Abstract
Objective
Two large randomized trials recently demonstrated efficacy of MRSA-active antibiotics for drained skin abscesses. We determine whether outcome advantages observed in one trial existed across lesion sizes and among subgroups with and without guideline recommended antibiotic indications.
Design, Setting, and Participants
We conducted a pre-planned subgroup analysis of a double-blind, randomized trial at 5 U.S. EDs demonstrating superiority of trimethoprim-sulfamethoxazole (320/1600 mg twice daily for 7 days) compared to placebo for patients >12 years of age with a drained skin abscess.
Methods
We determined between-group differences in rates of clinical (no new antibiotics) and composite cure (no new antibiotics or drainage) through 7–14 and 42–56 days after treatment among subgroups with and without abscess cavity or erythema diameter ≥5 cm, history of MRSA, fever, diabetes, and comorbidities. We also evaluated treatment effect by lesion size and culture result.
Results
Among 1057 mostly adult participants, median abscess cavity and erythema diameters were 2.5 cm (range, 0.1–16.0) and 6.5 cm (range, 1.0–38.5), respectively; 44.3% grew MRSA. Overall, for trimethoprim-sulfamethoxazole and placebo groups, clinical cure rate at 7–14 days was 92.9% and 85.7%, and composite cure rate at 7–14 was 86.5% and 74.3% and 82.4% and 70.2% at 42–56 days, respectively. For all outcomes, across lesion sizes and among subgroups with and without guideline antibiotic criteria, trimethoprim-sulfamethoxazole was associated with improved outcomes. Treatment effect was greatest with history of MRSA infection, fever, and MRSA etiology.
Conclusions
Treatment with trimethoprim-sulfamethoxazole was associated with improved outcomes regardless of lesion size or guideline antibiotic criteria.
Keywords: Abscess, small, uncomplicated, subgroup, randomized, antibiotics, trimethoprim-sulfamethoxazole, methicillin-resistant Staphylococcus aureus, MRSA
INTRODUCTION
Background
The primary treatment of an abscess is drainage.1 Past studies of adjunctive antibiotic treatment, conducted before and after the emergence of community-associated methicillin-resistant Staphylococcus aureus (MRSA),2 were small and did not clearly demonstrate benefit.3–12 Recently, two large U.S. randomized placebo-controlled trials demonstrated that treatment with an antibiotic possessing in vitro activity against MRSA was associated with improved outcomes among patients with a skin abscess that received drainage and who were treated as outpatients.13,14 The trial by Daum et al.13 enrolled 786 patients who were assigned 1:1:1 to treatment with clindamycin, trimethoprim-sulfamethoxazole, or placebo, and the trial by Talan et al.14 enrolled 1,265 patients assigned 1:1 to treatment with trimethoprim-sulfamethoxazole or placebo. Talan et al.14 found significantly fewer antibiotic-treated participants required a new antibiotic through 7–14 days following treatment as well as lower rates of subsequent surgical drainage procedures and infection at a new skin site through 42–56 days following treatment.
Practice guidelines for abscess treatment issued by the U.S. Centers for Disease Control and the Infectious Diseases Society of America prior to these trials stated that drainage is sufficient for many patients.15–17 Based primarily on expert opinion, the guidelines recommended adjunctive antibiotics for patients with specific associated conditions, including an infected site diameter >5 cm, cellulitis, Systemic Inflammatory Response Syndrome (SIRS), diabetes, and recurrent infection. The two recent randomized trials demonstrating antibiotic efficacy included patients with a wide range of abscess cavity and surrounding erythema dimensions as well as various comorbidities, including diabetes.13,14 The extent to which antibiotics are as efficacious for small and uncomplicated abscesses and the relative magnitude of the treatment effect in various subgroups is unclear.
Importance
Between 1993 and 2005, annual emergency department (ED) visits for skin and soft tissue infections in the U.S. increased from 1.2 to 3.4 million, primarily due to an increased incidence of abscesses.18,19 Determining optimal treatment for affected patients is crucial to assuring rapid recovery without need for further medical interventions and promoting good antibiotic stewardship.
Goals of this investigation
Our goals were to determine the degree to which outcome advantages observed with adjunctive antibiotics for all participants in the study by Talan et al.14 existed among subgroups with and without conditions for which antibiotics have been selectively recommended and identify patients who most benefit from treatment. Therefore, we conducted a pre-planned subgroup analysis to determine if, among these subgroups of patients, treatment with a seven-day course of trimethoprim-sulfamethoxazole was associated with higher cure rates than placebo such that no additional antibiotics or drainage procedures were required through 7–14 days and 42–56 days after treatment.
METHODS
Study design and setting
We conducted a pre-planned subgroup analysis among participants of a multicenter, double-blind, randomized trial demonstrating superiority of trimethoprim-sulfamethoxazole to placebo for treatment of patients with a skin abscess receiving incision and drainage and treated as an outpatient. The trial, full protocol, and statistical-analysis plan were previously published.14 The institutional review board at each site approved the trial.
Selection of Participants
Inclusion criteria
From April 2009 to April 2013, we enrolled patients older than 12 years presenting to any of 5 U.S. EDs with a cutaneous lesion suspected to be an abscess based on physical examination and ultrasound, or examination alone, and found to have purulent material upon surgical exploration. We enrolled only participants with a lesion present for <1 week and measuring ≥2.0 cm in diameter (i.e., from the borders of induration, if fluctuant, or borders of abscess cavity on ultrasound, if not fluctuant), for whom their treating clinician intended outpatient treatment and who agreed to return for reevaluation and provided written consent.
