Abstract
Aims
Patients with heart failure (HF) with reduced (HFrEF) or preserved (HFpEF) ejection fraction demonstrate an increased ventilatory equivalent for carbon dioxide (V̇E/V̇CO2) slope. The physiologic correlates of V̇E/V̇CO2 slope remain unclear in the two HF phenotypes. We hypothesized that changes in physiologic dead space to tidal volume ratio (VD/VT) and arterial CO2 tension (PaCO2) differentially contribute to V̇E/V̇CO2 slope in HFrEF versus HFpEF.
Methods and Results
Adults with HFrEF (N=32) and HFpEF (N=27) (LVEF: 22±7 and 61±9%; BMI: 28±4 and 33±6 kg/m2, P<0.01) performed cardiopulmonary exercise testing with breath-by-breath ventilation and gas-exchange measurements. PaCO2 was measured via radial arterial catheterization. We calculated V̇E/V̇CO2 slope via linear regression, and VD/VT=1−[(863×V̇CO2)/(V̇E×PaCO2)]. Resting VD/VT (0.48±0.08 vs. 0.41±0.11, P=0.04), but not PaCO2 (38±5 vs. 40±3 mm Hg, P=0.21) differed in HFrEF versus HFpEF, respectively. Peak exercise VD/VT (0.39±0.08 vs. 0.32±0.12, P=0.02) and PaCO2 (33±6 vs. 38±4 mm Hg, P<0.01) differed in HFrEF versus HFpEF, respectively. V̇E/V̇CO2 slope was higher in HFrEF compared to HFpEF (44±11 vs 35±8, P<0.01). Variance associated with V̇E/V̇CO2 slope in HFrEF and HFpEF was explained by peak exercise VD/VT (R2=0.30 vs. 0.50, respectively) and PaCO2 (R2=0.64 vs. 0.28, respectively), but with different relative contributions from each (all P<0.01).
Conclusions
Relationships between V̇E/V̇CO2 slope with both VD/VT and PaCO2 are robust, but differ in HFpEF compared to HFrEF. Increasing V̇E/V̇CO2 slope appears strongly explained by mechanisms influential to regulating PaCO2 in HFrEF, which contrasts the strong role of increased VD/VT in HFpEF.
Keywords: Cardiopulmonary exercise testing, exercise ventilatory efficiency, exercise capacity, exercise tolerance, exercise intolerance, HFpEF
Introduction
A steep slope of the ventilatory equivalent for carbon dioxide (V̇E/V̇CO2) as quantified during cardiopulmonary exercise testing (CPET) demonstrates robust prognostic power in heart failure (HF) patients with reduced (HFrEF) or preserved (HFpEF) ejection fraction (1–4). Ventilatory inefficiency is suggested to accompany an excessive rate-mediated rise in V̇E/V̇CO2 slope, which is likely both a consequence and key contributor to exercise intolerance in HF (2–5). In this context, despite the broad clinical and research use of V̇E/V̇CO2 slope to phenotype the magnitude of exercise ventilatory inefficiency in HF, the understanding of this index has not been clearly defined in patients with HFrEF or HFpEF.
The V̇E/V̇CO2 slope is determined by dynamic coupling of gas exchange and hemodynamics reflecting pulmonary, cardiac, musculoskeletal, and nervous system responses to exercise (4, 6–8). ‘Ideal’ alveolar gas and air equations implicate the proportion of tidal volume distributed to physiologic dead space (VD/VT) accompanied by mechanisms underpinning regulation of arterial carbon dioxide tension (PaCO2) as key contributors to V̇E/V̇CO2 slope (4, 6, 7, 9). While increased V̇E/V̇CO2 slope is likely to be the consequence of integrated factors including wasted alveolar ventilation (V̇A), atelectasis, and/or impaired neural ventilatory control, there is controversy as to which event is the principal mediator in HFrEF (4, 7, 9, 10), with even less information available on this topic in HFpEF. Further complicating this discussion is the evolving knowledge of what unique characteristics unequivocally define the HFpEF phenotype (2, 3, 5, 11, 12).
Guazzi et al. (4) demonstrated that high V̇E/V̇CO2 slope from rest to peak exercise is predictive of survival in HFrEF. Similar to data from Woods et al. (7) during submaximal exercise, Guazzi et al. (4) attributed increased V̇E/V̇CO2 slope to influences of impaired mechanisms of ventilatory control (e.g., low PaCO2 regulatory set-points) accompanied by contributions from elevated VD/VT. By contrast, without observations from specific tests of ventilatory control, Sullivan et al. (9) and others (10) suggest that ventilatory control related to regulation of PaCO2 is intact in HFrEF, whereas abnormal physiologic hemodynamic factors are more likely present in this population.
As a necessary hypothesis-generating step towards advancing the understanding and interpretation of V̇E/V̇CO2 slope in HF, this study aimed to demonstrate how V̇E/V̇CO2 slope is related to changes in VD/VT and PaCO2 during CPET in patients with HFrEF or HFpEF. We hypothesized that increased VD/VT accompanied by decreased PaCO2 would differentially explain the rise in V̇E/V̇CO2 slope in HFrEF compared to HFpEF.
Methods
Participants
Patients with HFrEF or HFpEF (N=59) were referred by their primary cardiologist for CPET as part of a comprehensive HF evaluation at Mayo Clinic, Rochester, MN. Using the European Society of Cardiology criteria, we studied 32 HFrEF (left ventricular ejection fraction [LVEF] ≤40%) and 27 HFpEF (LVEF >50%) (Characteristics, Table 1) (13).
Table 1.
