Abstract
Objective
Stress reactivity research has traditionally focused on the idea that exaggerated responses to stress may have adverse effects on health. Accumulating evidence suggests that attenuated responses to stress and delayed recovery may also be problematic.
Methods
This review focuses on the role of the stress response of the hypothalamic-pituitary-adrenocortical (HPA) axis, the endogenous opioid system (EOS), and the cardiovascular system in hypertension, pain perception, and addictive behaviors. Results from multiple methods of assessment and stress paradigms conducted in our laboratory over the past two decades are integrated with research from other investigators and with existing theories.
Results
Research indicates that exaggerated biological and physiological responses to stress and attenuated pain perception are associated with hypertension and risk for cardiovascular diseases. This research complements work linking reduced stress responses with enhanced pain sensitivity and discomfort. Multiple studies have also demonstrated that an attenuated stress response is linked to exacerbation of withdrawal symptoms and relapse in nicotine addiction. Evidence indicates important moderators (i.e., sex, personality traits, and early life adversity) and HPA- and EOS-related mechanisms in the altered response to stress. We integrate these findings in a conceptual model emphasizing that robust stress responses in the context of addiction and relapse should be considered as a marker of resiliency.
Conclusions
A blunted stress response may indicate long-term physiological dysregulation that could usher harmful consequences for cardiovascular disease, pain perception and addictive disorders. The impact of dysregulation is influenced by multiple individual and situational factors that should be considered in evaluating the clinical significance of stress response dysregulation.
Keywords: Stress, HPA response, endogenous opioid system, addiction, relapse, pain, craving, withdrawal
Introduction
Multiple biological, psychological, and behavioral mechanisms mediate the effects of “stress” on health and disease; and research has identified individual difference factors that increase vulnerability to the adverse impact of stress as well as factors that promote coping and resilience. In this paper, we use the term stress as an environmental challenge (also referred to as stressor) and distinguish this construct from the stress-response that has multiple psychological, behavioral and biological components. Although exaggerated stress response has been seen historically as a harmful outcome of exposure to stressful events, research provides strong evidence that a blunted stress response can also be harmful and that the direction of impact of the stress response is influenced by conditions, situations, and dispositional factors. Our research program has focused on understanding how stress produces its harmful effects on health, with a particular focus on hypertension, pain perception, and addictive processes.
Central to the focus of our research are the roles of two stress brain pathways: the hypothalamic-pituitary-adrenocortical (HPA) axis and the endogenous opioid system (EOS). After providing a brief definition of the stress response and a review of HPA-EOS interactions related to the stress response, we review several studies conducted by our laboratory and other research teams over the last two decades. Specifically, the following topics will be reviewed: (1) stress-reactivity hypothesis in hypertension and other cardiovascular diseases; (2) the role of stress reactivity in pain perception and analgesia; (3) the effects of exaggerated and blunted stress responses on nicotine addiction and relapse; and (4) moderators of the stress response in pain perception and nicotine addiction. The converging results of these studies are interpreted within a theoretical framework in which a blunted stress response is considered as a marker of adverse outcomes in addiction, while a robust stress response is considered as an indicator of addiction-related resiliency and effective coping.
The Stress Response
Definition of the stress response
Stressful events normally activate several biological systems, including the HPA axis and the sympathetic nervous system (1–5). The HPA axis performs a central function in directing the neuroendocrine response to stress and it plays a mediating role in stress effects on health (6–9). This axis involves three brain and peripheral structures: the hypothalamus, the pituitary gland, and the cortical part of the adrenals. The HPA axis is activated by the release of corticotropin-releasing factor (CRF) from neuronal cell bodies of the paraventricular nucleus (PVN) of the hypothalamus. CRF stimulates the release of adrenocorticotropic hormone (ACTH) from the pituitary and beta-endorphin into the systemic circulation. ACTH is transported via peripheral circulation to the adrenal cortex where it stimulates the synthesis and release of corticosteroids--most notably in humans, cortisol (10, 11). Cortisol triggers a negative feedback loop, where it regulates ACTH and CRF release exerting a bottom-up regulatory effects on the HPA (10, 11).
In addition to the HPA axis, stress activates the EOS. Endogenous opioids are naturally occurring, opiate-like substances that regulate mood, pain, and the reinforcing properties of many drugs of addiction (12, 13). Many of the brain structures that regulate physiological and behavioral components of stress responding, including cingulate cortex, amygdala, and periaqueductal gray, contain endogenous opioids (14–19) that are released in response to stress and that are directly involved in regulating the HPA stress response (12, 20–23). Preclinical studies have demonstrated that central opioid activity interacts with dopaminergic, serotonergic, adrenergic, and GABAergic pathways in the prefrontal cortex, limbic system, and brain stem (24–28). This wide network of activity demonstrates that this system is involved in multiple downstream effects related to HPA activity, stress response, mood changes, and drug reward.
HPA and opioid interaction
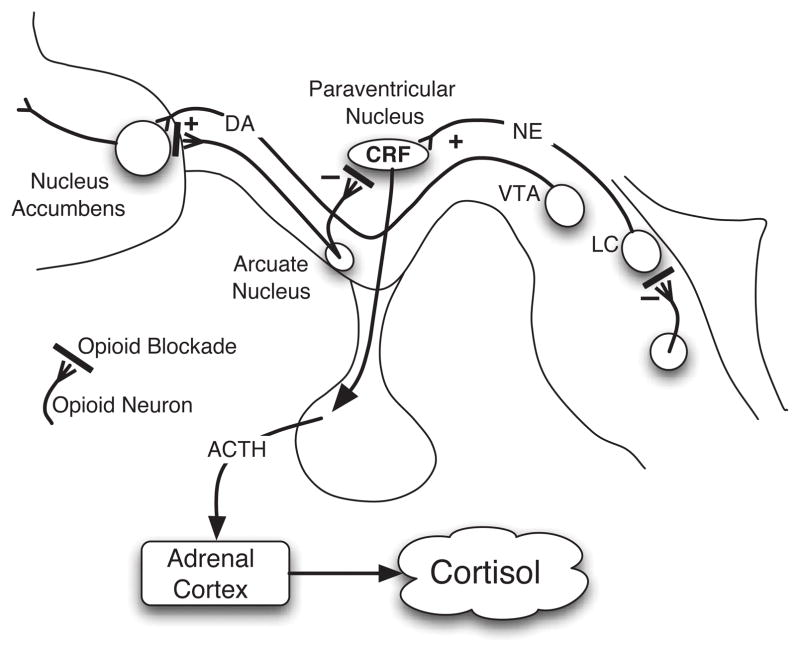
Central regulation of the HPA stress response by the EOS involves three points of interface (29–33) as indicated in Figure 1. First, CRF neurons in the hypothalamus receive direct inhibitory input from beta-endorphin neurons in the arcuate nucleus (34); this effect is mediated by activation of μ-opioid receptors (35, 36). Second, noradrenergic release from the locus coeruleus is under control of opioid neurons within the brainstem (37–39). Beta-endorphin and other opioid neurons indirectly inhibit CRF activity by inhibiting the CRF-stimulatory norepinephrine neurons (37, 40). Third, opioid neurons from the arcuate nucleus innervate the nucleus accumbens and prefrontal cortex, major structures involved in mesolimbic drug-reward pathway.
Figure 1.
The interaction of opioid system with multiple pathways involved in the stress response. The figure published originally by Lovallo (33) indicates that areas where opioid blockade acts in three areas: 1) the periventricular nucleus of the hypothalamus (PVN), the locus ceruleus, and the nucleus accumbens. Opioids normally stimulate DA release by the nucleus accumbens, inhibits CRF release from the PVN, and inhibit release of norepinephrine from the locus ceruleus (22). Reprinted from the International Journal of Psychophysiology, Vol. 59, Issue 3, Pages 195–202, William Lovallo, Cortisol secretion patterns in addiction and addiction risk (2006), with permission from Elsevier.
This innervation leads to activation of dopamine release in the mesolimbic pathway, which involves dopamine-producing neurons within the ventral tegmental area (VTA) projecting to the nucleus accumbens (NA) and engaging other structures involved in stress response regulation including the amygdala, prefrontal cortex (PFC), anterior cingulate cortex (ACC (41, 42). Reduced opioid stimulatory inputs to the mesolimbic dopaminergic system may lead to reduced basal dopamine activity, which may enhance the acute response and rewarding effects of dopamine-stimulating drugs, such as nicotine. Blocking opioid effects using opioid antagonists should lead to disinhibition of HPA regulatory effects, causing enhanced HPA output, as evidenced by increased ACTH and cortisol production. This pattern of hormonal release has been established as a reliable indicator to test the functional status of hypothalamic opioid tone (43–45). Decreased opioid tone is predicted to diminish effects of HPA axis opioid neurons, thereby attenuating HPA response to opioid blockade challenges. Reduced opioid tone may influence modulation of mesolimbic dopaminergic transmission; and observations that dysphoric states are usually associated with impaired endogenous opioid functions (46–49) suggest that reduced tone may also contribute to increased negative affect.
Stress Reactivity and Pain in Hypertension
Our research was initially grounded in the stress reactivity hypothesis, which is that enhanced physiological responses to acute stress increases risk for health problems, particularly cardiovascular diseases (50, 51); and we assumed that reduced response to stress would be beneficial to health. Work in this area assumes that responses to acute stressors in the laboratory provide an accurate indication of how individuals respond to stressful events in their daily life. Therefore, the stress reactivity hypothesis is typically studied using laboratory protocol wherein individuals are exposed to acute challenges, such as public speaking, mental arithmetic, or cold pressor tests, and their cardiovascular and physiological responses are measured (52–54).
Overall, there is mixed support regarding the generalizability of laboratory-based stress research (52, 53, 55), some longitudinal studies provide evidence of an association between the magnitude of responses to acute stress tasks (laboratory) and increases in blood pressure levels over time (56–60) as well as cardiac morphological changes, including increased left ventricular mass (51, 61, 62). Despite many researchers focusing on negative implications of enhanced stress response, and despite the underlying linear function implied by the stress reactivity hypothesis, much of the subsequent sections of this paper will address the need to consider the significance of a reduced or blunted stress response.
HPA stress response in hypertension
Early stress reactivity research focused primarily on autonomic and cardiovascular measures (e.g., (50, 63–65)), which tend to be responsive to various activating tasks rather than to specific tasks that are emotionally distressing. During the 1990s, more research was conducted focusing on other biological and neurochemical measures. Our research in the 1990s (e.g., (66–69)) contributed to expanding the definition of stress reactivity by incorporating new stress challenges (e.g., situational novelty, pharmacological challenges, and medical exam stress) and by incorporating additional biological measures, primarily the HPA hormones, prolactin, beta-endorphin, and dehydroepiandrosterone (DHEA) (70–74). The approach to expand the biomarkers of stress in the laboratory complemented an established approach to the assessment of sympathetic, hemodynamic, and cardiac structural changes associated with stress, especially in hypertensives and hypertension prone individuals (75–81).
The introduction of additional socially-relevant stressors to laboratory human research broadened the scope of our sampling of the stressful experiences people may encounter in daily life. This approach enhanced the generalizability of the results (64, 82–84) while reducing the artificial and academic natures of the acute laboratory stressors that dominated stress reactivity research up to that point (84–87). In addition, integration of multiple physiological systems in assessing the stress response enabled us to better capture the broad effects of stress and enhanced the validity of laboratory stress research.
For instance, in one study, we examined the effects of exposure to a novel experimental setting on resting, morning blood cortisol concentrations in men with borderline hypertension (66) to test the hypothesis that increased central nervous system activation among individuals with borderline hypertension would predict enhanced anticipation and cortisol secretion in a novel experimental situation. We found that participants with hypertension had higher cortisol concentrations than normotensive participants during the first and second days of exposure to the lab, but this difference disappeared on the third and fourth days. In another study, we assessed cortisol levels in individuals with hypertension before and after exposure to stressful challenges (67). Stress protocols included two levels of demand (intermittent reaction time with brief rests vs. reaction time alternating continuously with mental arithmetic); and we found that high-demand (continuous), but not moderate-demand (intermittent), mental stress produced enhanced cortisol response in participants with hypertension relative to normotensive participants.
To better define alterations of the psychobiological stress response in individuals with hypertension, we continued our research by evaluating the impact of a pharmacological challenge on the stress response in this group. In one study, we administered a dietary dose of caffeine (3.3 mg/kg, equivalent to 2 to 3 cups of coffee) and examined the effects of such a dose on HPA responses to stress in hypertensive and normotensive individuals (69). Although both hypertensive and normotensive participants showed significant cortisol responses to caffeine combined with the stress tasks, only participants with hypertension exhibited a significant cortisol response to the caffeine challenges during rest, indicating enhanced sensitivity of this stress hormone to this stimulant substance. We replicated and expanded these findings in a larger sample of normotensives who had a parental history for hypertension (88). In all, these observations reinforced the utility of adrenocortical measures in studies of stress reactivity among individuals at risk for cardiovascular diseases. They also demonstrated, for the first time, the combined effects of caffeine plus stress in hypertension-prone individuals.
Our research on the HPA axis in individuals with hypertension provided translational evidence of earlier work in animal models, as indicated by a review of the literature that integrated animal and human findings to evaluate the glucocorticoid hypothesis of hypertension (5). Overall, glucocorticoids (primarily corticosterone in rats and cortisol in humans) affect several blood pressure (BP) regulating processes and they influence expression of genes involved in BP (5). These effects may contribute to at least one subtype of the heterogeneous and multifactorial polygenic condition of hypertension. Furthermore, studies have examined cortisol tissue sensitivity, cortisol production and cortisol metabolic rate in hypertension-prone persons (89–91); the results of which provide support for the hypothesis that elevated cortisol levels may serve as an intermediate phenotype of hypertension risk. Incorporating HPA measures and non-invasive hemodynamic assessment in stress reactivity research and expanding the types of stress challenges used in the laboratory and in ambulatory settings (92–94) has enabled us to learn a great deal about manifestations of vulnerability and risk. This research also produced greater convergence of evidence related to the role of stress in hypertension risk.
