Abstract
Aim
Sphingosine-1-phosphate (S1P) influences resistance vessel function and is implicated in renal pathological processes. Previous studies revealed that S1P evoked potent vasoconstriction of the preglomerular microvasculature, but the underlying mechanisms remain incompletely defined. We postulated that S1P–mediated preglomerular microvascular vasoconstriction involves activation of voltage-dependent L-type calcium channels (L-VDCC) and the rho/rho kinase pathway.
Methods
Afferent arteriolar reactivity was assessed in vitro using the blood-perfused rat juxtamedullary nephron preparation and diameter was measured during exposure to physiological and pharmacological agents.
Results
Exogenous S1P (10−9-10−5 mol L−1) evoked concentration-dependent vasoconstriction of afferent arterioles. Superfusion with nifedipine, a L-VDCC blocker, increased arteriolar diameter by 39 ± 18% of baseline and significantly attenuated the S1P–induced vasoconstriction. Superfusion with the rho kinase inhibitor, Y-27632, increased diameter by 60 ± 12% of baseline and also significantly blunted vasoconstriction by S1P. Combined nifedipine and Y-27632 treatment significantly inhibited S1P–induced vasoconstriction over the entire concentration range tested. In contrast, depletion of intracellular Ca2+ stores with the Ca2+-ATPase inhibitors, thapsigargin or cyclopiazonic acid, did not alter the S1P–mediated vasoconstrictor profile. Scavenging reactive oxygen species (ROS) or inhibition of nicotinamide adenine dinucleotide phosphate oxidase activity significantly attenuated S1P–mediated vasoconstriction.
Conclusion
Exogenous S1P elicits potent vasoconstriction of rat afferent arterioles. These data also demonstrate that S1P–mediated preglomerular vasoconstriction involves activation of L-VDCC, the rho/rho kinase pathway and ROS. Mobilization of Ca2+ from intracellular stores is not required for S1P–mediated vasoconstriction. These studies reveal a potential role for S1P in modulation of renal microvascular tone.
Keywords: Ca2+ signalling, cyclopiazonic acid, reactive oxygen species, renal microvascular reactivity, tempol, thapsigargin
INTRODUCTION
Sphingosine-1-phosphate (S1P) is a bioactive metabolite of sphingomyeline that mediates multiple cellular functions.1, 2 S1P is synthesized in endothelial cells, erythrocytes and platelets upon stimulation.3, 4 Most biological functions of S1P are through activation of its five specific G-protein-coupled receptors (S1P1–5). Accumulating evidence implicates S1P as an important regulator of vascular tone and reactivity, especially in resistance vessels including the renal vasculature.5–11 A number of recent studies in animal models and human subjects indicate that S1P plays an important role in the development of many pathophysiological conditions including ischemia-reperfusion induced acute kidney injury, sepsis, diabetic nephropathy, hypertension and sickle cell disease2, 12–15 but the contribution of S1P to renal microvascular function is incompletely understood. We demonstrated that S1P potently vasoconstricted preglomerular microvessels via activation of S1P1 and S1P2 receptors while post-glomerular efferent arterioles were unresponsive.11 The downstream signalling mechanisms contributing to S1P–mediated vasoconstriction in renal microvascular reactivity remain to be determined.
Afferent arterioles play a major role in regulating renal vascular resistance which is crucial for maintaining a stable renal blood flow and glomerular filtration rate.16, 17 Afferent arteriolar reactivity is influenced by many bioactive vascular factors and hormones such as angiotensin II, nitric oxide, endothelin, vasopressin, norepinephrine and ATP.16, 18 Enhanced or attenuated renal microvascular reactivity to these vasoactive substances occurs in many pathophysiological conditions including hypertension, diabetic nephropathy and kidney failure19–23 and could contribute to kidney injury.
In non-renal vascular beds, S1P receptor activation is linked to multiple intracellular signalling pathways.5, 24–26 Stimulation of S1P receptors in vascular smooth muscle cells activates phospholipase-C which in turn triggers Ca2+ release from intracellular stores leading to vasoconstriction. S1P also activates voltage-dependent L-type calcium channels (L-VDCC) to allow Ca2+ influx or stimulates the rho/rho kinase pathway, leading to contraction of smooth muscle cells. Although our previous study showed that L-VDCC play a role in S1P–mediated afferent arteriolar vasoconstriction,11 the relative roles of Ca2+ mobilization from intracellular stores and the participation of the rho/rho kinase-dependent pathway in S1P–evoked vasoconstriction remain incompletely defined. Additionally, S1P is also linked to reactive oxygen species (ROS) generation in resistance arteries in response to pressure-stimulation6, 27, 28 but the role of ROS in S1P–mediated afferent arteriolar vasoconstriction is unknown. We hypothesized that S1P–mediated preglomerular microvascular vasoconstriction involves activation of L-VDCC and the rho/rho kinase pathway. In this study, we determined the signalling mechanisms underlying the afferent arteriolar response to exogenous S1P. Experiments were conducted to establish the impact of L-VDCC blockade, depletion of intracellular Ca2+ stores, rho/rho kinase inhibition, scavenging of ROS or inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase on the afferent arteriolar response to S1P.
