and no or poor quality ECG (n=2,333 person-visits). Additionally, Visit 1 data were dropped for all cohort members since the estimation model used the QTc value from the prior visit. These exclusion criteria resulted in a final sample of 36,602 person-visits for Visits 2–4 for 14,160 cohort members.
Racial composition is highly linked to geographic site in the ARIC cohort; Forsyth County is the only site with a mix of Caucasians and African-Americans, only African-Americans were enrolled in Jackson, and very few participants of other races besides Caucasian or African-American at any site. For each visit, we identified the following:
Use of one or more QTPM as categorized by CredibleMeds®, formally known as The Arizona Center for Education and Research on Therapeutics (AzCERT), which categorizes QTPMs based upon their proven lengthening of the QTc interval and their association with TdP as Known, Possible or Conditional [9]. The definitions of Known, Possible and Conditional categories and the complete list of medications used can be found in the Supplementary Index.
Participants were classified as Known if they received one or more Known and any number of Possible and/or Conditional QTc prolonging medications. Participants were classified as Possible if they received one or more Possible and any number of Conditional but no Known QTc prolonging medications. Participants were classified as Conditional if they received one or more Conditional but no Known or Possible QTc prolonging medications. Participants were classified as receiving ≥ 2 medications if they received two or more Known, Possible and/or Conditional QTc prolonging medications at a given visit.
Selected risk factors known to prolong QTc: age ≥ 65 years, female sex, left ventricular hypertrophy (LVH) and prolonged QTc (QTc > 500 ms) observed previously (i.e., at the prior visit for this analysis) [29,30]. QTc was not observed prior to Visit 1, so all Visit 1 observations were dropped from the analysis.
Corrected QT interval using Bazett (QTcBaz) and Framingham (QTcFram) formulas [31,32]. We used the Framingham formula for primary analysis given prior use in population-based studies and recommendation by American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Consensus Statement [33].
Comorbidities defined and measured by ARIC conventions. Hypertension was based on systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, self-report of hypertension medications, self-report of hypertension diagnosis or diagnosis of hypertension documented by a physician. Diabetes was defined as fasting blood glucose ≥ 126 mg/dL, non-fasting blood glucose ≥ 200 mg/dL, self-report of diabetes medications, self-report of diabetes diagnosis or diagnosis of diabetes by a physician. Coronary heart disease was defined as history of myocardial infarction, angioplasty or bypass surgery. Prevalent coronary heart disease at each visit was ascertained from self-report at the time of the examination at visit 1 and study-adjudicated hospitalized events occurring prior to each visit thereafter. LVH was defined as the ECG Cornell voltage score of more than 28 mm in men or more than 22 mm in women during ARIC visits [34].
Continuous variables are reported as mean ± standard deviation and discrete variables are reported as number (percentage). Ordinary least squares (OLS) regression was utilized for 36,602 person-visit observations (14,160 cohort members) from Visits 2 through 4 to estimate the association of the selected risk factors and QTPMs with QTc lengthening. The three dependent variables were QT interval, QTcBaz and QTcFram. The key explanatory variables were use of QTPMs with risk of TdP (Known, Conditional or Possible), individually and in combination with an indicator variable for having one or more risk factors (age ≥ 65 years, female gender, LVH, prior QTc > 500 ms). The assessment of the change due to the combination of risk factors and use of QTPM was calculated as the combined effects of the medication categorization, the risk factor and the interactive effect between the medication categorization and having one or more risk factors. All models used visit indicators to control for changes over time including changes in prescribing behaviors (or entry or exit of drugs to the market) separate from aging. Although use of QTPM was chosen by the participant or their medical provider, we did not adjust for treatment selection (confounding by indication) [35], so all estimations reflect statistical associations rather than causal effects. Cluster-robust standard errors were used to account for multiple observations per cohort member.
