Abstract
Introduction:
Experiences of racial discrimination have been associated with poor health outcomes. Little is known, however, about how perceived racial discrimination influences DNA methylation (DNAm) among African Americans (AAs). We examined the association of experiences of discrimination with DNAm among AA women in the Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure (InterGEN) study.
Methods:
The InterGEN study examines the effects of genetic and psychological factors on blood pressure among AA women and their children. Measures include the Major Life Discrimination (MLD) and the Race-Related Events (RES) scales. In the present analysis, we examined discrimination and DNAm at baseline in the InterGEN study. The 850K EPIC Illumina BeadChip was used for evaluating DNAm in this epigenome-wide association study (EWAS).
Results:
One hundred and fifty-two women contributed data for the RES-EWAS analysis and 147 for the MLD-EWAS analysis. Most were 30–39 years old, nonsmokers, had some college education, and had incomes <US$15,000/year. After controlling for age, smoking, and cell composition, MLD was significantly associated with DNAm at nine CpG (regions of DNA where a cytosine nucleotide is followed by a guanine nucleotide) sites (false discovery rate [FDR]-corrected p < .05). For the RES-EWAS analysis, no DNAm sites passed the epigenome-wide significance level after genomic control, though suggestive associations were observed at CpG sites after genomic control (raw p < 10−5).
Conclusion:
We observed significant epigenetic associations between disease-associated genes (e.g., schizophrenia, bipolar disorder, and asthma) and perceived discrimination as measured by the MLD Scale. Future health disparities research should include epigenetics in high-risk populations to elucidate functional consequences induced by the psychosocial environment.
Keywords: DNA methylation, epigenomics, African Americans, women, racism
Perceived experiences of discrimination have consistently been associated with stress and poor physical and mental health outcomes for adults in nursing and medical literature (Paradies et al., 2015; Williams & Mohammed, 2009) including increased risk of chronic illness such as hypertension (Orom, Sharma, Homish, Underwood, & Homish, 2017) and obesity (Cunningham et al., 2013) as well as preterm birth (Alhusen, Bower, Epstein, & Sharps, 2016) and depression (Molina & James, 2016). These experiences contribute to health disparities between racial/ethnic groups and adversely affect population health (Bailey et al., 2017). Experiences of perceived discrimination or racism have been defined as unfair treatment based on one’s race or ethnicity (Clark, Anderson, Clark, & Williams, 1999). This social classification is based on phenotype (Jones, 2001). Studies have increasingly focused on how experiences of interpersonal racism affect biomarkers for disease, such as allostatic load and hormonal dysregulation (Paradies et al., 2015; Williams & Mohammed, 2009). The mechanisms by which these psychosocial experiences affect physical and mental health, however, are still unclear (Brondolo et al., 2011).
One potential mechanism for how negative social exposures (such as perceived discrimination) based on phenotype affect health is through DNA methylation (DNAm) across the epigenome. DNAm, or the addition of methyl groups to promoter regions of genes, may effectively silence or activate gene expression and has been implicated in many disease processes, most notably in cancer (Liang & Weisenberger, 2017) but also in autoimmune diseases (Teruel & Sawalha, 2017), diabetes (Bansal & Pinney, 2017), schizophrenia (Shorter & Miller, 2015), and cardiovascular diseases (Muka et al., 2016). To our knowledge, there are no published studies that have examined how perceived racism and discrimination, as an environmental exposure is associated with DNAm across the epigenome among African American (AA) women. However, other studies have reported significant interactions between single-nucleotide polymorphisms (SNPs) and experiences of discrimination associated with blood pressure among AAs (Taylor et al., 2012, under review).
The experience of racism or discrimination is common for AAs (Stepanikova & Oates, 2017). In a recent national survey, the Pew Research Center (2016) found that 71% of AAs reported being treated unfairly because of their race, and 11% said this discrimination was a regular occurrence. In one large, longitudinal study of multiethnic women, researchers reported that AAs had the highest rates of perceived discrimination (35%) compared to Chinese (20%), Hispanic (12%), and Caucasian (3%) women (Jacobs et al., 2014). Considering that AAs carry a disproportionate burden of incidence, morbidity, and mortality from chronic diseases such as hypertension and obesity (Centers for Disease Control and Prevention, 2005), it is worthwhile to examine how perceived racism and discrimination affect the epigenome. The purpose of the present study was to examine the influence of perceived racism and discrimination on DNAm in a sample of AA mothers enrolled in the Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure (InterGEN) study.
Material and Method
The InterGEN study is an ongoing, longitudinal cohort study in Connecticut that examines the effects of genetic, epigenetic, and psychological factors on blood pressure. Recruitment began in April 2015, and 159 mother/child dyads had been enrolled as of April 2017. AA women were recruited from early childcare and education centers, primary care clinics, and community events (e.g., health fairs). Eligibility criteria are that women (a) are ≥21 years old, (b) identify as AA or Black (via self-report), (c) speak English, (d) not have a psychiatric or cognitive disorder that may limit accuracy of reporting of study data, and (e) have a biological child 3–5 years old. Full study procedures have been described elsewhere (Crusto, Barcelona de Mendoza, Connell, Sun, & Taylor, 2016; Taylor, Wright, Crusto, & Sun, 2016).
