Abstract
Purpose
To examine the diurnal variation of static and dynamic anterior segment parameters in young, healthy eyes by comparing anterior segment optical coherence tomography (AS-OCT) measurements obtained in the morning and evening and also in the light and dark.
Methods
Twenty-two subjects ranging from ages 19 to 47 years with no past ocular history were selected (12 male, 10 female; mean age 34.2 years). Imaging was performed with the Tomey CASIA2 AS-OCT device in two fixed lighting environments, light and dark, between the hours of 0830 to 1000 and 1730 to 1900. Four AS-OCT images were analyzed per eye. Pupil diameter (PD), iris area (IA), iris curvature (IC), anterior chamber depth (ACD), lens vault (LV), anterior chamber width (ACW), anterior chamber area (ACA), angle opening distance (AOD), angle recess area (ARA), trabecular iris space area (TISA), and trabecular iris angle (TIA) were measured.
Results
PD was similar between the AM and PM groups in the light (p = 0.89) and dark (p = 0.51). There was no significant difference between AM and PM measurement values for any of the static or dynamic parameters in the light (p > 0.39) and dark (p > 0.31). Intra-class correlation coefficients (ICC) demonstrated excellent agreement between AM and PM measurement values in the light (ICC > 0.81) and dark (ICC > 0.93). Additionally, there was no significant difference between AM and PM AOD500 measurement values in the light (p > 0.34) and dark (p > 0.40) when each of eight angle sectors was analyzed individually.
Conclusion
No significant diurnal variation of static or dynamic anterior segment parameter measurements was detected in the light and dark. Diurnal variation of these parameters does not regularly occur in young, healthy eyes.
Introduction
Anatomical structures in a healthy adult eye undergo changes in configuration and function throughout the day. Some changes are highly dynamic and occur on a moment-to-moment basis, the most obvious being those related to the pupillary light response. Other anatomical parameters, such as axial length, choroidal thickness, and aqueous outflow facility, undergo slower fluctuations, on the scale of minutes to hours.1–4
Anterior segment optical coherence tomography (AS-OCT) is a non-contact imaging modality that offers quantitative measurements of anterior segment structures and their biomechanical properties using a combination of static and dynamic parameters. AS-OCT measurements are highly reproducible between acquisitions, but this issue has been studied primarily using scans obtained in a narrow time window.5–7 The diurnal variation of the majority of AS-OCT parameters has not been studied. Therefore, it is not known if AS-OCT measurements taken at different times of the day are interchangeable or even comparable.
Static AS-OCT parameters describe the configuration of the anterior segment at a single time point. Measurements of some static parameters, such as iris curvature (IC) and lens vault (LV), have been implicated in disease processes such as acute angle closure (AAC).8 AAC most commonly occurs during the early evening hours and its incidence is also correlated with the number of hours without sunshine.9,10 While it is tempting to hypothesize that diurnal variation of static parameters plays a role in the pathogenesis of angle closure disease, such variation has never been clearly demonstrated in healthy or diseased eyes.
Dynamic AS-OCT parameters describe the magnitude of change that anterior segment structures, such as the iris, undergo in response to environmental influences.11 The dynamic response of the iris to light appears to be governed by intrinsic tissue properties that regulate fluid exchange between the aqueous humor and iris stroma. This exchange occurs in normal, healthy eyes, but varies among people of different ethnicities and is decreased in patients at risk for angle closure disease.11–17 It is not known if dynamic parameters that describe the magnitude of this behavioral difference undergo diurnal variation.
AS-OCT imaging offers a convenient alternative to gonioscopy in assessments of the anterior segment. With increasing clinical use of AS-OCT, it is important to ascertain whether measurements obtained at different times of day are interchangeable. Furthermore, it is important to assess if diurnal variation of static and dynamic parameters regularly occurs in normal, healthy eyes prior to studying the topic in angle closure eyes.. This study seeks to address these issues by comparing AS-OCT measurements obtained in the light and dark, and also in the morning and afternoon.
Methods
Image Acquisition
Twenty-two healthy volunteers from the Shiley Eye Institute and Hamilton Glaucoma Center in San Diego, California were recruited for participation in this study. Subjects had no history of ocular disease or prior eye procedures, including laser peripheral iridotomy and cataract surgery. Each subject received a standard baseline exam consisting of intraocular pressure (IOP) measurement by applanation tonometry, slit lamp examination of the anterior segment, gonioscopy with a 4-mirror lens, and undilated examination of the optic disc. Ethics committee approval was obtained from the University of California San Diego Institutional Review Board. All study procedures adhered to the recommendations of the Declaration of Helsinki.