Results
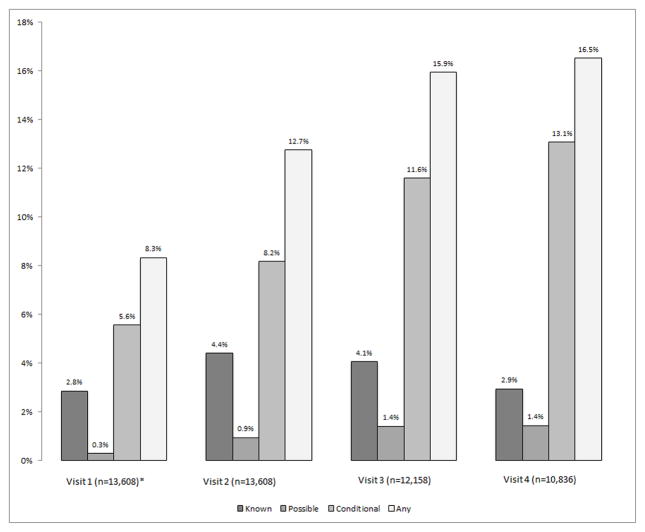
The use of QTPMs increased from 8% at Visit 1 to 17% at Visit 4 (Figure 1); this increase could be due to changes in prescribing patterns as well as aging. The use of QTPMs with varying risk of TdP (Known, Conditional and Possible) differed across visits. At Visits 1 through 4, prescribing of Conditional QTPMs was most common (6%, 8%, 12% and 13%, respectively) followed by Known and then Possible. Known was the most frequent QTPM among participants using two or more QTPMs and Possible QTPM was the most frequent QTPM among participants using two Possible or Conditional QTPMs but no Known medications.
Figure 1.
Use of QTc Interval Prolonging Medications over Time in the ARIC Study Cohort (1987–1998)
QTPM = QTc interval prolonging medication
Notes: The horizontal axis shows visit and sample size: visit 1 (1987–89), visit 2 (1990–92), visit 3 (1993–35) and visit 4 (1996–98). The vertical axis shows the percentage of QTc interval prolonging medication (QTPM) users by type of QTPM.
*The Visit 1 sample is limited to persons who also participated in Visit 2 (the first visit used in the regression)
Table 1 shows that at the first visit included in the regression analysis (Visit 2), study participants were 57 ± 6 years, 56% female and 75% white. The mean QTcFram was 422 ± 20 ms, and the QTcBaz was 428 ± 24 ms. The most common chronic diseases were hypertension (36%) and diabetes (15%); only 7% of study participants had coronary artery disease. Among person-visit observations from Visits 2 to 4, over 80% had at least one risk factor for QTc interval prolongation; 56% of the visits were among females, 25% were for those aged ≥ 65 years, 8% were among participants with LVH and 10% were among those with QTc interval > 450 ms in the prior visit (Table 2, Column 2).
Table 1.
Characteristics of the Study Sample at ARIC Visit 2
| Characteristic | n= 13,608 |
|---|---|
|
| |
| Age (years) | 57 ± 6 |
|
| |
| Sex: female | 7,597 (56%) |
|
| |
| Body mass index (kg/m2) | 28 ± 5 |
|
| |
| Race | |
| - White | 10,245 (75%) |
| - Black | 3,323 (24%) |
| - Other | 40 (<1%) |
|
| |
| Site | |
| - Minneapolis, MN | 3,662 (26.9%) |
| - Forsyth County, NC | 3,503 (25.7%) |
| - Jackson County, MS | 2,912 (21.4%) |
| - Washington Country, MD | 3,531 (25.9%) |
|
| |
| Comorbidities | |
| - Hypertension | 4,823 (36%) |
| - Diabetes mellitus | 2,017 (15%) |
| - Coronary heart disease | 925 (7%) |
| - Left ventricular hypertrophy (LVH) | 994 (7%) |
|
| |
| QT interval | |
| - QT uncorrected (ms) | 411 ± 28 |
| - QTcFram (ms) | 422 ± 20 |
| - QTcBaz (ms) | 428 ± 24 |
| - QRS (ms) | 91 ± 10 |
Note: Continuous variables are reported as mean (SD), and discrete variables are reported as number (percentage). QTcFram=QT interval corrected by using the Framingham formula; QTcBaz=QT interval corrected by using the Bazett formula.