In summary, we conducted a series of lab studies using different types of acute and natural stressors and using pharmacological challenges. The results of these studies demonstrate that vulnerable populations (primarily hypertensive and hypertension-prone individuals) consistently experience enhanced HPA activity and enhanced cardiovascular response to stress.
Stress and pain perception
The complex organization of the stress response involving multiple cortical, subcortical, and peripheral systems is also essential in pain reception and regulation of pain response. It is possible that processes involved within both stress response and pain regulation are integrated at multiple central nervous system (CNS) levels (95–97). Multiple brain structures, including the amygdala, prefrontal cortex, multiple nuclei in the hypothalamus, insula, anterior cingulate, locus coeruleus, and other structures within the limbic system are involved in both pain and stress response regulation (97–99). This integration may account for the reciprocal influences between stress and pain. Acute stress suppresses pain perception (100–102), and mechanisms may involve central opioid system (71, 103, 104) and peripheral baroreceptor activation (105, 106).
Stress, pain, and hypertension risk
Findings of exaggerated stress response in hypertensive and hypertension-prone individuals intersected with observations of blunted pain perception (i.e., hypoalgesia) in these populations (105, 107–109). This was particularly intriguing when examining the association of BP with pain perception in normal, healthy young individuals (108, 109) and in young individuals with parental history of hypertension (108, 109). To explain this phenomenon, we conducted a series of studies in collaboration with colleagues at Ohio University and the University of Birmingham, United Kingdome (110–114) in which we focused on two possible mechanisms.
One potential mechanism was enhanced baroreceptor stimulation (105, 106) that may result from increased cardiovascular response in this population (62, 69, 93, 115–117). We examined effects of baroreceptor stimulation on nociceptive responding in men and women with positive (high risk) or negative (low risk) parental history of hypertension. Subjective pain and nociceptive responding were evaluated during electrocutaneous sural nerve stimulation and under three baroreceptor conditions (stimulation, inhibition, and control). Although both stimulatory and inhibitory baroreceptor conditions were associated with reduced pain reports relative to control, this effect was comparable in low and high risk groups (112). This finding suggested that attenuated pain perception in hypertension-prone individuals may not be due to enhanced baroreceptor activity.
The second mechanism that we examined to explain hypoalgesia in hypertension-prone individuals was a blunting of normal opioidergic inhibitory regulation of hypothalamic activities. This work was based on previous opioid blockade research (118–120); therefore, we examined the effect of opiate blockade on HPA responses to a painful stressor in individuals with and without a parental history of hypertension. In this research, we used a double-blind, counterbalanced design to administer the blockade (placebo or naltrexone) prior to assessing nociceptive flexion reflex threshold. Results showed increases in salivary cortisol levels following pain assessment after the ingestion of naltrexone, but not after placebo. Although pain threshold and tolerance were higher among high-risk men relative to low-risk men, there was no difference in the influence of opioid blockade between the two groups (121).
In summary, these studies confirmed attenuated pain sensitivity in persons at high risk for hypertension, but they did not support a role for the EOS nor for enhanced baroreceptor activation as explanatory mechanisms for the hypoalgesia observed in hypertension-prone individuals. In light of the earlier observations that this group exhibits stress-response dysregulation, we speculated that alterations within the stress response systems could contribute to pain alteration in this population. Next, we briefly review studies that provided initial support for this hypothesis.
Stress response systems and pain
In our efforts to examine the role of the stress response in regulating pain perception, we conducted multiple studies. For example, we examined the extent to which basal cortisol concentrations and hemodynamics during rest predicted level of pain experienced during exposure to the hand cold pressor test (CPT) (122). To examine these relationships, we used a design that involved examining immediate and delayed pain, wherein participants were asked to rate their pain every 15 seconds during a 90-second CPT and during a 90-second post-CPT (recovery) period (109); participants also reported pain using the McGill Pain Questionnaire. Salivary cortisol samples and cardiovascular measures were collected prior to, during, and after the CPT. The results revealed that higher pre-CPT cortisol concentrations predicted lower pain reports during and after the CPT. We also found that systolic BP and stroke volume correlated negatively with pain.
Though our cardiovascular findings were consistent with previous research, the cortisol-pain connection was novel and suggested that HPA activation may contribute to, or be a marker of, attenuated pain perception seen during acute stress events. The role of increased CRF in pain has been proposed (123); and although early pharmacological studies indicated analgesic effects of CRF in dental patients (124), direct effects of CRF on pain sensitivity have not been consistently observed (125).
Our findings also extended previous observations of reduced adrenocortical activity in individuals with chronic pain conditions, such as fibromyalgia, rheumatoid arthritis (126, 127), chronic fatigue syndrome (128, 129), and child abdominal pain (130). Indeed, early research on chronic pain syndromes showed an association between ongoing pain and adrenocortical hyporesponsiveness (131–134). For instance, cortisol was inversely associated with pain intensity and number of pain sites (135); and chronic headache was associated with decreased cortisol concentrations in serum and cerebrospinal fluid (134). Other researchers found that women with chronic pelvic pain showed lower morning cortisol levels relative to normal controls (133); and others found that low back pain was associated with smaller cortisol variation between morning and midday levels (136). These findings, in combination, support the possibility of adrenocortical involvement in regulating pain and increasing risk for chronic pain syndromes. The immuno-regulatory effect of cortisol and its influence on inflammation, including its inhibitory effects on prostaglandins (137, 138), may play a role in explaining this phenomenon. Early experimental evidence with the Lewis rat, a model characterized by a genetically deficient glucocorticoid production, demonstrates consistent results, showing high susceptibility of this animal model to inflammatory diseases (139).
Stress-induced analgesia
One manifestation of the influence of stress on pain regulation is stress-induced analgesia. Stress (e.g., electric footshock) in rats suppresses pain behavior (140). Specifically, intermittent, uncontrollable footshock produces analgesia mediated by opioid mechanisms, whereas exposure to continuous footshock produces non-opioid analgesic effects (141). On the other hand, clinical research indicates the role of stress in exacerbating pain perception (142–144). Yet, research in healthy humans indicates the possibility that acute stress may attenuate pain perception (102, 145). These effects are likely mediated by physiological, peripheral, and descending opioid mechanisms. For example, there are indications that baroreceptor stimulation (105, 146), peripheral opioid peptides (147), and cardiovascular reactivity (148) may play a role (149).
Informed by this literature, we examined the extent to which psychophysiological and adrenocortical responses to acute stress predicted subsequent pain perception in a relatively large sample of healthy participants (100). Participants were assigned to one of two conditions: rest followed by pain induction (using the CPT) or stress (public-speaking challenge) followed by pain. Stress exposure was associated with reduced pain during CPT; and this effect was mediated by systolic BP level during stress. Mood changes were independent predictors of pain. This evidence further implicated stress-related pressor effects in altering pain perception. The findings provided additional support for the hypothesis that attenuated pain perception in hypertension may be due to altered stress response and BP changes.
We extended these findings by examining the role of endogenous opioid mechanisms in pain via a double-blind, counterbalanced study using placebo or 50 mg of naltrexone (for opioid blockade) (71). This study demonstrated consistent responses to opioid blockade as indicated by increased plasma cortisol, ACTH, beta-endorphin, prolactin, and salivary cortisol levels. One intriguing observation resulting from this study is that women in the blockade condition reported reduced pain and BP. This was opposite to the expected effects; and it indicates differential effects of the opioid system on pain perception and BP in men and women, further supporting the sex-specific pattern of interactions between hemodynamic and central regulation of pain and hypertension risk (71, 150, 151).
In summary, results from this series of studies indicate that differential baroreflex, endogenous opioid activation, and descending pain modulation did not explain observed hypoalgesia in hypertension-prone individuals. Studies examining the role of the stress response and systems involved in this response provide evidence that altered stress-response may be involved in modifying pain sensitivity among hypertension-prone individuals. These studies also provide evidence that attenuated stress response may be associated with adverse effects, including increased pain sensitivity and discomfort. Therefore, it is possible that reactivity to stress is not only a manifestation of demands on the body, but that it may also shape how we process and respond to subsequent stressors or emotional challenges. Next, we relate these findings to a growing literature on the clinical significance of the blunted stress response.
Blunted Stress Response as a Risk Factor for Addiction Relapse
The role of stress in the maintenance of and relapse to drug abuse is well established (6, 152–163). For example, stress is one of the most commonly cited triggers of tobacco use and relapse (6, 164–168). Stress increases the frequency of smoking and accelerates progression towards a full relapse among abstinent smokers (169–173). These risks are particularly high in the presence of other negative affective states, including anxiety, irritability, depression, and craving (174–178).
Observations from other literatures provide examples of when hypoactivation of the stress response systems can be associated with adverse symptoms, including negative affect, distress, and pain perception. For example, blunted HPA stress response has been observed in individuals with alcoholic addiction and other substance over-users (179–181). Our hypertension studies excluded heavy alcohol and other drug users and we applied specific dietary instructions to limit potential confounding effects on the stress response (69, 182, 183). We also excluded smokers to avoid the acute effects of tobacco, or the distressing effects of withdrawal (if smokers were asked to abstain from smoking), on the stress response. This led us to question the impact of abstinence from smoking and whether it could serve as a natural stressor for people who are dependent on tobacco. Therefore, we set out to conduct a series of studies to examine the effects of withdrawal stress on mood, psychobiological response to acute stress, and performance measures under these conditions.
Nicotine withdrawal as a stressor
Abstinence from smoking is associated with numerous distressing symptoms, including craving, anxiety, depression, restlessness, irritability, and difficulty concentrating (184). Intensity of withdrawal symptoms is influenced by multiple factors, including: 1) length of time since the last cigarette; 2) level of nicotine dependence; 3) situational demands (e.g., acute stressors, demanding challenges, or smoking-related cues); and 4) the person’s disposition and skills (e.g., coping resources, history of psychopathology). Smoking abstinence may also lead to acute changes in various neurobiological systems, although their characteristics and consequences are not well understood. The detrimental effects of abstinence on mood are possibly modulated by these biological systems and they likely exacerbate the experience of stress and increase the probability of smoking resumption (165, 185). Therefore, we decided to test the hypothesis that psychophysiological responses to behavioral challenges are enhanced by short-term abstinence from smoking.
In one study, we collected physiological, cognitive, and self-report measures during rest and in response to acute stress among smokers after a period of smoking abstinence (18 hours) or after ad libitum smoking (186). We found that withdrawal produced significant distress symptoms and greater BP responses to the acute speech challenges. Withdrawal was also associated with worse cognitive performance. This set of results demonstrated a stress-like cluster of symptoms during nicotine withdrawal, as evidenced by changes in mood, performance, and BP responses (186). Yet, it was not known how long-term smoking and dependence alter these stress response systems and how withdrawal may influence long-term stress-related changes.
Acutely, nicotine stimulates adrenocortical and cardiovascular functions (187–189); and an additive effect of nicotine and acute stress has been documented. Chronic nicotine administration may lead to prolonged HPA activation, but little was known about long-term effects on stress response or how these responses may be modified by short-term abstinence. The latter question is important for elucidating the role of stress-related physiological changes in exacerbating withdrawal symptoms and in altering the rewarding properties of nicotine (190). Therefore, we examined differences in adrenocortical, cardiovascular, and mood reports during rest and in response to acute psychological stress (a combination of public speaking and mental math) among three groups: abstinent smokers, smokers who continued to smoke at their normal rate, and non-smokers (191). We compared these data to those obtained during a control session, when participants rested for the entire time in the lab, and found that smokers (smoking ad libitum or abstinence) showed attenuated systolic BP responses to public-speaking stressor when compared to non-smokers. Compared to non-smokers, smokers also exhibited a blunted cortisol response to stress (191).
These diminished systolic BP and cortisol responses to stress in smokers, independent of acute exposure to nicotine, indicate alterations in the stress response resulting from chronic exposure to nicotine that are independent of withdrawal. Frequent and prolonged stimulation of the HPA by repeated exposure to nicotine may lead to enhanced HPA activation, but reduced sensitivity to effects of other stimuli not related to nicotine (192); therefore, disrupting the ability of the HPA system to mount a normal response to acute stress. Mechanisms mediating the effects of chronic tobacco use on stress response systems likely involve multiple processes, including reduction of the number or affinity of nicotinic receptors mediating effects of nicotine in the PVN and other central nervous system structures (191). It is not clear if such changes may be normalized by long-term abstinence.
In summary, these initial studies demonstrated that smoking deprivation was associated with deterioration of mood and with exaggerated stress response, indicating a stress-like effect of abstinence. Furthermore, the long-term effects of smoking appear to be associated with dysregulation of the stress response, characterized by blunted HPA and other biological responses to stress. The relevance of these processes within clinical contexts were not known. The role of dysregulated stress response in increasing vulnerability for smoking relapse was investigated subsequently.
Blunted stress response and addiction relapse
Building on research demonstrating altered stress response among smokers (191–196), we conducted a series of studies to examine the extent to which this altered stress response predicts early smoking relapse (197–202). In one study (203), we tested smokers interested in quitting during a day when they were still smoking at their usual rate (ad libitum) and during the first day of their abstinence. Salivary cortisol levels and mood reports were assessed during the evening and morning over 24 hours of ad libitum smoking and during the first 24 hours of abstinence. Not surprisingly, participants reported significant withdrawal symptoms on the first day that they were abstinent. Smokers who relapsed during the first week of abstinence reported greater craving for cigarettes and greater overall distress during the first 24-hour period of abstinence than those who maintained abstinence over the same period. Though all participants showed the expected diurnal changes in cortisol levels, smokers who relapsed within the first week of the quit attempt exhibited a greater decline in cortisol levels on the abstinence day compared to the ad libitum day.