All subjects underwent non-mydriatic AS-OCT imaging of both eyes using the CASIA2 swept-source spectral-domain OCT device (Tomey Corporation, Nagoya, Japan). Morning (AM) imaging was performed between the hours of 0830 to 1000 and evening (PM) imaging between the hours of 1730 to 1900. During both AM and PM imaging sessions, three consecutive scans were performed in ‘AC Angle’ mode under ambient room lighting conditions standardized to 27 cd/m2 at the imaging plane. Three consecutive scans were then performed after 5 minutes of adaptation under dark room conditions standardized to 1 cd/m2. Luminance was measured with a light meter (Light Meter 840021; Sper Scientific, Scottsdale, AZ). Each scan session produced 128 cross-sectional images spaced 1.4 degrees apart.
Measurement of Parameters
Data analysis was performed on images of both eyes obtained from each of the 22 subjects. Pupil diameter was measured along the horizontal (temporal-nasal) meridian in all three images of each eye obtained in the two lighting environments (light and dark) and time intervals (AM and PM). The AM and PM images closest in pupil diameter were selected for comparison for both light and dark. Comparisons in eyes for which pupil diameter differed by more than ten percent between all AM and PM scans were excluded from analysis to minimize the effects of pupil size on measurement values.
Two trained observers (B.Y.X and R.C.P.) masked to the identities and examination results of the subjects marked the scleral spurs and identified the angle structures in four images each spaced 45 degrees apart. If there was disagreement on the location of the scleral spur, adjacent images were reviewed and the image discussed until a consensus was reached. The scleral spur was defined as the inward protrusion of the sclera where a change in curvature of the corneoscleral junction was observed.18 Anterior segment parameter measurements were obtained from the AS-OCT images using built-in software (version 2A) provided by the manufacturer that automatically segmented the intraocular structures and generated measurement values after the scleral spurs were marked. Segmentation was confirmed and errors were corrected by the two observers.
In total, 15 parameters were analyzed. Seven described the anterior chamber and its structures: pupil diameter (PD), iris area (IA), iris curvature (IC), anterior chamber depth (ACD), lens vault (LV), anterior chamber width (ACW), and anterior chamber area (ACA). Eight described the angle: angle opening distance (AOD), angle recess area (ARA), trabecular iris space area (TISA), trabecular iris angle (TIA) at 500 um and 750 um from the scleral spur.8,19,20 Dynamic parameters were calculated by subtracting the light environment measurement from the dark environment measurement.
Statistical Analysis
The mean and standard deviation were calculated for each set of AM and PM measurement values by averaging across the four analyzed images. The distribution of each set of measurement values was assessed for normality using the Kolmorgorv-Smirnov test with significance defined as p < 0.05. A statistically significant difference between the measurement values was defined as p < 0.05 by Wilcoxan rank-sum test for non-normally distributed variables and paired t-test for normally distributed variables. Agreement between measurement values were assessed in the form of intra-class correlation coefficients (ICC). These analyses were repeated for AM and PM AOD500 measurement values obtained in the light and dark for each of the eight angle sectors. All data analysis was performed using MATLAB (Mathworks, Natick, MA).
Results
The age of the subjects ranged from 19 to 57 years (mean age 34.2 ± 8.4 years). There were 12 males and 10 females. Mean IOP was 15.6 ± 5.5 mmHg. All angles were open (modified Shaffer grade 2 or greater) in all four quadrants on gonioscopy.
Differences between mean AM and PM pupil diameter in the light and dark
Pupil diameter was measured in all three images of each eye obtained in the two lighting environments (light and dark) and time intervals (AM and PM). In the light, the mean AM pupil diameter was 4.85 ± 0.88 and PM pupil diameter was 4.93 ± 0.75 (PM). In the dark, the mean AM pupil diameter was 5.85 ± 0.67 and PM pupil diameter was 5.73 ± 0.75 (PM). All four distributions were non-normal (p < 1.0 × 109, KS test). There was no significant difference between AM and PM pupil diameter measurements in the light (p = 0.41, Wilcoxan rank-sum test) or dark (p = 0.31, Wilcoxan rank-sum test).
AM and PM measurement values of static parameters in the light and dark
The scleral spur was successfully identified in all of the images. Five of 44 eyes (11.4%) scanned in the light and three of 44 eyes (6.8%) scanned in the dark were excluded from analysis due to having a pupil diameter difference greater than ten percent between all AM and PM images.
All of the parameters measured in the light (Table 1) were non-normally distributed in the AM (p < 9.74 ×10−6, KS test) and PM (p < 1.03 × 10−5, KS test). There was no significant difference (p = 0.39 to 0.96, Wilcoxcan rank-sum test) between AM and PM measurement values in the light (Table 1). ICC demonstrated excellent correlation between AM and PM measurements for all parameters (ICC > 0.80).
