Abstract
Hepatitis B virus (HBV) chronically infects 250 million people worldwide, resulting in nearly one million deaths annually. Studies in recent years have significantly improved our knowledge on the mechanisms of HBV persistence. HBV uses multiple pathways to harness host innate immunity to enhance its replication. It can also take advantage of the developing immune system and the not yet stabilized gut microbiota of young children to facilitate its persistence and use maternal viral e antigen to educate immunity of the offspring to support its persistence after vertical transmission. The knowledge gained from these recent studies paves the way for the development of new therapies for the treatment of chronic HBV infection, which has so far been very challenging.
Keywords: Chronic hepatitis B, Interferon immune responses, Vertical transmission, Age and maternal effects, HBV e antigen
HBV Infection and Persistence
Hepatitis B virus (HBV) is a hepatotropic virus that can cause severe liver diseases including acute and chronic hepatitis, cirrhosis and hepatocellular carcinoma (HCC). Globally, there are approximately 250 million people suffering from chronic HBV infection, resulting in nearly one million deaths annually [1]. Most chronic HBV carriers acquired the virus from their carrier mothers early in life. In contrast to this vertical transmission (see Glossary), which usually leads to chronic infection, horizontal transmission such as through sex or the sharing of drug-injection needles often leads to self-limited acute infection [2–4]. Extensive studies have been conducted by different laboratories in the past to understand how HBV establishes chronic infection and these studies have led to many important findings. Here, we will review the current knowledge about how HBV evades and controls host immune responses to establish persistence.
HBV Genome and Life Cycle
HBV is an enveloped virus with a circular and partially double-stranded DNA genome of approximate 3.2-kb. After the infection of hepatocytes, the HBV genome is delivered into the nucleus where it is repaired to form a covalently closed circular DNA (cccDNA), which then serves as the template to direct viral RNA transcription [5]. The cccDNA is highly stable in the nucleus of infected hepatocyte, and that is the reason why chronic HBV infection is difficult to treat, as the cessation of the treatments often leads to the reappearance of the virus [6]. The viral genome is remarkably compact and encodes four overlapping genes named S, C, P and X. The S gene codes for the viral envelope proteins known as surface antigens (HBsAgs). The C gene codes for the 21-kDa core protein and the 25-kDa precore protein. The core protein packages its own mRNA, also known as the pregenomic RNA (pgRNA), to form the core particle that displays the core antigenic determinant (i.e., the core antigen, HBcAg). The precore protein contains the entire sequence of the core protein plus an amino-terminal extension of 29 amino acids (i.e., the “precore” sequence) [3, 7]. The first 19 amino acids of the precore protein constitute a signal peptide, which directs the precore protein to the endoplasmic reticulum (ER) for secretion. This signal peptide is removed by the signal peptidase located in the ER lumen to generate the 22-kDa precore protein derivative p22, which is further cleaved at its carboxy terminus by furin protease in Golgi. The secreted precore protein derivative is known as the e antigen (HBeAg). In contrast to the core protein, HBeAg is not essential for HBV replication, as mutations that abolish its expression do not negatively affect viral replication [8, 9]. The spontaneous loss of HBeAg and the development of antibodies against HBeAg, known as HBeAg seroconversion, have been observed in many chronic HBV patients and are often preceded by flares of hepatitis due to enhanced cytotoxic T lymphocyte (CTL) responses [10]. For that reason, HBeAg is thought to have immunomodulatory functions [11, 12]. The P gene codes for the viral DNA polymerase, which is also a reverse transcriptase [13], and the X gene codes for a regulatory protein that has many functions including the enhancement of viral gene expression and replication [14, 15]. After the formation of the viral core particle, the HBV pgRNA is reverse-transcribed by the viral DNA polymerase that is also packaged to become the circular and partially double-stranded DNA genome. The core particles subsequently interact with HBsAgs in intracellular membranes for the formation of mature viral particles, which are then released from infected hepatocytes. HBsAg can also be released from cells as empty subviral particles, often in vast excess of virions [16]. HBV is not a cytolytic virus and that is the reason why there are many asymptomatic HBV carriers who have little liver injury despite having high viral load.