The association of exaggerated adrenocortical and mood perturbations during the first 24 hours of abstinence with early relapse indicated that processes underlying these measures may contribute to perceived benefits of smoking (204), possibly enhancing the reinforcing value of smoking after abstinence and increasing risk for early relapse (205). This, in turn, may contribute to early relapse, possibly driven by motivation to self-medicate (169, 206). Although this study focused on important stress-response systems during a critical period of a quit attempt, and although it prospectively examined the extent to which changes in obtained measures were associated with early relapse, it did not directly address the stress response.
Thus, we performed a subsequent study to examine HPA responses to stress following the first 24 hours of a quit attempt in predicting relapse over a four-week period (198). In addition to ACTH plasma and salivary cortisol concentrations, we collected a battery of cardiovascular measures and mood reports during rest and in response to acute psychological stressors (public speaking and mental arithmetic). After the quit day, participants attended four weekly follow-up sessions to measure relapse and to verify maintained abstinence from smoking. Smokers who relapsed within four weeks showed attenuated ACTH, beta-endorphin, cortisol, and BP responses to stress relative to those who successfully abstained over the same period. Those who relapsed also reported exaggerated withdrawal symptoms and mood deterioration during the initial withdrawal period. The link between stress response and relapse is further moderated by several additional factors, some of which I review below.
Moderators of the Blunted Stress Response in Nicotine Addiction
To identify factors that moderate the link between stress response and intensity of withdrawal and risk for relapse, our research has focused on both biological and situational factors. Specifically, we have examined sex differences, individual differences in emotional dispositions, adverse early experiences, and exposure to multiple substances.
Sex differences
There is accumulated evidence demonstrating sex differences in hormonal and cardiovascular responses to stress (71, 151, 207). Clinical and epidemiological reports also indicate that stress is more frequently reported by women, compared to men, with addiction problems; and stress is more frequently cited as a reason for drug use and relapse by women than by men (208–211). Environmental context and smoking-related cues (e.g., smell and taste) play a greater role in determining the perception of smoking effects in women than in men (166, 178, 212–216). Compared to men, women who smoke are more likely to use smoking to cope with negative affect and discomfort (217, 218). Women also report more distress after exposure to acutely stressful situations and to smoking-specific stimuli (176, 177, 219–221); and they are less likely to maintain long-term abstinence (222–228). Sex differences in the role of the opioid system in the stress response (229–233) and in responses to opioid blockade have also been reported, with women exhibiting greater HPA response to opioid blockade challenges (45, 234). Preclinical evidence also indicates sex differences in the sensitivity, quantity, and ratio of different classes of opioid receptors (231, 233, 235).
Our laboratories have examined sex differences in HPA and emotional dysregulation within the area of nicotine addiction. Our findings indicate that during withdrawal (early abstinence), women experience greater negative symptoms than men, especially during exposure to stress; and men exhibit enhanced stress and cortisol response (198). Our studies also document sex-related moderation of relapse predictors. For instance, blunted ACTH and cortisol responses to stress predict relapse among men, but not among women (166). In a recent study with a relatively large sample of smokers, we demonstrated that elevated salivary cortisol during the early abstinence phase predicted quicker relapse for women, while the opposite was true for men (214).
Emotional dispositions
Knowing that drugs may be used to manage intense emotions, and informed by research indicating that smokers with high levels of hostility may use cigarettes to cope with anger-provoking situations (236–238), our laboratories examined the roles of various factors related to emotion regulation and dispositions in shaping the stress response among smokers and other stimulant users during withdrawal. Through this research, we found that smokers who have a high level of trait anger tend to experience more anger during withdrawal and in response to stress (200). We also found that high anger was associated with increased withdrawal symptoms and craving during the first 24 hours of abstinence as well as with blunted ACTH stress response. Furthermore, we found evidence that high trait anger was associated with increased risk for early relapse. These findings are consistent with acute effects of stress in smokers and other substance users (178, 239, 240); and the findings indicate that high levels of trait anger predispose individuals to experience greater emotional intensity during withdrawal, possibly contributing to mood difficulties and vulnerability for drug use and early relapse.
Life adversity
Considerable research has linked adverse childhood events to substance use and to long-term effects of stress (241–243); and our research has examined the influence of adversities on smoking relapse among habitual smokers using prospective observations. In this research, we followed participants from pre-quit ad libitum smoking to four weeks post-quit; and a non-smoking group was included for comparisons. The results provide evidence that early life adversity not only predicts smoking but also increases challenges (i.e., increased craving) in abstinence and vulnerability for relapse. In addition, we found sex differences in the connection between life adversity and risk for relapse such that female relapsers tended to report experiencing greater life adversity than their male counterparts (244).
Overall, this research complements our and others’ research on stress response dysregulation in this vulnerable group of smokers. Indeed, previous research demonstrated that individuals who have a history of high adversity exhibit dysregulated responses to stress, characterized by blunted HPA responses and blunted diurnal fluctuation (245–248). It is possible that this blunted response is accentuated by exposure to substances contributing to increased vulnerability to relapse.
Polysubstances use
Co-use of multiple substances is a highly prevalent clinical challenge. Indeed, in the area of tobacco addiction, co-use of other substances, such as alcohol, cannabis, and other stimulants, is a significant impediment for cessation and a risk for relapse (157, 166, 249–252). Our laboratories recently conducted a series of studies focusing on the adverse effects of concurrent use of tobacco and the psychostimulant khat, a plant widely used in east Africa, the Middle East, and among immigrant communities around the world (253), on the stress response, affect regulation, and intensity of withdrawal symptoms. These studies demonstrate parallel findings to studies that focused on tobacco smokers. Furthermore, these studies indicate that co-use of tobacco and khat is associated with significant neuropsychological deficits, particularly working memory and attention deficits (254–256). These deficits appear to be related to the hypoactive stress response systems in this co-using population (257–259).
Dysregulated Stress Response Mediating Nicotine Effects on Appetite
Neurobiological systems involved in the stress response may be involved in regulating appetite as well. During stress, the HPA axis exerts various regulatory actions that influence several survival functions, including appetite regulation and eating behavior. For example, glucocorticoids may influence the reinforcing effects of food by influencing appetite-regulating peptides, such as leptin, orexin, and neuropeptide Y (NPY) (260). In addition, evidence suggests that HPA and other reward-related circuitry may be involved in increasing consumption of calorie-dense food items under conditions of stress (260). As an example, observations in human studies show that participants who exhibit enhanced cortisol response to acute stress tend to consume more calories relative to low cortisol responders. Furthermore, negative affect responses to stress are associated with greater food consumption (261, 262). The mechanisms of this connection between stress and food selection may involve an interaction of reward and stress response pathways. Both stress and palatable food stimulate endogenous opioid release. Peptides modulating HPA axis activity, including the appetite-regulating peptides leptin, ghrelin, orexin, and NPY, are also associated with depressive mood, stress, and addictive behavior (263–266).
A related line of research has established convincing evidence of an inverse association between cigarette smoking and body weight (267–269). Failure to quit smoking is primarily attributed to the addictive properties of nicotine, but the fear of weight gain after cessation of smoking is often a contributing factor (270, 271). The mechanisms mediating the association between smoking cessation and weight gain likely include changes in 1) appetite–related neurobiological and peripheral regulation (272); 2) appetite that leads to increased intake of a high energy diet (e.g., fat and carbohydrates) (273, 274); and 3) metabolic rate (reductions) in the absence of nicotine (275)(276).
Informed by the literature on the long-term effects of nicotine and stress on appetite (277, 278), and encouraged by research linking smoking, craving, and mood with changes in appetite hormones (279, 280), we conducted a series of studies to examine the links between stress response, appetite hormones, and smoking relapse. The results of these studies provide evidence that leptin levels significantly increase following exposure to acute stress (281) and that leptin is associated positively with craving for cigarettes and with other mood states (279). These findings suggest that leptin levels may be a useful marker of the stress response and of craving for smoking. We also found that increased levels of the appetite reducing peptide YY after the first 24 hours of smoking abstinence is associated with decreases in reported craving and increases in positive affect, while higher ghrelin levels are associated with early relapse (282). When we examined changes in leptin from pre to 48 hours post smoking abstinence, we found increased leptin concentrations in women who were successful in cessation for four weeks (283).
Mechanisms for Dysregulated Stress Response in Addiction
Research that focuses on identifying pathways that explain blunted stress response in individuals with addictive problems is a critical step towards identifying potential therapeutic strategies. Our efforts to understand the blunted stress response were guided by three specific criteria: 1) a potential pathway must have a robust basic-science literature demonstrating its role in regulating the stress response, 2) such a pathway should also be directly involved in regulating the effects of substance use, and 3) such a pathway can be studied safely in humans and be subjected to experimental studies in the context of clinical research.
In addition to the HPA axis and the catecholaminergic system, stress activates the EOS (21, 71, 121, 284). Three key points of contact in the central nervous system play a role in the interactions between opioid neurons and structures involved in the stress response and reward regulation (22, 29, 43, 285). These include the locus coeruleus in the brainstem, the PVN of the hypothalamus, and the nucleus accumbens. Opioid neurons typically stimulate dopaminergic neurons, while inhibiting neurons within the PVN and the locus coeruleus, which leads to reduced CRF and catecholamine release (12, 286, 287). These effects indicate a regulatory effect of the EOS on the stress response, emotion regulation, and reward. Considering the role of the EOS in many addictive behaviors (19, 288), it is possible that chronic exposure to substances may alter the functions of this system and, consequently, may influence regulation of the stress response.
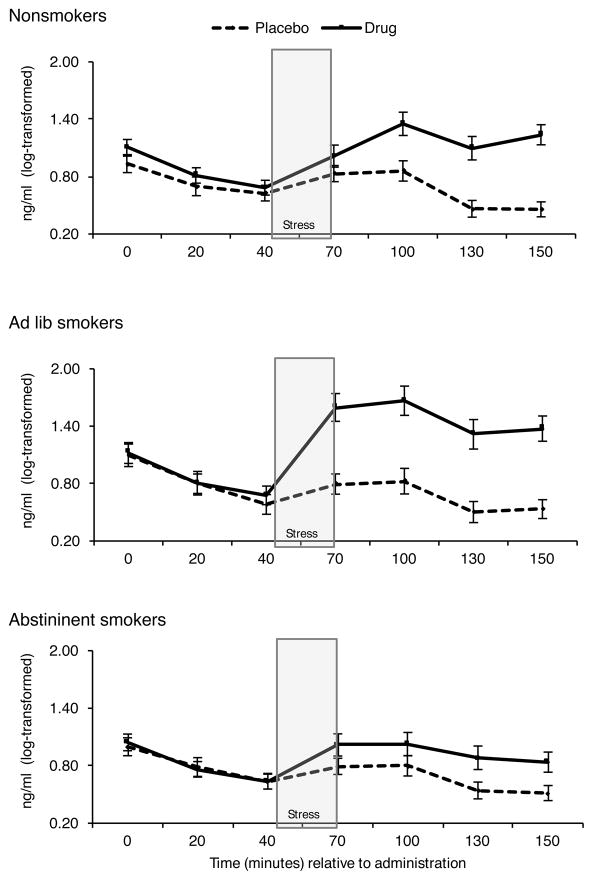
Guided by this research and our criteria (outlined above), we conducted a series of studies to examine the integrity of the EOS regulation of the HPA axis during exposure to pain and stress among dependent cigarette smokers and non-smoker controls (70–72, 110, 289–292). In all of these studies, we used the opioid blockade naltrexone within a double-blind crossover design. We hypothesized that because the EOS naturally inhibits HPA activity, naltrexone should increase HPA activity. This was the case across all studies; however, smokers exhibited a blunted HPA response to the EOS blockade, particularly during stress. Furthermore, blunted HPA responses to the EOS blockade and stress were particularly pronounced during smoking withdrawal. Interestingly, in a recent study, we found that smokers who were provided a cigarette to smoke during the laboratory protocol (so that they were not deprived from smoking) showed the most robust response to opioid blockade (see Figure 2). This suggests that availability of nicotine in the system may help normalize the EOS-HPA stress response regulation. Consistent with this idea, it is possible that a rewarding motivation for smoking is to normalize the stress response by normalizing the EOS’s regulation of this response.
Figure 2.
Effects of opioid blockade on cortisol response to stress was blunted in smokers who were deprived from nicotine, but it was enhanced in smokers who were not deprived from smoking relative to nonsmokers.
In addition to the role of the EOS, research has pointed to other factors that may contribute to a blunted stress response, including reduced effort to engage in stress challenges (293, 294), dampened central motivational processes (245, 295), and attenuated response to the rewarding effects of various activities (294). Theoretical discussions of the attenuated stress responses have also incorporated this stress-response dysregulation in the context of broader models of vulnerability (e.g., (296)), suggesting that blunted overall physiological arousal in emotion-related systems may be related to genetic and epigenetic variations that contribute to alterations in physiological systems involved in the stress response. These changes in the neurobiological systems of the stress response may disrupt affective, cognitive, and interpersonal processes, thereby increasing risk for maladaptive behaviors, such as substance use. Furthermore, social stress and early life adversity may exacerbate these risk-related processes. Early life adversity and certain traits, such as impulsivity, disinhibited temperament, and sensation seeking, may also contribute to both blunted response and to risky behaviors (245, 293–296).