Table 2.
Regression-Adjusted Contribution of Risk Factors and QTPMs to QTc Interval Lengthening in the ARIC Study Cohort
| Characteristics | n (%) | QTcBaz (ms) [95% CI] | QTcBaz Difference [95% CI] | QTcFram (ms) [95% CI] | QTcFram Difference [95% CI] |
|---|---|---|---|---|---|
| No risk factors or QTPMs‡ | 9,402 (26%) | 416 [416,417] | Reference | 413 [413,414] | Reference |
| Female (no QTPM) | 20,569 (56%) | 428 [428,429] | 12 [11,13]* | 423 [423,424] | 10 [10,11]* |
| LVH (no QTPM) | 2,847 (8%) | 421 [420,422] | 5 [4,6]* | 418 [417,419] | 5 [4,6]* |
| Age ≥ 65 years (no QTPM) | 9,051 (25%) | 419 [418,420] | 3 [2,4]* | 417 [416,417] | 3 [3,4]* |
| Prior QTc > 450–500 ms (no QTPM) | 3,669 (10%) | 444 [443,445] | 28 [27,29]* | 443 [441,444] | 30 [28,31]* |
| Prior QTc > 500 ms (no QTPM) | 107 (0.3%) | 456 [449,462] | 39 [33,46]* | 446 [436,457] | 33 [23,44]* |
| Known QTPM† (no risk factors) | 1,408 (4%) | 422 [419,424] | 5 [2,8]* | 417 [415,419] | 4 [2,6]* |
| Possible QTPM† (no risk factors) | 422 (1.2%) | 416 [411,421] | 0 [−5,5] | 414 [409,418] | 0 [−4,5] |
| Conditional QTPM† (no risk factors) | 3,629 (9.9%) | 418 [416,420] | 2 [0,4] | 413 [411,415] | 0 [−2,2] |
| ≥ 2 QTPM† (no risk factors) | 577 (1.6%) | 420 [418,422] | 4 [2,6]* | 414 [412,416] | 1 [−1,3] |
| ≥ 2 QTPM† (Female) | 461 (1.3%) | 432 [430,434] | 16 [14,18]* | 424 [422,426] | 11 [9,13]* |
| ≥ 2 QTPM† (LVH) | 56 (0.2%) | 425 [423,427] | 9 [7,11]* | 419 [417,421] | 6 [4,8]* |
| ≥ 2 QTPM† (Age ≥ 65 years) | 170 (0.5%) | 423 [421,425] | 7 [5,9]* | 418 [416,420] | 5 [3,7]* |
| ≥ 2 QTPM† (Prior QTc > 450–500 ms) | 102 (0.3%) | 448 [446,450] | 32 [30,34]* | 444 [441,446] | 31 [28,33]* |
| ≥ 2 QTPM† (Prior QTc > 500 ms) | 8 (0.02%) | 460 [453,466] | 43 [37,50]* | 447 [437,458] | 34 [24,45]* |
Full regression sample: n=36,602 person-visits occurring between Visit 2 through Visit 4 (1990–1998)
Difference is significant at p<0.05
- Known QTPM: participants received one or more known QTc prolonging medications.
- Possible QTPM: participants received one or more possible but no known QTc prolonging medications.
- Conditional QTPM: participants received one or more conditional but no known or possible QTc prolonging medications.
- ≥ 2 QTPM: Participants received two or more known, possible and/or conditional QTc prolonging medications
Patients with prior QTc < 450 ms and no QTPM are included in this group
The regression included three additional variables: interactions between the three medication categorizations and having one or more risk factor. (See Table 3)
LVH= Left ventricular hypertrophy; QTPM= QTc interval prolonging medication; QTcFram= QT interval corrected by using the Framingham formula; QTcBaz= QT interval corrected by using the Bazett formula.