Suppression of Innate Immune Response by HBV
Interactions between HBV, Pattern-recognition Receptors and Interferon Signaling
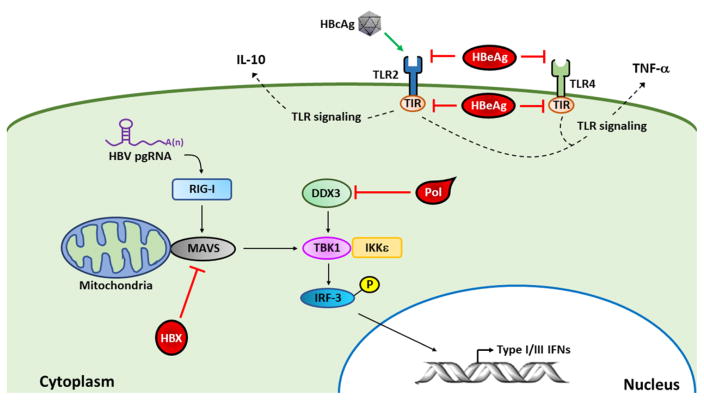
HBV was thought to be a stealth virus [17], as previous studies of patients with acute HBV infection indicated that its infection induced little type I interferon (IFN) immune response [18]. Similar to this observation, when the expression profiles of immune-response genes in the liver of chimpanzees infected by HBV were analyzed [19], no specific induction of IFN-stimulated genes (ISGs) was detected. In agreement with these findings, in a separate experiment using uPA-SCID mice grafted with human hepatocytes, only a slight increase of intrahepatic ISGs was detected after HBV infection [20]. These observations suggested that HBV could evade the innate immune response during natural infection. Sato et al. reported that the cytosolic pattern recognition receptor (PRR) RIG-I could bind to the ε stem-loop structure in the HBV pgRNA to stimulate a strong type III IFN response but only a weak type I IFN response in human hepatocytes [21] (Figure 1). HBV apparently can perturb the type I IFN immune responses in hepatocytes via multiple pathways. It had been shown that the HBV DNA polymerase could inhibit the induction of IFN-β by binding to DDX3 DEAD box RNA helicase to disrupt its interaction with TBK1/IKKε and consequently the phosphorylation and activation of IFN-regulatory factor 3 (IRF3) [22, 23], a transcription factor that activates the expression of IFN-β. The HBV X (HBx) protein had also been shown to inhibit the induction of type I IFNs by binding to the mitochondrial antiviral signaling (MAVS) protein, an important adaptor protein of the RIG-I signaling pathway, to suppress the activation of IRF3 [24–26]. Toll-like receptors (TLRs) are also PRRs that can recognize specific pathogen-associated molecular patterns (PAMPs) to trigger the innate immune responses to produce IFNs and pro-inflammatory cytokines [27, 28]. Indeed, the activation of various TLRs, with the exception of TLR2, using their specific ligands could induce intrahepatic IFN-α/β and suppress HBV replication in transgenic mice that carried an over-length HBV genome [29]. In contrast to other TLRs, TLR2 has been shown to support HBV persistence. By using an adenovirus-associated virus (AAV) vector to establish HBV persistence in mice, Li et al. found that the activation of TLR2 supported HBV persistence whereas the TLR2 knockout abolished HBV persistence [30]. They further found that HBV could upregulate the expression of TLR2 in Kupffer cells (i.e., hepatic macrophages), which could be activated by HBcAg to produce IL-10 and suppress CD8+ T cells for HBV persistence [30]. In addition to upregulating the expression of TLR2, Visvanathan et al. found that HBV could also down-regulate the expression of TLR2. They showed that the expression of TLR2 in hepatocytes, Kupffer cells and peripheral monocytes of chronic HBV patients was reduced in the presence of HBeAg and increased in the absence of it. To support this finding, they showed that HBV with and without the ability to express HBeAg would suppress and stimulate, respectively, the expression of TLR2 in HepG2 hepatoblastoma cells [31]. They further demonstrated that HBeAg, but not the related HBcAg, could suppress the expression of TNF-α in peripheral blood mononuclear cells (PBMC) induced by either TLR2 or TLR4 ligands, indicating that HBeAg could suppress TLR signaling. How HBeAg might achieve this suppressive effect remains unclear. The precore-specific sequence of HBeAg had been shown to bind to toll/IL-1 receptor (TIR) motif to interfere with the TIR-TIR homotypic interaction to disrupt TLR signaling [32]. While this mechanism may explain how HBeAg suppresses TLR signaling in HBV-infected cells, it cannot explain how the exogenously added HBeAg suppressed TLR signaling in PBMC. For the latter, it would appear more likely that HBeAg recognizes a specific cell surface receptor or a receptor resides in endosomes after its internalization by endocytosis. The results of Li et al. and Visvanathan et al. indicated a complicated interplay between HBV and TLR2 and that different HBV antigens could have different effects on TLR2.
Figure 1. Suppression of host innate immune response by HBV.
HBV DNA polymerase (Pol) binds to DDX3 to suppress the activation of IRF3 and, although HBV pgRNA activates RIG-I to induce type III IFN via MAVS, TBK1 and IRF3, the binding of HBx to MAVS disrupts the RIG-I signaling pathway. HBcAg can activate TLR2 in Kupffer cells to induce the expression of the anti-inflammatory cytokine IL-10, and HBeAg can suppress TLR2 and TLR4 signaling in PBMC to inhibit the expression of TNF-α. Intracellular HBeAg can also bind to the TIR motif of TLRs to suppress TLR signaling.