Discussion and Conceptual Model
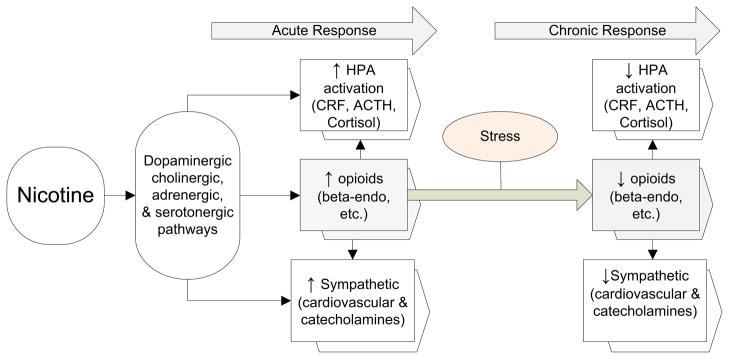
The research on stress and addiction reviewed above was guided by a heuristic model that links HPA dysregulation and opioid hypoactivity as a result of long-term exposure to nicotine. We hypothesize, as indicated in Figure 3, that acutely smoking and using other drugs of abuse increases indices of the HPA, endogenous opioid, and sympathetic systems.
Figure 3.
A heuristic model that depicts effects of drug use on the HPA, endogenous opioid, and sympathetic systems. Long-term effects of drug use may lead to neuronal adaptation that may eventually contribute to neurobiological vulnerability that increase drug use and risk for relapse.
After long-term, intermittent activation by ongoing drug use, changes ensue that lead to neuronal adaptation. This adaptation includes reduced opioid input to the PVN; enhanced basal HPA activation; and reduced stress (HPA), opioid, and sympathetic responses. These alterations become pronounced during cessation, with diminished activity in these systems increasing craving and exacerbating abstinence-related negative affect. This culminates in an increased vulnerability to relapse. Therefore, cessation may unmask underlying dysregulation of the stress response systems, leading to reduced HPA responses to stress and reduced opioid feedback to the PVN. The result is a maladaptive response to stress that may exacerbate withdrawal symptoms and reduce ability to cope effectively with acute stress. Increased sensitivity to negative affect and physical symptoms has recently been linked to low HPA response to stress (297, 298), possibly as a result of poorer counter-regulation by cortisol feedback to critical CRF- and other systems that regulate the stress response (299). These results are consistent with increased craving that may enhance the reinforcing properties of drug use.
In addition to this indirect evidence, our proposed model is supported by neurobiological studies of drug addiction that (a) indicate chronic drug use produces neuronal adaptations in multiple brain systems, and (b) suggest drug deprivation and neurobiological changes after long-term exposure to drugs may produce strong motivational states leading to maintenance of drug use (300–308).
Conclusions
Research reviewed here demonstrates that a blunted stress response may indicate a long-term, adverse effect of substance use on brain functions that may play an etiologic role in various mood, addictive, and cognitive processes. A blunted stress response is likely to contribute to maintenance of addictive behaviors and increase risk for relapse. Psychosocial stress and early life adversity may prime the brain to be sensitive to subsequent exposure to stress and to the impact of substance use. Research suggests that normalizing the biological stress response may help with regulating mood, cognition, and coping with pain and discomfort– all of which are issues associated with blunted stress response. We also propose that the ability to maintain a robust biological stress response in the face of psychosocial adversity or chronic exposure to substance use should be considered an indicator of resiliency. Indeed, our hormonal findings from multiple studies and populations indicate that a robust stress response buffers negative affect, withdrawal symptoms, craving, and risk for relapse. Accumulating preclinical evidence on neurobiological pathways mediating the stress response provides indirect evidence that a robust stress response is a marker of resiliency (309–314). Future work must probe the role of specific stress-response biological pathways (e.g., blunted HPA and EOS stress responses) in risk for, and maintenance of, substance use and in related mood disturbances. This should facilitate subsequent research into specific therapeutic approaches to address stress-response dysregulation and its impact on mental and behavioral health.
Table 1.
Key findings reviewed in this paper
|
Acknowledgments
Source of Funding:
This work was supported by the following NIH grants: NIH R01DA013435, NIH R01DA016351, NIH R21CA88272, NIH R01DA027232, NIH R03TW007219, NIH R21DA024626, and NIH R01HL64794. It was also supported by an AHA grant award #0355487Z.
List of acronyms
- DHEA
dehydroepiandrosterone
- CRF
corticotropin-releasing factor
- PVN
paraventricular nucleus
- BP
blood pressure
- VTA
ventral tegmental area
- ACC
anterior cingulate cortex
- NA
nucleus accumbens
- PFC
prefrontal cortex
- HPA
hypothalamic-pituitary-adrenocortical axis
- ACTH
adrenocorticotropic hormone
- GABA
gamma-aminobutyric acid
- EOS
endogenous opioid system
- CPT
cold pressor test
- NPY
neuropeptide Y
- DA
dopamine
- CNS
central nervous system
References
- 1.Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. AnnNYAcadSci. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. The brain is an important target of adrenal steroid actions. A comparison of synthetic and natural steroids. Ann N Y Acad Sci. 1997;823:201–13. doi: 10.1111/j.1749-6632.1997.tb48392.x. [DOI] [PubMed] [Google Scholar]
- 3.Chrousos GP, Gold PW. The Concepts of Stress and Stress System Disorders - Overview of Physical and Behavioral Homeostasis. Jama-Journal of the American Medical Association. 1992;267:1244–52. [PubMed] [Google Scholar]
- 4.Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–92. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 5.al’Absi M, Arnett DK. Adrenocortical responses to psychological stress and risk for hypertension. Biomed Pharmacother. 2000;54:234–44. doi: 10.1016/S0753-3322(00)80065-7. [DOI] [PubMed] [Google Scholar]
- 6.al’Absi M. Stress and Addiction: Biological and Psychological Mechanisms. London: Academic Press/Elsevier; 2007. [Google Scholar]
- 7.McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 8.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. BiolPsychiatry. 2000;48:755–65. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 9.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. EndocrRev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 10.Dallman M. Stress Update: Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends in Endocrinology and Metabolism. 1993;4:62–9. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- 11.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrinology Review. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 12.Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. ProgNeuropsychopharmacolBiolPsychiatry. 2001;25:729–41. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- 13.Martin del Campo AF, Dowson JH, Herbert J, Paykel ES. Effects of naloxone on diurnal rhythms in mood and endocrine function: a dose-response study in man. Psychopharmacology (Berl) 1994;114:583–90. doi: 10.1007/BF02244988. [DOI] [PubMed] [Google Scholar]
- 14.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. JAffectDisord. 2000;61:201–16. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 15.Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. AnnNYAcadSci. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- 16.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. EurJPain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Ableitner A, Schulz R. Neuroanatomical sites mediating the central actions of beta-endorphin as mapped by changes in glucose utilization: involvement of mu opioid receptors. JPharmacolExpTher. 1992;262:415–23. [PubMed] [Google Scholar]
- 18.Pasternak GW. Molecular biology of opioid analgesia. JPain SymptomManage. 2005;29:S2–S9. doi: 10.1016/j.jpainsymman.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. CurrTopMedChem. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- 20.Rushen J, Schwartze N, Ladewig J, Foxcroft G. Opioid modulation of the effects of repeated stress on A.C.T.H., cortisol, prolactin, and growth hormone in pigs. Physiology and Behavior. 1993;53:923–8. doi: 10.1016/0031-9384(93)90270-p. [DOI] [PubMed] [Google Scholar]
- 21.Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. AnnuRevNeurosci. 1984;7:223–55. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- 22.Wand GS, Schumann H. Relationship between plasma adrenocorticotropin, hypothalamic opioid tone, and plasma leptin. J ClinEndocrinolMetab. 1998;83:2138–42. doi: 10.1210/jcem.83.6.4900. [DOI] [PubMed] [Google Scholar]
- 23.Morris M, Salmon P, Steinberg H, Sykes EA, Bouloux P, Newbould E, McLoughlin L, Besser GM, Grossman A. Endogenous opioids modulate the cardiovascular response to mental stress. Psychoneuroendocrinology. 1990;15:185–92. doi: 10.1016/0306-4530(90)90029-9. [DOI] [PubMed] [Google Scholar]
- 24.Marinelli M. Dopaminergic reward pathways and effects of stress. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. London: Academic Press/Elsevier; 2007. pp. 41–83. [Google Scholar]
- 25.Piazza P, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have ste dependent stimulant effects on the mesencephalic dopaminergic transmission. Neurobiology. 1996;93:8716–20. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. EurJNeurosci. 2000;12:973–9. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- 27.Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. NeurochemInt. 1995;26:305–36. doi: 10.1016/0197-0186(94)00139-l. [DOI] [PubMed] [Google Scholar]
- 28.Cowen MS, Lawrence AJ. The role of opioid-dopamine interactions in the induction and maintenance of ethanol consumption. ProgNeuropsychopharmacolBiolPsychiatry. 1999;23:1171–212. doi: 10.1016/s0278-5846(99)00060-3. [DOI] [PubMed] [Google Scholar]
- 29.Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–58. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–72. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Avila CA, Oncken C, Van Kirk J, Wand G, Kranzler HR. Adrenocorticotropin and cortisol responses to a naloxone challenge and risk of alcoholism. BiolPsychiatry. 2002;51:652–8. doi: 10.1016/s0006-3223(01)01334-8. [DOI] [PubMed] [Google Scholar]
- 32.Munro CA, Oswald LM, Weerts EM, McCaul ME, Wand GS. Hormone responses to social stress in abstinent alcohol-dependent subjects and social drinkers with no history of alcohol dependence. Alcohol ClinExpRes. 2005;29:1133–8. doi: 10.1097/01.alc.0000172459.71517.05. [DOI] [PubMed] [Google Scholar]
- 33.Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol. 2006;59:195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellbach S, Gartner P, Deicke J, Fischer D, Hassan AH, Almeida OF. Inherent glucocorticoid response potential of isolated hypothalamic neuroendocrine neurons. FASEB J. 1998;12:199–207. doi: 10.1096/fasebj.12.2.199. [DOI] [PubMed] [Google Scholar]
- 35.Tsagarakis S, Rees LH, Besser M, Grossman A. Opiate receptor subtype regulation of CRF-41 release from rat hypothalamus in vitro. Neuroendocrinology. 1990;51:599–605. doi: 10.1159/000125397. [DOI] [PubMed] [Google Scholar]
- 36.Henderson G. Electrophysiological analysis of opioid action in the central nervous system. BrMedBull. 1983;39:59–64. doi: 10.1093/oxfordjournals.bmb.a071792. [DOI] [PubMed] [Google Scholar]
- 37.Valentino RJ, Van Bockstaele E. Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology (Berl) 2001;158:331–42. doi: 10.1007/s002130000673. [DOI] [PubMed] [Google Scholar]
- 38.Singewald N, Philippu A. Release of neurotransmitters in the locus coeruleus. ProgNeurobiol. 1998;56:237–67. doi: 10.1016/s0301-0082(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 39.Christie MJ. Mechanisms of opioid actions on neurons of the locus coeruleus. ProgBrain Res. 1991;88:197–205. doi: 10.1016/s0079-6123(08)63809-1. [DOI] [PubMed] [Google Scholar]
- 40.Jackson RV, Grice JE, Jackson AJ, Hockings GI. Naloxone-induced ACTH release in man is inhibited by clonidine. ClinExpPharmacolPhysiol. 1990;17:179–84. doi: 10.1111/j.1440-1681.1990.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 41.Herkenham M, Edley SM, Stuart J. Cell clusters in the nucleus accumbens of the rat, and the mosaic relationship of opiate receptors, acetylcholinesterase and subcortical afferent terminations. Neuroscience. 1984;11:561–93. doi: 10.1016/0306-4522(84)90045-9. [DOI] [PubMed] [Google Scholar]
- 42.Devine DP, Leone P, Wise RA. Mesolimbic dopamine neurotransmission is increased by administration of mu-opioid receptor antagonists. EurJPharmacol. 1993;243:55–64. doi: 10.1016/0014-2999(93)90167-g. [DOI] [PubMed] [Google Scholar]
- 43.Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The Micro-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31:204–11. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- 44.Uhart M, Oswald L, McCaul ME, Chong R, Wand GS. Hormonal responses to psychological stress and family history of alcoholism. Neuropsychopharmacology. 2006;31:2255–63. doi: 10.1038/sj.npp.1301063. [DOI] [PubMed] [Google Scholar]
- 45.Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31:642–52. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Burnett FE, Scott LV, Weaver MG, Medbak SH, Dinan TG. The effect of naloxone on adrenocorticotropin and cortisol release: evidence for a reduced response in depression. J AffectDisord. 1999;53:263–8. doi: 10.1016/s0165-0327(98)00127-x. [DOI] [PubMed] [Google Scholar]
- 47.Facchinetti F, Fioroni L, Martignoni E, Sances G, Costa A, Genazzani AR. Changes of opioid modulation of the hypothalamo-pituitary-adrenal axis in patients with severe premenstrual syndrome. PsychosomMed. 1994;56:418–22. doi: 10.1097/00006842-199409000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Fioroni L, Martignoni E, Facchinetti F. Changes of neuroendocrine axes in patients with menstrual migraine. Cephalalgia. 1995;15:297–300. doi: 10.1046/j.1468-2982.1995.1504297.x. [DOI] [PubMed] [Google Scholar]
- 49.Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000;23:411–8. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 50.Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: a review and methodologic critique. PsycholBull. 1984;96:435–64. [PubMed] [Google Scholar]