Table 2 also provides the predicted QTc intervals and the estimated difference in QTc associated with the individual risk factors or medication categories, holding all else constant. Among participants using no QTPMs, the risk factor associated with the greatest QTc interval lengthening was prior visit QTc > 500 ms (QTcBaz increased by 39 ms [95% confidence interval (CI): 33, 46]), followed by female sex, LVH and age ≥ 65 years. As measured at each visit, in the absence of risk factors Known QTPMs versus no QTPMs were associated with the highest magnitude of QTc lengthening (QTcBaz increased by 5 ms [95% CI: 2, 8]), followed by ≥2 QTPM (QTcBaz increased by 4 ms, [95% CI: 2, 6]). Possible and conditional QTPM use without any risk factors were not associated with a statistically significant increase in QTcBaz. In the presence of risk factors, ≥ 2 QTPM further increases QTc lengthening. Table 2 shows that the estimated effects for QTcFram were generally less than for QTcBaz and only statistically significant for Known QTPMs.
Table 3 and Figure 2 show the combined effects and 95% confidence intervals from having risk factors in each of the QTPM medication categories, including an interactive effect between having one or more risk factor and the medication category. For each QTPM category, every risk factor was associated with increased QTc interval lengthening beyond that which was observed with QTPMs and no risk factors. Substantial portions of the effect on QTc interval lengthening come from the individual risk factors (effects shown in Table 2) rather than the medication effect or interaction effects. The highest magnitudes of QTc interval lengthening were observed in participants receiving QTPMs in addition to having prior QTc > 500 ms (increases in QTc from 40 to 46, depending on QTc medication category and correction formula).
Table 3.
Regression-Adjusted Contribution of QTPM Categories in Combination with Risk Factors to QTc Interval Lengthening in the ARIC Study Cohort
| Medications | Risk Factors | QTcBaz (ms) [95% CI] | QTcBaz Difference‡ [95% CI] | QTcFram (ms) [95% CI] | QTcFram Difference‡ [95% CI] |
|---|---|---|---|---|---|
| Known QTPM† (n= 1,408) | No Risk Factor | 422 [419, 424] | Reference | 417 [415, 419] | Reference |
| Female | 435 [433, 436] | 13 [10, 16]* | 429 [427, 430] | 12 [9, 14]* | |
| LVH | 427 [425, 429] | 6 [2, 9]* | 423 [422, 425] | 6 [3, 9]* | |
| Age ≥ 65years | 425 [424, 427] | 4 [1, 7]* | 422 [420, 423] | 5 [2, 8]* | |
| Prior QTc > 500 ms | 462 [456, 468] | 40 [33, 48]* | 452 [441, 462] | 35 [24, 45]* | |
| Possible QTPM† (n= 422) | No Risk Factor | 416 [411, 421] | Reference | 414 [409, 418] | Reference |
| Female | 435 [432, 438] | 19 [13, 24]* | 429 [426, 431] | 15 [10, 20]* | |
| LVH | 428 [425, 431] | 11 [6, 17]* | 423 [421, 426] | 10 [5, 15]* | |
| Age ≥ 65 years | 426 [423, 428] | 9 [4, 15]* | 422 [419, 425] | 8 [3, 14]* | |
| Prior QTc > 500 ms | 462 [456, 469] | 46 [38, 54]* | 452 [441, 463] | 38 [27, 50]* | |
| Conditional QTPM† (n= 3,630) | No Risk Factor | 418 [416, 420] | Reference | 413 [411, 415] | Reference |
| Female | 433 [432, 434] | 15 [13, 17]* | 426 [425, 427] | 13 [11, 15]* | |
| LVH | 426 [424, 427] | 8 [5, 10]* | 420 [419, 422] | 7 [5, 9]* | |
| Age ≥ 65 years | 424 [423, 425] | 6 [3, 8]* | 419 [418, 420] | 6 [4, 8]* | |
| Prior QTc > 500 ms | 460 [454, 467] | 42 [36, 49]* | 449 [438, 459] | 36 [25, 46]* |
Coefficient estimates come from the same regression as in Table 2: n=36,602 person-visits occurring between Visit 2 through Visit 4 (1990–1998)
Difference is significant at p<0.05
- Known QTPM: participants received one or more known QTc prolonging medications.
- Possible QTPM: participants received one or more possible but no known QTc prolonging medications.