Type I IFNs bind to the IFN-α/β receptor (IFNAR), which leads to the activation of IFNAR-associated JAK1 and TYK2 tyrosine kinases and the phosphorylation and activation of STAT proteins. HBV can induce the expression of protein phosphatase 2Ac (PP2Ac), the catalytic subunit of PP2A, in cell cultures [33, 34]. This induction of PP2Ac inhibits protein arginine methyltransferase I (PRMT1), resulting in the hypomethylation of STAT1 and its binding and inactivation by protein inhibitor of activated STAT1 (PIAS1) [33, 34]. The upregulation of PP2Ac was also observed in the liver tissues of many chronic hepatitis B patients [33].
These findings indicated that HBV had developed multiple strategies to interfere with PRR signaling and interferon immune responses (Figure 1). This likely plays an important role for its persistence in patients.
HBV and Natural Killer Cells
Natural killer (NK) cells are important effectors of innate immunity. They provide rapid responses to viral infections. The activities of NK cells are impaired in patients with persistent HBV replication [35]. The expression of NKG2D and 2B4, two activating receptors on NK cells, as well as DAP10 and SAP, which are intracellular adaptor proteins of NKG2D and 2B4, respectively, were found to be reduced in NK cells of chronic HBV patients [36]. This reduction impaired the ability of NK cells to produce IFN-γ and mediate cytotoxicity and was due to the increased level of circulating transforming growth factor-β1 (TGF-β1), which was shown to down-regulate the expression of NKG2D and 2B4 in NK cells ex vivo [36]. HBV also suppresses the expression of major histocompatibility complex class I-related molecules A and B (MICA and MICB), which are ligands of NKG2D, in chronic HBV patients [37, 38]. The suppression of MICA and MICB was apparently mediated by HBsAg, as it could be accomplished by the expression of HBsAg alone [38]. In contrast, the expression of NKG2A, an inhibitor of NK cell activity, was found to be elevated in NK cells of chronic HBV patients with high viral load [35, 39]. The ability of HBV to induce the expression of NKG2A was confirmed in a mouse model with persistent HBV replication [39]. The expression of TIM-3, an important immune checkpoint molecule, is also increased in circulating NK cells of chronic HBV patients, and blocking TIM-3 signaling with the anti-TIM3 antibody was found to enhance the cytotoxicity of NK cells isolated from chronic HBV patients [40]. These observations indicated that HBV could also evade immune responses mediated by NK cells via the regulation of expression of their positive and negative regulators.
Suppression of Host Immunity by HBsAg
HBsAg has immunosuppressive functions and its high level in serum has also been suspected to cause T-cell exhaustion [41]. Dendritic cells (DCs), which play an important role in the induction of T- cell responses, are functionally impaired in chronic HBV patients [42]. Myeloid DCs (mDCs) treated with HBsAg displayed a tolerogenic phenotype with a decreased T-cell stimulatory capacity as well as reduced interleukin-12 (IL-12) production [43]. HBsAg can also bind to monocytes/macrophages and selectively inhibit TLR2 ligand and TLR4 ligand-induced production of IL-12 and IL-18 by interfering with signaling pathways [44, 45]. Thus, HBsAg can suppress monocytes and DCs by direct interaction. This direct interaction was similarly found to suppress NK cells and NK T (NKT) cells [41]. The ability of HBsAg to suppress these immune cells clearly plays an important role in HBV persistence in patients.
Impact of Viral Load on HBV Persistence
The ability of HBV to establish persistence is also affected by the size of the viral inoculum. By using chimpanzees as a model, it was demonstrated that the infection of chimpanzees with a low-dose inoculum of 100 or 101 genome-equivalent (GE) of HBV would lead to massive spread of the virus to 100% of hepatocytes and viral persistence [46]. The infection of chimpanzees with 1010 GE of HBV led to massive spread of the virus in the liver and the delayed clearance of the virus. In contrast, the infection of chimpanzees with 104 or 107 GE of HBV led to limited spread of the virus to less than 0.1% of hepatocytes followed by abrupt viral clearance. An early peripheral HBV-specific CD4+ T cell response was predictive of HBV clearance, and the depletion of CD4+ T cells before and during HBV infection led to HBV persistence even when the animals were inoculated with 104 GE of HBV, which led to self-limited acute infection. Such depletion of CD4+ T cells six weeks after HBV infection had no impact on the outcome of HBV infection, confirming the importance of early CD4+ T cell response in the control of HBV infection. Interestingly, in a recent study, which used the AAV vector to establish HBV persistence in mice, HBV persistence was due to the sustained production of IFN-γ by hepatic CD4+ T cells, which promoted the secretion of chemokine CXCL9 from Kupffer cells to retain HBV-specific CD4+ T cells in the liver via CXCR3 (i.e., the receptor for CXCL9). This led to their subsequent apoptosis and HBV persistence [47]. The HBV clearance in chimpanzees is associated with a synchronized CD8+ T cell response to HBV, and viral persistence is associated with a poor CD8+ T cell response. These results also indicated an important role of CD8+ T cells in viral clearance.