Trained research assistants approached mothers for recruitment, conducted screening to verify eligibility, and obtained written, informed consent per established study protocols. InterGEN consists of four study visits approximately 6 months apart over the span of 2 years. During the baseline (Time 1) visit, study personnel take clinical measurements of blood pressure, height, and weight and collect saliva for DNA analysis from both mother and child. Researchers collect repeated clinical data and psychological measures at the three follow-up visits. Demographic information, health history, and psychological measures (including parenting, experiences of perceived racism and discrimination, and depression) are collected through mother’s report using Audio Computer-Assisted Self-Interview software (ACASI, version 16). Although an InterGEN research assistant is present for data collection, social desirability bias is minimized through the use of computer-based data collection. For the present analysis, we examined the association between discrimination (measured by Major Life Discrimination [MLD] and Race-Related Events [RES] scales) and DNAm (epigenome-wide association study [EWAS]) at baseline (Time 1 in the InterGEN cohort study). Yale University’s Institutional Review Board reviewed and approved the study procedures (approval #1311012986). Data are available via written request to the InterGEN study investigators.
Experiences of perceived racism and discrimination were measured using two scales. The 22-item RES assesses exposure to stressful and potentially traumatizing experiences of race-related stress in adults (Waelde et al., 2010). These experiences include being treated unfairly, harassed, or hurt because of one’s race. Respondents indicate whether they have ever experienced each event (yes/no), and all 22 items are summed (0 = no, 1 = yes) for a total RES score, ranging from 0 to 22. The RES has demonstrated good reliability (α = .78 to .88) in a diverse sample of caregivers recruited from Head Start centers (Crusto, Dantzler, Roberts, & Hooper, 2015).
The 9-item MLD Scale assesses experiences of unfair treatment in adults (Krieger, Smith, Naishadham, Hartman, & Barbeau, 2005; Williams, Yan, Jackson, & Anderson, 1997). Respondents indicate whether they have ever experienced each listed major discrimination event, such as being unfairly fired, denied a bank loan, or stopped and searched by the police (yes/no). They further attribute each event to one main reason from 11 options, including gender, race, age, skin color, religion, weight or height, and others. The 9 items are summed (0 = no, 1 = yes) for a total MLD score ranging from 0 to 9.
Researchers collected saliva samples for DNA from mothers and children using the Oragene-500 format tubes (Bahlo et al., 2010), which requires participants to spit into the tube until the contents reach the fill line (2 ml). Detailed DNA collection and analysis procedures have been described elsewhere (Taylor et al., 2016). Samples were transported from the field to the research laboratory where they were refrigerated at 4°C until DNA extraction and analysis were completed. Standard protocol for DNA extraction and purification was conducted as indicated in the standard operating procedure guidelines using ReliaPrep kits; more details on DNA and methylation processing can be found in Taylor, Wright, Crusto, and Sun (2016). All tubes and plates that contained an individual’s DNA were labeled with a barcode to ensure precise sample tracking and recorded in the laboratory’s computerized freezer inventory via barcodes upon arrival to the laboratory. All DNA pipetting was performed with robotic workstations that incorporate barcode scanning to track the transfer of the biological material from tube to tube, tube to plate, and plate to plate. These customized barcode scanning programs were integrated with the robotics deck configuration and networked to the laboratory. This concurrent electronic sample tracking ensured that an individual’s DNA in each well of every plate generated could be identified for correct merging to genotype calls. These quality control assurance procedures were monitored frequently and updated as needed for continued improvement of sample management.
The Illumina Infinium Methylation EPIC (850K) BeadChip was used for epigenome-wide DNAm measurement. Quantile normalization of β values for autosomal CpG sites was performed. All individual samples passed laboratory-based quality control procedures (missing rate <10% and no sex mismatch). CpG sites were excluded if they had detection p value greater than .01 (n = 71), had a missing rate greater than 10% (n = 514), overlapped with SNPs (n = 14,184), or were listed in the recent Illumina quality notice (https://mkt.illumina.com/rs/600-XEX-927/images/PQN0223_Methylation%20EPIC.pdf?mkt_tok=eyJpIjoiTURSa01ESm1aRGRtT0RnNCIsInQiOiJCcFBMc0pEUkFqUVwvc3g1OFJuOUdTYW1NWThIXC9EenVaemI4QkRzcXJDUGtcL0J0T1NkM05SaGxWcHNsZElEY0RyXC9jVFNNVnFGZ01seFVnZHlOSjFGMjdmVFwvVWxtR3; n = 977). A total of 831,658 autosomal and 19,007 X-chromosomal CpG sites were included in the association analyses.
Participants self-reported age in years, whether they smoked cigarettes (yes/no) and other demographic data at the initial interview. We controlled for age and maternal smoking, which are accepted confounders in epigenetic studies (Klebaner et al., 2016). We also adjusted for batch effects and potential heterogeneity in cell proportions from saliva using the reference-free EWAS method (Houseman, Molitor, & Marsit, 2014).
We examined all variables using univariate analyses and descriptive statistics including mean and standard deviation for continuous variables and frequencies for categorical. We calculated the correlation coefficient between the two discrimination variables and used the following linear regression model to study the influence of perceived discrimination on DNAm:
where each discrimination variable (D) was modeled separately, controlling for the confounding factors of maternal smoking and age, and the error term e to predict the dependent variable (Y), the β value of DNAm. We applied the genomic control approach (Devlin & Roeder, 1999) to address residual unmeasured confounding and inflation of Type I error and used false discovery rate (FDR) to correct for multiple comparisons. We conducted all analyses in the R statistical computing environment (http://www.R-project.org) using a selection of packages from Bioconductor.