- Conditional QTPM: participants received one or more conditional but no known or possible QTc prolonging medications.
Differences were calculated as the coefficient for the risk factor plus the coefficient for the interaction between the QTPM category and having one or more risk factors. For example, the change for females taking a known QTPM was calculated by adding the coefficient for female and the coefficient for the interaction of known QTPM with having one or more risk factor. In this example, the reference group is individuals with no risk factors that are taking a known QTPM.
LVH= Left ventricular hypertrophy; QTPM= QTc interval prolonging medication; QTcFram= QT interval corrected by using the Framingham formula; QTcBaz= QT interval corrected by using the Bazett formula.
Figure 2.
Association of the Use of QTc Prolonging Medications with Change in QTc Interval (ms)
LVH = Left ventricular hypertrophy, QTc = corrected QT, QTPM = QTc interval prolonging medication
* Difference from zero is significant at p<0.05
†The Visit 1 sample is limited to persons who also participated in Visit 2 (the first visit used in the regression)
The error bars show the 95% confidence interval.
The horizontal axis shows the three different QTPM categories and no QTPM. The vertical axis shows the change in the QTc interval from the prior visit in milliseconds. The changes were calculated by combining coefficients from the QTPM category, the risk factor, and an interaction of the QTPM category with having one or more risk factor. For example, the change for females taking a known QTPM was calculated by adding the coefficient for known QTPMs, the coefficient for female, and the coefficient for the interaction of known QTPM with having one or more risk factors. The reference group for all changes is individuals with no risk factors that are not taking any QTPMs.
Discussion
Our study is the first to determine the relative associations of selected risk factors for QTc interval lengthening with different categorizations of QTPMs on the QTc interval in a community-based cohort. Approximately 80% of participants had at least one risk factor for QTc interval lengthening with prior QTc > 500 ms being associated with the most substantial lengthening. In addition, our results suggest that approximately 8 to 17% of community-dwelling adults use at least one QTPM and use increases with age. Our findings complement prior literature showing that nearly 5 to 10% of community-dwelling adults receive at least one QTPM [10,11].
Conditional QTPMs were the most commonly utilized QTPMs in this study sample, followed by Known and Possible QTPMs. In participants with no risk factors, use of Known QTPMs or ≥ 2 QTPMs was associated with the highest magnitude of QTc lengthening. In combination with risk factors, the association of all QTPM categories with QTc lengthening was greater than QTPMs alone.
Although risk factors had a greater association with QTc interval lengthening than QTPMs alone, the magnitude of QTc lengthening increases when risk factors are combined with QTPMs. History of prolonged QTc interval was associated with the greatest degree of QTc interval lengthening. These findings should assist prescribers of QTPMs and more narrowly focus screening and monitoring to selected patients at high risk.
Our study has several limitations. First, we identified QTPM use from 1987 (ARIC Visit 1) through 1998 (ARIC Visit 4), during which time several QTPMs were withdrawn from the U.S. market or became less widely used as first line agents to treat specific conditions. However, nearly all medications that have been observed in extant studies to be associated with QTc interval lengthening inhibit the rapid component of the delayed rectifier potassium channel, which prolongs the myocardial action potential that is manifested as lengthening of the QTc interval on the ECG [36]. Therefore, our findings likely can be applied to QTPMs more recently approved by the FDA. Another limitation is that only select risk factors were available in the ARIC database at all four visits. Some other important risk factors known to prolong QTc interval including electrolyte disturbance were collected at Visits 1 and 2 only. A cross-sectional analysis based on Visit 2 data showed that hypokalemia was associated with 9 ms increase in QTc interval (95% CI: 7, 13) and hypomagnesaemia was associated with a 4 ms increase in QTc interval (95% CI: 2, 5). Those data were not available at subsequent visits and therefore could not be included in the analyses. Our analysis did not assess the effect of pharmacokinetic drug interactions that may have contributed to the observed QTc interval prolongation; such interactions may increase the exposure to QTPMs through pharmacokinetic mechanisms. The lack of a statistically significant difference between the Possible and Conditional QTPMs in patients without risk factors may have been due to the limited number of participants using Possible QTPMs. Due to the nature of the study, we were not able to confirm the duration or dose of any QTPMs. The possible effect of inherited QTc syndromes on QTc lengthening could not be eliminated since this analysis did not incorporate any genetic data. Finally, the associations identified in this analysis should not be interpreted as causal effects, as the estimates may be affected by confounding by indication (i.e., bias that may occur when a drug treatment serves as a marker for something that triggers the use of the treatment and that, at the same time, increases the risk of the outcome under study) [35].