Table 1.
Diurnal variation of select AS-OCT parameter measurements in the light. Mean AM and PM measurement values shown with standard deviations. P-values derived from Wilcoxan rank-sum test. ICC values shown with 95% confidence intervals. PD, pupillary diameter. IA, iris area. IC, iris curvature. ACD, anterior chamber depth. LV, lens vault. ACW, anterior chamber width. ACA, anterior chamber area. AOD500/750, angle opening distance at 500/750um. TISA500/750, trabecular iris space area at 500/750um. SSAngle750, scleral spur angle at 500/750um.
| AM | PM | P-value | ICC | |
|---|---|---|---|---|
| PD (mm) | 5.77 ± 1.04 | 5.73 ± 1.00 | 0.89 | 0.98 (0.95–0.99) |
| IA (mm2) | 1.47 ± 0.22 | 1.45 ± 0.24 | 0.76 | 0.91 (0.83–0.95) |
| IC (mm) | 0.09 ± 0.10 | 0.07 ± 0.11 | 0.39 | 0.81 (0.65–0.90) |
| ACD (mm) | 3.14 ± 0.27 | 3.13 ± 0.25 | 0.96 | 0.98 (0.96–0.99) |
| LV (mm) | 0.09 ± 0.22 | 0.11 ± 0.23 | 0.92 | 0.96 (0.92–0.98) |
| ACW (mm) | 12.07 ± 0.47 | 12.08 ± 0.47 | 0.90 | 0.99 (0.98–0.99) |
| ACA (mm2) | 25.29 ± 3.52 | 25.35 ± 3.40 | 0.88 | 0.99 (0.99–1.00) |
| AOD500 (mm) | 0.45 ± 0.14 | 0.46 ± 0.15 | 0.66 | 0.94 (0.88–0.97) |
| AOD750 (mm) | 0.62 ± 0.18 | 0.64 ± 0.20 | 0.78 | 0.94 (0.88–0.97) |
| ARA500 (mm2) | 0.17 ± 0.07 | 0.17 ± 0.07 | 0.79 | 0.96 (0.92–0.98) |
| ARA750 (mm2) | 0.30 ± 0.11 | 0.31 ± 0.12 | 0.61 | 0.95 (0.91–0.98) |
| TISA500 (mm2) | 0.16 ± 0.06 | 0.16 ± 0.06 | 0.73 | 0.95 (0.90–0.97) |
| TISA750 (mm2) | 0.29 ± 0.10 | 0.29 ± 0.10 | 0.60 | 0.95 (0.90–0.97) |
| TIA500 (degrees) | 38.16 ± 8.02 | 38.58 ± 9.01 | 0.69 | 0.89 (0.80–0.94) |
| TIA750 (degrees) | 37.14 ± 7.70 | 37.51 ± 8.44 | 0.87 | 0.92 (0.85–0.96) |
All of the parameters measured in the dark Table 2) were non-normally distributed in the AM (p < 1.47 ×10−5, KS test) and PM (p < 1.37 × 10−5, KS test). There was also no significant difference (p = 0.31 0.97, Wilcoxan rank-sum test) between AM and PM measurement values in the dark (Table 2). ICC demonstrated excellent correlation between AM and PM measurements for all parameters (ICC > 0.89).
Table 2.
Diurnal variation of select AS-OCT parameter measurements in the dark. P-values derived from Wilcoxan rank-sum test except for ACW. P-value for ACW derived from paired t-test.
| AM | PM | P-value | ICC | |
|---|---|---|---|---|
| PD (mm) | 6.73 ± 0.87 | 6.59 ± 0.87 | 0.31 | 0.96 (0.92–0.98) |
| IA (mm2) | 1.31 ± 0.25 | 1.33 ± 0.26 | 0.40 | 0.95 (0.91–0.97) |
| IC (mm) | 0.04 ± 0.14 | 0.06 ± 0.13 | 0.58 | 0.94 (0.89–0.97) |
| ACD (mm) | 3.13 ± 0.29 | 3.13 ± 0.29 | 0.96 | 1.00 (0.99–1.00) |
| LV (mm) | 0.07 ± 0.26 | 0.08 ± 0.27 | 0.94 | 0.99 (0.98–0.99) |
| ACW (mm) | 12.03 ± 0.46 | 12.03 ± 0.46 | 0.97 | 0.99 (0.98–1.00) |
| ACA (mm2) | 26.11 ± 3.65 | 25.93 ± 3.59 | 0.84 | 1.00 (0.99–1.00) |
| AOD500 (mm) | 0.53 ± 0.23 | 0.50 ± 0.22 | 0.64 | 0.96 (0.92–0.98) |
| AOD750 (mm) | 0.74 ± 0.32 | 0.70 ± 0.30 | 0.56 | 0.97 (0.94–0.98) |
| ARA500 (mm2) | 0.20 ± 0.10 | 0.19 ± 0.09 | 0.67 | 0.96 (0.93–0.98) |
| ARA750 (mm2) | 0.36 ± 0.17 | 0.34 ± 0.16 | 0.63 | 0.96 (0.93–0.98) |
| TISA500 (mm2) | 0.18 ± 0.08 | 0.17 ± 0.08 | 0.65 | 0.95 (0.91–0.98) |
| TISA750 (mm2) | 0.34 ± 0.15 | 0.32 ± 0.14 | 0.63 | 0.96 (0.92–0.98) |
| TIA500 (degrees) | 41.31 ± 13.30 | 40.12 ± 11.77 | 0.68 | 0.93 (0.87–0.96) |
| TIA750 (degrees) | 40.37 ± 12.27 | 39.13 ± 11.10 | 0.56 | 0.95 (0.91–0.97) |
AM and PM measurement values of dynamic parameters
Seven of 44 eyes (15.9%) scanned were excluded from analysis due to having a pupil diameter difference greater than ten percent between all AM and PM images in either the light or dark.