Results
Of the 159 InterGEN participants contributing data for this analysis, 6 had missing data for the RES discrimination variable, 11 were missing data for the MLD variable, and three were missing maternal smoking status. CpG sites with ≥50% missing rate were excluded, and all subjects with complete discrimination data were included in the analyses, leaving N = 152 for the RES-EWAS analysis and N = 147 for the MLD-EWAS analysis. Most participants were between the ages of 30 and 39 years old, nonsmokers, had achieved at least some college education or higher, and had an annual household income of less than US$15,000/year. Other demographic information is presented in Table 1. The correlation coefficient between the two discrimination variables was .62, indicating moderate correlation between them.
Table 1.
Participant Characteristics.
| Primary Covariate | n (%) |
|---|---|
| Age, years | |
| 20–29 | 64 (40.2) |
| 30–39 | 77 (48.4) |
| 40–49 | 18 (11.3) |
| Maternal smoker | |
| No | 124 (79.5) |
| Yes | 32 (20.5) |
| Education | |
| <High school | 8 (5.1) |
| High school graduate | 57 (36.3) |
| Some college/graduate | 92 (58.6) |
| Annual household income | |
| ≤US$15,000 | 71 (47.6) |
| >US$15,000–34,999 | 53 (35.5) |
| >US$35,000 | 25 (16.7) |
| Health insurance type | |
| Private | 19 (12.7) |
| Medicaid | 96 (64.0) |
| Government/ACA | 23 (15.3) |
| None | 12 (8.0) |
| Ever received diagnosis of high blood pressure | |
| No | 131 (84.0) |
| Yes | 25 (16.0) |
| Current high blood pressure medication use | |
| No | 148 (94.3) |
| Yes | 9 (5.7) |
| BMI category | |
| Underweight (<18.5 kg/m2) | 9 (5.7) |
| Normal weight (18.5–24.9 kg/m2) | 40 (25.1) |
| Overweight (25–29.9 kg/m2) | 44 (27.7) |
| Obese (≥30 kg/m2) | 66 (41.5) |
Note. N = 159. Frequencies do not sum to 159 for all variables due to missing data. ACA = Affordable Care Act; BMI = body mass index.
In the EWAS analyses, DNAm at nine CpG sites were associated with MLD after genomic control (FDR-corrected p < .05, which indicates that these associations should not be false positives assuming a .05 FDR for the whole epigenome; Figure 1A and B). Furthermore, we observed reduced DNAm (hypomethylation) with increasing MLD at most of the significantly associated CpG sites (77.8%; Table 2). However, for the RES-EWAS analysis, no DNAm site passed the epigenome-wide significance level after genomic control. We observed marginal negative and positive associations between DNAm and RES at six and five CpG sites after genomic control (p < 10−5), respectively. We identified no common DNAm site in comparing the top 20 epigenetic associations of RES with the epigenome-wide significant associations of MLD.
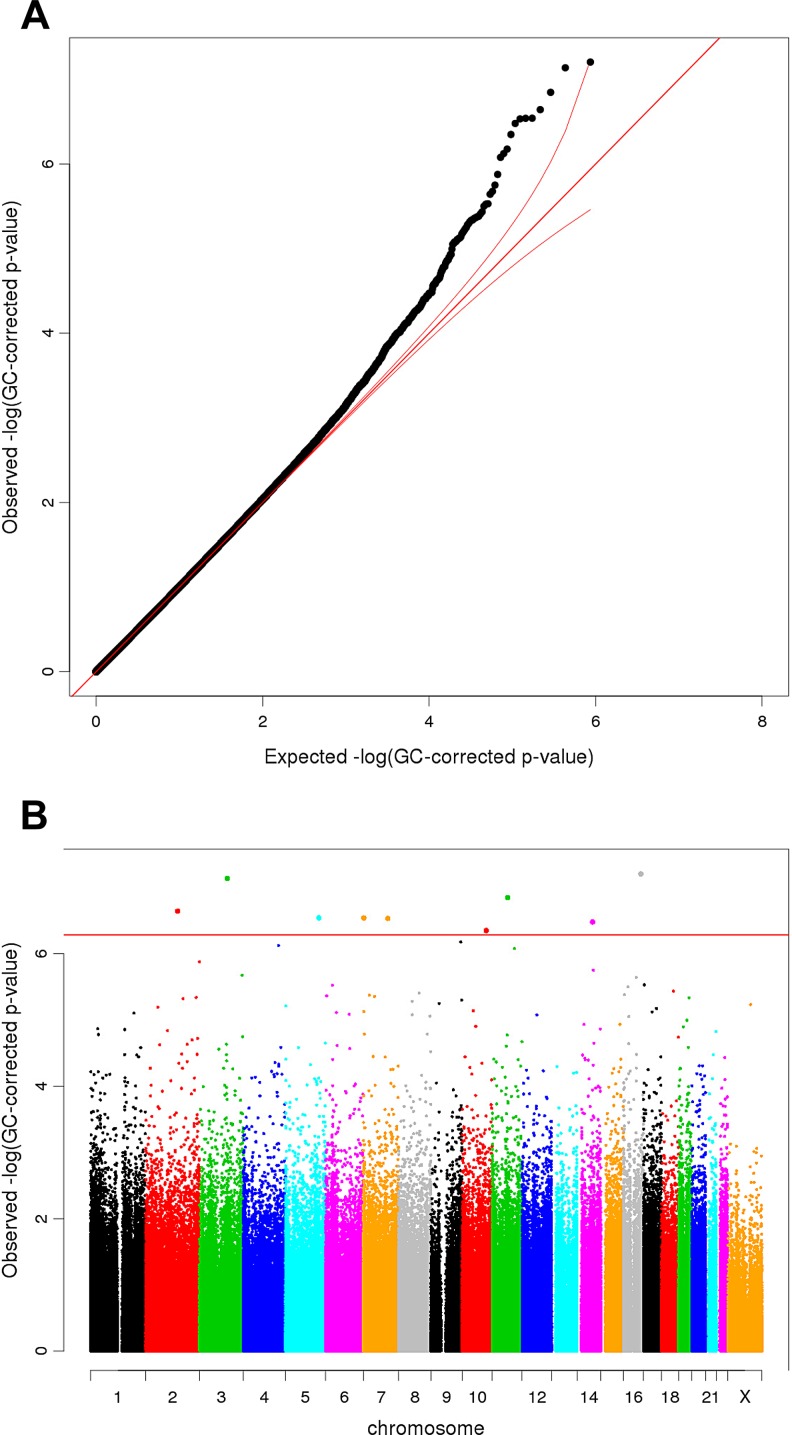
Figure 1.