Exclusion criteria
We excluded patients with the following conditions: indwelling device; suspected osteomyelitis or septic arthritis; diabetic foot, decubitus, or ischemic ulcer; mammalian bite; wound with organic foreign body; infection of another organ system or skin site; perirectal, perineal or paronychial location; intravenous drug use within previous month with fever; underlying skin condition; long-term care residence; incarceration; immunodeficiency (e.g., absolute neutrophil count <500/mm3, immunosuppressive drugs, active chemotherapy, or known AIDS assessed by subject history); creatinine clearance <50 mL/min; cardiac condition with risk of endocarditis; allergy or intolerance to trimethoprim-sulfamethoxazole; taking warfarin, phenytoin, or methotrexate; known G-6-PD or folic acid deficiency; pregnant or lactating; trimethoprim-sulfamethoxazole treatment within 24 hours; concurrent treatment with topical or systemic antibiotic; or enrolled in the study within 12 weeks. The treating clinician decided if any laboratory testing was done.
Interventions
We randomized participants to receive a seven-day course of trimethoprim-sulfamethoxazole (4 single-strength pills, 80 mg/400 mg each, twice daily) or placebo (4 pills twice daily). Clinicians had training in standardized incision and drainage technique.14
Methods and Measurements
We collected participant demographic and clinical information, including history of MRSA infection and fever, co-morbidities, measured temperature in the ED, and lesion size (Table 1).
Table 1.
Baseline Characteristics of Participants with a Drained Skin Abscess Treated with Trimethoprim-Sulfamethoxazole or Placebo in the Per-Protocol Population.
| Characteristic | Trimethoprim- sulfamethoxazole (n=524) | Placebo (n=533) |
|---|---|---|
|
| ||
| Median age, years (IQR, range)* | 35 (26–47.5, 14–69) | 35 (26–48, 16–73) |
|
| ||
| Male Sex – n (%) | 303 (57.8) | 308 (57.8) |
|
| ||
| Race – n (%) | ||
| White | 244 (46.6) | 252 (47.3) |
| Black | 232 (44.3) | 233 (43.7) |
| Asian | 5 (1.0) | 2 (0.4) |
| Hawaiian/Pacific Islander | 0 (0.0) | 1 (0.2) |
| American Indian/Alaskan Native | 3 (0.6) | 2 (0.4) |
| Multi-Racial | 22 (4.2) | 22 (4.1) |
| Other/Unknown | 18 (3.4) | 21 (3.9) |
|
| ||
| Hispanic Ethnicity - n (%) | 178 (34.0) | 180 (33.8) |
|
| ||
| Median (IQR) number of days symptoms | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) |
|
| ||
| History of methicillin-resistant Staphylococcus aureus (MRSA) infection | 45 (8.6) | 40 (7.5) |
|
| ||
| Comorbidities† – n (%) | ||
| Cancer | 6 (1.1) | 3 (0.6) |
| CHF | 5 (1.0) | 4 (0.8) |
| COPD | 6 (1.1) | 6 (1.1) |
| Chronic edema | 3 (0.6) | 1 (0.2) |
| Diabetes | 58 (11.1) | 57 (10.7) |
| Eczema | 22 (4.2) | 14 (2.6) |
| HIV+ | 7 (1.3) | 10 (1.9) |
| Any of the above | 92 (17.6) | 88 (16.5) |
|
| ||
| Abscess related to IV drug use – n (%) | 20 (3.8) | 13 (2.4) |
|
| ||
| History of prior antibiotic treatment for skin and soft tissue infection – n (%) | 2 (0.4) | 4 (0.8) |
|
| ||
| Close household contact‡ – n (%) | 37/520 (7.1) | 39/529 (7.4) |
|
| ||
| Fever§ - n (%) | 102/520 (19.6) | 98/528 (18.6) |
|
| ||
| Abscess location – n (%) | ||
| Head/neck | 68 (13.0) | 78 (14.6) |
| Trunk/Abdomen/Back | 116 (22.1) | 108 (20.3) |
| Groin/Buttocks | 113 (21.6) | 102 (19.1) |
| Upper Extremity | 117 (22.3) | 118 (22.1) |
| Lower Extremity | 110 (21.0) | 127 (23.8) |
|
| ||
| Median abscess dimension by probe of abscess cavity, cm (IQR, range) | ||
| Length|| | 2.5 (2.0–3.5,0.5–13.0) | 2.5 (2.0–3.5,0.1–16.0) |
| Width | 2.0 (1.5–3.0,0.3–11.0) | 2.0 (1.5–3.0,0.1–10.0) |
| Depth | 1.5 (1.0–2.0,0.3–5.5) | 1.5 (1.0–2.0, 0.1–5.0) |
|
| ||
| Abscess cavity maximal dimension|| ≥ 5 cm – n (%) | 78/523 (14.9) | 72/533 (13.5) |
|
| ||
| Median erythema dimension, cm (IQR, range) | ||
| Length | 6.8 (4.5–10.0,1.0–32.0) | 6.5 (4.0–10.0,2.0–38.5) |
| Width | 5.0 (3.2–8.0,1.0–40.0) | 5.0 (3.4–8.0,1.0–28.5) |
| Area||¶ | 19.7 (7.0–54.4,0.0–582.8) | 21.2 (7.1–55.4,0.0–520.9) |
|
| ||
| Area|| of erythema >75 cm2 – n (%) | 107 (20.4) | 110 (20.6) |
|
| ||
| Erythema maximal dimension|| ≥ 5 cm – n (%) | 390 (74.4) | 399 (74.9) |
|
| ||
| Median dimensions of induration/swelling, cm (IQR, range) | ||
| Length | 4.5 (3.5–6.5,1.0–19.0) | 4.5 (3.2–6.0,0.5–20.0) |
| Width | 4.0 (3.0–5.0,1.0–16.0) | 4.0 (3.0–5.0,0.9–29.0) |
| Area¶ | 7.5 (2.8–18.3,0.0–198.5) | 8.2 (2.8–18.1,0.0–396.6) |
|
| ||
| Baseline wound culture results | ||
| Methicillin-resistant Staphylococcus aureus | 219 (41.8) | 249 (46.7) |
| Methicillin-susceptible S. aureus | 86 (16.4) | 86 (16.1) |
| Coagulase-negative staphylococci | 68 (13.0) | 59 (11.1) |
| Streptococcal species** | 36 (6.9) | 14 (2.6) |
| Other# | 87 (16.6) | 63 (11.8) |
| No growth | 80 (15.3) | 93 (17.4) |
| Not done | 3 (0.6) | 3 (0.6) |
Participants with missing data were excluded from relevant analyses.