All of the parameters measured were non-normally distributed in the AM (p < 0.042, KS test) and PM (p < 0.03, KS test) except ACW (p = 0.34 in the AM; p = 0.16 in the PM) (Table 3, Figure 1). There was no significant difference (p = 0.35 to 0.99, Wilcoxcan rank-sum or paired t-test) between AM and PM measurement values in the light (Table 3). ICC demonstrated excellent correlation between AM and PM measurements for all parameters (ICC > 0.87).
Table 3.
Diurnal variation of dynamic AS-OCT parameter measurements. P-values derived from Wilcoxan rank-sum test.
| AM | PM | P-value | ICC | |
|---|---|---|---|---|
| ΔIA (mm2) | −0.16 ± 0.24 | −0.11 ± 0.25 | 0.41 | 0.89 (0.78–0.94) |
| ΔIC (mm) | −0.05 ± 0.16 | −0.01 ± 0.17 | 0.35 | 0.89 (0.80–0.95) |
| ΔACD (mm) | −0.02 ± 0.44 | −0.01 ± 0.42 | 0.99 | 0.99 (0.98–1.00) |
| ΔLV (mm) | −0.01 ± 0.31 | −0.02 ± 0.32 | 0.98 | 0.96 (0.93–0.98) |
| ΔACW (mm) | −0.03 ± 0.65 | −0.05 ± 0.64 | 0.91 | 0.99 (0.98–0.99) |
| ΔACA (mm2) | 0.73 ± 5.43 | 0.47 ± 5.28 | 0.80 | 0.99 (0.99–1.00) |
| ΔPD (mm) | 0.96 ± 1.24 | 0.88 ± 1.26 | 0.61 | 0.97 (0.94–0.98) |
| ΔAOD500 (mm) | 0.07 ± 0.25 | 0.04 ± 0.25 | 0.75 | 0.95 (0.89–0.97) |
| ΔAOD750 (mm) | 0.10 ± 0.35 | 0.06 ± 0.35 | 0.66 | 0.96 (0.92–0.98) |
| ΔARA500 (mm2) | 0.02 ± 0.10 | 0.01 ± 0.10 | 0.75 | 0.94 (0.88–0.97) |
| ΔARA750 (mm2) | 0.04 ± 0.17 | 0.03 ± 0.17 | 0.70 | 0.95 (0.90–0.97) |
| ΔTISA500 (mm2) | 0.02 ± 0.08 | 0.01 ± 0.09 | 0.76 | 0.94 (0.88–0.97) |
| ΔTISA750 (mm2) | 0.04 ± 0.16 | 0.03 ± 0.16 | 0.72 | 0.95 (0.90–0.97) |
| ΔTIA500 (degrees) | 2.37 ± 14.67 | 1.37 ± 14.18 | 0.96 | 0.92 (0.85–0.96) |
Figure 1.