The low-dose inoculation of HBV, which led to prolonged HBV persistence, might be related to the response of HBV to type I IFNs. Previous studies using mouse models indicated that, although IFN-α/β suppressed HBV replication when viral load was high, they stimulated HBV gene expression when viral load was low, leading to prolonged viral persistence [48, 49]. This stimulation of HBV gene expression was via the activation of STAT3, which interacted with hepatocyte nuclear factor 3γ (HNF3γ) to stimulate the activity of enhancer I in the HBV genome. This observation indicated that HBV might actually use the host IFN response to stimulate its own replication and spread in the early stage of viral infection. Recently, it was reported that enhancing the exposure of encapsidated HBV DNA replicative intermediates would lead to the activation of cGAS, a PRR that is activated by DNA, and its downstream effectors STING-TBK1-IRF3, and this activation of the cGAS signaling pathway was associated with an enhancement of viral gene expression and replication in the early stage of HBV infection when the viral level was low and the suppression of viral gene expression and replication after the viral replication had reached its peak [50]. These results further supported a role of IFNs in supporting HBV replication in the early stage of viral replication.
Age-Dependent Immune Response to HBV Infection
As mentioned above, people who are exposed to HBV via horizontal transmission would often develop self-limited acute infection. In contrast, people who acquired the virus from their mothers via vertical transmission would frequently become chronic HBV carriers. As horizontal transmission usually occurs between adults, and vertical transmission occurs early in life, it was conceivable that this difference of the infection outcome might be due to the difference of immunity between adults and young children. Indeed, in studies conducted using woodchucks inoculated with the woodchuck hepatitis B virus (WHV), a virus closely related to HBV, it was found that neonate woodchucks were more susceptible to chronic infection than 8-week old weanlings, and no adult woodchucks developed chronic infection after WHV infection [51]. By adoptive transfer of splenocytes from adult C57BL/6 mice into adult (8–10 weeks old) and young Rag1−/− C57BL/6 mice (3–4 weeks old) that carried either the HBsAg transgene or the 1.3mer HBV genome, Publicover et al. found that the liver of adult mice produced higher levels of HBV-dependent IL-21 than that of young mice [52]. This IL-21 was produced primarily by T follicular helper (TFH) cells and was apparently critical for CD8+ T cell and B cell responses. In support of these mouse studies, they also observed that the PBMCs of patients with acute HBV infection who subsequently recovered from the infection produced higher levels of IL-21 than the PBMCs of chronic hepatitis B patients [52]. They further demonstrated that the age-dependent expression of CXCL13, which is important for B cell trafficking and lymphoid architecture and development, in Kupffer cells played a critical role in directing an effective immune response against HBV [53].
The gut microbiota, which affects local and systemic immune responses [54, 55], provided yet another possible explanation to the mechanism of age-dependent effect of HBV persistence. Chou et al. found that the gut microbiota of C3H/HeN mice was stabilized only after 9 weeks of age [56]. When hydrodynamic injection was used to introduce the AAV-HBV DNA into the liver of 12-week old adult mice, HBV was cleared from the mice within 6 weeks after DNA injection. If gut microbiota was depleted by antibiotics, adaptive humoral and cellular immunity as well as the ability of the mice to clear HBV was impaired [56]. In contrast, if the AAV-HBV DNA was introduced into the liver of 6-week old mice, before the stabilization of gut microbiota, it would lead to viral persistence for up to 26 weeks. Their results indicated an important role of stabilized gut microbiota in the regulation of host immunity for HBV clearance. As the mutation in TLR4 or the depletion of Kupffer cells in young mice prior to HBV DNA injection led to rapid HBV clearance from these mice, Chou et al. suggested that the immune tolerance in young mice was likely mediated by Kupffer cells via the activation of TLR4 by its ligand lipopolysaccharide (LPS), which is produced primarily by Gram-negative bacteria. Thus, the age difference of gut microbiota may also affect host immunity and HBV persistence.
Maternal Effect on HBV Persistence
Although age may affect the host immune responses to HBV and render small children more susceptible to HBV persistence, recent studies indicated another important factor for HBV persistence. This factor is the effect exerted by HBV carrier mothers on the immune system of their offspring [57]. By crossing female hemizygous HBV transgenic mice to naive male mice, HBV-negative mouse pups were produced. When the 1.3mer HBV genomic DNA was introduced into the liver of these mice at 9 weeks of age (i.e., adult mice), HBV replication in these mice persisted for up to 28 weeks. In contrast, the repeat of HBV DNA injection using age-matched mice that were born to HBV-negative mothers led to HBV clearance within 4 weeks. These results indicated that young age was not an essential factor for HBV to establish persistence, but rather it was the HBV positivity in the mother that dictated whether or not HBV could establish persistence in the offspring. Further analysis indicated that the HBV persistence in the offspring born to HBV-positive mothers was due to the impaired CD8+ T cell response to HBV cause by the increased expression of programmed death 1 (PD-1) in HBV-specific CD8+ T cells and PD-L1 in Kupffer cells. PD-1 is a major regulator for T-cell exhaustion and can negatively modulate CTL response to HBV in mice [58, 59] and in chronic HBV patients [60, 61]. PD-L1 is the ligand of PD-1. Interaction between PD-1 and PD-L1 of these two cell types was responsible for the suppression of HBV-specific CD8+ T cell activities, as the blocking of the interaction between PD-1 and PD-L1 or the depletion of Kupffer cells restored the CD8+ T cell response to HBV and led to HBV clearance [57]. These results were consistent with the previous observation that HBV-specific CTL response was weak in chronic hepatitis B patients, compared with patients who recovered from HBV infection [62].