Participant characteristics
| All | HFrEF | HFpEF | P-value | |
|---|---|---|---|---|
|
|
|
|||
| Variable | N = 59 | N = 32 | N = 27 | |
| Age (years) | 62 ± 13 | 55 ± 10 | 71 ± 11 | <0.001 |
| Sex (male/female) | 46/13 | 30/2 | 16/11 | <0.001 |
| LV ejection fraction (%) | 40 ± 21 | 22 ± 7 | 61 ± 9 | <0.001 |
| NYHA class, n (%) | ||||
| II | 20 (34) | 13 (41) | 7 (26) | 0.07 |
| III | 39 (66) | 19 (59) | 20 (74) | 0.19 |
| Height (cm) | 172 ± 9 | 174 ± 8 | 170 ± 10 | 0.14 |
| Weight (kg) | 92 ± 20 | 86 ± 15 | 96 ± 23 | 0.05 |
| Body mass index (kg/m2) | 30 ± 6 | 28 ± 4 | 33 ± 6 | <0.01 |
| Body surface area (m2) | 2.07 ± 0.26 | 2.03 ± 0.21 | 2.12 ± 0.31 | 0.19 |
| Peak V̇O2 (% of pred.) | 39 ± 14 | 31 ± 7 | 50 ± 14 | <0.001 |
| Peak V̇O2 (mL/kg/min) | 8.6 ± 2.3 | 8.3 ± 2.2 | 8.8 ± 2.4 | 0.46 |
| Weber-Janicki class [peak V̇O2], n (%) | ||||
| C, 10–16 mL/kg/min | 14 (24) | 7 (22) | 7 (26) | 0.88 |
| D, <10 mL/kg/min | 45 (76) | 25 (78) | 20 (74) | 0.75 |
| Hemoglobin (g/dL) | 13.3 ± 1.7 | 13.9 ± 1.6 | 12.5 ± 1.4 | 0.01 |
| Creatinine (mg/dL) | 1.33 ± 0.42 | 1.39 ± 0.40 | 1.26 ± 0.43 | 0.23 |
| eGFR (mL/min per 1.73 m2) | 56 ± 15 | 56 ± 15 | 55 ± 16 | 0.80 |
| Drug Therapy, n (%) | ||||
| ACE inhibitor | 37 (63) | 25 (78) | 12 (44) | <0.001 |
| ARBs | 9 (15) | 2 (6) | 7 (26) | <0.001 |
| Antiarrhythmic | 15 (25) | 10 (31) | 5 (19) | 0.09 |
| β-blocker (β1 or non-select.) | 45 (76) | 28 (88) | 17 (63) | 0.04 |
| Ca2+ channel blocker | 7 (12) | 1 (3) | 6 (22) | <0.001 |
| Digoxin | 25 (42) | 23 (72) | 2 (7) | <0.001 |
| Nitrate (oral, SL, or topical) | 19 (32) | 9 (28) | 10 (33) | 0.52 |
| Aspirin | 43 (73) | 22 (69) | 21 (78) | 0.46 |
| Diuretics | 40 (68) | 29 (91) | 11 (41) | <0.001 |
| Echocardiography | ||||
| LA volume (mL) | 104 ± 43 | 115 ± 42 | 84 ± 40 | 0.03 |
| LA volume index (mL/m2) | 51 ± 20 | 56 ± 20 | 43 ± 20 | 0.04 |
| Mitral E-wave VEL (cm/s) | 89.3 ± 30.1 | 89.3 ± 40.1 | 89.3 ± 22.2 | 0.58 |
| Mitral A-wave VEL (cm/s) | 68.9 ± 29.7 | 59.0 ± 32.8 | 77.5 ± 24.2 | 0.01 |
| Mitral E/A ratio | 1.4 ± 0.8 | 1.7 ± 0.9 | 1.2 ± 0.6 | 0.11 |
| Mitral septal tissue Doppler VEL (e′) (cm/s) | 5.3 ± 1.8 | 4.5 ± 1.7 | 6.1 ± 1.7 | <0.001 |
| Mitral E/e′ ratio | 19.1 ± 9.8 | 22.1 ± 10.9 | 16.2 ± 7.7 | 0.02 |
| IV septum thickness (mm) | 9.9 ± 1.6 | 9.5 ± 1.6 | 10.2 ± 1.6 | 0.06 |
| Posterior wall thickness (mm) | 9.8 ± 1.7 | 9.7 ± 1.5 | 9.9 ± 1.9 | 0.96 |
Data are mean ± SD, n, or percentage (%).
Patients were excluded based on the following criteria: significant coronary artery disease (stenosis ≥50%), cor pulmonale, diagnosed pulmonary disease secondary to HF, primary renal or hepatic disease, valvular heart disease (any stenosis, >mild regurgitation, etc.), hypertrophic or infiltrative cardiomyopathy, constrictive pericarditis, or deep vein thrombosis. All aspects of this study were approved by the Mayo Clinic Institutional Review Board while conforming to principles outlined in the Declaration of Helsinki. All authors have read and agreed to the manuscript as written.
Echocardiography
Resting two-dimensional and tissue Doppler echocardiography according to guidelines of the American Society of Echocardiography were used to assess LVEF, morphology, and function; as well as early transmitral flow velocity (E), late transmitral flow velocity (A), E/A ratio, early diastolic mitral annular velocity (e′), peak E to e′ ratio, and left atrial volumes (Table 1) (13).
CPET protocol
Patients performed CPET in the morning in a fasted state while remaining on standard pharmacologic therapy. Patients exercised in a semi-recumbent position at an initial workload of 20-watts (W) while pedaling at 60–65 rpm, thereafter increasing by 10-W every 3 min until volitional fatigue. Heart rate and rhythm were continuously monitored using a 12-lead electrocardiogram.
Ventilation and gas exchange
Breath-by-breath open-circuit spirometry (CPX/D, MGC Diagnostics, St.Paul, MN) was used to continuously collect ventilation and gas exchange data throughout CPET. The final 30-s of rest and the final completed stage (i.e., peak pulmonary oxygen uptake, V̇O2peak) were used for data analyses. Data were acquired for variables: V̇O2, V̇CO2, respiratory exchange ratio (RER), respiratory rate (fB), VT, V̇E, end-tidal CO2 tension (PETCO2), and end-tidal O2 tension (PETO2). Percent (%) of predicted V̇O2peak was calculated from Hansen et al. (14).
Arterial gas measurement and parameters
Continuous monitoring and periodic sampling of arterial blood gases occurred using standard technique via percutaneous insertion of a 20-gauge catheter at the left radial artery. Arterialized blood drawn into heparinized 3-mL glass syringes occurred so as to approximately align with breath-by-breath periods (i.e., final ~30-s of each CPET stage), with the final draw of the last completed stage used to describe peak exercise. Arterialized samples were chilled on ice and immediately sent to our institutional core laboratory where standard processing techniques occurred for: PaCO2, oxygen tension (PaO2), pH (pHa), and oxygen saturation (SaO2). From these measurements, we used standard physiologic and blood biochemical equations (described in Appendix) to derive bicarbonate (HCO3−) and base excess (BE) of arterialized blood.
Derived ventilation and gas exchange parameters
By using arterialized gas measurements combined with acquired basic ventilatory and gas exchange responses, ‘ideal’ alveolar gas and air equations with associated parameters were used to calculate: V̇A, alveolar volume (VA), alveolar CO2 tension (PACO2), alveolar O2 tension (PAO2), alveolar-to-arterial O2 difference (PA-aO2), indirect simplified estimate of whole lung diffusing capacity for O2 (DLO2) via Fick’s law of diffusion, and VD ventilation (V̇D) (described in Appendix), whereas VD/VT equaled (4, 6–10),
where 863 converts gas concentration to partial pressure while correcting for the fact that V̇CO2 is expressed as a dry gas volume at standard temperature (0°C), pressure (760 mm Hg), and dry (0% water vapor) (STPD), whereas ventilation is expressed as a wet gas volume at body temperature, pressure, and saturation (BTPS).
Mixed-expired CO2 (PECO2) equaled the quotient of 863 and V̇E/V̇CO2 (15). All data from rest to peak exercise was used to calculate V̇E/V̇CO2 slope via linear regression, which provided optimal data interpretability and temporal consistency across participants (16).
Statistical Analyses
Parametric data are presented as mean±SD. Results of Shapiro-Wilk and Levene’s tests did not suggest data were significantly skewed or variance was heterogeneous. Between group baseline differences were compared using independent Student’s t-tests for parametric data or χ2-tests for categorical variables. Repeated measures ANOVA models with group-by-time interaction terms were used to test between-within group differences at rest and peak exercise. When F-tests from group-by-time interaction terms were significant, post-hoc Tukey-Kramer tests were used to assess between-within pairwise differences.
Ordinary least squares univariate linear regressions were used to test relationships between dependent (ordinate, V̇E/V̇CO2 slope) and independent (abscissa, VD/VT, PaCO2, etc.) variables. Coefficient of determination (R2) and regression equations, yest= a+bx, were computed from these models. Interpretation of R2 were based on standards of Cohen (17): modest=0.02; moderate=0.15; or strong≥0.25. Two-tailed significance was determined using an alpha level set at 0.05. Statistical analyses were performed using SAS statistical software v.9.4 (SAS Institute, Cary, NC).