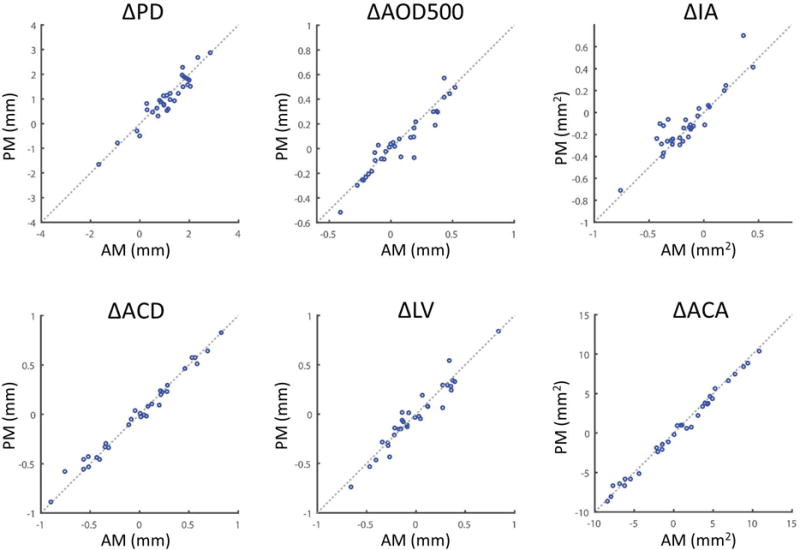
Diurnal variation of select dynamic AS-OCT parameter measurements. Δ Measurement defined as: Dark environment measurement – Light environment measurement. Positive values indicate a larger value in the dark than in the light. Scatter plots for AM and PM measurement values (blue dots) are plotted for each parameter (heading). The equivalence line (x = y, dotted line) is shown for each comparison. PD, pupil diameter. AOD500, angle opening distance at 500 um from scleral spur. IA, iris area. ACD, anterior chamber depth. LV, lens vault. ACA, anterior chamber area.
Sectoral differences between AM and PM measurement values for the AOD500 parameter
AS-OCT images obtained in the light and dark from each of the eight angle sectors was analyzed separately to assess for sectoral differences in diurnal variation (Table 4). All of the parameters measured in the light and dark were non-normally distributed in the AM (p < 1.13 ×10−5, KS test) and PM (p < 1.57 × 10−5, KS test). The superonasal sector demonstrated the smallest mean measurement values in the light and dark and AM and PM. The temporal sector was the widest in the light and inferotemporal sector the widest in the dark. None of the sectors demonstrated a significant difference between AM and PM values in the light (p > 0.34, Wilcoxcan rank-sum test) or dark (p > 0.40, Wilcoxcan rank-sum test).
Table 4.
Diurnal variation of eight sectoral AOD500 measurements in the light and dark. P-values derived from Wilcoxan rank-sum test.
| AM | PM | P-value | ICC | |
|---|---|---|---|---|
| Nasal | 0.47 ± 0.13 | 0.48 ± 0.15 | 0.84 | 0.82 (0.68–0.91) |
| Inferonasal | 0.35 ± 0.12 | 0.36 ± 0.14 | 0.65 | 0.88 (0.78–0.94) |
| Inferior | 0.39 ± 0.14 | 0.39 ± 0.16 | 0.82 | 0.86 (0.73–0.93) |
| Inferotemporal | 0.42 ± 0.13 | 0.41 ± 0.15 | 0.59 | 0.79 (0.62–0.89) |
| Temporal | 0.49 ± 0.16 | 0.49 ± 0.19 | 0.70 | 0.78 (0.61–0.88) |
| Superotemporal | 0.46 ± 0.13 | 0.50 ± 0.15 | 0.34 | 0.79 (0.63–0.89) |
| Superior | 0.46 ± 0.17 | 0.47 ± 0.18 | 0.74 | 0.83 (0.68–0.91) |
| Superonasal | 0.43 ± 0.15 | 0.47 ± 0.17 | 0.46 | 0.80 (0.64–0.89) |
| Dark | AM | PM | P-value | ICC |
| Nasal | 0.52 ± 0.18 | 0.49 ± 0.21 | 0.56 | 0.79 (0.64–0.89) |
| Inferonasal | 0.41 ± 0.18 | 0.41 ± 0.17 | 0.83 | 0.89 (0.79–0.94) |
| Inferior | 0.46 ± 0.20 | 0.43 ± 0.18 | 0.40 | 0.88 (0.78–0.94) |
| Inferotemporal | 0.51 ± 0.22 | 0.49 ± 0.18 | 0.75 | 0.87 (0.76–0.93) |
| Temporal | 0.57 ± 0.22 | 0.57 ± 0.21 | 0.98 | 0.93 (0.86–0.96) |
| Superotemporal | 0.63 ± 0.29 | 0.59 ± 0.24 | 0.43 | 0.91 (0.83–0.95) |
| Superior | 0.57 ± 0.27 | 0.53 ± 0.27 | 0.51 | 0.87 (0.76–0.93) |
| Superonasal | 0.53 ± 0.24 | 0.51 ± 0.23 | 0.99 | 0.91 (0.84–0.95) |
Discussion
This study utilized AS-OCT to study the diurnal variation of static and dynamic anterior segment parameters in young, healthy eyes. On average, there was no diurnal variation of PD in two fixed lighting environments. Additionally, there were no significant differences between AM and PM measurements in the light and dark for any of the static or dynamic parameters. Finally, there were no significant differences between AM and PM AOD500 measurements obtained in the dark or light when each of eight angle sectors was analyzed independently.