Further studies indicated that the ability of HBV to establish persistence in mice born to HBV-positive mothers was dependent on the expression of HBeAg in both the mother and the offspring [57]. This finding was consistent with the observation of Okada et al. who reported more than 40 years ago that there was a correlation between the HBeAg positivity of the mother and the HBV carrier state of the offspring [63]. Subsequent studies indicated that maternal HBeAg could condition Kupffer cells of the offspring, which would undergo M2 anti-inflammatory polarization when they were stimulated by HBeAg again after birth [57]. Interestingly, Bility et al. also found that immunodeficient NSG mice grafted with human hematopoietic stem cells and liver progenitor cell could support HBV infection and persistence, and this HBV persistence was associated with the accumulation of M2-polarized macrophages in the liver, which was also observed in the liver of chronic HBV patients [64]. These results together supported an immunosuppressive role of M2 macrophages in HBV persistence. In mouse studies, without the conditioning by maternal HBeAg, Kupffer cells would undergo M1 pro-inflammatory polarization when stimulated by HBeAg or other HBV antigens [57]. Thus, HBeAg apparently displays a functional dichotomy in HBV infection: it promotes M2-like anti-inflammatory polarization of Kupffer cells during HBV persistence and M1-like pro-inflammatory polarization of the same cells during viral clearance. These findings provide a mechanism to explain how HBV exploits Kupffer cells to create an immunosuppressive microenvironment for its persistence, and highlight a potential role of HBeAg and Kupffer cells in the exhaustion of HBV-specific CD8+ T cells.
The conditioning of Kupffer cells by maternal HBeAg might take place in utero, as previously suggested [65]. This possibility is also supported by the observation that HBeAg could cross the placenta [66]. Kupffer cells mature in fetal liver and can maintain themselves after birth and during adulthood without the replenishment by blood monocytes [67]. Their long lifespan and their ability to suppress HBV-specific CD8+ T cells could explain why HBV could persist in patients for decades after vertical transmission. It is conceivable that the activation of HBV-specific CD8+ T cells and the development of hepatic flares during chronic infection may be due in part to the loss of Kupffer cells conditioned by maternal HBeAg. Note that the possibility that maternal HBeAg may also condition Kupffer cells immediately after birth such as through colostrum feeding cannot be ruled out [68]. Recently, Hong et al. analyzed the umbilical cord blood of babies born to healthy and HBV+ mothers and found that HBV could train fetal immunity in utero, which led to the alteration of cytokine profiles in the cord blood characterized by low IL-10 and high IL-12p40 and IFN-α2, enhanced maturation and activation of CD14+ monocytes, a robust Th1 polarization response, and better immune responses to unrelated pathogens [69]. Their results, which indicated HBV could train fetal immunity of newborns were consistent with the notion that maternal HBeAg could train fetal Kupffer cells in utero to promote HBV persistence after birth.
Concluding Remarks
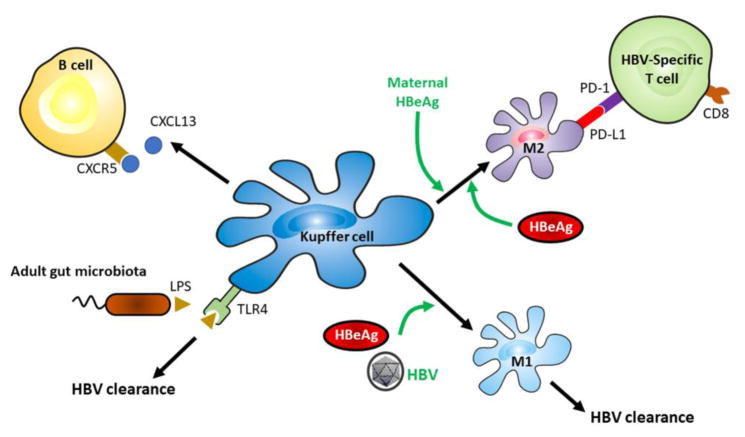
HBV establishes chronic infection in approximately 250 million people worldwide. The research in recent years has generated significant amount of information for understanding how HBV evades host immunity to establish persistence. HBV can harness type I IFN responses and suppress NK cells to enhance its own replication. It can also take advantage of the developing immune system of young children and the gut microbiota as well as educate fetal immunity to facilitate its persistence in patients after its vertical transmission. The studies by various groups pointed to an important role of Kupffer cells in promoting HBV clearance and persistence, which is illustrated in Figure 2 (Key Figure). The current treatments for HBV infection include IFN-α or its pegylated derivative and nucleoside/nucleotide analogs. These drugs are less than satisfactory, as they generate sustained response in only a small fraction of patients. The improvement of our knowledge on the mechanisms of HBV persistence provides us with a unique possibility to tackle these mechanisms to abolish HBV persistence. For example, the mouse models indicated an important role of Kupffer cells in the suppression of HBV-specific CD8+ T cells for HBV persistence in the offspring of carrier mothers [57]. Thus, the disruption of the interaction between Kupffer cells and HBV-specific CD8+ T cells offers a possible approach for the treatment of HBV patients (see Outstanding Questions). Indeed, the treatment of woodchucks chronically infected by WHV with the anti-PD-L1 antibody to block the PD-1 and PD-L1 interaction in combination with the nucleoside analog entecavir and WHV DNA vaccination had been show to potently enhance the WHV-specific CD8+ T cell activity and WHV clearance [70]. Immunotherapy like this offers promising alternatives for the treatments of chronic HBV patients in the future.