- 51.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Turner JR, Ward MM, Gellman MD, Johnston DW, Light KC, van Doornen LJ. The relationship between laboratory and ambulatory cardiovascular activity: current evidence and future directions. AnnBehavMed. 1994;16:12–23. [Google Scholar]
- 53.Kamarck TW, Debski TT, Manuck SB. Enhancing the laboratory-to-life generalizability of cardiovascular reactivity using multiple occasions of measurement. Psychophysiology. 2000;37:533–42. [PubMed] [Google Scholar]
- 54.Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: conceptual and measurement considerations. PsychosomMed. 2003;65:9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- 55.Kamarck TW, Schwartz JE, Janicki DL, Shiffman S, Raynor DA. Correspondence between laboratory and ambulatory measures of cardiovascular reactivity: a multilevel modeling approach. Psychophysiology. 2003;40:675–83. doi: 10.1111/1469-8986.00069. [DOI] [PubMed] [Google Scholar]
- 56.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–32. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 57.Everson SA, Kaplan GA, Goldberg DE, Salonen JT. Anticipatory blood pressure response to exercise predicts future high blood pressure in middle-aged men. Hypertension. 1996;27:1059–64. doi: 10.1161/01.hyp.27.5.1059. [DOI] [PubMed] [Google Scholar]
- 58.Markovitz JH. Cardiovascular risk factors in rapidly developing countries. AnnEpidemiol. 1998;8:1–2. doi: 10.1016/s1047-2797(97)00176-2. [DOI] [PubMed] [Google Scholar]
- 59.Carroll D, Ring C, Hunt K, Ford G, Macintyre S. Blood pressure reactions to stress and the prediction of future blood pressure: effects of sex, age, and socioeconomic position. Psychosom Med. 2003;65:1058–64. doi: 10.1097/01.psy.0000097330.58739.26. [DOI] [PubMed] [Google Scholar]
- 60.Matthews KA, Zhu S, Tucker DC, Whooley MA. Blood pressure reactivity to psychological stress and coronary calcification in the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2006;47:391–5. doi: 10.1161/01.HYP.0000200713.44895.38. [DOI] [PubMed] [Google Scholar]
- 61.al’Absi M, Devereux RB, Rao DC, Kitzman D, Oberman A, Hopkins P, Arnett DK. Blood pressure stress reactivity and left ventricular mass in a random community sample of African-American and caucasian men and women. American Journal of Cardiology. United States. 2006:240–4. doi: 10.1016/j.amjcard.2005.07.134. [DOI] [PubMed] [Google Scholar]
- 62.al’Absi M, Devereux RB, Lewis CE, Kitzman DW, Rao DC, Hopkins P, Markovitz J, Arnett DK. Blood pressure responses to acute stress and left ventricular mass (The Hypertension Genetic Epidemiology Network Study) Am J Cardiol. 2002;89:536–40. doi: 10.1016/s0002-9149(01)02305-0. [DOI] [PubMed] [Google Scholar]
- 63.Cinciripini P. Cognitive stress and cardiovascular reactivity. II. Relationship to atherosclerosis, arrhythmias, and cognitive control. American Heart journal. 1986;112:1051–65. doi: 10.1016/0002-8703(86)90320-0. [DOI] [PubMed] [Google Scholar]
- 64.Van Egeren LF, Sparrow AW. Labortory stress testing to assess real-life cardiovascular reactivity. Psychosom Med. 1989;51:1–9. doi: 10.1097/00006842-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Emmons KM, Weidner G. The effects of cognitive and physical stress on cardiovascular reactivity among smokers and oral contraceptive users. Med Clin North Am. 1988;76:289–303. doi: 10.1111/j.1469-8986.1988.tb00981.x. [DOI] [PubMed] [Google Scholar]
- 66.al’Absi M, Lovallo WR. Cortisol concentrations in serum of borderline hypertensive men exposed to a novel experimental setting. Psychoneuroendocrinology. 1993;18:355–63. doi: 10.1016/0306-4530(93)90011-9. [DOI] [PubMed] [Google Scholar]
- 67.al’Absi M, Lovallo WR, McKey BS, Pincomb GA. Borderline hypertensives produce exaggerated adrenocortical responses to mental stress. Psychosom Med. 1994;56:245–50. doi: 10.1097/00006842-199405000-00011. [DOI] [PubMed] [Google Scholar]
- 68.al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–75. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- 69.al’Absi M, Lovallo WR, Pincomb GA, Sung BH, Wilson MF. Adrenocortical effects of caffeine at rest and during mental stress in borderline hypertensive men. Int J Behav Med. 1995;2:263–75. doi: 10.1207/s15327558ijbm0203_5. [DOI] [PubMed] [Google Scholar]
- 70.Shaw D, al’Absi M. Blunted opiate modulation of prolactin response in smoking men and women. Pharmacol Biochem Behav. 2010;95:1–5. doi: 10.1016/j.pbb.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66:198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- 72.Ceballos NA, France CR, al’Absi M. Influence of naltrexone administration on dehydroepiandrosterone sulfate levels in male and female participants. Biological Psychology. Netherlands. 2007:414–6. doi: 10.1016/j.biopsycho.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Ceballos NA, al’Absi M. Dehydroepiandrosterone sulfate, cortisol, mood state and smoking cessation: relationship to relapse status at 4-week follow-up. Pharmacol Biochem Behav. 2006;85:23–8. doi: 10.1016/j.pbb.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 74.Shaw D, al’Absi M. Attenuated beta endorphin response to acute stress is associated with smoking relapse. 2008;90:357–62. doi: 10.1016/j.pbb.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Julius S, Nesbitt S. Sympathetic overactivity in hypertension. A moving target. American Journal of Hypertension. 1996;9:113S–20S. doi: 10.1016/0895-7061(96)00287-7. [DOI] [PubMed] [Google Scholar]
- 76.Marcus R, Krause L, Weder AB, Dominguez-Meja A, Schork NJ, Julius S. Sex-specific determinants of increased left ventricular mass in the Tecumseh Blood Pressure Study. Circulation. 1994;90:928–36. doi: 10.1161/01.cir.90.2.928. [DOI] [PubMed] [Google Scholar]
- 77.Julius S, Petrin J. Autonomic nervous and behavioral factors in hypertension: A rationale for treatment. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. New York: Raven Press; 1990. pp. 2083–90. [Google Scholar]
- 78.Schneider RH, Julius S, Karunas R. Ambulatory blood preasure monitering and laboratory reactivity in Type A behavior and components. Psychosom Med. 1989;51:290–305. doi: 10.1097/00006842-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 79.Julius S. Transition from high cardiac output to elevated vascular resistance in hypertension. American Heart journal. 1988;116:600–6. doi: 10.1016/0002-8703(88)90557-1. [DOI] [PubMed] [Google Scholar]
- 80.Julius S, Schork N, Schork A. Sympathetic hyperactivity in early stages of hypertension: The Ann Arbor data set. Journal of Cardiovascular Pharmacology. 1988;12(suppl):S121–S9. [PubMed] [Google Scholar]
- 81.Schneider RH, Egan BM, Johnson EH, Drobny H, Julius S. Anger and anxiety in borderline hypertension. Psychosom Med. 1986;48:242–8. doi: 10.1097/00006842-198603000-00009. [DOI] [PubMed] [Google Scholar]
- 82.Schwartz AR, Gerin W, Davidson KW, Pickering TG, Brosschot JF, Thayer JF, Christenfeld N, Linden W. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. PsychosomMed. 2003;65:22–35. doi: 10.1097/01.psy.0000046075.79922.61. [DOI] [PubMed] [Google Scholar]
- 83.van Doornen LJ, van Blokland RW. The relationship between cardiovascular and catecholamine reactions to laboratory and real-life stress. Psychophysiology. 1992;29:173–81. doi: 10.1111/j.1469-8986.1992.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 84.Matthews KA, Owens JF, Allen MT, Stoney CM. Do cardiovascular responses to laboratory stress relate to ambulatory blood pressure levels?: Yes, in some of the people, some of the time. PsychosomMed. 1992;54:686–97. doi: 10.1097/00006842-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 85.Kapuku GK, Treiber FA, Davis HC, Harshfield GA, Cook BB, Mensah GA. Hemodynamic function at rest, during acute stress, and in the field: predictors of cardiac structure and function 2 years later in youth. Hypertension. 1999;34:1026–31. doi: 10.1161/01.hyp.34.5.1026. [DOI] [PubMed] [Google Scholar]
- 86.van Doornen LJ, Turner JR. The ecological validity of laboratory stress testing. In: Turner JR, Sherwood A, Light KC, editors. Indivudial Differences in Cardiovascular Responce to Stress. New York: Plenum Press; 1992. pp. 63–83. [Google Scholar]
- 87.Zanstra YJ, Johnston DW. Cardiovascular reactivity in real life settings: measurement, mechanisms and meaning. BiolPsychol. 2011;86:98–105. doi: 10.1016/j.biopsycho.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.al’Absi M, Lovallo WR, McKey B, Sung BH, Whitsett TL, Wilson MF. Hypothalamic-pituitary-adrenocortical responses to psychological stress and caffeine in men at high and low risk for hypertension. Psychosom Med. 1998;60:521–7. doi: 10.1097/00006842-199807000-00021. [DOI] [PubMed] [Google Scholar]
- 89.Walker BR, Phillips DI, Noon JP, Panarelli M, Andrew R, Edwards HV, Holton DW, Seckl JR, Webb DJ, Watt GC. Increased glucocorticoid activity in men with cardiovascular risk factors. Hypertension. 1998;31:891–5. doi: 10.1161/01.hyp.31.4.891. [DOI] [PubMed] [Google Scholar]
- 90.Mulatero P, Panarelli M, Schiavone D, Rossi A, Mengozzi G, Kenyon CJ, Chiandussi L, Veglio F. Impaired cortisol binding to glucocorticoid receptors in hypertensive patients. Hypertension. 1997;30:1274–8. doi: 10.1161/01.hyp.30.5.1274. [DOI] [PubMed] [Google Scholar]
- 91.Walker BR. Abnormal glucocorticoid activity in subjects with risk factors for cardiovascular disease. Endocr Res. 1996;22:701–8. doi: 10.1080/07435809609043765. [DOI] [PubMed] [Google Scholar]
- 92.Shepard JD, al’Absi M, Whitsett TL, Passey RB, Lovallo WR. Additive pressor effects of caffeine and stress in male medical students at risk for hypertension. AmJHypertens. 2000;13:475–81. doi: 10.1016/s0895-7061(99)00217-4. [DOI] [PubMed] [Google Scholar]
- 93.al’Absi M, Everson S, Lovallo WR. Hypertension risk factors and cardiovascular reactivity to mental stress in men. International Journal of Psychophysiology. 1995;20:155–60. doi: 10.1016/0167-8760(95)00029-1. [DOI] [PubMed] [Google Scholar]
- 94.al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–75. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- 95.Chapman CR, Bradshaw DH, Donaldson GW, Jacobson RC, Nakamura Y. Central noradrenergic mechanisms and the acute stress response during painful stimulation. J Psychopharmacol. 2014;28:1135–42. doi: 10.1177/0269881114543718. [DOI] [PubMed] [Google Scholar]
- 96.Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain. 2008;9:122–45. doi: 10.1016/j.jpain.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26:303–7. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 98.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 99.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 100.al’Absi M, Petersen KL. Blood pressure but not cortisol mediates stress effects on subsequent pain perception in healthy men and women. Pain. 2003;106:285–95. doi: 10.1016/S0304-3959(03)00300-2. [DOI] [PubMed] [Google Scholar]
- 101.Butler RK, Finn DP. Stress-induced analgesia. ProgNeurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 102.Janssen SA, Spinhoven P, Brosschot JF. Experimentally induced anger, cardiovascular reactivity, and pain sensitivity. J Psychosom Res. 2001;51:479–85. doi: 10.1016/s0022-3999(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 103.al’Absi M, Wittmers L, Hatsukami D, Westra R. Blunted Opiate Modulation of Hypothalamic-Pituitary-Adrenocortical Activity in Men and Women Who Smoke. 2008;70:928. doi: 10.1097/PSY.0b013e31818434ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–85. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 105.France CR. Decreased pain perception and risk for hypertension: considering a common physiological mechanism. Psychophysiology. 1999;36:683–92. [PubMed] [Google Scholar]
- 106.Guasti L, Zanotta D, Mainardi LT, Petrozzino MR, Grimoldi P, Garganico D, Diolisi A, Gaudio G, Klersy C, Grandi AM, Simoni C, Cerutti S. Hypertension-related hypoalgesia, autonomic function and spontaneous baroreflex sensitivity. AutonNeurosci. 2002;99:127–33. doi: 10.1016/s1566-0702(02)00052-8. [DOI] [PubMed] [Google Scholar]
- 107.Ghione S. Hypertension-associated hypalgesia. Evidence in experimental animals and humans, pathophysiological mechanisms, and potential clinical consequences. Hypertension. 1996;28:494–504. doi: 10.1161/01.hyp.28.3.494. [DOI] [PubMed] [Google Scholar]
- 108.France C, Ditto B, Adler P. Pain sensitivity in offspring of hypertensives at rest and during baroreflex stimulation. Journal of Behavioral Medicine. 1991;14:513–25. doi: 10.1007/BF00845108. [DOI] [PubMed] [Google Scholar]
- 109.al’Absi M, Buchanan T, Lovallo WR. Pain perception and cardiovascular responses in men with positive parental history for hypertension. Psychophysiology. 1996;33:655–61. doi: 10.1111/j.1469-8986.1996.tb02361.x. [DOI] [PubMed] [Google Scholar]
- 110.France CR, al’Absi M, Ring C, France JL, Harju A, Wittmers LE. Nociceptive flexion reflex and pain rating responses during endogenous opiate blockade with naltrexone in healthy young adults. Biol Psychol. 2007;75:95–100. doi: 10.1016/j.biopsycho.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ring C, France CR, al’Absi M, Beesley L, Edwards L, McIntyre D, Carroll D, Martin U. Effects of opioid blockade with naltrexone and distraction on cold and ischemic pain in hypertension. J Behav Med. 2007;30:59–68. doi: 10.1007/s10865-006-9084-1. [DOI] [PubMed] [Google Scholar]
- 112.al’Absi M, France CR, Ring C, France J, Harju A, McIntyre D, Wittmers LE. Nociception and baroreceptor stimulation in hypertension-prone men and women. Psychophysiology. 2005;42:83–91. doi: 10.1111/j.1469-8986.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 113.Ring C, France CR, al’Absi M, Edwards L, McIntyre D, Carroll D, Martin U. Effects of naltrexone on electrocutaneous pain in patients with hypertension compared to normotensive individuals. Biol Psychol. 2008;77:191–6. doi: 10.1016/j.biopsycho.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edwards L, Ring C, France CR, al’Absi M, McIntyre D, Carroll D, Martin U. Nociceptive flexion reflex thresholds and pain during rest and computer game play in patients with hypertension and individuals at risk for hypertension. Biol Psychol. 2007;76:72–82. doi: 10.1016/j.biopsycho.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lovallo WR, al’Absi M. Hemodynamics during rest and behavioral stress in normotensive men at high risk for hypertension. Psychophysiology. 1998;35:47–53. [PubMed] [Google Scholar]