Eight (0.8%) participants were age 13 to 17 years.
Co-morbidities were those present in >0.5%.
A close household contact was defined as someone living in the same household with similar skin infection in last month.
Fever was defined as patient report of history of fever in prior week or temperature >38°C in the ED.
Largest measurement of length or width was defined as the maximal dimension.
Areas of erythema and induration/swelling were calculated using formula for an ellipse (1/4 x πx length x width) minus area of probe measurements of length and width of abscess area.
Streptococcal species included: Group A streptococcus, Group B streptococcus, S. anginosus, beta-hemolytic group C streptococcus, beta-hemolytic group F streptococcus, beta-hemolytic group G streptococcus, Non-group A and B beta-hemolytic streptococcus, Viridans group streptococcus, and Alpha-hemolytic streptococcus.
Other isolates included: Actinomyces species, Bacteroides species, Diphtheroid bacilli, Eikenella corrodens, Enterobacter species, Enterococcus species, Escherichia coli, Fusobacterium species, Haemophilus species, Klebsiella species, Lactobacillus species, Peptostreptococcus species, Porphyromonas species, Prevotella species, Proteus mirabilis, and Veillonella species.
We measured abscess cavity and erythema dimension according to the following methods. After the abscess cavity was opened with a scalpel, the internal cavity was probed with the wooden end of a cotton swab or with an instrument such as a hemostat. This broke up loculations in the abscess and allowed the investigator to estimate the internal dimensions of the abscess cavity by noting how far the probe went to the edge of the abscess cavity from the center. Adding the length of the probe from the center to the edge of the abscess cavity in four directions, allowed the length and width of the abscess cavity to be measured. The depth of the abscess cavity was determined by measuring the depth to which the probe went to the bottom of the abscess cavity from the outer skin level. Some patients enrolled with an abscess estimated to be ≥2 cm were smaller upon surgical exploration.
We took all measurements of erythema while the infected area was in a nondependent position, e.g., if infection was on the leg, the subject was lying down, not sitting or standing. The border of erythema was marked with a pen. Maximal dimensions of erythema, both width and length, were recorded in centimeters. Investigators attempted to find the infection edge that best distinguished erythema from non-erythematous skin. Erythema was measured in the dimension of maximal length. The maximal width was measured perpendicular to the axis of the maximal length. The maximal width measurement did not have to be in the center if the area of erythema was irregular.
We sent purulent material from all abscesses for wound culture and susceptibility testing at local site laboratories.
Outcomes
We defined the primary outcome as the difference in abscess clinical cure rates between patients receiving trimethoprim-sulfamethoxazole and those receiving placebo. We defined clinical cure as resolution of all symptoms and signs of infection, or improvement such that no new antibiotics were prescribed through 7–14 days after the end of treatment. In the original trial, primary outcomes were reported in the modified intention-to-treat (mITT) and per-protocol (PP) populations.14 In both the mITT and PP populations, the clinical cure rate of the trimethoprim-sulfamethoxazole group was significantly higher than that of the placebo group by about the same magnitude, i.e., 7 percentage points. Thus, for this subgroup analysis, we evaluated outcomes in the PP population, i.e., participants who returned for follow-up and had ≥75% medication adherence, whom could be most accurately assessed for outcomes and treatment effect.
We conducted subgroup analyses for three outcomes; 1 - clinical cure at 7–14 days after the end of treatment, as described above; 2 - composite cure, defined as resolution of all symptoms and signs of infection, or improvement such that no additional antibiotics and/or surgical drainage procedure were prescribed through 7–14 days after the end of treatment, and 3 - composite cure through 42–56 days after the end of treatment.
We evaluated these outcomes among subgroups defined by presence or absence of the following characteristics: abscess cavity maximal dimension ≥5 cm; erythema maximal dimension ≥5 cm; history of MRSA infection at any time in the past defined by patient report; fever defined as history of subjective or measured fever in the preceding week by patient report or measured temperature >38°C (100.4°F) in the ED; diabetes; major co-morbidities, defined as relevant serious medical conditions present in >0.5% of the PP population (i.e., diabetes, eczema or chronic edema, chronic obstructive pulmonary disease, congestive heart failure, human immunodeficiency virus infection, and cancer); and culture positive for MRSA or methicillin-susceptible S. aureus (MSSA). Among this population of ED patients who were felt to be stable for outpatient care, measured temperature >38°C (100.4°F) was present in <1%. Also, few patients were elderly. Therefore, we could not evaluate subgroups with SIRS and advanced age.
Analysis
We conducted separate analyses for each characteristic for each of the three outcomes described above. We pre-planned secondary assessment of the association of the presence or absence of certain conditions specified by guidelines to determine use of adjunctive antibiotics. As such, we considered all analyses exploratory and unrelated to any specific hypothesis or formal power assessment. Results are presented as point measures of the individual treatment effects (i.e., difference in cure rate among participants treated with trimethoprim-sulfamethoxazole as compared to that of participants treated with placebo) and the associated 95% confidence interval (CI). The between-group difference in cure rates as a function of maximal abscess and erythema linear dimension are presented. We plotted between-group percentage point differences in cure rates by 1 cm increments of abscess cavity or erythema dimension. For each increment, we calculated cure rates for those that had the same or smaller size lesion. We completed calculations using Microsoft® Excel 2016 (Redmond, WA) and the methods of Fleiss.20 We excluded participants with missing values from relevant analyses.