(A) Quantile–quantile plot of epigenome-wide association analysis with Major Life Discrimination (MLD) Scale score among n = 147 African American (AA) mothers. GC approach was applied on raw p values. Inflation factor = 1.00. (B) Manhattan plot of epigenome-wide association analysis with MLD score among 147 AA mothers. GC approach was applied on raw p values. Red line indicates false discovery rate–adjusted p value = .05. GC = genomic control.
Table 2.
Epigenome-Wide Significant Associations With Scores on the Major Life Discrimination (MLD; n = 147) and Race-Related Events (RES; n = 152) Scales Among African American Mothers.
| CpG Site | Chr | bp | Gene | Exposure | β | SE | T Stat | p Value After GC | FDR-Adjusted p Valuea |
|---|---|---|---|---|---|---|---|---|---|
| cg09437479 | 16 | 78539954 | WWOX | RES | −1.73E-03 | 7.47E-04 | −2.32 | 3.56E-02 | .994 |
| MLD | −1.13E-02 | 1.88E-03 | −6.03 | 6.25E-08 | .031 | ||||
| cg16143945 | 3 | 126168382 | ZXDC | RES | 5.96E-04 | 1.36E-04 | 4.38 | 1.02E-04 | .766 |
| MLD | 2.27E-03 | 3.79E-04 | 6.00 | 7.30E-08 | .031 | ||||
| cg03317714 | 11 | 69912795 | LOC101928443 | RES | −1.85E-03 | 5.65E-04 | −3.28 | 3.16E-03 | .941 |
| MLD | −8.69E-03 | 1.49E-03 | −5.85 | 1.43E-07 | .035 | ||||
| cg19958721 | 2 | 144455439 | ARHGAP15 | RES | −1.45E-03 | 5.67E-04 | −2.56 | 2.07E-02 | .981 |
| MLD | −8.23E-03 | 1.43E-03 | −5.74 | 2.28E-07 | .035 | ||||
| cg05711042 | 5 | 150913871 | FAT2 | RES | −2.59E-03 | 6.59E-04 | −3.93 | 4.50E-04 | .823 |
| MLD | −1.14E-02 | 2.00E-03 | −5.69 | 2.88E-07 | .035 | ||||
| cg02578462 | 7 | 2028754 | MAD1L1 | RES | −1.59E-03 | 4.42E-04 | −3.60 | 1.24E-03 | .888 |
| MLD | −6.49E-03 | 1.14E-03 | −5.69 | 2.89E-07 | .035 | ||||
| cg07726178 | 7 | 110748868 | LRRN3; IMMP2L | RES | −1.62E-03 | 4.10E-04 | −3.95 | 4.27E-04 | .808 |
| MLD | −6.58E-03 | 1.16E-03 | −5.68 | 2.94E-07 | .035 | ||||
| cg15209133 | 14 | 70020151 | RES | 2.21E-03 | 7.21E-04 | 3.06 | 5.76E-03 | .957 | |
| MLD | 1.13E-02 | 1.99E-03 | 5.66 | 3.32E-07 | .035 | ||||
| cg04928761 | 10 | 108756503 | SORCS1 | RES | −3.16E-03 | 1.01E-03 | −3.13 | 4.87E-03 | .954 |
| MLD | −1.46E-02 | 2.62E-03 | −5.59 | 4.49E-07 | .042 |
Note. bp = base pair; Chr = chromosome; GC = genomic control.
aFalse discovery rate (FDR) adjustment applied after GC approach.
Discussion
In the present study, we found a significant inverse relationship between perceived racial discrimination measured by the MLD Scale and DNAm in a sample of AA women enrolled in the InterGEN study. Women who reported greater perceived discrimination as measured by the MLD Scale had decreased DNAm on seven genes/CpG sites. However, we found no significant associations between score on the RES and DNAm.