Results
Participant characteristics
Both HFrEF and HFpEF demonstrated age, sex distribution, and LVEF consistent with currently recognized clinical phenotypes (Table 1). Despite greater %predicted V̇O2peak in HFpEF versus HFrEF, there were no differences in V̇O2peak (mL/kg/min). New York Heart Association and Weber-Janicki functional classifications aligned in HFpEF, but to a lesser extent in HFrEF. Compared to HFpEF, Hb was higher in HFrEF whereas creatinine and eGFR were similar. Frequency of therapies including ACE inhibitors, β-blockers (HFrEF, 6 and 22 on non-selective and β1-selective, respectively; HFpEF, 17/17 on β1-selective), digoxin, and diuretics were higher in HFrEF versus HFpEF. Resting echocardiography indicated there were increased LV filling pressures in HFrEF and HFpEF. Participants completed all aspects of this study in the absence of demonstrating adverse events (e.g., no signs/symptoms of pulmonary embolism, which could contribute to impaired ventilatory function).
Workload and basic ventilation and gas exchange
Resting V̇O2, V̇CO2, RER, V̇E, RR, VT, PETO2, and PECO2/PETCO2 were similar between groups, whereas PETCO2 and PECO2 were lower in HFrEF versus HFpEF (Table 2). Despite no differences in peak W and RER, HFrEF demonstrated lower V̇O2 and V̇CO2 versus HFpEF, whereas differences in V̇E/V̇CO2, PETCO2, and PECO2 persisted from rest. Except for V̇E/V̇CO2 (↓HFpEF), PETCO2 (↓HFrEF), and PECO2 (↑HFpEF), changes in other metrics from rest to peak exercise within HFrEF and HFpEF were similar (Table 2).
Table 2.
Basic ventilatory and gas exchange responses at rest and peak exercise
| All | HFrEF | HFpEF | P-value | |
|---|---|---|---|---|
|
|
|
|||
| Rest | N = 59 | N = 32 | N = 27 | |
| V̇O2 (L/min) | 0.24 ± 0.06 | 0.23 ± 0.05 | 0.25 ± 0.07 | 0.97 |
| V̇CO2 (L/min) | 0.21 ± 0.06 | 0.20 ± 0.05 | 0.21 ± 0.07 | 0.99 |
| RER | 0.87 ± 0.10 | 0.88 ± 0.10 | 0.85 ± 0.09 | 0.63 |
| V̇E (L/min) | 8.6 ± 2.7 | 9.0 ± 2.2 | 8.1 ± 3.3 | 0.95 |
| fB (breaths/min) | 17 ± 4 | 17 ± 4 | 16 ± 5 | 0.91 |
| VT (L) | 0.54 ± 0.18 | 0.54 ± 0.16 | 0.53 ± 0.20 | 0.99 |
| V̇E/V̇CO2 | 42 ± 8 | 45 ± 7 | 38 ± 6 | <0.001 |
| PETO2 (mm Hg) | 103 ± 6 | 103 ± 6 | 102 ± 5 | 0.98 |
| PETCO2 (mm Hg) | 35 ± 6 | 33 ± 5 | 39 ± 5 | <0.001 |
| PECO2 (mm Hg) | 21 ± 4 | 20 ± 3 | 23 ± 4 | <0.01 |
| PECO2/PETCO2 | 0.60 ± 0.06 | 0.61 ± 0.06 | 0.60 ± 0.06 | 0.98 |
| Peak exercise | ||||
| Workload, W | 38 ± 11 | 39 ± 11 | 36 ± 12 | 0.39 |
| V̇O2 (L/min) | 0.76 ± 0.22* | 0.70 ± 0.18* | 0.82 ± 0.25* | 0.04 |
| V̇CO2 (L/min) | 0.82 ± 0.23* | 0.77 ± 0.19* | 0.89 ± 0.25* | 0.04 |
| RER | 1.10 ± 0.08* | 1.10 ± 0.09* | 1.10 ± 0.06* | 0.92 |
| V̇E (L/min) | 32 ± 9* | 33 ± 9* | 35 ± 9* | 0.43 |
| fB (breaths/min) | 31 ± 8* | 30 ± 7* | 31 ± 10* | 0.97 |
| VT (L) | 1.08 ± 0.33* | 1.13 ± 0.35* | 1.03 ± 0.31* | 0.43 |
| V̇E/V̇CO2 | 40 ± 10 | 44 ± 10 | 35 ± 7* | <0.001 |
| PETO2 (mm Hg) | 112 ± 7* | 113 ± 7* | 111 ± 6* | 0.62 |
| PETCO2 (mm Hg) | 34 ± 8 | 30 ± 6* | 39 ± 7 | <0.001 |
| PECO2 (mm Hg) | 23 ± 5 | 20 ± 4 | 25 ± 5* | <0.01 |
| PECO2/PETCO2 | 0.68 ± 0.05* | 0.69 ± 0.04* | 0.66 ± 0.06* | 0.14 |
Data are mean ± SD. Oxygen consumption (V̇O2); carbon dioxide output (V̇CO2); respiratory exchange ratio (RER); minute ventilation (V̇E); respiratory rate (fB); tidal volume (VT); end-tidal oxygen (PETO2); end-tidal carbon dioxide (PETCO2); mixed expired carbon dioxide (PECO2).
Within group, rest vs. peak exercise, P<0.05. Table P-values and symbols represent significance after Tukey-Kramer post-hoc testing from repeated measures ANOVA models.
Alveolar equation and blood biochemistry parameters
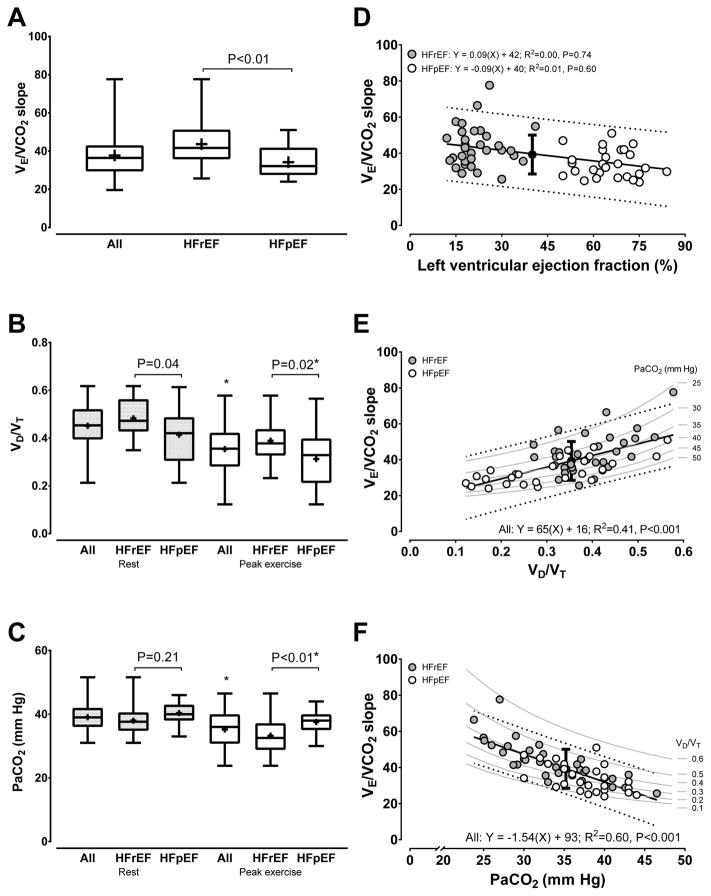
Excluding lower resting VD/VT in HFpEF versus HFrEF (Figure 1), parameters associated with alveolar equations or blood biochemistry were similar between groups (Table 3). By contrast, consistent with higher V̇E/V̇CO2 slope in HFrEF (Figure 1A), HFrEF demonstrated higher VD, V̇D, and VD/VT (Figure 1B) as well as lower PaCO2 (Figure 1C) and PACO2 accompanied by higher pHa versus HFpEF at peak exercise (Table 3). Absence of between group differences for other metrics at rest persisted to peak exercise. For within group differences from rest to peak exercise, HFrEF and HFpEF demonstrated similar directional changes for variables in Table 3 except for PaO2, which increased within HFrEF but not within HFpEF.