RESULTS
Characteristics of study subjects
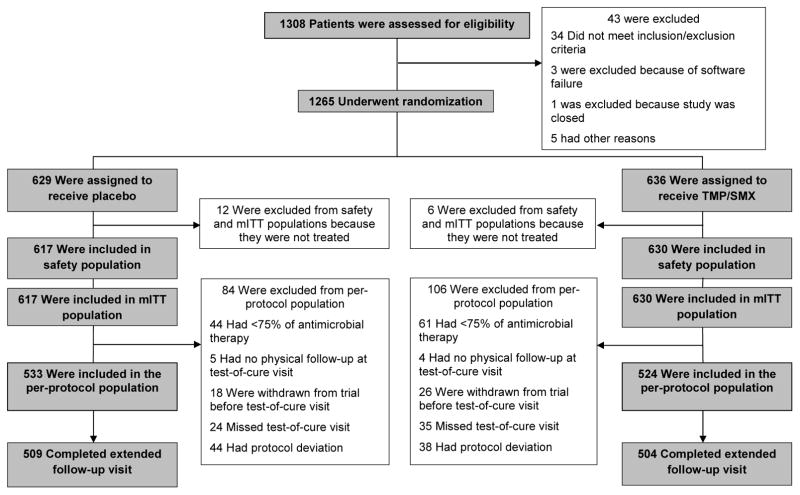
Of 1,265 enrolled patients 1,247 (98.6%) were randomly assigned to trimethoprim-sulfamethoxazole or placebo and received ≥1 dose, and 1,057 (83.6%) participants qualified for the PP population (Figure 1). Participant characteristics in the PP population are summarized in Table 1. Median age was 35 years, (range: 14–73) and 57.8% were male. Diabetes was present in 115 participants (10.9%) and 180 (17.0%) had a major comorbidity. Eighty-five (8.0%) participants reported a history of MRSA infection and 197 (18.6%) reported a history of fever within the preceding week while 8 (0.8%) had a temperature >38°C in the ED. Median abscess length and width were 2.5 cm (IQR, 2.0–3.5, range: 0.1–16.0) and 2.0 cm (IQR, 1.5–3.0, range: 0.1–11.0), respectively. Median length and width of erythema were 6.5 cm (IQR, 4.2–10.0, range: 1.0–38.5) and 5.0 cm (IQR 3.3–8.0, range: 1.0–40.0), respectively. MRSA was cultured from the abscess of 468 (44.3%) participants and MSSA from 172 (16.3%) participants; 461 (98.5%) MRSA isolates and 166 (96.5%) MSSA isolates demonstrated in vitro susceptibility to trimethoprim-sulfamethoxazole.
Figure 1.
Enrollment, Randomization, and Follow-Up of Patients with a Drained Skin Abscess Treated with Trimethoprim-Sulfamethoxazole or Placebo.
Main results
Overall, for the primary outcome, clinical cure (no new antibiotics prescribed) through 7–14 days after the end of treatment occurred in 487 of 524 participants (92.9%) in the trimethoprim-sulfamethoxazole group and 457 of 533 participants (85.7%) in the placebo group (difference, 7.2; 95% CI 3.2–11.2).14 Composite cure (neither a new antibiotic nor an additional surgical drainage procedure) through 7–14 days occurred in 453 of 524 participants (86.5%) in the trimethoprim-sulfamethoxazole group and in 396 of 533 participants (74.3%) in the placebo group (difference, 12.2; 95% CI 7.2–17.1). Composite cure through 42–56 days occurred in 416 of 505 participants (82.4%) in the trimethoprim-sulfamethoxazole group and in 358 of 510 participants (70.2%) in the placebo group (difference, 12.2; 95%CI 6.8–17.6).
Analysis of subgroup of participants with and without high-risk conditions for which antibiotics have been recommended for rates of clinical and composite cure through 7–14 days and composite cure through 42–56 days in the trimethoprim-sulfamethoxazole and placebo groups are shown in Tables 2a, b, and c, respectively. For all three outcomes, cure rates were higher for participants treated with trimethoprim-sulfamethoxazole than for those treated with placebo in all subgroups, i.e., presence or absence of abscess cavity dimension ≥5 cm, erythema dimension ≥5 cm, past MRSA infection, fever, diabetes, or a major comorbidity. The magnitude of the difference in cure rates between the trimethoprim-sulfamethoxazole and placebo groups for all three outcomes was greater than for the whole population among subgroups with a history of MRSA infection and fever. For example, for composite cure through 42–56 days after treatment, the treatment effect for the whole PP population was 12.2 percentage points whereas it was 22.9 percentage points among participants with a history of MRSA and 16.9 percentage points among those with fever. Based on culture results, the greatest treatment effect was associated with lesions that grew MRSA, and to a lesser degree, MSSA, as opposed to other organisms or no growth. No outcome advantage was associated with trimethoprim-sulfamethoxazole among participants who grew neither MRSA nor MSSA.
Table 2.