The genes associated with increased reports of perceived racism and discrimination and hypomethylation were as follows: WWOX, LOC101928443 (uncharacterized in HapMap), ARHGAP15, FAT atypical cadherin 2 (FAT2), mitotic arrest deficient like 1 (MAD1L1), leucine-rich repeat neuronal 3 (LRRN3), and sortilin-related VPS10 domain-containing receptor 1 (SORCS1). The WW domain-containing oxidoreductase (WWOX) is a protein-coding tumor suppressor gene that has been implicated in various cancers including osteosarcoma (Wen et al., 2017), colorectal (Tian et al., 2017), and bone and breast cancer (Maroni, Matteucci, Bendinelli, & Desiderio, 2017) among others. Epigenetic mechanisms of WWOX have been implicated in influencing the phenotype of certain cancers (Maroni et al., 2017). The functional pathways for WWOX include the transcriptional regulation by the AP-2 family of transcription factors and gene expression (Gene Cards, 2017) and have also been associated with lung function (Burdett et al., 2017). ARHGAP15 is also a protein-coding gene and has been associated with cancers and cognitive function in animal models (Sun et al., 2017; Zamboni et al., 2016). The ARHGAP15 gene is associated with three pathways: G-protein-coupled receptor (GPCR) downstream signaling, signaling by GPCR, and signal transduction (Path Cards, 2017). ARHGAP15 is also associated with asthma, and interestingly, with educational attainment (Burdett et al., 2017), suggesting that education could be used as a proxy phenotype to study genetic influences on mental illness (Okbay et al., 2016). FAT2 is another protein-coding tumor suppressor gene, which has been linked with skin and spinal cancer and slower growth of other carcinomas (Cao et al., 2016). MAD1L1 is yet another tumor suppression protein-coding gene that has been associated with various cancers and that, when hypomethylated, is also associated with recurrence of tumors (Cui et al., 2016). This locus is also associated with schizophrenia and bipolar disease (Burdett et al., 2017). However, diverse DNAm profiles have been documented on MAD1L1 across differing multiethnic populations residing in the same city (Giuliani et al., 2016). This diversity suggests that the DNAm profile of individuals is influenced by genetic ancestry as well as environmental and demographic stressors.
Leucine-rich repeat neuronal 3 (LRRN3) is a well-known protein-coding gene that has been significantly associated with inflammation, cigarette smoking, and chronic obstructive pulmonary disease (Martin, Talikka, Hoeng, & Peitsch, 2015; Obeidat et al., 2016; Poussin et al., 2017). The LRRN3 gene has also recently been linked with differential gene expression by age after traumatic brain injury (TBI; Cho et al., 2016). Lastly, the (SORCS1) is a protein-coding gene that is also associated with a brain disorder; however, unlike LRRN3, it is not TBI but narcolepsy and neurodegeneration in Alzheimer’s (Knight et al., 2016; Printy, Verma, Cowperthwaite, Markey, & Alzheimer’s Disease Neuroimaging Initiative, 2014; Scheinfeldt et al., 2015).
There were two genes in which the significant association between MLD and DNAm was not inversely correlated: one uncharacterized gene and the ZXD family zinc finger C (ZXDC). The ZXDC gene is a protein-coding gene that has been linked with cardiovascular-related diseases such as cerebral arterial disease (Shoemaker et al., 2015). Although the relationship between these genes and cardiovascular diseases requires further examination and study, these genes hold promise for improving our understanding of the complex and multigenic response to environmental stimuli and how these responses may affect health.
Researchers in previous studies have examined how perceived racism and discrimination relate to genetics and the development of chronic diseases on the population level, with much of the focus on hypertension and other common, chronic diseases. One such study investigated how SNPs on genes associated with hypertension interacted with experiences of discrimination to influence blood pressure among AAs enrolled in the Jackson Heart Study (N = 2,937; Taylor et al., 2017). In that study, investigators identified two SNPs that interacted significantly with MLD on both systolic and diastolic blood pressure in AAs. These SNPs are located near the sodium bicarbonate cotransporter gene (SLC4A5). Others have examined the relationship between genetic polymorphisms and self-reported skin color (as a proxy for racial discrimination) on high blood pressure in a sample of 137 AA women from Detroit (Taylor et al., 2012). They found that one SNP–skin color interaction was significant, also on the SLC4A5 gene.
Researchers have also examined how family environment influences DNAm in AA populations. Brody, Miller, Yu, Beach, and Chen (2016) studied family environments (including parent–child conflict, parental emotional support, and chaotic home environment) and epigenetic aging in adolescents from Georgia using two replication cohorts. They found that supportive family environments buffered epigenetic aging and DNAm among AA adolescents exposed to high levels of discrimination. They also observed that adolescents in less supportive family environments had faster epigenetic aging in immune system cells in peripheral blood. Wright and colleagues (2017) observed that higher parenting stress was associated with differential DNAm among a sample of AA women (N = 74) also enrolled in the InterGEN study, especially in pathways associated with stress signaling.
In the present study, we measured discrimination in two ways, and interestingly, we observed statistically significant results only with the MLD variable. The MLD measures all experiences of discrimination over the life span, while the RES captures only those related to race. It may be that the cumulative effects of discrimination measured by the MLD resulted in longer and larger effects on the epigenome, which made it easier to detect epigenetic modifications. In a large study of AA and Latino adults (N = 617) from New York, researchers reported an interaction between age and lifetime experiences of discrimination on ambulatory blood pressure, noting that they did not observe these effects of discrimination in younger participants (Beatty Moody et al., 2016). These findings support the idea that the accumulation of experiences of discrimination may be more important than the type of discrimination for predicting long-term health effects. The measurement of discrimination can also include influences of structural racism as well as other, less studied indices, which increase the possibility that health disparities research can produce meaningful interventions (Riley, 2017). Further research that accounts for perceived racism and discrimination and DNAm effects when exploring specific health outcomes in this population is needed to truly understand and address health disparities.