- 116.Marrero AF, al’Absi M, Pincomb GA, Lovallo WR. Men at risk for hypertension show elevated vascular resistance at rest and during mental stress. Int J Psychophysiol. 1997;25:185–92. doi: 10.1016/s0167-8760(96)00737-4. [DOI] [PubMed] [Google Scholar]
- 117.al’Absi M, Wittmers LE. Enhanced adrenocortical responses to stress in hypertension-prone men and women. Ann Behav Med. 2003;25:25–33. doi: 10.1207/S15324796ABM2501_04. [DOI] [PubMed] [Google Scholar]
- 118.McCubbin JA, Bruehl S. Do endogenous opioids mediate the relationship between blood pressure and pain sensitivity in normotensives? Pain. 1994;57:63–7. doi: 10.1016/0304-3959(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 119.McCubbin JA. Stress induced endogenous opioids: Behavioral and circulatory interactions. Biological Psychology. 1993;35:91–122. doi: 10.1016/0301-0511(93)90008-v. [DOI] [PubMed] [Google Scholar]
- 120.McCubbin JA, Cheung R, Montgomery TB, Bulbulian R, Wilson JF. Opiodergic inhibition of circulatory and endocrine stress responses in cynomolgus monkeys: A preliminary study. Psychosomatic Medicine. 1993;55:23–8. doi: 10.1097/00006842-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 121.al’Absi M, France C, Harju A, France J, Wittmers L. Adrenocortical and nociceptive responses to opioid blockade in hypertension-prone men and women. Psychosom Med. 2006;68:292–8. doi: 10.1097/01.psy.0000203240.64965.bd. [DOI] [PubMed] [Google Scholar]
- 122.al’Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002 doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- 123.Lariviere WR, Melzack R. The role of corticotropin-releasing factor in pain and analgesia. Pain. 2000;84:1–12. doi: 10.1016/S0304-3959(99)00193-1. [DOI] [PubMed] [Google Scholar]
- 124.Hargreaves KM, Flores CM, Dionne RA, Mueller GP. The role of pituitary beta-endorphin in mediating corticotropin-releasing factor-induced antinociception. American Journal of Physiology. 1990:258. doi: 10.1152/ajpendo.1990.258.2.E235. [DOI] [PubMed] [Google Scholar]
- 125.Lautenbacher S, Roscher S, Kohl G, Vedder H, Krieg J. Corticotropin-releasing-hormone lacks analgesic properties: an experimental study in humans, using non-inflammatory pain. Pain. 1999;83:1–7. doi: 10.1016/s0304-3959(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 126.Griep EN, Boersma JW, de Kloet ER. Altered reactivity of the hypothalamic-pituitary-adrenal axis in the primary fibromyalgia syndrome. J Rheumatol. 1993;20:469–74. [PubMed] [Google Scholar]
- 127.Crofford LJ, Pillemer SR, Kalogeras KT, Cash JM, Michelson D, Kling MA, Sternberg EM, Gold PW, Chrousos GP, Wilder RL. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum. 1994;37:1583–92. doi: 10.1002/art.1780371105. [DOI] [PubMed] [Google Scholar]
- 128.Demitrack MA, Crofford LJ. Evidence for and pathophysiologic implications of hypothalamic-pituitary-adrenal axis dysregulation in fibromyalgia and chronic fatigue syndrome. AnnNYAcadSci. 1998;840:684–97. doi: 10.1111/j.1749-6632.1998.tb09607.x. [DOI] [PubMed] [Google Scholar]
- 129.Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJ, Chrousos GP, Gold PW. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. 1991;73:1224–34. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- 130.Alfven G, de la Torre B, Uvnas-Moberg K. Depressed concentrations of oxytocin and cortisol in children with recurrent abdominal pain of non-organic origin. Acta Paediatr. 1994;83:1076–80. doi: 10.1111/j.1651-2227.1994.tb12989.x. [DOI] [PubMed] [Google Scholar]
- 131.Geiss A, Varadi E, Steinbach K, Bauer HW, Anton F. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neurosci Lett. 1997;237:65–8. doi: 10.1016/s0304-3940(97)00810-0. [DOI] [PubMed] [Google Scholar]
- 132.Lentjes EG, Griep EN, Boersma JW, Romijn FP, de Kloet ER. Glucocorticoid receptors, fibromyalgia and low back pain. Psychoneuroendocrinology. 1997;22:603–14. doi: 10.1016/s0306-4530(97)00061-9. [DOI] [PubMed] [Google Scholar]
- 133.Heim C, Ehlert U, Hanker JP, Hellhammer DH. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom Med. 1998;60:309–18. doi: 10.1097/00006842-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 134.Elwan O, Abdella M, el Bayad AB, Hamdy S. Hormonal changes in headache patients. J Neurol Sci. 1991;106:75–81. doi: 10.1016/0022-510x(91)90197-f. [DOI] [PubMed] [Google Scholar]
- 135.Morrison MF, Redei E, TenHave T, Parmelee P, Boyce AA, Sinha PS, Katz IR. Dehydroepiandrosterone sulfate and psychiatric measures in a frail, elderly residential care population. Biol Psychiatry. 2000;47:144–50. doi: 10.1016/s0006-3223(99)00099-2. [DOI] [PubMed] [Google Scholar]
- 136.Theorell T, Hasselhorn HM, Vingrd E, Andersson B. Interleukin 6 and cortisol in acute musculoskeletal disorders: results from a case-referent study in Sweden. Stress Medicine. 2000;16:27–35. [Google Scholar]
- 137.Nicol GD, Klingberg DK, Vasko MR. Prostaglandin E2 increases calcium conductance and stimulates release of substance P in avian sensory neurons. J Neurosci. 1992;12:1917–27. doi: 10.1523/JNEUROSCI.12-05-01917.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hingtgen CM, Vasko MR. Prostacyclin enhances the evoked-release of substance P and calcitonin gene-related peptide from rat sensory neurons. Brain Research. 1994;655:51–60. doi: 10.1016/0006-8993(94)91596-2. [DOI] [PubMed] [Google Scholar]
- 139.Sternberg EM, Chrousos GP, Wilder RL, Gold PW. The stress response and the regulation of inflammatory disease. Ann Intern Med. 1992;117:854–66. doi: 10.7326/0003-4819-117-10-854. [DOI] [PubMed] [Google Scholar]
- 140.Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: activation by stress. Science. 1984;226:1270–7. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- 141.Maier SF, Sherman JE, Lewis JW, Terman GW, Liebeskind JC. The opioid/nonopioid nature of stress-induced analgesia and learned helplessness. J Exp Psychol Anim Behav Process. 1983;9:80–90. [PubMed] [Google Scholar]
- 142.Caceres C, Burns JW. Cardiovascular reactivity to psychological stress may enhance subsequent pain sensitivity. Pain. 1997;69:237–44. doi: 10.1016/S0304-3959(96)03289-7. [DOI] [PubMed] [Google Scholar]
- 143.Bansevicius D, Westgaard RH, Jensen C. Mental stress of long duration: EMG activity, perceived tension, fatigue, and pain development in pain-free subjects. Headache. 1997;37:499–510. doi: 10.1046/j.1526-4610.1997.3708499.x. [DOI] [PubMed] [Google Scholar]
- 144.Lundberg U. Stress responses in low-status jobs and their relationship to health risks: musculoskeletal disorders. AnnNYAcadSci. 1999;896:162–72. doi: 10.1111/j.1749-6632.1999.tb08113.x. [DOI] [PubMed] [Google Scholar]
- 145.al Absi M, Rokke PD. Can anxiety help us tolerate pain? Pain. 1991;46:43–51. doi: 10.1016/0304-3959(91)90032-S. [DOI] [PubMed] [Google Scholar]
- 146.Sheps DS, Maixner W, Hinderliter AL, Herbst MC, Bragdon EE, Herdt J, Kropp S, Adams KF, Koch G, Ekelund LG. Relationship between systolic blood pressure, ventricular volume and ischemic pain perception in patients with angina pectoris: a potential role for baroreceptors. IsrJMed Sci. 1989;25:482–7. [PubMed] [Google Scholar]
- 147.Randich A, Maixner W. The role of sinoaortic and cardiopulmonary baroreceptor reflex arcs in nociception and stress-induced analgesia. AnnNYAcadSci. 1986;467:385–401. doi: 10.1111/j.1749-6632.1986.tb14642.x. [DOI] [PubMed] [Google Scholar]
- 148.France CR, Stewart KM. Parental history of hypertension and enhanced cardiovascular reactivity are associated with decreased pain ratings. Psychophysiology. 1995;52:571–8. doi: 10.1111/j.1469-8986.1995.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 149.Janssen SA. Negative affect and sensitization to pain. ScandJ Psychol. 2002;43:131–7. doi: 10.1111/1467-9450.00278. [DOI] [PubMed] [Google Scholar]
- 150.al’Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. Netherlands. 2002:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- 151.al’Absi M, Buchanan TW, Marrero A, Lovallo WR. Sex differences in pain perception and cardiovascular responses in persons with parental history for hypertension. Pain. 1999;83:331–8. doi: 10.1016/s0304-3959(99)00122-0. [DOI] [PubMed] [Google Scholar]
- 152.Jaremko KM, Sterling RC, Van Bockstaele EJ. Psychological and physiological stress negatively impacts early engagement and retention of opioid-dependent individuals on methadone maintenance. J Subst Abuse Treat. 2015;48:117–27. doi: 10.1016/j.jsat.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev [Review] 2005;49:505–28. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 154.al’Absi M, Nakajima M, Grabowski J. Stress response dysregulation and stress-induced analgesia in nicotine dependent men and women. Biol Psychol. 2013;93:1–8. doi: 10.1016/j.biopsycho.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neurosci Biobehav Rev. 2012;36:1418–41. doi: 10.1016/j.neubiorev.2012.02.015. [Research Support, N.I.H., Extramural Research Support, Non-U.S Gov’t Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koob GF, Le MM. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 157.Heffner JL, Blom TJ, Anthenelli RM. Gender differences in trauma history and symptoms as predictors of relapse to alcohol and drug use. Am J Addict. 2011;20:307–11. doi: 10.1111/j.1521-0391.2011.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 159.Kassel JD, Veilleux JC, Wardle MC, Yates MC, Greenstein JE, Evatt DP, Roesch LL. Negative affect and addiction. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. London: Academic Press/Elsevier; 2007. pp. 171–90. [Google Scholar]
- 160.Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol. 2010;5:393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Erb S. Neurobiology of stress and risk for relapse. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. London: Academic Press/Elsevier; 2007. pp. 147–69. [Google Scholar]
- 162.Goeders NE. The hypothalamic-pituitary-adrenocortical axis and addiction. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. London: Academic Press/Elsevier; 2007. pp. 21–40. [Google Scholar]
- 163.Kosten TA, kehoe P. Early life stress and vulnerability to addiction. Stress and Addiction: Biological and Psychological Mechanisms. London: Academic Press/Elsevier; 2007. pp. 105–26. [Google Scholar]
- 164.Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, Niaura R, Price LH. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine TobRes. 2009;11:493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shiffman S. J Pers. United States. 2005. Dynamic influences on smoking relapse process; pp. 1715–48. [DOI] [PubMed] [Google Scholar]
- 166.al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–27. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 167.Heishman SJ. Behavioral and cognitive effects of smoking: relationship to nicotine addiction. NicotineTobRes. 1999;1(Suppl 2) doi: 10.1080/14622299050011971. [DOI] [PubMed] [Google Scholar]
- 168.Matheny KB, Weatherman KE. Predictors of smoking cessation and maintenance. JClinPsychol. 1998;54:223–35. doi: 10.1002/(sici)1097-4679(199802)54:2<223::aid-jclp12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 169.Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. JConsult ClinPsychol. 2002;70:216–27. [PubMed] [Google Scholar]
- 170.Koval JJ, Pederson LL. Stress-coping and other psychosocial risk factors: a model for smoking in grade 6 students. Addict Behav. 1999;24:207–18. doi: 10.1016/s0306-4603(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 171.Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J Consult Clin Psychol. 1996;64:993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- 172.Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology (Berl) 1995;119:171–8. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- 173.Carey MP, Kalra DL, Carey KB, Halperin S, Richards CS. Stress and unaided smoking cessation: a prospective investigation. J Consult Clin Psychol. 1993;61:831–8. doi: 10.1037//0022-006x.61.5.831. [DOI] [PubMed] [Google Scholar]
- 174.Swan GE, Ward MM, Jack LM. Abstinence effects as predictors of 28-day relapse in smokers. Addict Behav. 1996;21:481–90. doi: 10.1016/0306-4603(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 175.Nakajima M, al’Absi M. Patterns of change in affect and adrenocortical activity over an extended period of smoking abstinence. Psychol Addict Behav. 2013;27:1189–95. doi: 10.1037/a0033879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine TobRes. 2005;7:91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- 177.Xu J, Azizian A, Monterosso J, Domier CP, Brody AL, Fong TW, London ED. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. NicotineTobRes. 2008;10:1653–61. doi: 10.1080/14622200802412929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nakajima M, al’Absi M. Predictors of risk for smoking relapse in men and women: a prospective examination. Psychol Addict Behav. 2012;26:633–7. doi: 10.1037/a0027280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol ClinExpRes. 2000;24:651–8. [PubMed] [Google Scholar]
- 180.Bernardy NC, King AC, Parsons OA, Lovallo WR. Altered cortisol response in sober alcoholics: an examination of contributing factors. Alcohol. 1996;13:493–8. doi: 10.1016/0741-8329(96)00043-2. [DOI] [PubMed] [Google Scholar]
- 181.Errico AL, Parsons OA, King AC, Lovallo WR. Attenuated cortisol response to biobehavioral stressors in sober alcoholics. Journal of Studies on Alcohol. 1993;54:393–8. doi: 10.15288/jsa.1993.54.393. [DOI] [PubMed] [Google Scholar]