| Table 2a. Subgroup Analysis of Clinical Cure* Rates Through 7–14 days for Participants with a Drained Skin Abscess Treated with Trimethoprim-Sulfamethoxazole or Placebo by Presence or Absence of High-Risk Conditions. | |||
|---|---|---|---|
| Condition | Cure (n/total no. [%]) | Difference (95% CI) | |
| Trimethoprim- sulfamethoxazole | Placebo | ||
| Abscess cavity ≥5 cm | 69/77 (89.6) | 54/71 (76.1) | 14.7 (1.4 – 28.1) |
| Abscess cavity <5 cm† | 418/446 (93.7) | 403/462 (87.2) | 6.1 (2.1 – 10.1) |
| Erythema ≥5 cm | 357/390 (91.5) | 338/399 (84.7) | 6.8 (2.1 – 11.6) |
| Erythema <5 cm† | 130/134 (97.0) | 119/134 (88.8) | 8.2 (1.4 – 15.0) |
| History of MRSA infection | 43/45 (95.6) | 32/40 (80.0) | 15.6 (−0.6 – 31.7) |
| No history of MRSA infection | 444/479 (92.7) | 425/493 (86.2) | 6.5 (2.4 – 10.5) |
| Fever‡ | 93/102 (91.2) | 78/98 (79.6) | 14.3 (3.7 – 24.9) |
| No fever | 391/418 (93.5) | 374/430 (87.0) | 5.9 (1.7 – 10.1) |
| Diabetes | 50/58 (86.2) | 47/57 (82.5) | 3.8 (−11.3 – 18.8) |
| No diabetes | 437/466 (93.8) | 410/476 (86.1) | 7.6 (3.6 – 11.7) |
| Co-morbidity§ | 83/92 (90.2) | 73/88 (83.0) | 8.4 (−2.8 – 19.6) |
| No co-morbidity | 404/432 (93.5) | 384/445 (86.3) | 7.0 (2.8 – 11.2) |
| MRSA|| | 203/219 (92.7) | 202/249 (81.1) | 11.6 (5.2 – 18.0) |
| MSSA | 78/86 (90.7) | 71/86 (82.6) | 8.1 (−3.1 – 19.4) |
| No MRSA or MSSA | 203/216 (94.0) | 181/195 (92.8) | 1.2 (−4.1 – 6.5) |
| Table 2b. Subgroup Analysis of Composite Cure* Rates Through 7–14 days Among Participants with a Drained Skin Abscess Treated with Trimethoprim-Sulfamethoxazole or Placebo by Presence or Absence of High-Risk Conditions. | |||
|---|---|---|---|
| Condition | Cure (n/total no. [%]) | Difference (95% CI) | |
| Trimethoprim- sulfamethoxazole | Placebo | ||
| Abscess cavity ≥5 cm | 62/77 (80.5) | 51/71 (71.8) | 8.5 (−6.3 – 23.4) |
| Abscess cavity <5 cm† | 391/446 (87.7) | 345/462 (74.7) | 12.2 (7.0 – 17.4) |
| Erythema ≥5 cm | 335/390 (85.9) | 295/399 (73.9) | 11.5 (5.7 – 17.2) |
| Erythema <5 cm† | 118/134 (88.1) | 101/134 (75.4) | 11.9 (2.1 – 21.8) |
| History of MRSA infection | 40/45 (88.9) | 24/40 (60.0) | 28.9 (8.8 – 49.0) |
| No history of MRSA infection | 413/479 (86.2) | 372/493 (75.5) | 10.2 (5.1 – 15.2) |
| Fever‡ | 83/102 (81.4) | 64/98 (65.3) | 18.4 (5.4 – 31.3) |
| No fever | 368/418 (88.0) | 329/430 (76.5) | 10.2 (4.9 – 15.5) |
| Diabetes | 44/58 (75.9) | 36/57 (63.2) | 10.9 (−7.4 – 29.3) |
| No diabetes | 409/466 (87.8) | 360/476 (75.6) | 11.7 (6.7 – 16.8) |
| Co-morbidity§ | 71/92 (77.2) | 55/88 (62.5) | 13.5 (−0.8 – 27.9) |
| No co-morbidity | 382/432 (88.4) | 341/445 (76.6) | 11.3 (6.2 – 16.5) |
| MRSA|| | 186/219 (84.9) | 163/249 (65.5) | 18.3 (10.3 – 26.2) |
| MSSA | 72/86 (83.7) | 63/86 (73.3) | 10.5 (−2.9 – 23.8) |
| No MRSA or MSSA | 192/216 (88.9) | 167/195 (85.6) | 3.24 (−3.7 – 10.2) |
| Table 2c. Subgroup Analysis of Composite Cure* Rates Through 42–56 days Among Participants with a Drained Skin Abscess Treated with Trimethoprim-Sulfamethoxazole or Placebo by Presence or Absence of High-Risk Conditions. | |||
|---|---|---|---|
| Condition | Cure (n/total no. [%]) | Difference (95% CI) | |
| Trimethoprim-sulfamethoxazole | Placebo | ||
| Abscess cavity ≥5 cm | 55/70 (78.6) | 47/70 (67.1) | 11.4 (−4.61 – 27.5) |
| Abscess cavity <5 cm† | 361/434 (83.2) | 311/440 (70.7) | 12.5 (6.75 – 18.2) |
| Erythema >5 cm | 307/376 (81.6) | 269/383 (70.2) | 11.4 (5.13 – 17.7) |
| Erythema <5 cm† | 109/129 (84.5) | 89/127 (70.1) | 14.4 (3.51 – 25.3) |
| History of MRSA infection | 33/43 (76.7) | 21/39 (53.8) | 22.9 (0.34 – 45.4) |
| No history of MRSA infection | 383/462 (82.9) | 337/471 (71.5) | 11.4 (5.81 – 16.9) |
| Fever‡ | 76/98 (77.6) | 57/94 (60.6) | 16.9 (2.99 – 30.8) |
| No fever | 338/403 (83.9) | 299/412 (72.6) | 11.3 (5.44 – 17.2) |
| Diabetes | 38/56 (67.9) | 36/56 (64.3) | 3.57 (−15.7 – 22.9) |
| No diabetes | 378/449 (84.2) | 322/454 (70.9) | 13.3 (7.67 – 18.9) |
| Co-morbidity§ | 61/88 (69.3) | 54/86 (62.8) | 6.53 (−8.7 – 21.7) |
| No co-morbidity | 355/417 (85.1) | 304/424 (71.7) | 13.4 (7.71 – 19.2) |
| MRSA|| | 166/208 (79.8) | 139/235 (59.2) | 20.7 (11.9 – 29.4) |
| MSSA | 71/85 (83.5) | 57/82 (69.5) | 14.0 (0.11 – 27.9) |
| No MRSA or MSSA | 176/209 (84.2) | 160/190 (84.2) | 0.0 (−7.71 – 7.71) |
Abbreviations: MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-susceptible S. aureus
Overall, for trimethoprim-sulfamethoxazole and placebo groups, clinical cure rate at 7–14 days was 92.9% and 85.7% (difference, 7.2; 95% CI 3.2–11.2), composite cure rate at 7–14 was 86.5% and 74.3% (difference, 12.2; 95% CI 7.2–17.1) and extended composite cure at 42–56 days was 82.4% and 70.2% (difference, 12.2; 95% CI 6.8–17.6), respectively. Outcomes were defined as follows: clinical cure was defined as resolution of all symptoms and signs of infection, or improvement such that no new antibiotics were prescribed (through 7–14 days after the end of treatment); composite cure was defined as resolution of all symptoms and signs of infection, or improvement such that no additional antibiotics and/or surgical drainage procedure were required (through 7–14 days and 42–56 days after the end of treatment).