Figure 1.
Measurements or univariate linear regressions involving the ventilatory equivalent for carbon dioxide (V̇E/V̇CO2) slope, physiologic dead space to tidal volume ratio (VD/VT), or arterial carbon dioxide tension (PaCO2). Data presented in panels A to C are interquartile range with mean represented as (+). Variables presented on the abscissa in panels D to F are at rest, peak exercise, and peak exercise, respectively. For panels D to F representative of all participants: filled circle is mean ± SD of V̇E/V̇CO2 slope at the mean of the variable set on the abscissa, solid line is goodness of fit line of the model fit equation, dotted lines are 95% prediction bands of the model fit equation, and grey bands are isopleths representing linked changes in observed V̇E/V̇CO2 slope and PaCO2 or VD/VT when either PaCO2 or VD/VT are theoretically constrained values. Interpretation of R2: modest=0.02; moderate=0.15; or strong≥0.25. The Y-intercept in panels D to F differed from 0.0 (P<0.01). Differences in R2: panel D vs. E or F, P<0.001; panel E vs. F, P=0.16. Heart failure with reduced (HFrEF, N=32) or preserved (HFpEF, N=27) ejection fraction. *Following post-hoc Tukey-Kramer testing, different within group for both HFrEF and HFpEF or all HF, rest to peak exercise, P<0.05.
Table 3.
Alveolar equation, related derivatives, and blood biochemistry parameters
| All | HFrEF | HFpEF | P-value | |
|---|---|---|---|---|
|
|
|
|||
| Rest | N = 59 | N = 32 | N = 27 | |
| VD (L) | 0.24 ± 0.10 | 0.26 ± 0.08 | 0.22 ± 0.13 | 0.64 |
| V̇D (L/min) | 4.0 ± 1.7 | 4.3 ± 1.3 | 3.5 ± 2.2 | 0.79 |
| VA (L) | 0.29 ± 0.11 | 0.28 ± 0.11 | 0.30 ± 0.11 | 0.98 |
| V̇A (L/min) | 4.64 ± 1.43 | 4.67 ± 1.32 | 4.61 ± 1.57 | 0.99 |
| PaO2 (mm Hg) | 71 ± 12 | 71 ± 14 | 72 ± 11 | 0.99 |
| PAO2 (mm Hg) | 96 ± 7 | 98 ± 8 | 94 ± 6 | 0.11 |
| PA-aO2 (mm Hg) | 25 ± 12 | 26 ± 13 | 22 ± 11 | 0.54 |
| PA-aO2/V̇O2 (mm Hg/mL/kg/min) | 9.6 ± 5.0 | 10.5 ± 5.7 | 8.5 ± 3.8 | 0.41 |
| DLO2 (mL/min/mm Hg) | 12 ± 7 | 12 ± 8 | 13 ± 7 | 0.98 |
| PACO2 (mm Hg) | 39 ± 4 | 38 ± 5 | 40 ± 4 | 0.45 |
| SaO2 (%) | 94 ± 3 | 94 ± 3 | 95 ± 3 | 0.88 |
| pHa | 7.41 ± 0.04 | 7.42 ± 0.04 | 7.39 ± 0.05 | 0.19 |
| HCO3− (mEq/L) | 24.33 ± 2.52 | 24.23 ± 2.78 | 24.46 ± 2.22 | 0.99 |
| Base excess (mEq/L) | −0.50 ± 2.60 | −0.44 ± 2.71 | −0.56 ± 2.50 | 0.98 |
| Peak exercise | ||||
| VD (L) | 0.38 ± 0.15* | 0.43 ± 0.15* | 0.31 ± 0.12* | <0.01 |
| V̇D (L/min) | 11.4 ± 4.8* | 12.9 ± 4.3* | 9.7 ± 4.9* | <0.01 |
| VA (L) | 0.71 ± 0.28* | 0.70 ± 0.26* | 0.72 ± 0.30* | 0.98 |
| V̇A (L/min) | 21 ± 7* | 20 ± 6* | 21 ± 7* | 0.97 |
| PaO2 (mm Hg) | 76 ± 15 | 78 ± 14* | 74 ± 15 | 0.59 |
| PAO2 (mm Hg) | 109 ± 6* | 111 ± 7* | 107 ± 5* | 0.10 |
| PA-aO2 (mm Hg) | 33 ± 14* | 33 ± 14* | 33 ± 14* | 0.99 |
| PA-aO2/V̇O2 (mm Hg/mL/kg/min) | 4.3 ± 2.7* | 4.3 ± 2.6* | 4.3 ± 2.9* | 0.99 |
| DLO2 (mL/min/mm Hg) | 29 ± 21* | 27 ± 18* | 31 ± 25* | 0.81 |
| PACO2 (mm Hg) | 35 ± 6* | 34 ± 6* | 37 ± 5* | 0.03 |
| SaO2 (%) | 95 ± 4 | 95 ± 3 | 94 ± 5 | 0.63 |
| pHa | 7.40 ± 0.05 | 7.42 ± 0.04 | 7.38 ± 0.05 | 0.02 |
| HCO3− (mEq/L) | 21.62 ± 3.23* | 21.19 ± 3.75* | 22.13 ± 2.47* | 0.61 |
| Base excess (mEq/L) | −3.04 ± 3.21* | −3.21 ± 3.62* | −2.83 ± 2.70* | 0.96 |
Data are mean ± SD. Physiologic dead space (VD); VD ventilation (V̇D); alveolar volume (VA); alveolar ventilation (V̇A); arterial oxygen tension (PaO2); alveolar oxygen tension (PAO2); alveolar-to-arterial O2 gradient (PA-aO2); pulmonary oxygen uptake (V̇O2); estimated pulmonary diffusing capacity for oxygen (DLO2); alveolar carbon dioxide tension (PACO2); arterial oxygen saturation (SaO2); arterial pH (pHa); bicarbonate (HCO3−);.
Within group, rest vs. peak exercise, P<0.05. Table P-values and symbols represent significance after Tukey-Kramer post-hoc testing from repeated measures ANOVA models.
In sub-analyses including only HFrEF (N=22) and HFpEF (N=17) on β1-blockers, between-within differences for V̇E/V̇CO2 slope (F=13.7, P<0.01), VD/VT (F=20.7, P<0.01), and PaCO2 (F=5.12, P<0.01) mirrored outcomes illustrated in Figure 1(A–C). Within HFpEF, there were no differences in V̇E/V̇CO2 slope (P=0.96), peak exercise VD/VT (P=0.69), and peak exercise PaCO2 (P=0.90) between patients on versus not on β1-blockers.