The present study had both strengths and limitations. Strengths included the use of measures of discrimination as an environmental factor that has not traditionally been studied in the context of epigenetics, and in an all AA sample. The choice of saliva for EWAS analysis is both a strength, as it is an accessible and acceptable tissue type, and a potential limitation, as its generalizability to other tissues may be limited due to tissue/cell type specificity. Other limitations are that our sample was relatively small, which may have resulted in inadequate power to fully examine the effects of the RES on DNAm. It is encouraging that we identified several significant CpG sites after stringent controls for multiple testing, confounding, and genomic inflation for MLD. Future replication studies with similar samples are needed to further validate the findings.
In conclusion, this study adds to the sparse literature examining the influence of perceived racial discrimination on DNAm in AAs. This research is important for nursing practice, as a better understanding of social determinants of health and their interplay with epigenetics could lead to improved, individualized treatments for chronic illness as well as a better delineation of disease risk. Future health disparities research should include how environmental factors contribute to epigenetic changes in this high-risk population. Nursing research can and should incorporate both genome-wide and epigenome-wide methodologies to explore -omic interactions with perceived racism and discrimination on health outcomes among AAs.
Footnotes
Author Contribution: V. Barcelona de Mendoza contributed to conception, analysis, and interpretation; drafted the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Y. Huang contributed to analysis and interpretation, drafted the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. C. Crusto contributed to conception and design, critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Y. Sun contributed to conception, design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. J. Taylor contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health, National Institute of Nursing Research [R01NR013520].
References
- Alhusen J. L., Bower K. M., Epstein E., Sharps P. (2016). Racial discrimination and adverse birth outcomes: An integrative review. Journal of Midwifery & Women’s Health, 61, 707–720. doi:10.1111/jmwh.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahlo M., Stankovich J., Danoy P., Hickey P. F., Taylor B. V., Browning S. R.…Rubio J. P. (2010). Saliva-derived DNA performs well in large-scale, high-density single-nucleotide polymorphism microarray studies. Cancer Epidemiology, Biomarkers & Prevention, 19, 794–798. doi:10.1158/1055-9965.EPI-09-0812 [DOI] [PubMed] [Google Scholar]
- Bailey Z. D., Krieger N., Agenor M., Graves J., Linos N., Bassett M. T. (2017). Structural racism and health inequities in the USA: Evidence and interventions. Lancet (London), 389, 1453–1463. doi:S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- Bansal A., Pinney S. E. (2017). DNA methylation and its role in the pathogenesis of diabetes. Pediatric Diabetes, 18, 167–177. doi:10.1111/pedi.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty Moody D. L., Waldstein S. R., Tobin J. N., Cassells A., Schwartz J. C., Brondolo E. (2016). Lifetime racial/ethnic discrimination and ambulatory blood pressure: The moderating effect of age. Health Psychology, 35, 333–342. doi:10.1037/hea0000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G. H., Miller G. E., Yu T., Beach S. R., Chen E. (2016). Supportive family environments ameliorate the link between racial discrimination and epigenetic aging: A replication across two longitudinal cohorts. Psychological Science, 27, 530–541. doi:10.1177/0956797615626703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondolo E., Hausmann L. R., Jhalani J., Pencille M., Atencio-Bacayon J., Kumar A.…Schwartz J. (2011). Dimensions of perceived racism and self-reported health: Examination of racial/ethnic differences and potential mediators. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 42, 14–28. doi:10.1007/s12160-011-9265 -1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett T., Hall P. N., Hastings E., Hindorff L. A., Junkins H. A., Klemm A. K.…Welter D. (2017). The NHGRI-EBI catalog of published genome-wide association studies. Retrieved from https://www.ebi.ac.uk/gwas [DOI] [PMC free article] [PubMed]
- Cao L. L., Riascos-Bernal D. F., Chinnasamy P., Dunaway C. M., Hou R., Pujato M. A.…Sibinga N. E. (2016). Control of mitochondrial function and cell growth by the atypical cadherin Fat1. Nature, 539, 575–578. doi:10.1038/nature20170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2005). Health disparities experienced by Black or African Americans—United States. MMWR Morbidity and Mortality Weekly Report, 54, 1–3. [PubMed] [Google Scholar]
- Cho Y. E., Latour L. L., Kim H., Turtzo L. C., Olivera A., Livingston W. S.…Gill J. (2016). Older age results in differential gene expression after mild traumatic brain injury and is linked to imaging differences at acute follow-up. Frontiers in Aging Neuroscience, 8, 168 doi:10.3389/fnagi.2016.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Anderson N. B., Clark V. R., Williams D. R. (1999). Racism as a stressor for African Americans. A biopsychosocial model. American Psychologist, 54, 805–816. [DOI] [PubMed] [Google Scholar]
- Crusto C. A., Barcelona de Mendoza V., Connell C. M., Sun Y. V., Taylor J. Y. (2016). The intergenerational impact of genetic and psychological factors on blood pressure study (InterGEN): Design and methods for recruitment and psychological measures. Nursing Research, 65, 331–338. doi:10.1097/NNR.0000000000000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusto C. A., Dantzler J., Roberts Y. H., Hooper L. M. (2015). Psychometric evaluation of data from the race-related events scale. Measurement and Evaluation in Counseling and Development, 48, 285–296. doi:10.1177/0748175615578735 [Google Scholar]