Static biometric parameters describing the position of the lens, such as ACD and LV, have emerged as important risk factors for angle closure disease.21–23 The incidence of acute angle closure differs greatly between morning and early evening hours.9 The simplest explanation for this observation is that there are diurnal effects on lens position that are most pronounced at these two time points, which we chose for AS-OCT imaging. We elected to study young, healthy eyes with open angles since diurnal variation of the majority of AS-OCT parameters had not been characterized in these eyes, let alone eyes with angle closure. Previous studies of eyes with open angles utilizing slit lamp photography and a Scheimflug camera system found small, conflicting changes in ACD between the morning and evening.2,24 Our results, obtained using modern imaging methods, clearly demonstrate that AM and PM measurements of lens position are closely correlated in young, healthy eyes. This provides a useful comparator in future AS-OCT studies of diurnal variation in angle closure eyes.
Our results did not find any diurnal variation among static parameters that describe the dimensions of the angle. These results are supported by a recent study that used AS-OCT to examine diurnal variations of AOD and TISA measurements from the inferior angle in young subjects with open angles.25 In contrast to that study, we standardized AS-OCT measurements according to PD, since even small changes in PD can have a large impact on AS-OCT measurements.26 We also elected to analyze diurnal variation of AOD500 in each of eight sectors rather than assume that all portions of the angle behave in a similar manner. Given the extensive anatomical variation throughout the angle, it was conceivable that different sectors could exhibit different amounts of diurnal variation, even though our data demonstrates that this is not the case.27,28
Measurements of dynamic parameters, such as iris volume changes secondary to the pupillary light response, have gained attention due to associations between these parameters and angle closure disease.8,11 We hypothesized that changes in hydration status or extended time in the upright position throughout the day could affect the fluid capacity and compliance of the iris. Based on our data, however, this does not appear to be the case, as changes in IA and IC between light and dark were the same in the morning and evening. Whatever intrinsic tissue property determines the relationship between iris area and pupil diameter does not appear affected by the time of day.
The results of this study suggest that AS-OCT measurements obtained at different times of the day can be compared directly or used interchangeably so long as PD is held constant. Prior to this study, it was unclear if time of day influenced the pupillary response to a fixed light intensity. In order to address this issue, we compared the average PD of three consecutive AM and PM scans obtained in the light or dark. There was no significant difference between average AM and PM PD in the light or dark, which suggests the average pupillary response to a fixed light intensity is static throughout the day. However, it was common for one or more pairs of AM and PM PD measurements to differ by more than 10%, likely due to transient changes in illumination during blinks. This highlights an important point, that controlling for lighting conditions is not necessarily equivalent to controlling for PD during AS-OCT imaging.
On average, angle parameter (AOD, ARA, TISA, TIA) measurements from our study population reflected angle widening with pupillary dilation, which is contrary to the expected effect. However, PD and IA measurements increased and decreased, respectively, as expected. While these findings appear to cast doubt on the validity of our results, we speculate that the observed response is related to the young age, lack of cataracts, and widely open angles of our subject population. A study on anterior chamber changes after physiologic dilation in older subjects (mean 64 years) reported less shallowing of the angle in open compared to narrow angle subjects.29 Another study in older subjects (mean 71.9 years) described a relationship between LV and change in AOD500 upon pharmacologic dilation whereby a significant number of subjects with LV values less than 0.76 mm experienced deepening of the angle.30 Dynamic parameter measurements of AOD500 in Figure 1 show that approximately half of our subjects experienced shallowing of the angle upon dilation even though their average LV was less than 0.1 mm.
We recognize that our study has several limitations. While our objective was to characterize diurnal changes in young, healthy eyes, this limits the generalizability of our findings. The question remains as to the specific mechanism by which angle closure most commonly occurs during the early evening hours. Our findings do not preclude the possibility that diurnal variation is variable among subject populations and is only significant in individuals at risk for angle closure disease. Therefore, a future study in subjects with occludable angles or prior episodes of AAC is warranted. Another limitation is that we only scanned subjects at two time points during the day. These time points were selected due to the difference in reported incidence of AAC during those times, but it is possible that significant variation occurs at other times of the day.9
In summary, diurnal variation associated with static or dynamic anterior segment parameter was not observed. AS-OCT measurements obtained at different times of the day are highly reproducible and appear to be interchangeable. Also, diurnal variation of AS-OCT parameters does not appear to be a regular physiologic occurrence in young, healthy eyes, although such changes may be present in individuals at risk for angle closure disease.