Predictors of V̇E/V̇CO2 slope
Resting LVEF was unrelated to V̇E/V̇CO2 slope when stratified by HF group (Figure 1D), whereas combining all HF resulted in a modest relationship (y=−0.21x+48, R2=0.16, P=0.01). In comparison, peak exercise VD/VT and PaCO2 strongly explained the variance in V̇E/V̇CO2 slope across all HF (Figure 1E and 1F).
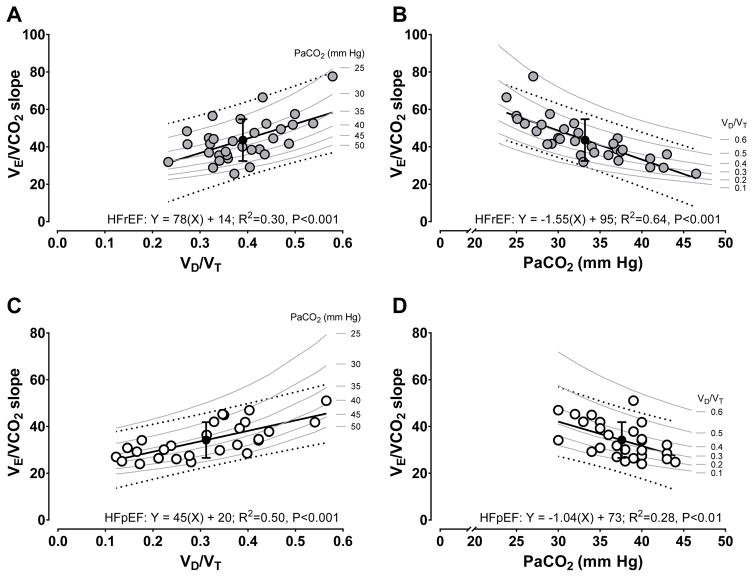
Consistent with plots of HFrEF versus HFpEF in Figures 1E and 1F, unique contributions from each group to pooled regressions are illustrated in detail in Figure 2. While decreasing PaCO2 strongly explained the variance associated with increasing V̇E/V̇CO2 slope in HFrEF (Figure 2B), VD/VT was more strongly related to elevated V̇E/V̇CO2 slope in HFpEF (Figure 2C). Although there was significant linearity between increasing VD/VT and V̇E/V̇CO2 slope in HFrEF (Figure 2A) as well as PaCO2 and V̇E/V̇CO2 slope (Figure 2D, inverse relationship) in HFpEF, variance explained in Figures 2A and 2D was approximately half of Figures 2B and 2C in HFrEF and HFpEF, respectively. In the absence of VD/VT (Y-intercept not different from 0.0, P=0.14; Figure 2A), and not until VD/VT >0.2, was V̇E/V̇CO2 slope elevated except for the influence of PaCO2 in HFrEF (Figure 2B). In contrast, VD/VT accompanied by lesser effects from PaCO2 contributed to increased V̇E/V̇CO2 slope at VD/VT ≥1.0 in HFpEF (Figure 2C; and Figure 2D isopleths).
Figure 2.
Univariate linear regressions involving the ventilatory equivalent for carbon dioxide (V̇E/V̇CO2) slope (dependent, ordinate), peak exercise physiologic dead space to tidal volume ratio (VD/VT) (independent, abscissa), or peak exercise arterial CO2 tension (PaCO2) (independent, abscissa). For all panels: filled circle is mean ± SD of V̇E/V̇CO2 slope at the mean of the variable set on the abscissa, solid line is goodness of fit line of the model fit equation, dotted lines are 95% prediction bands of the model fit equation, and grey bands are isopleths representing linked changes in observed V̇E/V̇CO2 slope and PaCO2 or VD/VT when either PaCO2 or VD/VT are theoretically constrained values. Interpretation of R2: modest=0.02; moderate=0.15; or strong≥0.25. The Y-intercept in panel A did not differ from 0.0 (P=0.14), whereas the Y-intercept in panels B to D differed from 0.0 (P<0.01). Differences in R2: panel A vs. B, P=0.06; panel A vs. C, P=0.33; panel B vs. D, P=0.06; panel C vs. D, P=0.30. Heart failure with reduced (HFrEF, N=32) or preserved (HFpEF, N=27) ejection fraction.
Finally, while increasing V̇D and PAO2 accompanied by decreasing PACO2 demonstrated significant linearity with increasing V̇E/V̇CO2 slope in HFrEF and HFpEF, in an inverse manner, HCO3− and BE explained the variance in V̇E/V̇CO2 slope in HFrEF, whereas this did not occur in HFpEF (Table 4).
Table 4.
Univariate predictors of V̇E/V̇CO2 slope
| b (95% CL) | β (95% CL) | Intercept (95% CL) | R2 (95% CL) | P-value | |
|---|---|---|---|---|---|
|
| |||||
| HFrEF | |||||
| V̇D | 33 (8, 59) | 0.44 (0.11, 0.68) | 29 (18, 41) | 0.20 (−0.03, 0.43) | 0.01 |
| V̇A | 0.26 (−0.40, 0.92) | 0.14 (−0.22, 0.47) | 38 (24, 52) | 0.02 (−0.07, 0.11) | 0.43 |
| PaO2 | 0.11 (−0.18, 0.40) | 0.14 (−0.22, 0.47) | 35 (12, 58) | 0.02 (−0.07, 0.11) | 0.45 |
| PAO2 | 1.29 (0.89, 1.69) | 0.77 (0.58, 0.88) | −100 (−144, −55) | 0.59 (0.39, 0.79) | <0.001 |
| PA-aO2 | 0.19 (−0.10, 0.49) | 0.24 (−0.12, 0.54) | 37 (26, 48) | 0.06 (−0.09, 0.21) | 0.19 |
| DLO2 | 1.41 (−0.16, 2.99) | 0.32 (−0.03, 0.60) | 37 (29, 45) | 0.10 (−0.08, 0.28) | 0.08 |
| PACO2 | −1.37 (−1.87, −0.87) | −0.72 (−0.85, −0.50) | 90 (73, 107) | 0.51 (0.28, 0.74) | <0.001 |
| HCO3− | −1.70 (−2.64, −0.76) | −0.56 (−0.76, −0.26) | 79 (59, 100) | 0.31 (0.06, 0.56) | <0.01 |
| Base excess | −1.44 (−2.48, −0.40) | −0.46 (−0.70, −0.13) | 39 (34, 44) | 0.21 (−0.02, 0.44) | <0.01 |
| HFpEF | |||||
| V̇D | 31 (8, 53) | 0.49 (0.14, 0.73) | 25 (17, 32) | 0.24 (−0.02, 0.50) | <0.01 |
| V̇A | 0.00 (−0.42, 0.44) | 0.01 (−0.37, 0.39) | 34 (25, 44) | 0.00 (0.0, 0.0) | 0.98 |
| PaO2 | −0.03 (−0.24, 0.18) | −0.06 (−0.43, 0.33) | 36 (21, 52) | 0.00 (−0.04, 0.04) | 0.77 |
| PAO2 | 0.92 (0.38, 1.47) | 0.57 (0.24, 0.78) | −65 (−124, −7) | 0.33 (0.06, 0.60) | <0.01 |
| PA-aO2 | 0.13 (−0.08, 0.35) | 0.25 (−0.14, 0.58) | 30 (22, 37) | 0.06 (−0.10, 0.22) | 0.21 |
| DLO2 | 0.95 (−0.09, 1.98) | 0.35 (−0.03, 0.64) | 30 (25, 35) | 0.13 (−0.09, 0.35) | 0.07 |
| PACO2 | −0.80 (−1.40, −0.20) | −0.48 (−0.73, −0.12) | 64 (41, 86) | 0.23 (−0.03, 0.49) | 0.01 |
| HCO3− | −0.40 (−1.66, 0.87) | −0.13 (−0.49, 0.26) | 43 (15, 71) | 0.02 (−0.08, 0.12) | 0.53 |
| Base excess | −0.04 (−1.21, 1.12) | −0.02 (−0.40, 0.36) | 34 (30, 39) | 0.00 (−0.01, 0.01) | 0.94 |
Data are model parameters from univariate linear regressions predicting V̇E/V̇CO2 slope from rest to peak exercise in heart failure with reduced (HFrEF, N=32) or preserved (HFpEF, N=27) ejection fraction. Standardized beta (β). See definition of variables in Table 3 caption.