Abscess cavity and erythema dimensions were defined as the maximal dimension (length or width).
Fever was defined as history of fever within the preceding week or temperature >38°C measured in the ED (0.8%).
Co-morbidities were defined as those present in >0.5% of study participants: diabetes (10.9%); eczema or other chronic skin condition (3.4%); chronic obstructive pulmonary disease (1.1%); congestive heart failure (0.9%); human immunodeficiency virus infection (1.6%); and cancer (0.9%).
Culture results were those based on the organism isolated from primary wound specimen.
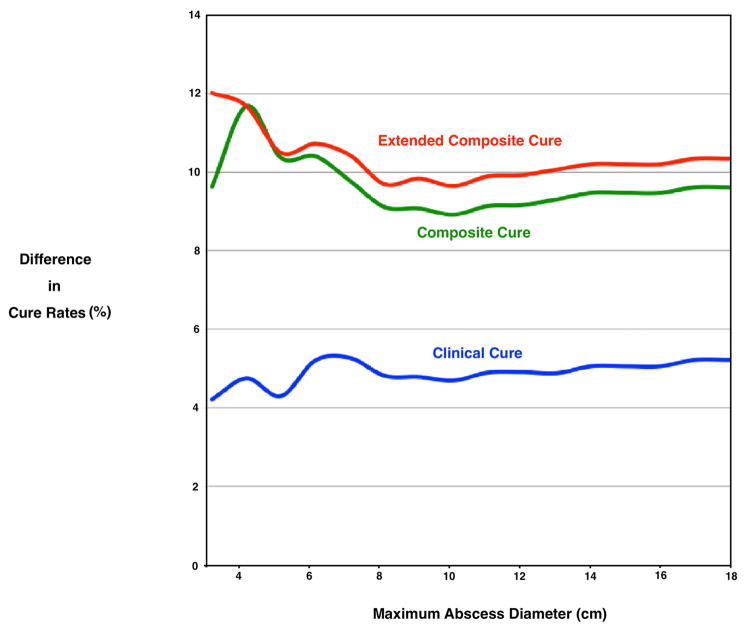
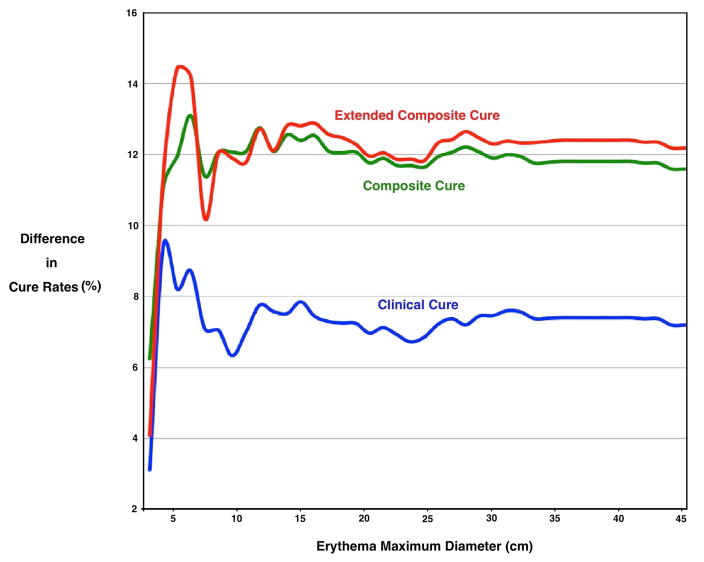
Figures 2 and 3 show the between -group percentage point differences in cure rates among the treatment groups by abscess cavity and erythema maximal dimension, further illustrating that the outcome advantage associated with trimethoprim-sulfamethoxazole existed across all lesion sizes.
Figure 2.
Difference in Cure Rates* by Abscess Cavity Diameter† Among Participants with a Drained Skin Abscess Treated with Trimethoprim-Sulfamethoxazole Compared to Placebo.
Overall, for trimethoprim-sulfamethoxazole and placebo groups, clinical cure rate at 7–14 days was 92.9% and 85.7% (difference, 7.2; 95% CI 3.2–11.2), composite cure rate at 7–14 was 86.5% and 74.3% (12.2; 95% CI 7.2–17.1) and extended composite cure at 42–56 days was 82.4% and 70.2% (difference, 12.2; 95% CI 6.8–17.6), respectively.
* The between–group percentage point differences in cure rates were plotted by 1 cm increments of abscess cavity or erythema dimension. For each increment, cure rates were calculated for those that had the same or smaller size lesion. Outcomes were defined as follows: clinical cure was defined as resolution of all symptoms and signs of infection, or improvement such that no new antibiotics were prescribed (through 7–14 days after the end of treatment); composite cure was defined as resolution of all symptoms and signs of infection, or improvement such that no additional antibiotics and/or surgical drainage procedure were required (through 7–14 days and 42–56 days after the end of treatment).
†Diameter was defined as the maximal linear dimension (length or width) of the abscess cavity, by probe after incision, and erythema, measured on the skin.
Figure 3.
Difference in Cure Rates* by Erythema Diameter† Among Participants with a Drained Skin Abscess Treated with Trimethoprim-Sulfamethoxazole Compared to Placebo.
Overall, for trimethoprim-sulfamethoxazole and placebo groups, clinical cure rate at 7–14 days was 92.9% and 85.7% (difference, 7.2; 95% CI 3.2–11.2), composite cure rate at 7–14 was 86.5% and 74.3% (12.2; 95% CI 7.2–17.1) and extended composite cure at 42–56 days was 82.4% and 70.2% (difference, 12.2; 95% CI 6.8–17.6), respectively.