Acknowledgments
This work was supported by Vision Research Core Grant P30EY022589 from the National Eye Institute, National Institutes of Health, Bethesda, MD and by an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY.
Footnotes
- Benjamin Y. Xu: none
- Rafaella C. Penteado: none
- Robert N. Weinreb: C: Aerie Pharmaceuticals, Alcon, Allergan, Bausch & Lomb, Eyenovia, Novartis, Valeant; F: Centeruve, Heidelberg Engineering, Carl Zeiss Meditec, Genentech, Konan, Optos, Optovue, Quark, Tomey, Topcon
References
- 1.Liu JHK, Sit AJ, Weinreb RN. Variation of 24-hour intraocular pressure in healthy individuals: right eye versus left eye. Ophthalmology. 2005;112(10):1670–1675. doi: 10.1016/j.ophtha.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Read SA, Collins MJ, Iskander DR. Diurnal Variation of Axial Length, Intraocular Pressure, and Anterior Eye Biometrics. Investig Opthalmology Vis Sci. 2008;49(7):2911. doi: 10.1167/iovs.08-1833. [DOI] [PubMed] [Google Scholar]
- 3.Tan CS, Ouyang Y, Ruiz H, et al. Diurnal Variation of Choroidal Thickness in Normal, Healthy Subjects Measured by Spectral Domain Optical Coherence Tomography. Investig Opthalmology Vis Sci. 2012;53(1):261. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 4.Nau CB, Malihi M, McLaren JW, Hodge DO, Sit AJ. Circadian variation of aqueous humor dynamics in older healthy adults. Invest Ophthalmol Vis Sci. 2013;54(12):7623–7629. doi: 10.1167/iovs.12-12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cumba RJ, Radhakrishnan S, Bell NP, et al. Reproducibility of Scleral Spur Identification and Angle Measurements Using Fourier Domain Anterior Segment Optical Coherence Tomography. J Ophthalmol. 2012;2012:1–14. doi: 10.1155/2012/487309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maram J, Pan X, Sadda S, Francis B, Marion K, Chopra V. Reproducibility of angle metrics using the time-domain anterior segment optical coherence tomography: intra-observer and inter-observer variability. Curr Eye Res. 2015;40(5):496–500. doi: 10.3109/02713683.2014.930155. [DOI] [PubMed] [Google Scholar]
- 7.Marion KM, Maram J, Pan X, et al. Reproducibility and Agreement Between 2 Spectral Domain Optical Coherence Tomography Devices for Anterior Chamber Angle Measurements. J Glaucoma. 2015;24(9):642–646. doi: 10.1097/IJG.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghimi S, Chen R, Hamzeh N, Khatibi N, Lin SC. Qualitative evaluation of anterior segment in angle closure disease using anterior segment optical coherence tomography. J Curr Ophthalmol. 2016;28(4):170–175. doi: 10.1016/j.joco.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark CV, Mapstone R. Diurnal variation in onset of acute closed angle glaucoma. Br Med J (Clin Res Ed) 1986;292(6528):1106. doi: 10.1136/bmj.292.6528.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teikari JM, O’Donnell J, Nurminen M, Raivio I. Acute closed angle glaucoma and sunshine. J Epidemiol Community Health. 1991;45(4):291–293. doi: 10.1136/jech.45.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quigley HA, Silver DM, Friedman DS, et al. Iris cross-sectional area decreases with pupil dilation and its dynamic behavior is a risk factor in angle closure. J Glaucoma. 2009;18(3):173–179. doi: 10.1097/IJG.0b013e31818624ce. [DOI] [PubMed] [Google Scholar]
- 12.Aptel F, Denis P, Yamamoto T, Kitazawa Y. Optical coherence tomography quantitative analysis of iris volume changes after pharmacologic mydriasis. Ophthalmology. 2010;117(1):3–10. doi: 10.1016/j.ophtha.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Li SZ, Li L, He MG, Thomas R, Wang NL. Dynamic Iris Changes as a Risk Factor in Primary Angle Closure Disease. Investig Opthalmology Vis Sci. 2016;57(1):218. doi: 10.1167/iovs.15-17651. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Li SZ, Li L, He MG, Thomas R, Wang NL. Quantitative analysis of iris changes after physiologic and pharmacologic mydriasis in a rural Chinese population. Invest Ophthalmol Vis Sci. 2014;55(7):4405–4412. doi: 10.1167/iovs.13-13782. [DOI] [PubMed] [Google Scholar]