Figure 2. Key role of Kupffer cells in HBV clearance and persistence.
Kupffer cells can be activated via TLR4 by LPS produced by stabilized gut microbiota in adults to promote HBV clearance. They also express CXCL13 in an age-dependent manner to facilitate B cell trafficking and lymphoid development to direct immune responses against HBV. If Kupffer cells are not trained by maternal HBeAg, they will undergo M1 polarization to promote HBV clearance after the exposure to HBV antigens. However, if they are trained by maternal HBeAg, they will undergo M2 polarization in the presence of HBeAg to suppress HBV-specific CD8+ T cells to support HBV persistence.
Outstanding Questions.
How does the size of viral inoculum affect the spread and persistence of HBV? Is it due to the dichotomous effects of type I IFNs on HBV?
HBV is more likely to establish persistence in young mice during the maturation process of immunity. What is the role of HBeAg in this process?
Does HBeAg affect the expression of cytokines and chemokines in young mice?
Is TLR4 the only PRR that mediates the effect of gut microbiota to facilitate HBV persistence?
Maternal HBeAg can train Kupffer cells of the offspring. Does this training have to be in utero or can it also take place perinatally?
How do Kupffer cells recognize HBeAg? Do they have a specific receptor for HBeAg?
How does maternal HBeAg modify Kupffer cells of the offspring? How is the memory of Kupffer cells maintained?
Can the disruption of the interaction between Kupffer cells and HBV-specific CD8+ T cells be an effective therapy for treating chronic HBV patients?
Trends Box.
HBV frequently causes chronic infection after vertical transmission but mostly self-limited acute infection after horizontal transmission.
HBV harnesses type I interferon immune response to enhance its own replication.
HBV persistence is affected by the age of infection, size of viral inoculum, and gut microbiota.
Maternal HBeAg can train Kupffer cells of the offspring, likely in utero, to suppress HBV-specific CD8+ T cells in the presence of HBeAg after birth.
Glossary
- Gut microbiota
community of microorganisms living in digestive tracts
- HBeAg seroconversion
serological change from HBeAg-positive to anti-HBeAg antibody-positive during chronic HBV infection
- Horizontal transmission
transmission of the virus between two individuals, usually between adults
- Hydrodynamic injection
injection of high volume of saline via the tail vein of mice within a short duration of 5–8 seconds
- Kupffer cells
resident macrophages of the liver
- T-cell exhaustion
the progressive loss of T cell functions
- uPA-SCID mice
transgenic mice with severe combined immunodeficiency and hepatocyte-specific expression of urokinase-type plasminogen activator
- Vertical transmission
transmission of the virus from a mother to her children
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. Global Hepatitis Report 2017. World Health Organization; Geneva: 2017. [Google Scholar]
- 2.Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003;38(5):1075–86. doi: 10.1053/jhep.2003.50453. [DOI] [PubMed] [Google Scholar]
- 3.Ou JH. Molecular biology of hepatitis B virus e antigen. J Gastroenterol Hepatol. 1997;12(9–10):S178–87. doi: 10.1111/j.1440-1746.1997.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 4.Shin EC, et al. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol. 2016;16(8):509–23. doi: 10.1038/nri.2016.69. [DOI] [PubMed] [Google Scholar]
- 5.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 6.Shih C, et al. Control and Eradication Strategies of Hepatitis B Virus. Trends Microbiol. 2016;24(9):739–49. doi: 10.1016/j.tim.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Ou JH, et al. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc Natl Acad Sci U S A. 1986;83(6):1578–82. doi: 10.1073/pnas.83.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HS, et al. The precore gene of the woodchuck hepatitis virus genome is not essential for viral replication in the natural host. J Virol. 1992;66(9):5682–4. doi: 10.1128/jvi.66.9.5682-5684.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamberts C, et al. Precore-mediated inhibition of hepatitis B virus progeny DNA synthesis. J Virol. 1993;67(7):3756–62. doi: 10.1128/jvi.67.7.3756-3762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liaw YF, et al. Hepatitis B e antigen seroconversion: a critical event in chronic hepatitis B virus infection. Dig Dis Sci. 2010;55(10):2727–34. doi: 10.1007/s10620-010-1179-4. [DOI] [PubMed] [Google Scholar]
- 11.Chen MT, et al. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc Natl Acad Sci U S A. 2004;101(41):14913–8. doi: 10.1073/pnas.0406282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidotti LG, et al. Host-virus interactions in hepatitis B virus infection. Curr Opin Immunol. 2015;36:61–6. doi: 10.1016/j.coi.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones SA, Hu J. Hepatitis B virus reverse transcriptase: diverse functions as classical and emerging targets for antiviral intervention. Emerg Microbes Infect. 2013;2(9):e56. doi: 10.1038/emi.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang H, et al. Molecular functions and biological roles of hepatitis B virus x protein. Cancer Sci. 2006;97(10):977–83. doi: 10.1111/j.1349-7006.2006.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kew MC. Hepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26(Suppl 1):144–52. doi: 10.1111/j.1440-1746.2010.06546.x. [DOI] [PubMed] [Google Scholar]