- 182.Lovallo WR, al’Absi M, Pincomb GA, Everson SA, Sung BH, Passey RB, Wilson MF. Caffeine and behavioral stress effects on blood pressure in borderline hypertensive Caucasian men. Health Psychol. 1996;15:11–7. doi: 10.1037//0278-6133.15.1.11. [DOI] [PubMed] [Google Scholar]
- 183.al’Absi M, Lovallo WR, Pincomb GP, et al. Interactive effects of caffeine and behavioral stress on adrenocortical responses in hypertension-prone men. International Journal of Behavioral Medicine. 1995;2:263–75. doi: 10.1207/s15327558ijbm0203_5. [DOI] [PubMed] [Google Scholar]
- 184.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 185.Shiffman S. Coping with temptations to smoke. J Consult Clin Psychol. 1984;52:261–7. doi: 10.1037//0022-006x.52.2.261. [DOI] [PubMed] [Google Scholar]
- 186.al’Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of abstinence and behavioral challenges in habitual smokers. Pharmacology Biochemistry & Behavior. 2002;72:707–16. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- 187.Davis MC. Oral contraceptive use and hemodynamic, lipid, and fibrinogen responses to smoking and stress in women. Health Psychol. 1999;18:122–30. doi: 10.1037//0278-6133.18.2.122. [DOI] [PubMed] [Google Scholar]
- 188.Pomerleau OF, Pomerleau CS. Research on stress and smoking: progress and problems. British J of Addiction. 1991;86:599–603. doi: 10.1111/j.1360-0443.1991.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 189.Houlihan ME, Pritchard WS, Robinson JH. A double blind study of the effects of smoking on heart rate: is there tachyphylaxis? Psychopharmacology (Berl) 1999;144:38–44. doi: 10.1007/s002130050974. [DOI] [PubMed] [Google Scholar]
- 190.Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- 191.al’Absi M, Wittmers LE, Erickson J, Hatsukami DK, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–10. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- 192.Kirschbaum C, Scherer G, Strasburger CJ. Pituitary and adrenal hormone responses to pharmacological, physical, and psychological stimulation in habitual smokers and nonsmokers. Clinical Investigator. 1994;72:804–10. doi: 10.1007/BF00180552. [DOI] [PubMed] [Google Scholar]
- 193.Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacology, Biochemistry, and Behavior. 1993;44:527–31. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- 194.Tsuda A, Steptoe A, West R, Fieldman G, Kirschbaum C. Cigarette smoking and psychophysiological stress responsiveness: effects of recent smoking and temporary abstinence. Psychopharmacology (Berl) 1996;126:226–33. doi: 10.1007/BF02246452. [DOI] [PubMed] [Google Scholar]
- 195.Gilbert DG, Meliska CJ, Plath LC. Noise stress does not modulate effects of smoking/nicotine on β-endorphin, cortisol, ACTH, glucose, and mood. Psychopharmacology. 1997;130:197–202. doi: 10.1007/s002130050229. [DOI] [PubMed] [Google Scholar]
- 196.Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. PharmacolBiochemBehav. 1993;44:527–31. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- 197.al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. IntJ Psychophysiol. 2006;59:218–27. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 198.al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181:107–17. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- 199.Shaw D, al’Absi M. Attenuated beta endorphin response to acute stress is associated with smoking relapse. Pharmacol Biochem Behav. 2008;90:357–62. doi: 10.1016/j.pbb.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.al’Absi M, Carr SB, Bongard S. Anger and psychobiological changes during smoking abstinence and in response to acute stress: prediction of smoking relapse. Int J Psychophysiol. 2007;66:109–15. doi: 10.1016/j.ijpsycho.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.al’Absi M, Carr SB, Bongard S. Anger and psychobiological changes during smoking abstinence and in response to acute stress: prediction of smoking relapse. International Journal of Psychophysiology. Netherlands. 2007:109–15. doi: 10.1016/j.ijpsycho.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Allen AM, Allen SS, Widenmier J, Al’absi M. Patterns of cortisol and craving by menstrual phase in women attempting to quit smoking. Addict Behav. 2009;34:632–5. doi: 10.1016/j.addbeh.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 2004;73:267–78. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 204.West R. Beneficial effects of nicotine: reprise. Addiction. 1994;89:144–6. [Google Scholar]
- 205.Perkins K, Epstein L, Grobe J, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacology Biochemistry and Behavior. 1994;47:107–12. doi: 10.1016/0091-3057(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 206.Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. Journal of Abnormal Psychology. 2003;112:14–27. [PubMed] [Google Scholar]
- 207.Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex specific effects of social support on cortisol, heart rate, and subjective responses to acute psychological stress. Pschyosomatic Medicine. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 208.Najavits LM, Gastfriend DR, Barber JP, Reif S, Muenz LR, Blaine J, Frank A, Crits-Christoph P, Thase M, Weiss RD. Cocaine dependence with and without PTSD among subjects in the National Institute on Drug Abuse Collaborative Cocaine Treatment Study. AmJPsychiatry. 1998;155:214–9. doi: 10.1176/ajp.155.2.214. [DOI] [PubMed] [Google Scholar]
- 209.Green CA, Polen MR, Lynch FL, Dickinson DM, Bennett MD. Gender differences in outcomes in an HMO-based substance abuse treatment program. JAddictDis. 2004;23:47–70. doi: 10.1300/J069v23n02_04. [DOI] [PubMed] [Google Scholar]
- 210.Alati R, Kinner S, Najman JM, Fowler G, Watt K, Green D. Gender differences in the relationships between alcohol, tobacco and mental health in patients attending an emergency department. Alcohol and Alcoholism. 2004;39:463–9. doi: 10.1093/alcalc/agh080. [DOI] [PubMed] [Google Scholar]
- 211.Green CA. Gender and use of substance abuse treatment services. Alcohol ResHealth. 2006;29:55–62. [PMC free article] [PubMed] [Google Scholar]
- 212.Perkins KA, Gerlach D, Broge M, Grobe JE, Sanders M, Fonte C, Vender J, Cherry C, Wilson A. Dissociation of nicotine tolerance from tobacco dependence in humans. JPharmacolExpTher. 2001;296:849–56. [PubMed] [Google Scholar]
- 213.al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. International Journal of Psychophysiology. Netherlands. 2006:218–27. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 214.al’Absi M, Nakajima M, Allen S, Lemieux A, Hatsukami D. Sex Differences in Hormonal Responses to Stress and Smoking Relapse: A Prospective Examination. Nicotine & Tobacco Research. 2015 doi: 10.1093/ntr/ntu340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl) 2002;163:194–201. doi: 10.1007/s00213-002-1168-1. [DOI] [PubMed] [Google Scholar]
- 216.Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. NicotineTobRes. 2008;10:1245–50. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- 217.File SE, Fluck E, Leahy A. Nicotine has calming effects on stress-induced mood changes in females, but enhances aggressive mood in males. IntJNeuropsychopharmacol. 2001;4:371–6. doi: 10.1017/S1461145701002577. [DOI] [PubMed] [Google Scholar]
- 218.Dicken C. Sex roles, smoking, and smoking cessation. J Health Soc Behav. 1978;19:324–34. [PubMed] [Google Scholar]
- 219.Swan GE, Ward MM, Jack LM, Javitz HS. Cardiovascular reactivity as a predictor of relapse in male and female smokers. Health Psychol. 1993;12:451–8. doi: 10.1037//0278-6133.12.6.451. [DOI] [PubMed] [Google Scholar]
- 220.Abrams DB, Monti PM, Pinto RP, Elder JP, Brown RA, Jacobus SI. Psychosocial stress and coping in smokers who relapse or quit. Health Psychology. 1987;6:289–303. doi: 10.1037//0278-6133.6.4.289. [DOI] [PubMed] [Google Scholar]
- 221.al’Absi M, Nakajima M, Allen S, Lemieux A, Hatsukami D. Sex differences in hormonal responses to stress and smoking relapse: a prospective examination. Nicotine Tob Res. 2015;17:382–9. doi: 10.1093/ntr/ntu340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.D’Angelo ME, Reid RD, Brown KS, Pipe AL. Gender differences in predictors for long-term smoking cessation following physician advice and nicotine replacement therapy. CanJPublic Health. 2001;92:418–22. doi: 10.1007/BF03404531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bowen DJ, McTiernan A, Powers D, Feng Z. Recruiting women into a smoking cessation program: who might quit? Women Health. 2000;31:41–58. doi: 10.1300/j013v31n04_03. [DOI] [PubMed] [Google Scholar]
- 224.Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67:555–62. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- 225.Ward KD, Klesges RC, Zbikowski SM, Bliss RE, Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addict Behav. 1998;22:521–33. doi: 10.1016/s0306-4603(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 226.McKee SA, Potenza MN, Kober H, Sofuoglu M, Arnsten AF, Picciotto MR, Weinberger AH, Ashare R, Sinha R. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J Psychopharmacol. 2015;29:300–11. doi: 10.1177/0269881114562091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Smith PH, Kasza KA, Hyland A, Fong GT, Borland R, Brady K, Carpenter MJ, Hartwell K, Cummings KM, McKee SA. Gender differences in medication use and cigarette smoking cessation: results from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2015;17:463–72. doi: 10.1093/ntr/ntu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Weinberger AH, Smith PH, Allen SS, Cosgrove KP, Saladin ME, Gray KM, Mazure CM, Wetherington CL, McKee SA. Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation. Nicotine Tob Res. 2015;17:407–21. doi: 10.1093/ntr/ntu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74:541–9. doi: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- 230.Cicero TJ, Nock B, O’Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol ExpTher. 2002;300:695–701. doi: 10.1124/jpet.300.2.695. [DOI] [PubMed] [Google Scholar]
- 231.Hammer RP, Jr, Zhou L, Cheung S. Gonadal steroid hormones and hypothalamic opioid circuitry. HormBehav. 1994;28:431–7. doi: 10.1006/hbeh.1994.1040. [DOI] [PubMed] [Google Scholar]
- 232.Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. AmJ Psychiatry. 1999;156:842–8. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 233.Sershen H, Hashim A, Lajtha A. Gender differences in kappa-opioid modulation of cocaine-induced behavior and NMDA-evoked dopamine release. Brain Res. 1998;801:67–71. doi: 10.1016/s0006-8993(98)00546-0. [DOI] [PubMed] [Google Scholar]
- 234.Chong RY, Uhart M, Wand GS. Endogenous opiates, addiction, and stress response. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. London: Academic Press/Elsevier; 2007. pp. 85–104. [Google Scholar]
- 235.Hammer RP., Jr The sex hormone-dependent development of opiate receptors in the rat medial preoptic area. Brain Res. 1985;360:65–74. doi: 10.1016/0006-8993(85)91221-1. [DOI] [PubMed] [Google Scholar]
- 236.Kahler CW, Strong DR, Niaura R, Brown RA. Hostility in smokers with past major depressive disorder: relation to smoking patterns, reasons for quitting, and cessation outcomes. NicotineTobRes. 2004;6:809–18. doi: 10.1080/1462220042000282546. [DOI] [PubMed] [Google Scholar]
- 237.Jamner LD, Shapiro D, Jarvik ME. Nicotine reduces the frequency of anger reports in smokers and nonsmokers with high but not low hostility: an ambulatory study. ExpClinPsychopharmacol. 1999;7:454–63. doi: 10.1037//1064-1297.7.4.454. [DOI] [PubMed] [Google Scholar]
- 238.Whiteman MC, Fowkes FG, Deary IJ, Lee AJ. Hostility, cigarette smoking and alcohol consumption in the general population. SocSciMed. 1997;44:1089–96. doi: 10.1016/s0277-9536(96)00236-5. [DOI] [PubMed] [Google Scholar]
- 239.Bongard S, Olson L, Nakajima M, al’Absi M. Anger Expression Style Predicts the Domain of the First Smoking Relapse After a Quit Attempt. Subst Use Misuse. 2016;51:1810–4. doi: 10.1080/10826084.2016.1197259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bongard S, al’Absi M, Khalil NS, Al Habori M. Khat use and trait anger: effects on affect regulation during an acute stressful challenge. Eur Addict Res. 2011;17:285–91. doi: 10.1159/000330317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Edwards VJ, Anda RF, Gu D, Dube SR, Felitti VJ. Adverse childhood experiences and smoking persistence in adults with smoking-related symptoms and illness. Perm J. 2007;11:5–13. doi: 10.7812/tpp/06-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sacco KA, Head CA, Vessicchio JC, Easton CJ, Prigerson HG, George TP. Adverse childhood experiences, smoking and mental illness in adulthood: a preliminary study. Ann Clin Psychiatry. 2007;19:89–97. doi: 10.1080/10401230701334762. [DOI] [PubMed] [Google Scholar]
- 243.Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282:1652–8. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- 244.Lemieux A, Olson L, Nakajima M, Schulberg L, al’Absi M. Life adversity is associated with smoking relapse after a quit attempt. Addict Behav. 2016;60:71–7. doi: 10.1016/j.addbeh.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biol Psychiatry. 2012;71:344–9. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Caspi A, Moffitt TE, Arseneault L. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol Psychiatry. 2011;70:1016–23. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Voellmin A, Winzeler K, Hug E, Wilhelm FH, Schaefer V, Gaab J, La Marca R, Pruessner JC, Bader K. Blunted endocrine and cardiovascular reactivity in young healthy women reporting a history of childhood adversity. Psychoneuroendocrinology. 2015;51:58–67. doi: 10.1016/j.psyneuen.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 248.Lovallo WR. Early life adversity reduces stress reactivity and enhances impulsive behavior: implications for health behaviors. Int J Psychophysiol. 2013;90:8–16. doi: 10.1016/j.ijpsycho.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gulliver SB, Hughes JR, Solomon LJ, Dey AN. An investigation of self-efficacy, partner support and daily stresses as predictors of relapse to smoking in self-quitters. Addiction. 1995;90:767–72. doi: 10.1046/j.1360-0443.1995.9067673.x. [DOI] [PubMed] [Google Scholar]