Conclusion
Risk factors, particularly female sex and history of prolonged QTc interval, have stronger associations with QTc interval lengthening than QTPMs alone. In the absence of risk factors, QTPMs categorized as Known risk of TdP were associated with the greatest risk of QTc lengthening, as was using ≥ 2 QTPMs. All QTPM categories augmented QTc interval lengthening associated with risk factors.
Supplementary Material
Acknowledgments
Funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions. There are no relationships with industry.
Footnotes
Conflict of interest: Dr. Tisdale is a volunteer member of the Advisory Board for the QT drugs list on CredibleMeds.org. All other authors have no conflict of interest to disclose.
References
- 1.Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047–1060. doi: 10.1161/CIRCULATIONAHA.109.192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83(6):1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 3.Kozik TM, Wung S-F. Cardiac arrest from acquired long QT syndrome: a case report. Heart Lung. 2009;38(3):238–242. doi: 10.1016/j.hrtlng.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Post WS, Blasco-Colmenares E, Dalal D, Tomaselli GF, Guallar E. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology. 2011;22(5):660–670. doi: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekker JM, Crow RS, Hannan PJ, Schouten EG, Folsom AR. Heart rate-corrected QT interval prolongation predicts risk of coronary heart disease in black and white middle-aged men and women: the ARIC study. J Am Coll Cardiol. 2004;43(4):565–571. doi: 10.1016/j.jacc.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 6.Dekker JM, Schouten EG, Klootwijk P, Pool J, Kromhout D. Association between QT interval and coronary heart disease in middle-aged and elderly men. The Zutphen Study. Circulation. 1994;90(2):779–785. doi: 10.1161/01.cir.90.2.779. [DOI] [PubMed] [Google Scholar]
- 7.Benoit SR, Mendelsohn AB, Nourjah P, Staffa JA, Graham DJ. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur J Cardiovasc Prev Rehabil. 2005;12(4):363–368. doi: 10.1097/01.hjr.0000173110.21851.a9. [DOI] [PubMed] [Google Scholar]
- 8.Kannankeril PJ, Roden DM. Drug-induced long QT and torsade de pointes: recent advances. Curr Opin Cardiol. 2007;22(1):39–43. doi: 10.1097/HCO.0b013e32801129eb. [DOI] [PubMed] [Google Scholar]
- 9.QTDrugs Lists. [Accessed June 14, 2015];For Credible Meds. https://www.crediblemeds.org/new-drug-list/
- 10.Curtis LH, Østbye T, Sendersky V, et al. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. Am J Med. 2003;114(2):135–141. doi: 10.1016/s0002-9343(02)01455-9. [DOI] [PubMed] [Google Scholar]
- 11.Allen LaPointe NM, Curtis LH, Chan KA, et al. Frequency of high-risk use of QT-prolonging medications. Pharmacoepidemiol Drug Saf. 2006;15(6):361–368. doi: 10.1002/pds.1155. [DOI] [PubMed] [Google Scholar]
- 12.Mandyam MC, Soliman EZ, Alonso A, et al. The QT interval and risk of incident atrial fibrillation. Heart Rhythm. 2013;10(10):1562–1568. doi: 10.1016/j.hrthm.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Accessed October 2, 2015];FDA Drug Safety Communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide) http://www.fda.gov/Drugs/DrugSafety/ucm269086.htm.
- 14. [Accessed October 2, 2015];FDA Drug Safety Communication: Abnormal heart rhythms may be associated with use of Zofran (ondansetron) http://www.fda.gov/Drugs/DrugSafety/ucm271913.htm.