- Cui C., Lu Z., Yang L., Gao Y., Liu W., Gu L.…Sun Z. S. (2016). Genome-wide identification of differential methylation between primary and recurrent hepatocellular carcinomas. Molecular Carcinogenesis, 55, 1163–1174. doi:10.1002/mc.22359 [DOI] [PubMed] [Google Scholar]
- Cunningham T. J., Berkman L. F., Kawachi I., Jacobs D. R., Jr, Seeman T. E., Kiefe C. I., Gortmaker S. L. (2013). Changes in waist circumference and body mass index in the US CARDIA cohort: Fixed-effects associations with self-reported experiences of racial/ethnic discrimination. Journal of Biosocial Science, 45, 267–278. doi:10.1017/S0021932012000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin B., Roeder K. (1999). Genomic control for association studies. Biometrics, 55, 997–1004. [DOI] [PubMed] [Google Scholar]
- Gene Cards. (2017). WWOX gene. Retrieved from http://www.genecards.org/cgi-bin/carddisp.pl?gene=WWOX
- Giuliani C., Sazzini M., Bacalini M. G., Pirazzini C., Marasco E., Fontanesi E.…Garagnani P. (2016). Epigenetic variability across human populations: A focus on DNA methylation profiles of the KRTCAP3 , MAD1L1 and BRSK2 genes. Genome Biology and Evolution, 8, 2760–2773. doi:10.1093/gbe/evw186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman E. A., Molitor J., Marsit C. J. (2014). Reference-free cell mixture adjustments in analysis of DNA methylation data. Bioinformatics (Oxford, England), 30, 1431–1439. doi:10.1093/bioinformatics/btu029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E. A., Rathouz P. J., Karavolos K., Everson-Rose S. A., Janssen I., Kravitz H. M.…Powell L. H. (2014). Perceived discrimination is associated with reduced breast and cervical cancer screening: The Study of Women’s Health Across the Nation (SWAN). Journal of Women’s Health, 23, 138–145. doi:10.1089/jwh.2013.4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. P. (2001). Invited commentary: “Race,” racism, and the practice of epidemiology. American Journal of Epidemiology, 154, 299–304; discussion 305–306. [DOI] [PubMed] [Google Scholar]
- Klebaner D., Huang Y., Hui Q., Taylor J. Y., Goldberg J., Vaccarino V., Sun Y. V. (2016). X chromosome-wide analysis identifies DNA methylation sites influenced by cigarette smoking. Clinical Epigenetics, 8, 20 doi:10.1186/s13148-016-0189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E. M., Ruiz H. H., Kim S. H., Harte J. C., Hsieh W., Glabe C.…Gandy S. (2016). Unexpected partial correction of metabolic and behavioral phenotypes of Alzheimer’s APP/PSEN1 mice by gene targeting of diabetes/Alzheimer’s-related Sorcs1. Acta Neuropathologica Communications, 4, 16 doi:10.1186/s40478-016-0282-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N., Smith K., Naishadham D., Hartman C., Barbeau E. M. (2005). Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science & Medicine, 61, 1576–1596. doi:S0277-9536(05)00097-3 [DOI] [PubMed] [Google Scholar]
- Liang G., Weisenberger D. J. (2017). DNA methylation aberrancies as a guide for surveillance and treatment of human cancers. Epigenetics, 12, 416–432. doi:10.1080/15592294.2017.1311434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroni P., Matteucci E., Bendinelli P., Desiderio M. A. (2017). Functions and epigenetic regulation of WWOX in bone metastasis from breast carcinoma: Comparison with primary tumors. International Journal of Molecular Sciences, 18, 75 doi:10.3390/ijms18010075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F., Talikka M., Hoeng J., Peitsch M. C. (2015). Identification of gene expression signature for cigarette smoke exposure response—From man to mouse. Human & Experimental Toxicology, 34, 1200–1211. doi:10.1177/0960327115600364 [DOI] [PubMed] [Google Scholar]
- Molina K. M., James D. (2016). Discrimination, internalized racism, and depression: A comparative study of African American and Afro-Caribbean adults in the US. Group Processes & Intergroup Relations: GPIR, 19, 439–461. doi:10.1177/1368430216641304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muka T., Koromani F., Portilla E., O’Connor A., Bramer W. M., Troup J.…Franco O. H. (2016). The role of epigenetic modifications in cardiovascular disease: A systematic review. International Journal of Cardiology, 212, 174–183. doi:10.1016/j.ijcard.2016.03.062 [DOI] [PubMed] [Google Scholar]
- Obeidat M., Ding X., Fishbane N., Hollander Z., Ng R. T., McManus B.…Sin D. D. (2016). The effect of different case definitions of current smoking on the discovery of smoking-related blood gene expression signatures in chronic obstructive pulmonary disease. Nicotine & Tobacco Research, 18, 1903–1909. doi:10.1093/ntr/ntw129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A., Beauchamp J. P., Fontana M. A., Lee J. J., Pers T. H., Rietveld C. A.…Benjamin D. J. (2016). Genome-wide association study identifies 74 loci associated with educational attainment. Nature, 533, 539–542. doi:10.1038/nature17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orom H., Sharma C., Homish G. G., Underwood W., Homish D. L. (2017). Racial discrimination and stigma consciousness are associated with higher blood pressure and hypertension in minority men. Journal of Racial and Ethnic Health Disparities, 4, 819–826. doi:10.1007/s40615-016-0284-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies Y., Ben J., Denson N., Elias A., Priest N., Pieterse A.…Gee G. (2015). Racism as a determinant of health: A systematic review and meta-analysis. PloS One, 10, e0138511 doi:10.1371/journal.pone.0138511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Path Cards. (2017). Pathway network for signaling by GPCR. Retrieved from http://pathcards.genecards.org/card/signaling_by_gpcr