RESULTS
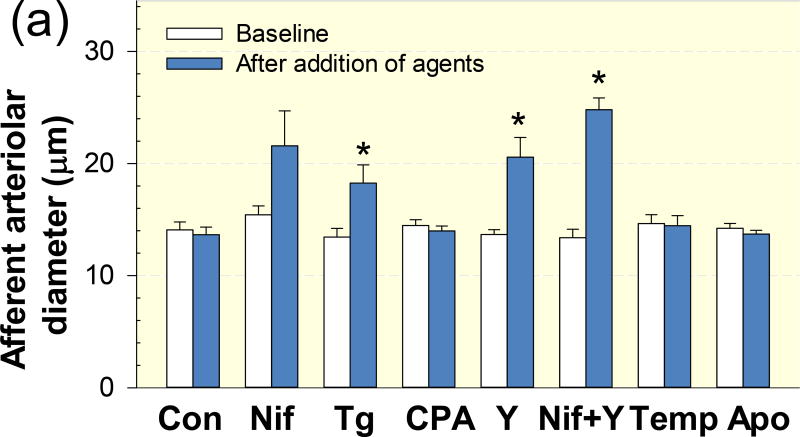
Figure 1 summarizes baseline diameters of afferent arterioles prior to and during exposure to pharmacological agent(s) before conducting S1P concentration-responses. Baseline diameters of afferent arterioles during the initial 5-min period were similar across all groups (Fig. 1a). Afferent arteriolar diameter was relatively stable during the 10-min incubation with 1% BSA in the time-control group (P > 0.05) but significantly increased during exposure to nifedipine (39 ± 18%), thapsigargin (36 ± 10%), Y-27632 (60 ± 12%) or a combination of nifedipine and Y-27632 (87 ± 8%) compared to each of their respective baseline diameters (Fig. 1b, P < 0.05). Baseline arteriolar diameters were unaltered by tempol or apocynin (P > 0.05). Administration of CPA caused a transit reduction of afferent arteriole diameter by 8 ± 3% 1–2 min after exposure to CPA that returned to near baseline in 2–3 min.
Figure 1. Effect of pharmacological agents on baseline diameters of afferent arterioles.
(a): afferent arteriolar diameters prior to exposure to pharmacological agents (baseline, white columns) and after exposure to pharmacological agent(s) but before administration of S1P (blue columns). (b): the same data normalized as a percentage of the baseline diameter for each group. Con, control group; Nif, nifedipine group; Tg, thapsigargin; CPA, cyclopiazonic acid; Y, Y-27632; Nif+Y, nifedipine+Y-27632; Temp, tempol; Apo, apocynin. Values are expressed as mean ± SEM. *P < 0.05 vs. baseline in the same group.
Effect of L-VDCC blockade on S1P–evoked vasoconstriction of afferent arterioles
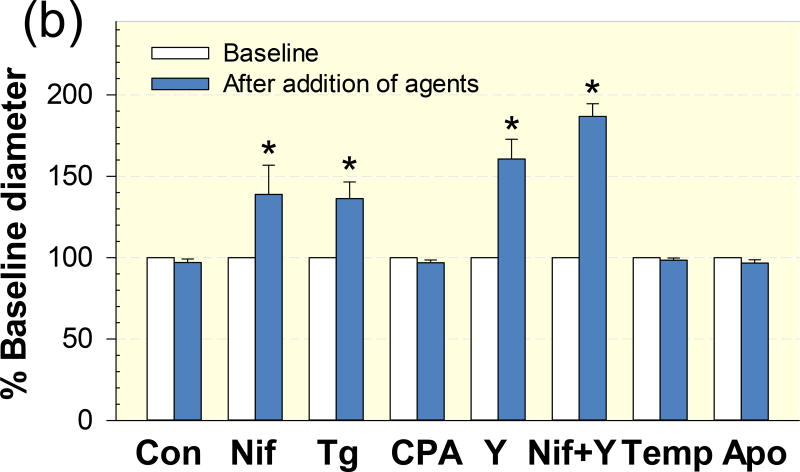
Figure 2 depicts the effect of the selective L-VDCC blocker, nifedipine, on afferent arteriolar responses to S1P compared to responses from the control group. In control kidneys, superfusion of exogenous S1P evoked concentration-dependent vasoconstriction of afferent arterioles (Fig. 2). The diameter of afferent arterioles declined to 94 ± 1, 85 ± 3, 75 ± 4, 60 ± 4 and 45 ± 4% of the control diameter (Fig. 2b) in response to increasing concentrations of S1P from 10−9 to 10−5 mol L−1, respectively. The magnitude of the S1P–concentration dependent vasoconstriction is similar to our previous report 11. In contrast, blockade of L-VDCC with nifedipine significantly blunted the afferent arteriolar vasoconstrictor responses to S1P. The diameter of afferent arterioles decreased to 99 ± 1%, 98 ± 1%, 90 ± 3%, 79 ± 3% and 52 ± 5% of the control diameter over a range of S1P from 10−9 to 10−5 mol L−1, respectively (Fig. 2b, P < 0.05 against S1P alone group).