- 16.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350(11):1118–29. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 17.Wieland SF, Chisari FV. Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol. 2005;79(15):9369–80. doi: 10.1128/JVI.79.15.9369-9380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn C, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137(4):1289–300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 19.Wieland S, et al. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101(17):6669–74. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutgehetmann M, et al. Hepatitis B virus limits response of human hepatocytes to interferon-alpha in chimeric mice. Gastroenterology. 2011;140(7):2074–83. 2083 e1–2. doi: 10.1053/j.gastro.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, et al. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. 2015;42(1):123–32. doi: 10.1016/j.immuni.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Ryu WS. Hepatitis B virus polymerase blocks pattern recognition receptor signaling via interaction with DDX3: implications for immune evasion. PLoS Pathog. 2010;6(7):e1000986. doi: 10.1371/journal.ppat.1000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu S, et al. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKKepsilon and DDX3. J Gen Virol. 2010;91(Pt 8):2080–90. doi: 10.1099/vir.0.020552-0. [DOI] [PubMed] [Google Scholar]
- 24.Kumar M, et al. Hepatitis B virus regulatory HBx protein binds to adaptor protein IPS-1 and inhibits the activation of beta interferon. J Virol. 2011;85(2):987–95. doi: 10.1128/JVI.01825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, et al. Hepatitis B virus X protein suppresses virus-triggered IRF3 activation and IFN-beta induction by disrupting the VISA-associated complex. Cell Mol Immunol. 2010;7(5):341–8. doi: 10.1038/cmi.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei C, et al. The hepatitis B virus X protein disrupts innate immunity by downregulating mitochondrial antiviral signaling protein. J Immunol. 2010;185(2):1158–68. doi: 10.4049/jimmunol.0903874. [DOI] [PubMed] [Google Scholar]
- 27.Akira S, et al. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Zhang E, Lu M. Toll-like receptor (TLR)-mediated innate immune responses in the control of hepatitis B virus (HBV) infection. Med Microbiol Immunol. 2015;204(1):11–20. doi: 10.1007/s00430-014-0370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isogawa M, et al. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79(11):7269–72. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, et al. Kupffer Cells Support Hepatitis B Virus-Mediated CD8+ T Cell Exhaustion via Hepatitis B Core Antigen-TLR2 Interactions in Mice. J Immunol. 2015;195(7):3100–9. doi: 10.4049/jimmunol.1500839. [DOI] [PubMed] [Google Scholar]
- 31.Visvanathan K, et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007;45(1):102–10. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 32.Lang T, et al. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll-like receptor signaling pathway. J Hepatol. 2011;55(4):762–9. doi: 10.1016/j.jhep.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 33.Christen V, et al. Inhibition of alpha interferon signaling by hepatitis B virus. J Virol. 2007;81(1):159–65. doi: 10.1128/JVI.01292-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, et al. Inhibition of STAT1 methylation is involved in the resistance of hepatitis B virus to Interferon alpha. Antiviral Res. 2010;85(3):463–9. doi: 10.1016/j.antiviral.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Tjwa ET, et al. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol. 2011;54(2):209–18. doi: 10.1016/j.jhep.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Sun C, et al. TGF-beta1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012;8(3):e1002594. doi: 10.1371/journal.ppat.1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koumbi L, et al. Hepatitis B viral replication influences the expression of natural killer cell ligands. Ann Gastroenterol. 2016;29(3):348–57. doi: 10.20524/aog.2016.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, et al. Hepatitis B surface antigen inhibits MICA and MICB expression via induction of cellular miRNAs in hepatocellular carcinoma cells. Carcinogenesis. 2014;35(1):155–63. doi: 10.1093/carcin/bgt268. [DOI] [PubMed] [Google Scholar]
- 39.Li F, et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2013;144(2):392–401. doi: 10.1053/j.gastro.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 40.Ju Y, et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol. 2010;52(3):322–9. doi: 10.1016/j.jhep.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Kondo Y, et al. Hepatitis B surface antigen could contribute to the immunopathogenesis of hepatitis B virus infection. ISRN Gastroenterol. 2013;2013:935295. doi: 10.1155/2013/935295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Molen RG, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40(3):738–46. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- 43.Op den Brouw ML, et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126(2):280–9. doi: 10.1111/j.1365-2567.2008.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S, et al. Hepatitis B virus surface antigen selectively inhibits TLR2 ligand-induced IL-12 production in monocytes/macrophages by interfering with JNK activation. J Immunol. 2013;190(10):5142–51. doi: 10.4049/jimmunol.1201625. [DOI] [PubMed] [Google Scholar]