- Pew Research Center. (2016). On views of race and inequality, Whites and Blacks are worlds apart. Retrieved from http://www.pewsocialtrends.org/2016/06/27/on-views-of-race-and-inequality-blacks-and-whites-are-worlds-apart/
- Poussin C., Belcastro V., Martin F., Boue S., Peitsch M. C., Hoeng J. (2017). Crowd-sourced verification of computational methods and data in systems toxicology: A case study with a heat-not-burn candidate modified risk tobacco product. Chemical Research in Toxicology, 30, 934–945. doi:10.1021/acs.chemrestox.6b00345 [DOI] [PubMed] [Google Scholar]
- Printy B. P., Verma N., Cowperthwaite M. C., Markey M. K., & Alzheimer’s Disease Neuroimaging Initiative. (2014). Effects of genetic variation on the dynamics of neurodegeneration in Alzheimer’s disease. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference, 2014, 2464–2467. doi:10.1109/EMBC.2014.6944121 [DOI] [PubMed] [Google Scholar]
- Riley A. R. (2017). Neighborhood disadvantage, residential segregation, and beyond—Lessons for studying structural racism and health. Journal of Racial and Ethnic Health Disparities. doi:10.1007/s40615-017-0378-5 [DOI] [PubMed] [Google Scholar]
- Scheinfeldt L. B., Gharani N., Kasper R. S., Schmidlen T. J., Gordon E. S., Jarvis J. P.…Christman M. F. (2015). Using the Coriell personalized medicine collaborative data to conduct a genome-wide association study of sleep duration. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 168, 697–705. doi:10.1002/ajmg.b.32362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker L. D., Clark M. J., Patwardhan A., Chandratillake G., Garcia S., Chen R.…Steinberg G. K. (2015). Disease variant landscape of a large multiethnic population of moyamoya patients by exome sequencing. G3 (Bethesda, Md.), 6, 41–49. doi:10.1534/g3.115.020321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter K. R., Miller B. H. (2015). Epigenetic mechanisms in schizophrenia. Progress in Biophysics and Molecular Biology, 118, 1–7. doi:10.1016/j.pbiomolbio.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanikova I., Oates G. R. (2017). Perceived discrimination and privilege in health care: The role of socioeconomic status and race. American Journal of Preventive Medicine, 52, S86–S94. doi:S0749-3797(16)30466-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Zhang B., Wang C., Fu T., Li L., Wu Q.…Wang J. (2017). Forkhead box P3 regulates ARHGAP15 expression and affects migration of glioma cells through the Rac1 signaling pathway. Cancer Science, 108, 61–72. doi:10.1111/cas.13118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. Y., Sun Y. V., Barcelona de Mendoza V., Ifatunji M., Rafferty J., Fox E.…Jackson J. S. (2017). The combined effects of genetic risk and perceived discrimination on blood pressure among African Americans in the Jackson Heart Study. Medicine, 96, e8369 doi:10.1097/MD.0000000000008369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. Y., Wright M. L., Crusto C. A., Sun Y. V. (2016). The Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure (InterGEN) study: Design and methods for complex DNA analysis. Biological Research for Nursing, 18, 521–530. doi:10.1177/1099800416645399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. Y., Wu C. Y., Darling D., Sun Y. V., Kardia S. L., Jackson J. S. (2012). Gene-environment effects of SLC4A5 and skin color on blood pressure among African American women. Ethnicity & Disease, 22, 155–161. [PMC free article] [PubMed] [Google Scholar]
- Taylor J. Y., Sun Y. V., Barcelona de Mendoza V., Ifatunji M., Rafferty J., Fox E., Musani S., Sims M., Jackson J. S. (2017). The Combined Effects of Genetic Risk and Perceived Discrimination on Blood Pressure among African Americans in the Jackson Heart Study. Medicine, 96(43), e8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruel M., Sawalha A. H. (2017). Epigenetic variability in systemic lupus erythematosus: What we learned from genome-wide DNA methylation studies. Current Rheumatology Reports, 19, 32 doi:10.1007/s11926-017-0657-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T., Chen C., Yang F., Tang J., Pei J., Shi B.…Zhang J. (2017). Establishment of apoptotic regulatory network for genetic markers of colorectal cancer and optimal selection of traditional Chinese medicine target. Saudi Journal of Biological Sciences, 24, 634–643. doi:10.1016/j.sjbs.2017.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelde L. C., Pennington D., Mahan C., Mahan R., Kabour M., Marquett R. (2010). Psychometric properties of the race-related events scale. Psychological Trauma: Theory, Research, Practice and Policy, 2, 4–11. [Google Scholar]
- Wen J., Xu Z., Li J., Zhang Y., Fan W., Wang Y.…Li J. (2017). Decreased WWOX expression promotes angiogenesis in osteosarcoma. Oncotarget, 8, 60917–60932. doi:10.18632/oncotarget.17126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. R., Mohammed S. A. (2009). Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine, 32, 20–47. doi:10.1007/s10865-008-9185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. R., Yan Y., Jackson J. S., Anderson N. B. (1997). Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology, 2, 335–351. doi:10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- Wright M. L., Huang Y., Hui Q., Newhall K., Crusto C., Sun Y., Taylor J. Y. (2017). Parenting stress and DNA methylation among African Americans in the InterGEN study. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni V., Armentano M., Saro G., Ciraolo E., Ghigo A., Germena G.…Merlo G. R. (2016). Disruption of ArhGAP15 results in hyperactive Rac1, affects the architecture and function of hippocampal inhibitory neurons and causes cognitive deficits. Scientific Reports, 6, 34877 doi:10.1038/srep34877 [DOI] [PMC free article] [PubMed] [Google Scholar]