* The between-group percentage point differences in cure rates were plotted by 1 cm increments of abscess cavity or erythema dimension. For each increment, cure rates were calculated for those that had the same or smaller size lesion. Outcomes were defined as follows: clinical cure was defined as resolution of all symptoms and signs of infection, or improvement such that no new antibiotics were prescribed (through 7–14 days after the end of treatment); composite cure was defined as resolution of all symptoms and signs of infection, or improvement such that no additional antibiotics and/or surgical drainage procedure were required (through 7–14 days and 42–56 days after the end of treatment).
†Diameter was defined as the maximal linear dimension (length or width) of the abscess cavity, by probe after incision, and erythema, measured on the skin.
LIMITATIONS
This trial has limitations. The original trial was not powered to detect a treatment effect in groups other than in the primary outcome population. A significant treatment effect was generally observed among participants with and without high-risk conditions, however, in a few subgroups, the 95%CI of the difference in cure rates crossed 0, which may be a result of small subgroup sizes. Further, multiple comparisons were conducted, which leads to a greater likelihood of observing positive associations due to chance. Analyses were conducted using the PP population for whom follow-up status was available. Although the participant characteristics and magnitude of the treatment effect in the PP and mITT populations were similar, it is possible that the PP population was affected by post-randomization bias. Only patients with a maximal abscess cavity diameter estimated to be ≥2 cm based on physical examination or ultrasound were enrolled. Therefore, our results do not apply to patients with smaller lesions and other conditions that led to ineligibility.
DISCUSSION
Two recent large randomized trials demonstrated the efficacy of adjunctive antibiotics possessing activity against methicillin-resistant Staphylococcus aureus (MRSA) for treatment of patients with a drained skin abscess.13,14 This subgroup analysis was pre-planned to test the validity of guidelines for use of adjunctive antibiotics for a drained skin abscess among the largest reported trial population, which mostly consisted of adults.14 Although exploratory, these results support adjunctive antibiotic treatment for the small uncomplicated skin abscesses estimated to be at least 2 cm diameter by physical examination or ultrasound. A consistent treatment effect was associated with trimethoprim-sulfamethoxazole treatment among patients with small abscess and erythema size and lacking any condition for which antibiotics have been selectively recommended,15–17 including history of MRSA infection, fever, diabetes, and other co-morbidities. The findings of a treatment effect among participants with an abscess size <5 cm and those without diabetes are consistent with the results of the trial by Daum et al.13, in which pediatric and adult patients with small abscesses of no minimum size were enrolled but those with larger abscesses (i.e., >5 cm for adults and >3–4 cm for children) and diabetes were excluded. The treatment effect was observed across all dimensions of abscess cavity and associated erythema. Together, the two trials suggest that adjunctive antibiotics improve cure rates for a drained uncomplicated skin abscess of any size.
Trimethoprim-sulfamethoxazole is highly active against staphylococci, with 97.4% of study MRSA isolates tested demonstrating in vitro susceptibility.14 The treatment effect was highly associated with culture of MRSA, and to a lesser degree, MSSA. No treatment effect was found with lesions that grew other organisms or had no growth, a result also found in the trial by Daum et al., supporting the role of antibiotics to treat the bacterial tissue infection once the abscess has been drained. Non-S. aureus lesions may largely represent inflamed but non-infected cysts, with negative cultures or growth representing skin contamination. Daum et al.13 also found that the cure rate of participants treated with clindamycin was lower among those for which the staphylococcal isolate demonstrated in vitro resistance as opposed to susceptibility, further supporting this biological model. Specimen Gram stain or more recently available rapid polymerase chain reaction-based assays of drainage material to identify staphylococcal infections potentially could guide treatment decisions.
Among all participants, through 42–56 days after treatment, recurrent infection at a new site occurred in 19.1% of the placebo group compared to 10.9% of the trimethoprim-sulfamethoxazole group.14 The composite cure outcome through 42–56 days after treatment captured all initial treatment failures and subsequent infections that required either a new antibiotic or drainage procedure. The treatment effect associated with trimethoprim-sulfamethoxazole extended through 42–56 days in all clinical subgroups. Further, history of MRSA infection and abscesses that grew MRSA were associated with a greater magnitude of treatment benefit compared to that of the entire study population. These observations suggest that trimethoprim-sulfamethoxazole may reduce MRSA colonization for several weeks following initial treatment.
The outcome advantage associated with trimethoprim-sulfamethoxazole existed across the range of abscess or erythema dimensions. However, the presence of certain characteristics identified by guidelines15–17 as criteria for antibiotic treatment, i.e., history of MRSA infection and fever, was associated with the greatest treatment effect. For the entire study population, there was a 12.2 percentage point higher rate of neither requiring a new antibiotic nor drainage procedure through 42–56 days after treatment. This difference was 22.9 percentage points among participants with history of MRSA infection and 16.9 percentage points among those with history of fever in the preceding week or measured elevated temperature in the ED.
Further studies could attempt to validate these subgroup treatment effect associations and further inform shared decisionmaking with patients about benefit, risk, and cost associated with the decision to provide adjunctive antibiotics. However, it may be difficult to accrue a sufficient number of patients in these subgroups. Trimethoprim-sulfamethoxazole is off-patent and, currently, a full course costs about $5 USD. Its use was associated with only slightly more mild gastrointestinal side effects than placebo14, although, this antibiotic is rarely associated with serious adverse reactions, such as Stephens-Johnson syndrome.21 The trial had inadequate power to detect either an increased rate of rare antibiotic-related adverse effects or a decreased rate of subsequent invasive infections compared to placebo. The study by Daum et al. demonstrated efficacy of clindamycin 150 mg three times daily and trimethoprim-sulfamethoxazole 160 mg/800 mg twice daily compared to placebo.13 The trimethoprim-sulfamethoxazole dose was half that used in our trial, and the Daum et al. treated for 10 days, while we treated for 7 days. This investigation also found that adverse events were approximately twice as frequent with clindamycin compared to trimethoprim-sulfamethoxazole. Neither trial detected any cases of Clostridium difficile colitis. The costs and risks associated with adjunctive antibiotic treatment must be weighed against those associated with the care and consequences of treatment failure and recurrence.