- 45.Cheng J, et al. Recombinant HBsAg inhibits LPS-induced COX-2 expression and IL-18 production by interfering with the NFkappaB pathway in a human monocytic cell line, THP-1. J Hepatol. 2005;43(3):465–71. doi: 10.1016/j.jhep.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 46.Asabe S, et al. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol. 2009;83(19):9652–62. doi: 10.1128/JVI.00867-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng Z, et al. Interferon-gamma facilitates hepatic antiviral T cell retention for the maintenance of liver-induced systemic tolerance. J Exp Med. 2016;213(6):1079–93. doi: 10.1084/jem.20151218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian Y, et al. Effects of interferon-alpha/beta on HBV replication determined by viral load. PLoS Pathog. 2011;7(7):e1002159. doi: 10.1371/journal.ppat.1002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian Y, et al. Viral-load-dependent effects of liver injury and regeneration on hepatitis B virus replication in mice. J Virol. 2012;86(18):9599–605. doi: 10.1128/JVI.01087-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui X, et al. Viral DNA-Dependent Induction of Innate Immune Response to Hepatitis B Virus in Immortalized Mouse Hepatocytes. J Virol. 2015;90(1):486–96. doi: 10.1128/JVI.01263-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cote PJ, et al. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology. 2000;31(1):190–200. doi: 10.1002/hep.510310128. [DOI] [PubMed] [Google Scholar]
- 52.Publicover J, et al. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest. 2011;121(3):1154–62. doi: 10.1172/JCI44198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Publicover J, et al. Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J Clin Invest. 2013;123(9):3728–39. doi: 10.1172/JCI68182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol. 2013;14(7):646–53. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molloy MJ, et al. Intestinal microbiota: shaping local and systemic immune responses. Semin Immunol. 2012;24(1):58–66. doi: 10.1016/j.smim.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chou HH, et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc Natl Acad Sci U S A. 2015;112(7):2175–80. doi: 10.1073/pnas.1424775112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian Y, et al. Maternal-Derived Hepatitis B Virus e Antigen Alters Macrophage Function in Offspring to Drive Viral Persistence after Vertical Transmission. Immunity. 2016;44(5):1204–14. doi: 10.1016/j.immuni.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzeng HT, et al. PD-1 blockage reverses immune dysfunction and hepatitis B viral persistence in a mouse animal model. PLoS One. 2012;7(6):e39179. doi: 10.1371/journal.pone.0039179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isogawa M, et al. CD40 activation rescues antiviral CD8(+) T cells from PD-1-mediated exhaustion. PLoS Pathog. 2013;9(7):e1003490. doi: 10.1371/journal.ppat.1003490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wenjin Z, et al. Longitudinal fluctuations in PD1 and PD-L1 expression in association with changes in anti-viral immune response in chronic hepatitis B. BMC Gastroenterol. 2012;12:109. doi: 10.1186/1471-230X-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bengsch B, et al. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol. 2014;61(6):1212–9. doi: 10.1016/j.jhep.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Chisari FV, et al. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris) 2010;58(4):258–66. doi: 10.1016/j.patbio.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okada K, et al. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med. 1976;294(14):746–9. doi: 10.1056/NEJM197604012941402. [DOI] [PubMed] [Google Scholar]
- 64.Bility MT, et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10(3):e1004032. doi: 10.1371/journal.ppat.1004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Milich DR, et al. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A. 1990;87(17):6599–603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang JS, Zhu QR. Infection of the fetus with hepatitis B e antigen via the placenta. Lancet. 2000;355(9208):989. doi: 10.1016/S0140-6736(00)90021-7. [DOI] [PubMed] [Google Scholar]
- 67.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin HH, et al. Hepatitis B virus in the colostra of HBeAg-positive carrier mothers. J Pediatr Gastroenterol Nutr. 1993;17(2):207–10. doi: 10.1097/00005176-199308000-00014. [DOI] [PubMed] [Google Scholar]
- 69.Hong M, et al. Trained immunity in newborn infants of HBV-infected mothers. Nat Commun. 2015;6:6588. doi: 10.1038/ncomms7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10(1):e1003856. doi: 10.1371/journal.ppat.1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]