- 15.Wysowski DK, Corken A, Gallo-Torres H, Talarico L, Rodriguez EM. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol. 2001;96(6):1698–1703. doi: 10.1111/j.1572-0241.2001.03927.x. [DOI] [PubMed] [Google Scholar]
- 16.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350(10):1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 17.Hondeghem LM, Dujardin K, Hoffmann P, Dumotier B, De Clerck F. Drug-induced QTC prolongation dangerously underestimates proarrhythmic potential: lessons from terfenadine. J Cardiovasc Pharmacol. 2011;57(5):589–597. doi: 10.1097/FJC.0b013e3182135e91. [DOI] [PubMed] [Google Scholar]
- 18.Simons FE, Kesselman MS, Giddins NG, Pelech AN, Simons KJ. Astemizole-induced torsade de pointes. Lancet (London, England) 1988;2(8611):624. doi: 10.1016/s0140-6736(88)90656-3. [DOI] [PubMed] [Google Scholar]
- 19.Macinko J, Starfield B, Shi L. Quantifying the health benefits of primary care physician supply in the United States. Int J Health Serv. 2007;37(1):111–126. doi: 10.2190/3431-G6T7-37M8-P224. [DOI] [PubMed] [Google Scholar]
- 20.Kronman AC, Ash AS, Freund KM, Hanchate A, Emanuel EJ. Can primary care visits reduce hospital utilization among Medicare beneficiaries at the end of life? J Gen Intern Med. 2008;23(9):1330–1335. doi: 10.1007/s11606-008-0638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarganas G, Garbe E, Klimpel A, Hering RC, Bronder E, Haverkamp W. Epidemiology of symptomatic drug-induced long QT syndrome and Torsade de Pointes in Germany. Europace. 2014;16(1):101–108. doi: 10.1093/europace/eut214. [DOI] [PubMed] [Google Scholar]
- 22.Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351(11):1089–1096. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- 23.Straus SMJM, Bleumink GS, Dieleman JP, et al. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med. 2004;164(12):1293–1297. doi: 10.1001/archinte.164.12.1293. [DOI] [PubMed] [Google Scholar]
- 24.Straus SMJM, Sturkenboom MCJM, Bleumink GS, et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;26(19):2007–2012. doi: 10.1093/eurheartj/ehi312. [DOI] [PubMed] [Google Scholar]
- 25.Allen LaPointe NM, Chen A, Hammill B, DeLong E, Kramer JM, Califf RM. Evaluation of the dofetilide risk-management program. Am Heart J. 2003;146(5):894–901. doi: 10.1016/S0002-8703(03)00409-5. [DOI] [PubMed] [Google Scholar]
- 26.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 27.Rautaharju PM, Park LP, Chaitman BR, Rautaharju F, Zhang ZM. The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol. 1998;31(3):157–187. [PubMed] [Google Scholar]
- 28.Rautaharju PM, MacInnis PJ, Warren JW, Wolf HK, Rykers PM, Calhoun HP. Methodology of ECG interpretation in the Dalhousie program; NOVACODE ECG classification procedures for clinical trials and population health surveys. Methods Inf Med. 1990;29(4):362–374. [PubMed] [Google Scholar]
- 29.Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of Torsade de Pointes. Dtsch Ärzteblatt Int. 2011;108(41):687–693. doi: 10.3238/arztebl.2011.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet (London, England) 2000;355(9209):1048–1052. doi: 10.1016/s0140-6736(00)02035-3. [DOI] [PubMed] [Google Scholar]
- 31.Bazett HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–367. [Google Scholar]
- 32.Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study) Am J Cardiol. 1992;70(7):797–801. doi: 10.1016/0002-9149(92)90562-d. [DOI] [PubMed] [Google Scholar]
- 33.Rautaharju PM, Surawicz B, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias C. J Am Coll Cardiol. 2009;53(11):982–991. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 34.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 35.Psaty BM, Koepsell TD, Lin D, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47(6):749–754. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 36.Redfern WS, Carlsson L, Davis AS, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58(1):32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.