CONCLUSIONS
This subgroup analysis of the largest reported trial comparing antibiotics to placebo for patients with a drained skin abscess found that trimethoprim-sulfamethoxazole treatment was associated with improved outcomes regardless of abscess or erythema size or the presence or absence of conditions for which antibiotics have been selectively recommended, including history of MRSA infection, fever, diabetes, and major comorbidities. The magnitude of the treatment effect was greatest among subgroups with a history of MRSA infection or fever, and for those with an abscess due to MRSA.
Acknowledgments
Funding: Supported by a contract from the National Institutes of Allergy and Infectious Diseases (HHSN272200700032C to Drs. Talan and Moran). The National Institutes of Allergy and Infectious Diseases had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Disclosure of Potential Conflicts of Interest:
Dr. Talan reports personal fees for consultation from Allergan, GlaxoSmithKline, and Paratek Pharmaceuticals and research grants from Allergan, Debiopharm, the Centers for Disease Control and Prevention, and the Patient Centered Outcomes Research Institute.
Dr. Moran reports personal fees for consultation and research grants from Allergan, and research grants from Allergan, Cempra, ContraFect, YES Biotechnology, the Centers for Disease Control and Prevention, and the Patient Centered Outcomes Research Institute.
Dr. Krishnadasan reports research grants from the Centers for Disease Control and Prevention, and the Patient Centered Outcomes Research Institute.
Dr. Lovecchio reports research grants from Allergan, NIH and the Centers for the Disease Control and Prevention.
Dr. Abrahamian reports personal fees from participation on speakers' bureaus for Merck, Allergan, and The Medicines Company, consultation for Cempra, Summit Therapeutics, Tetraphase Pharmaceuticals, Paratek Pharmaceuticals, and Janssen Research & Development, and research grants from the Centers for Disease Control and Prevention, Merck, and Cempra.
Dr. Karras has no potential conflicts of interest to report.
Dr. Steele reports research grants from Allergan and the Centers for the Disease Control and Prevention.
Dr. Rothman reports personal fees for consultation and research grant from Allergan, and research grants from Gilead (FOCUS Program) the Centers for the Disease Control and Prevention, NIH (NIAID), and HHS (BARDA). Dr. Rothman also participated in a 1 day consultation for Allergan with travel costs paid for by Allergan.
Dr. Mower reports research grants from Allergan, the Centers for Disease Control and Prevention, and the Patient Centered Outcomes Research Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Eng J Med. 2014;370:1039–1047. doi: 10.1056/NEJMra1212788. [DOI] [PubMed] [Google Scholar]
- 2.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 3.Macfie J, Harvey J. The treatment of acute superficial abscesses: a prospective clinical trial. Br J Surg. 1977;64:264–266. doi: 10.1002/bjs.1800640410. [DOI] [PubMed] [Google Scholar]
- 4.Meislin HW, Lerner SA, Graves MH, et al. Cutaneous abscesses (anaerobic and aerobic bacteriology and outpatient management) Ann Intern Med. 1977;87:145–149. doi: 10.7326/0003-4819-87-2-145. [DOI] [PubMed] [Google Scholar]
- 5.Llera JL, Levy RC, Staneck JL. Cutaneous abscesses: natural history and management in an outpatient facility. J Emerg Med. 1984;1:489–493. doi: 10.1016/0736-4679(84)90002-7. [DOI] [PubMed] [Google Scholar]
- 6.Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15–19. doi: 10.1016/s0196-0644(85)80727-7. [DOI] [PubMed] [Google Scholar]
- 7.Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123–17. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 8.Ruhe J, Smith N, Bradsher R, et al. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy. Clin Infect Dis. 2007;44:777–784. doi: 10.1086/511872. [DOI] [PubMed] [Google Scholar]
- 9.Szumowski JD, Cohen DE, Kanaya F, et al. Treatment and outcomes of infections by -resistant Staphylococcus aureus at an ambulatory clinic. Antimicrob Agents Chemother. 2007;51:423–428. doi: 10.1128/AAC.01244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044–4048. doi: 10.1128/AAC.00377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med. 2010;55:401–407. doi: 10.1016/j.annemergmed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283–287. doi: 10.1016/j.annemergmed.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Daum RS, Miller L, Immergluck L, et al. Placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376:2545–2555. doi: 10.1056/NEJMoa1607033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374:823–832. doi: 10.1056/NEJMoa1507476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorwitz RJ, Jernigan DB, Powers JH, Jernigan JA. Participants in the Centers for Disease Control and Prevention-Convened Experts’ Meeting on Management of MRSA in the Community. Strategies for Clinical Management of MRSA in the Community: Summary of an Experts’ Meeting Convened by the Centers for Disease Control and Prevention; March 2006; pp. 1–24. [Google Scholar]
- 16.Liu C, Bayer A, Cosgrove SE, Daum RS, et al. Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 17.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10–52. doi: 10.1093/cid/ciu444. [DOI] [PubMed] [Google Scholar]
- 18.Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA., Jr Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2008;51:291–298. doi: 10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Qualls ML, Mooney MM, Camargo CA, Jr, Zucconi T, Hooper DC, Pallin DJ. Emergency department visit rates for abscess versus other skin infections during the emergence of community-associated methicillin-resistant Staphylococcus aureus, 1997–2007. Clin Infect Dis. 2012;55:103–105. doi: 10.1093/cid/cis342. [DOI] [PubMed] [Google Scholar]
- 20.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Propositions. 3. John Wiley and Sons, Incorporated; Hoboken, New Jersey: 2003. [Google Scholar]
- 21.Chan HL, Stern RS, Arndt KA, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: a population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126:43–47. [PubMed] [Google Scholar]