Discussion
These data suggest increased V̇E/V̇CO2 slope in both HFrEF and HFpEF patients is fundamentally linked to elevated VD/VT accompanied by altered regulation of PaCO2 during CPET. Both HFrEF and HFpEF demonstrated V̇E/V̇CO2 slope at levels consistent with worsened prognosis (1–4). However, moderate-to-strong relationships between increasing VD/VT and decreasing PaCO2 with rising V̇E/V̇CO2 slope differed in HFrEF compared to HFpEF. Decreasing PaCO2 at peak exercise in HFrEF explained >2× the variance in V̇E/V̇CO2 slope compared to the same relationship tested in HFpEF. By contrast, despite demonstrating lower peak exercise VD/VT compared to HFrEF, variance explained between increasing VD/VT and V̇E/V̇CO2 slope in HFpEF was >1.5× that of HFrEF. These data are consistent with the hypothesis that increased V̇E/V̇CO2 slope is not exclusively a function of central hemodynamic limitations in patients with HFrEF or HFpEF (4, 5, 18–20).
While abnormal VD/VT and PaCO2 contribute to increased V̇E/V̇CO2 (slope/ratio) in HFrEF (4, 7, 9, 10), these relationships have not been tested in HFpEF. These data in HFrEF are consistent with work from Guazzi et al. (4) and Woods et al. (7) who during peak and submaximal CPET, demonstrated low PaCO2 and high VD/VT paralleled increased V̇E/V̇CO2 in HFrEF. By comparison, these observations are in contrast to interpretations of others (9, 10) suggesting PaCO2 coupled to ventilatory control mechanisms are not altered in HFrEF; instead, suggesting increased V̇E/V̇CO2 slope is related to high VD/VT provoked by impaired pulmonary perfusion and, hence, V̇A/Q̇ >1.0. Although this study did not include specific tests of ventilatory control related to chemoreflexes and/or group III/IV muscle afferents, these data align with several related studies in HF suggesting neurophysiologic control pathways abnormally activated during increased energy demand may play an appreciable role in excessive ventilatory responses to exercise in HF (4, 7, 18–21).
Energy demand in-excess of oxidative capabilities leads to a marked rise in CO2 production followed by an acidotic blood milieu if accumulated CO2 blood content is not adequately compensated (6, 8). In healthy adults, metabolic-driven production of CO2 leads to intrinsic activation of pathways relating to mechanisms of HCO3− formation accompanied by increased ventilation. Timely coordination of these events during exercise functions well as a buffering mechanism (Hb binds H+) to help maintain blood acid-base balance while facilitating decreased blood CO2 content as Na+ binds HCO3− (and to a lesser extent Hb binds CO2) to be transported to the pulmonary system for expiration. However, it remains unclear how these interconnected compensatory mechanisms of breathing and acid-base buffering equilibrate in conditions where both the rate and absolute level of CO2 accumulation in blood may become accelerated, such as might occur in HF.
Patients with HF demonstrate greater reliance on nonoxidative energy demand leading to increased CO2 production (20). Both HFrEF and HFpEF may demonstrate skeletal muscle fiber shifts leading to an increased proportion of type II (glycolytic) relative type I fibers, decreased mitochondrial density and enzymatic activity, and exacerbated central chemoreceptor and/or group III/IV afferent activation via elevated circulating H+ (18–21). Thus, these and other pathologic events proposed in HF are compatible with augmented blood CO2 content and low pHa, which could be expected to provoke augmented ventilatory compensation.
The rate of increase in V̇E relative to metabolic demand was exaggerated across our HF patients. Likewise, a formidable drop in HCO3− from rest to peak exercise accompanied by negative BE is consistent with the presence of metabolic acidosis and base deficit (6, 8, 22, 23). The concurrent decrease in PaCO2 linked to patterned responses in HCO3−, BE, V̇E, and pHa is consistent with the notion that ventilatory control pathways may function at an abnormally high gain coupled with a low PaCO2 (apneic) set-point in HF (4). In this context, our group and others have demonstrated that metabolically activated muscle afferents during exercise contribute to increased ventilation and V̇E/V̇CO2 slope in HFrEF (18, 19, 21). While this study did not include tests of muscle afferent activation linked to ventilatory function, assuming skeletal muscle pathology occurs secondary to HF (20), this musculoskeletal neural pathway connecting systemic blood biochemical changes to brainstem centers of ventilatory control is a candidate mechanism contributing to increased V̇E/V̇CO2 slope in HF.
Although PaCO2 and VD/VT are interrelated, the shift in the relationship between increased V̇E/V̇CO2 slope from PaCO2 in HFrEF to VD/VT in HFpEF suggests there may have been an influence of sub-clinical pathology of mechanical and/or structural pulmonary features on increased V̇E/V̇CO2 slope in HFpEF (4, 9, 15, 24). However, if sub-clinical pulmonary disease occurring independently or secondary to HF were to have been present in HFpEF, we hypothesize that while inhomogeneous distribution of V̇A/Q̇ >1.0 lung units may have contributed to increased VD/VT and V̇E/V̇CO2 slope (9, 10), the stronger influence of V̇A/Q̇ on high VD/VT would have derived from effects of V̇A/Q̇ <1.0 (suggested by PECO2/PETCO2 ≤0.70 (15)). Even with the potential for moderately reduced (~20% drop from normal) lung diffusing capacity in HFpEF (24), because of distinct properties of CO2 (e.g., high blood solubility and diffusion rate) it could be suggested that any perfusion (V̇A/Q̇ >0.0) of normally ventilated alveoli will result in little interference in CO2 transfer (6, 8). In contrast, it is likely that with inhomogeneous distribution of V̇A/Q̇ <1.0, V̇A, and DLO2 throughout lungs that effects of venous admixture and moderately reduced gas transfer could be expected to nominally influence PaCO2 while effectively diluting arterial O2 content and increase PA-aO2. Inasmuch as HFpEF demonstrated alignment between PaCO2 and PACO2 suggesting no impairment in CO2 diffusing capacity, PA-aO2 simultaneously extended beyond normal limits (6, 8). These combined observations are consistent with the suggestion that V̇A/Q̇ <1.0 lung areas demonstrate stronger effects on PaO2 compared to effects of V̇A/Q̇ >1.0 lung sectors on impaired CO2 exchange and, hence, PaCO2 (6, 8). Thus, although peak exercise PaCO2 inversely related to V̇E/V̇CO2 slope in HFpEF, it does not appear that effects of abnormal pulmonary CO2 transfer and whole lung V̇A/Q̇ >1.0 played an appreciable role in this relationship.