- 15.Quigley HA, Foster PJ, Friedman DS, et al. The iris is a sponge: a cause of angle closure. Ophthalmology. 2010;117(1):1–2. doi: 10.1016/j.ophtha.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Friedman DS, Gazzard G, Foster P, et al. Ultrasonographic Biomicroscopy, Scheimpflug Photography, and Novel Provocative Tests in Contralateral Eyes of Chinese Patients Initially Seen With Acute Angle Closure. Arch Ophthalmol. 2003;121(5):633. doi: 10.1001/archopht.121.5.633. [DOI] [PubMed] [Google Scholar]
- 17.Nongpiur ME, Ku JYF, Aung T. Angle closure glaucoma: a mechanistic review. Curr Opin Ophthalmol. 2011;22(2):96–101. doi: 10.1097/ICU.0b013e32834372b9. [DOI] [PubMed] [Google Scholar]
- 18.Ho S-W, Baskaran M, Zheng C, et al. Swept source optical coherence tomography measurement of the iris-trabecular contact (ITC) index: a new parameter for angle closure. Graefes Arch Clin Exp Ophthalmol. 2013;251(4):1205–1211. doi: 10.1007/s00417-012-2158-6. [DOI] [PubMed] [Google Scholar]
- 19.Aptel F, Chiquet C, Gimbert A, et al. Anterior Segment Biometry Using Spectral-Domain Optical Coherence Tomography. J Refract Surg. 2014;30(5):354–360. doi: 10.3928/1081597X-20140326-01. [DOI] [PubMed] [Google Scholar]
- 20.Tun TA, Baskaran M, Zheng C, et al. Assessment of trabecular meshwork width using swept source optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2013;251(6):1587–1592. doi: 10.1007/s00417-013-2285-8. [DOI] [PubMed] [Google Scholar]
- 21.Nongpiur ME, He M, Amerasinghe N, et al. Lens Vault, Thickness, and Position in Chinese Subjects with Angle Closure. Ophthalmology. 2011;118(3):474–479. doi: 10.1016/j.ophtha.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Ozaki M, Nongpiur ME, Aung T, He M, Mizoguchi T. Increased lens vault as a risk factor for angle closure: confirmation in a Japanese population. Graefe’s Arch Clin Exp Ophthalmol. 2012;250(12):1863–1868. doi: 10.1007/s00417-012-2011-y. [DOI] [PubMed] [Google Scholar]
- 23.Aung T, Nolan WP, Machin D, et al. Anterior chamber depth and the risk of primary angle closure in 2 East Asian populations. Arch Ophthalmol (Chicago, Ill 1960) 2005;123(4):527–532. doi: 10.1001/archopht.123.4.527. [DOI] [PubMed] [Google Scholar]
- 24.Mapstone R, Clark CV. Diurnal Variation in the Dimensions of the Anterior Chamber. Arch Ophthalmol. 1985;103(10):1485–1486. doi: 10.1001/archopht.1985.01050100061019. [DOI] [PubMed] [Google Scholar]
- 25.Akil H, Dastiridou A, Marion K, Francis BA, Chopra V. Effects of diurnal, lighting, and angle-of-incidence variation on anterior segment optical coherence tomography (AS-OCT) angle metrics. BMC Ophthalmol. 2017;17(1):31. doi: 10.1186/s12886-017-0425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung CK, Cheung CYL, Li H, et al. Dynamic Analysis of Dark–Light Changes of the Anterior Chamber Angle with Anterior Segment OCT. Investig Opthalmology Vis Sci. 2007;48(9):4116. doi: 10.1167/iovs.07-0010. [DOI] [PubMed] [Google Scholar]
- 27.Xu BY, Israelsen P, Pan BX, Wang D, Jiang X, Varma R. Benefit of Measuring Anterior Segment Structures Using an Increased Number of Optical Coherence Tomography Images: The Chinese American Eye Study. Invest Ophthalmol Vis Sci. 2016;57(14):6313–6319. doi: 10.1167/iovs.16-19755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blieden LS, Chuang AZ, Baker LA, et al. Optimal number of angle images for calculating anterior angle volume and iris volume measurements. Invest Ophthalmol Vis Sci. 2015;56(5):2842–2847. doi: 10.1167/iovs.14-15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aptel F, Chiquet C, Beccat S, et al. Biometric Evaluation of Anterior Chamber Changes after Physiologic Pupil Dilation Using Pentacam and Anterior Segment Optical Coherence Tomography. Investig Opthalmology Vis Sci. 2012;53(7):4005. doi: 10.1167/iovs.11-9387. [DOI] [PubMed] [Google Scholar]
- 30.Arimura S, Takamura Y, Takihara Y, Matsumura T, Tomomatsu T, Inatani M. Determinants of anterior chamber angle narrowing after mydriasis in the patients with cataract. Graefe’s Arch Clin Exp Ophthalmol. 2015;253(2):307–312. doi: 10.1007/s00417-014-2817-x. [DOI] [PubMed] [Google Scholar]