- 250.Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. NicotineTobRes. 2008;10:507–17. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Shmulewitz D, Stohl M, Keyes KM, Brown Q, Saha TD, Hasin D. Effects of State-Level Tobacco Environment on Cigarette Smoking are Stronger Among Those With Individual-Level Risk Factors. Nicotine Tob Res. 2016 doi: 10.1093/ntr/ntw114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Becker J, Schaub MP, Gmel G, Haug S. Cannabis use and other predictors of the onset of daily cigarette use in young men: what matters most? Results from a longitudinal study. BMC Public Health. 2015;15:843. doi: 10.1186/s12889-015-2194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.al’Absi M, Grabowski J. Concurrent use of tobacco and khat: added burden on chronic disease epidemic. Addiction. 2012;107:451–2. doi: 10.1111/j.1360-0443.2011.03684.x. [DOI] [PubMed] [Google Scholar]
- 254.Hoffman R, al’Absi M. Working memory and speed of information processing in chronic khat users: preliminary findings. Eur Addict Res. 2013;19:1–6. doi: 10.1159/000338285. [DOI] [PubMed] [Google Scholar]
- 255.Hoffman R, al’Absi M. Khat use and neurobehavioral functions: Suggestions for future studies. JEthnopharmacol. 2010;132:554–63. doi: 10.1016/j.jep.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hoffman R, Al’absi M. Concurrent use of khat and tobacco is associated with verbal learning and delayed recall deficits. Addiction. 2013;108:1855–62. doi: 10.1111/add.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Nakajima M, Hoffman R, Al’absi M. Poor Working Memory and Reduced Blood Pressure Levels in Concurrent Users of Khat and Tobacco. Nicotine Tob Res. 2013 doi: 10.1093/ntr/ntt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.al’Absi M, Nakajima M, Dokam A, Sameai A, Alsoofi M, Saem Khalil N, Al Habori M. Concurrent tobacco and khat use is associated with blunted cardiovascular stress response and enhanced negative mood: a cross-sectional investigation. Hum Psychopharmacol. 2014;29:307–15. doi: 10.1002/hup.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.al’Absi M, Khalil NS, Al Habori M, Hoffman R, Fujiwara K, Wittmers L. Effects of chronic khat use on cardiovascular, adrenocortical, and psychological responses to stress in men and women. Am J Addict. 2013;22:99–107. doi: 10.1111/j.1521-0391.2013.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–58. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 261.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 262.Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. PsychosomMed. 2000;62:853–65. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 263.Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. PharmacolBiochemBehav. 2004;79:671–89. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 264.Kiefer F, Jahn H, Otte C, Demiralay C, Wolf K, Wiedemann K. Increasing leptin precedes craving and relapse during pharmacological abstinence maintenance treatment of alcoholism. JPsychiatrRes. 2005;39:545–51. doi: 10.1016/j.jpsychires.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 265.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. ProcNatlAcadSciUSA. 2005;102:19168–73. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hillemacher T, Kraus T, Rauh J, Weiss J, Schanze A, Frieling H, Wilhelm J, Heberlein A, Groschl M, Sperling W, Kornhuber J, Bleich S. Role of appetite-regulating peptides in alcohol craving: an analysis in respect to subtypes and different consumption patterns in alcoholism. Alcohol ClinExpRes. 2007;31:950–4. doi: 10.1111/j.1530-0277.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 267.French SA, Jeffery RW, Klesges LM, Forster JL. Weight concerns and change in smoking behavior over two years in a working population. AmJPublic Health. 1995;85:720–2. doi: 10.2105/ajph.85.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Borrelli B, Spring B, Niaura R, Hitsman B, Papandonatos G. Influences of gender and weight gain on short-term relapse to smoking in a cessation trial. JConsult ClinPsychol. 2001;69:511–5. doi: 10.1037//0022-006x.69.3.511. [DOI] [PubMed] [Google Scholar]
- 269.Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. NEnglJMed. 1991;324:739–45. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 270.Mitchell SL, Perkins KA. Interaction of stress, smoking, and dietary restraint in women. Physiol Behav. 1998;64:103–9. doi: 10.1016/s0031-9384(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 271.Kos J, Hasenfratz M, Battig K. Effects of a 2-day abstinence from smoking on dietary, cognitive, subjective, and physiologic parameters among younger and older female smokers. Physiology and Behavior. 1997;61:671–8. doi: 10.1016/s0031-9384(96)00518-5. [DOI] [PubMed] [Google Scholar]
- 272.Volkow ND, Wise RA. How can drug addiction help us understand obesity? NatNeurosci. 2005;8:555–60. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 273.Caan B, Coates A, Schaefer C, Finkler L, Sternfeld B, Corbett K. Women gain weight 1 year after smoking cessation while dietary intake temporarily increases. JAmDietAssoc. 1996;96:1150–5. doi: 10.1016/S0002-8223(96)00296-9. [DOI] [PubMed] [Google Scholar]
- 274.Jorenby DE, Hatsukami DK, Smith SS, Fiore MC, Allen S, Jensen J, Baker TB. Characterization of tobacco withdrawal symptoms: transdermal nicotine reduces hunger and weight gain. Psychopharmacology (Berl) 1996;128:130–8. doi: 10.1007/s002130050118. [DOI] [PubMed] [Google Scholar]
- 275.Perkins KA, Epstein LH, Pastor S. Changes in energy balance following smoking cessation and resumption of smoking in women. JConsult ClinPsychol. 1990;58:121–5. doi: 10.1037//0022-006x.58.1.121. [DOI] [PubMed] [Google Scholar]
- 276.Pisinger C, Jorgensen T. Weight concerns and smoking in a general population: the Inter99 study. PrevMed. 2007;44:283–9. doi: 10.1016/j.ypmed.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 277.Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry. 2013;73:827–35. doi: 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Koopmann A, Dinter C, Grosshans M, von der Goltz C, Hentschel R, Dahmen N, Gallinat J, Wagner M, Gründer G, Thürauf N, Wienker T, Brinkmeyer J, Mobascher A, Spreckelmeyer KN, Clepce M, de Millas W, Wiedemann K, Winterer G, Kiefer F. Psychological and hormonal features of smokers at risk to gain weight after smoking cessation--results of a multicenter study. Horm Behav. 2011;60:58–64. doi: 10.1016/j.yhbeh.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 279.al’Absi M, Hooker S, Fujiwara K, Kiefer F, von der Goltz C, Cragin T, Wittmers LE. Circulating leptin levels are associated with increased craving to smoke in abstinent smokers. Pharmacol Biochem Behav. 2011;97:509–13. doi: 10.1016/j.pbb.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kiefer F, Jahn H, Kellner M, Naber D, Wiedemann K. Leptin as a possible modulator of craving for alcohol. ArchGenPsychiatry. 2001;58:509–10. doi: 10.1001/archpsyc.58.5.509. [DOI] [PubMed] [Google Scholar]
- 281.Potretzke S, Nakajima M, Cragin T, al’Absi M. Changes in circulating leptin levels during acute stress and associations with craving in abstinent smokers: a preliminary investigation. Psychoneuroendocrinology. 2014;47:232–40. doi: 10.1016/j.psyneuen.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.al’Absi M, Lemieux A, Nakajima M. Peptide YY and ghrelin predict craving and risk for relapse in abstinent smokers. Psychoneuroendocrinology. 2014;49:253–9. doi: 10.1016/j.psyneuen.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lemieux A, Nakajima M, Hatsukami DK, Allen S, al’Absi M. Changes in circulating leptin levels during the initial stage of cessation are associated with smoking relapse. Psychopharmacology (Berl) 2015;232:3355–61. doi: 10.1007/s00213-015-3989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.al’Absi M, France C, Harju A, France J, Wittmers L. Adrenocortical and nociceptive responses to opioid blockade in hypertension-prone men and women. Psychosomatic Medicine. United States. 2006:292–8. doi: 10.1097/01.psy.0000203240.64965.bd. [DOI] [PubMed] [Google Scholar]
- 285.Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–14. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 286.Bruijnzeel AW. kappa-Opioid receptor signaling and brain reward function. Brain Res Rev. 2009;62:127–46. doi: 10.1016/j.brainresrev.2009.09.008. [Research Support, N.I.H., Extramural Research Support, Non-U.S Gov’t Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. England. 2008:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gianoulakis C. Implications of endogenous opioids and dopamine in alcoholism: human and basic science studies. Alcohol Alcohol Suppl. 1996;1:33–42. [PubMed] [Google Scholar]
- 289.al’Absi M, Bongard S. Neuroendocrine and behavioral mechanisms mediating the relationship between anger expression and cardiovascular risk: assessment considerations and improvements. J Behav Med. 2006;29:573–91. doi: 10.1007/s10865-006-9077-0. [DOI] [PubMed] [Google Scholar]
- 290.France CR, al’absi M, Ring C, France JL, Brose J, Spaeth D, Harju A, Nordehn G, Wittmers LE. Assessment of opiate modulation of pain and nociceptive responding in young adults with a parental history of hypertension. Biol Psychol. 2005;70:168–74. doi: 10.1016/j.biopsycho.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 291.France CR, al’absi M, Ring C, France JL, Brose J, Spaeth D, Harju A, Nordehn G, Wittmers LE. Assessment of opiate modulation of pain and nociceptive responding in young adults with a parental history of hypertension. Biological Psychology. Netherlands. 2005:168–74. doi: 10.1016/j.biopsycho.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 292.al’Absi M, Wittmers LE, Hatsukami D, Westra R. Blunted opiate modulation of hypothalamic-pituitary-adrenocortical activity in men and women who smoke. Psychosom Med. 2008;70:928–35. doi: 10.1097/PSY.0b013e31818434ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Phillips AC, Ginty AT, Hughes BM. The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. Int J Psychophysiol. 2013;90:1–7. doi: 10.1016/j.ijpsycho.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 294.Ginty AT, Gianaros PJ, Derbyshire SW, Phillips AC, Carroll D. Blunted cardiac stress reactivity relates to neural hypoactivation. Psychophysiology. 2013;50:219–29. doi: 10.1111/psyp.12017. [DOI] [PubMed] [Google Scholar]
- 295.Phillips AC, Hunt K, Der G, Carroll D. Blunted cardiac reactions to acute psychological stress predict symptoms of depression five years later: evidence from a large community study. Psychophysiology. 2011;48:142–8. doi: 10.1111/j.1469-8986.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 296.AS Physiological arousal and its dysregulation in child maladjustment. Current Directions in Psychological Science. 2015;24:345–51. [Google Scholar]
- 297.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000:25. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 298.Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–37. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 299.Lovallo W. Individual differences in response to stress and risk for addiction. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. London: Academic Press/Elsevier; 2007. pp. 265–84. [Google Scholar]
- 300.Besson M, Granon S, Mameli-Engvall M, Cloez-Tayarani I, Maubourguet N, Cormier A, Cazala P, David V, Changeux JP, Faure P. Long-term effects of chronic nicotine exposure on brain nicotinic receptors. ProcNatlAcadSciUSA. 2007;104:8155–60. doi: 10.1073/pnas.0702698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Anderson KL, Pinkerton KE, Uyeminami D, Simons CT, Carstens MI, Carstens E. Antinociception induced by chronic exposure of rats to cigarette smoke. NeurosciLett. 2004;366:86–91. doi: 10.1016/j.neulet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 302.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–9. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 303.Koob GF, Le Moal M. Neurobiology of Addiction. New York: Elsevier; 2005. [Google Scholar]
- 304.Buczek Y, Le AD, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144:183–8. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- 305.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–82. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 306.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. NatRevNeurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 307.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. AmJPsychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 308.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 309.Anacker C, Scholz J, O’Donnell KJ, Allemang-Grand R, Diorio J, Bagot RC, Nestler EJ, Hen R, Lerch JP, Meaney MJ. Neuroanatomic Differences Associated With Stress Susceptibility and Resilience. Biol Psychiatry. 2016;79:840–9. doi: 10.1016/j.biopsych.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry. 2014;76:542–9. doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Heller EA, Cates HM, Peña CJ, Sun H, Shao N, Feng J, Golden SA, Herman JP, Walsh JJ, Mazei-Robison M, Ferguson D, Knight S, Gerber MA, Nievera C, Han MH, Russo SJ, Tamminga CS, Neve RL, Shen L, Zhang HS, Zhang F, Nestler EJ. Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors. Nat Neurosci. 2014;17:1720–7. doi: 10.1038/nn.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Reul JM, Collins A, Saliba RS, Mifsud KR, Carter SD, Gutierrez-Mecinas M, Qian X, Linthorst AC. Glucocorticoids, epigenetic control and stress resilience. Neurobiol Stress. 2015;1:44–59. doi: 10.1016/j.ynstr.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–84. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]