Limitations
Information regarding the long-term prognostic translation of the present observations is not yet available in this limited sample size of HF patients, and causality cannot be deduced from these data. Increased V̇E/V̇CO2 slope in HFpEF of this study represents the higher limit in this population. A possible explanation for this observation is a secondary effect of aging as these data are consistent with reports of Haykowski et al. (5) and Borlaug et al. (12) in HFpEF of similar age, whereas younger HFpEF in Shafiq et al. (2) and Guazzi et al. (1) demonstrated lesser V̇E/V̇CO2 slope. Additional effects of obesity might also be considered impactful to increased V̇E/V̇CO2 slope secondary to attenuated pulmonary perfusion as others have demonstrated direct correlation between BMI and degree of diastolic dysfunction independent of comorbidities commonly recognized in HFpEF (25). While this study did not include age and body sized matched controls with respect to each HF group, elucidating the independent influences of aging and obesity on ventilatory inefficiency is important in this line of study as both factors contribute to the HFpEF phenotype.
Discussions regarding the influence of pulmonary perfusion on V̇E/V̇CO2 slope and VD/VT are hypothesis generating interpretations without direct measurements of central hemodynamics. Despite this limitation, the study design based upon ‘ideal’ alveolar equations accounts for cardiopulmonary hemodynamic interactions in addition to influences of peripheral perfusion physiology on the derivation of V̇E/V̇CO2 slope (4, 6–9). Although PaCO2 is taken as a cross-sectional measurement, whereas V̇E/V̇CO2 slope is quantified as a continuous variable, we suggest breath-to-breath variability in PaCO2 (i.e., negligible compared to inter-breath variability in PETCO2) would not have an appreciable effect on relationships demonstrated in this study.
We acknowledge that β-blocker selectivity may potentially impact the interpretation of V̇E/V̇CO2 slope in HFrEF (26). This study was not powered to test the effect of β-blocker selectivity on our outcomes, and therefore additional studies are needed to clarify the potential role of β-blocker selectivity on exercise ventilation in HF. The immediate translation of these data to a specific therapeutic strategy to improve ventilatory efficiency in HF is also not yet available. However, based on recent observations in HFpEF suggesting exercise training attenuates pathologic processes of skeletal muscle remodeling (leading to improved oxidative metabolism (27)) and exercise accompanied by inorganic nitrite supplementation improves central—peripheral hemodynamics (e.g., V̇A/Q̇ ~1.0) (12, 28), we hypothesize that questions tested in this study applied to those paradigms can be used to advance the understanding of how to interpret V̇E/V̇CO2 slope while also lending an ability to properly manage ventilatory function in HF. Lastly, our suggestion of a possible role of impaired skeletal muscle afferents on abnormal ventilatory control that contributes to increased V̇E/V̇CO2 slope in HFpEF has not been formally tested in the setting of the present aims. However, because of skeletal muscle pathophysiology (e.g., shift from type I to II fibers (20)), HFpEF may be predisposed to abnormal skeletal muscle afferent function similar to that recognized in HFrEF (18, 19, 21).
Conclusions
These data suggest patients with HF demonstrate abnormally increased V̇E/V̇CO2 slope, which can be explained by both VD/VT and PaCO2 during CPET. However, variance in V̇E/V̇CO2 slope is more strongly explained by VD/VT than PaCO2 in HFpEF as compared to HFrEF patients, in whom variance in V̇E/V̇CO2 slope can be primarily accounted for by decreased PaCO2. These hypothesis-generating data emphasize the need to refine the interpretation and clinical utility of V̇E/V̇CO2 slope in HF. Advanced studies focusing on the integrated pathophysiology of abnormal V̇E/V̇CO2 slope, VD/VT, and PaCO2 are warranted in HF, particularly those examining the unique relevance of peripheral musculoskeletal pathologies in contributing to the variable HF phenotype which has come to the forefront in discussions of HFpEF (1–3, 5, 11, 12, 20).
Acknowledgments
The authors would like to acknowledge and thank all participants who volunteered for this study.
Sources of Funding
Funding for this work was supported by National Institutes of Health [HL126638 to TPO; HL071478 to BDJ; and HL128526 and U10 HL110262 to BAB]; and American Heart Association [16POST30260021 to EHV].
Appendix: Calculation methods for derived blood biochemistry and ‘ideal’ alveolar (gas/air) equation parameters
In using arterialized blood draws as described in the above text (methods), the Henderson-Hasselbalch equation was used to derive bicarbonate (HCO3−) in blood as,
where at standard body temperature of 37°C and pHa at 7.4, s equals 0.0307 mmol/L/mm Hg, which is the plasma solubility coefficient of CO2, whereas pK′ equals 6.0907, which is the acid-dissociation constant of carbonic acid (29, 30). Both s and pK′ were corrected for temperature (T) and pHa as follows (29, 30):
Base excess of arterialized blood (BE) was calculated using the Van Slyke equation derived by Siggaard-Andersen from BE and buffer base curves at body temperature and corrected for SaO2 (22, 23),
where Z describes the distribution of HCO3− between red blood cells and plasma depending on hemoglobin (Hb) concentration equal to, 1–0.0143×Hb; 24.4 is the plasma HCO3− concentration at a reference pHa of 7.4; β is the slope constant of the HCO3− vs. pHa plot equal to, 7.7+1.43×Hb; and the final bracketed term is to correct for differences in SaO2 from ideal oxygenation at 1.0.
Derived alveolar gas and air equation parameters
By using arterial blood gas measurements and acquired basic ventilation and gas exchange responses, ‘ideal’ alveolar gas and air equations and associated parameters could be used to calculate the following in addition to VD/VT (as explained above in methods) (4, 6, 7, 9, 10): alveolar ventilation and volume,
alveolar CO2 tension,
alveolar O2 tension,
ventilation of VD (V̇D),
and alveolar-to-arterial O2 difference (PA-aO2) was calculated as the difference between PAO2 and PaO2. To account for potential effects of differences in absolute V̇O2 on the interpretability of PA-aO2, we also provide PA-aO2 standardized to V̇O2 (PA-aO2/V̇O2).
Because of complexities involved in properly deriving end-pulmonary capillary O2 tension, we provide an indirect simplified estimate of whole lung diffusion capacity for O2 via Fick’s law of diffusion,
For above parameters related to ‘ideal’ alveolar gas and air equations: V̇E is minute ventilation, VD is physiologic dead space, VT is tidal volume, fB is respiratory rate, V̇CO2 is carbon dioxide output, 863 is the correction factor needed when computing partial pressure from fractional concentration involving both volumes/gas (STPD) and volumes/flows (BTPS, body temperature and pressure, saturated) standards of measurement, PIO2 is the inspired tension of O2, 0.21 is inspired O2 fraction (room air), RER is the respiratory exchange ratio at the lung, V̇O2 is pulmonary O2 uptake, and PaO2 is arterial O2 tension.
Footnotes
All aspects of the work in this manuscript were performed at Mayo Clinic, Rochester MN
Positions, institution, and location of authors:
Erik H. Van Iterson, PhD: Research Fellow in Cardiovascular Medicine, Mayo Clinic, Rochester MN
Bruce D. Johnson, PhD: Professor of Medicine and Professor of Physiology, Mayo Clinic, Rochester MN
Barry A. Borlaug, MD: Associate Professor of Medicine, Mayo Clinic, Rochester MN
Thomas P. Olson, PhD: Associate Professor of Medicine, Mayo Clinic, Rochester MN
Conflicts of Interest: none declared.
References
- 1.Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol. 2005 Nov 15;46(10):1883–1890. doi: 10.1016/j.jacc.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Shafiq A, Brawner CA, Aldred HA, Lewis B, Williams CT, Tita C, Schairer JR, Ehrman JK, Velez M, Selektor Y, Lanfear DE, Keteyian SJ. Prognostic value of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. The Henry Ford HospITal CardioPulmonary EXercise Testing (FIT-CPX) project. Am Heart J. 2016 Apr;174:167–172. doi: 10.1016/j.ahj.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nedeljkovic I, Banovic M, Stepanovic J, Giga V, Djordjevic-Dikic A, Trifunovic D, Nedeljkovic M, Petrovic M, Dobric M, Dikic N, Zlatar M, Beleslin B. The combined exercise stress echocardiography and cardiopulmonary exercise test for identification of masked heart failure with preserved ejection fraction in patients with hypertension. Eur J Prev Cardiol. 2016 Jan;23(1):71–77. doi: 10.1177/2047487315604836. [DOI] [PubMed] [Google Scholar]
- 4.Guazzi M, Reina G, Tumminello G, Guazzi MD. Exercise ventilation inefficiency and cardiovascular mortality in heart failure: the critical independent prognostic value of the arterial CO2 partial pressure. Eur Heart J. 2005 Mar;26(5):472–480. doi: 10.1093/eurheartj/ehi060. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 5.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011 Jul 12;58(3):265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley RL, Cournand A. Analysis of factors affecting partial pressures of oxygen and carbon dioxide in gas and blood of lungs; theory. J Appl Physiol. 1951 Aug;4(2):77–101. doi: 10.1152/jappl.1951.4.2.77. [DOI] [PubMed] [Google Scholar]
- 7.Woods PR, Olson TP, Frantz RP, Johnson BD. Causes of breathing inefficiency during exercise in heart failure. J Card Fail. 2010 Oct;16(10):835–842. doi: 10.1016/j.cardfail.2010.05.003. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasserman K, Van Kessel AL, Burton GG. Interaction of physiological mechanisms during exercise. J Appl Physiol. 1967 Jan;22(1):71–85. doi: 10.1152/jappl.1967.22.1.71. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan MJ, Higginbotham MB, Cobb FR. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation. 1988 Mar;77(3):552–559. doi: 10.1161/01.cir.77.3.552. [DOI] [PubMed] [Google Scholar]
- 10.Wensel R, Francis DP, Georgiadou P, Scott A, Genth-Zotz S, Anker SD, Coats AJ, Piepoli MF. Exercise hyperventilation in chronic heart failure is not caused by systemic lactic acidosis. European journal of heart failure. 2005;7(7):1105–1111. doi: 10.1016/j.ejheart.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 11.De Keulenaer GW, Brutsaert DL. Systolic and Diastolic Heart Failure Are Overlapping Phenotypes Within the Heart Failure SpectrumResponse to De Keulenaer and Brutsaert. Circulation. 2011;123(18):1996–2005. doi: 10.1161/CIRCULATIONAHA.110.981431. [DOI] [PubMed] [Google Scholar]
- 12.Borlaug BA, Melenovsky V, Koepp KE. Inhaled Sodium Nitrite Improves Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. Circ Res. 2016 Sep 16;119(7):880–886. doi: 10.1161/CIRCRESAHA.116.309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. European journal of heart failure. 2012;14(8):803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 14.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984 Feb;129(2 Pt 2):S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 15.Hansen JE, Ulubay G, Chow BF, Sun XG, Wasserman K. Mixed-expired and end-tidal CO2 distinguish between ventilation and perfusion defects during exercise testing in patients with lung and heart diseases. Chest. 2007 Sep;132(3):977–983. doi: 10.1378/chest.07-0619. [DOI] [PubMed] [Google Scholar]
- 16.Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Technical considerations related to the minute ventilation/carbon dioxide output slope in patients with heart failure. Chest. 2003 Aug;124(2):720–727. doi: 10.1378/chest.124.2.720. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J. A power primer. Psychol Bull. 1992 Jul;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 18.Ponikowski PP, Chua TP, Francis DP, Capucci A, Coats AJ, Piepoli MF. Muscle ergoreceptor overactivity reflects deterioration in clinical status and cardiorespiratory reflex control in chronic heart failure. Circulation. 2001 Nov 6;104(19):2324–2330. doi: 10.1161/hc4401.098491. [Clinical Trial Controlled Clinical Trial Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 19.Scott AC, Francis DP, Davies LC, Ponikowski P, Coats AJ, Piepoli MF. Contribution of skeletal muscle ‘ergoreceptors’ in the human leg to respiratory control in chronic heart failure. J Physiol. 2000;529(3):863–870. doi: 10.1111/j.1469-7793.2000.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014 May;306(9):H1364–1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson TP, Joyner MJ, Eisenach JH, Curry TB, Johnson BD. Influence of locomotor muscle afferent inhibition on the ventilatory response to exercise in heart failure. Exp Physiol. 2014 Feb;99(2):414–426. doi: 10.1113/expphysiol.2013.075937. [Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 22.Andersen OS. Blood acid-base alignment nomogram. Scales for pH, pCO2 base excess of whole blood of different hemoglobin concentrations, plasma bicarbonate, and plasma total-CO2. Scand J Clin Lab Invest. 1963;15:211–217. doi: 10.3109/00365516309079734. [DOI] [PubMed] [Google Scholar]
- 23.Siggaard-Andersen O. The van Slyke equation. Scand J Clin Lab Invest Suppl. 1977;146(sup146):15–20. doi: 10.3109/00365517709098927. [DOI] [PubMed] [Google Scholar]
- 24.Olson TP, Johnson BD, Borlaug BA. Impaired Pulmonary Diffusion in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016 Jun;4(6):490–498. doi: 10.1016/j.jchf.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. Journal of the American College of Cardiology. 2011;57(12):1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witte KK, Thackray S, Nikitin NP, Cleland JG, Clark AL. The effects of long-term β-blockade on the ventilatory responses to exercise in chronic heart failure. European journal of heart failure. 2005;7(4):612–617. doi: 10.1016/j.ejheart.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry JD. Exercise training in patients with heart failure and preserved ejection fraction: a meta-analysis of randomized control trials. Circulation: Heart Failure. 2014 doi: 10.1161/CIRCHEARTFAILURE.114.001615. CIRCHEARTFAILURE. 114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borlaug BA, Koepp KE, Melenovsky V. Sodium Nitrite Improves Exercise Hemodynamics and Ventricular Performance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2015 Oct 13;66(15):1672–1682. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 29.Van Slyke DD, Sendroy J, Hastings AB, Neill JM. STUDIES OF GAS AND ELECTROLYTE EQUILIBRIA IN BLOOD X. THE SOLUBILITY OF CARBON DIOXIDE AT 38° IN WATER, SALT SOLUTION, SERUM, AND BLOOD CELLS. Journal of Biological Chemistry. 1928;78(3):765–799. [Google Scholar]
- 30.Severinghaus JW. Blood gas calculator. J Appl Physiol. 1966 May;21(3):1108–1116. doi: 10.1152/jappl.1966.21.3.1108. [DOI] [PubMed] [Google Scholar]