Figure 2. Effect of nifedipine on S1P–induced afferent arteriolar vasoconstriction.
(a): afferent arteriolar responses to increasing concentrations of S1P in the absence (diamonds) and presence of the voltage-dependent L-type calcium channel blocker, nifedipine (10−6 mol L−1, squares). Data represent the actual diameters from each group. Con, control diameters averaged from the last 5-min incubation period in the absence or presence of nifedipine but prior to administration of S1P. (b): the same data normalized as a percentage of the control diameter for each group. Values are expressed as mean ± SEM. *P < 0.05 vs. control diameter in the same group. †P < 0.05 vs. vasoconstriction with S1P alone at the same concentration.
Effect of depletion of intracellular Ca2+ stores on S1P–evoked vasoconstriction of afferent arterioles
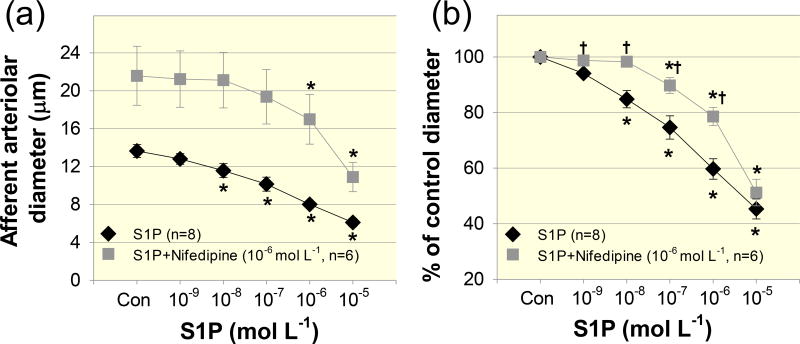
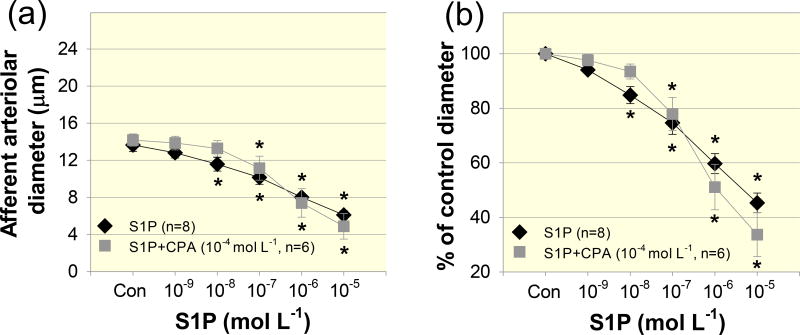
To determine the role of intracellular Ca2+ stores in S1P–evoked afferent arteriolar vasoconstriction, we applied the chemically distinct Ca2+-ATPase inhibitors, thapsigargin and CPA. Although thapsigargin caused vasorelaxation of afferent arterioles, the S1P–induced vasoconstrictor profile was similar to that without thapsigargin (Fig.3). Afferent arteriolar diameter declined to 99 ± 3, 95 ± 5, 85 ± 10, 60 ± 13 and 32 ± 8% of the control diameter in response to S1P from 10−9 to 10−5 mol L−1, respectively, (Fig. 3b, P > 0.05 against S1P alone group). To confirm the results with thapsigargin, we used a structurally different Ca2+-ATPase inhibitor, CPA. Similar to the effect of thapsigargin, administration of CPA did not significantly alter S1P–induced afferent arteriolar vasoconstriction (Fig. 4). Arteriolar diameter decreased to 97 ± 2, 93 ± 2, 75 ± 5, 53 ± 6 and 36 ± 5% of the control diameter in response to S1P (10−9 – 10−5 mol L−1) during continuous superfusion of CPA (Fig.4b, P > 0.05 against S1P alone group). These data suggest that mobilization of intracellular Ca2+ stores does not contribute significantly to vasoconstriction induced by exogenous S1P.
Figure 3. Effect of thapsigargin on S1P–induced afferent arteriolar vasoconstriction.
(a): afferent arteriolar responses to increasing concentrations of S1P in the absence (diamonds) and presence of the Ca2+-ATPase inhibitor, thapsigargin (10−6 mol L−1, squares). Data represent the actual diameters from each group. The S1P alone data are taken from Figure 2. Con, control diameters averaged from the last 5-min incubation period in the absence or presence of thapsigargin but prior to administration of S1P. (b): the same data normalized as a percentage of the control diameter for each group. Values are expressed as mean ± SEM. *P < 0.05 vs. control diameter in the same group.
Figure 4. Effect of cyclopiazonic acid on S1P–induced afferent arteriolar vasoconstriction.
(a): afferent arteriolar responses to increasing concentrations of S1P in the absence (diamonds) and presence of the selective Ca2+-ATPase inhibitor, cyclopiazonic acid (CPA, 10−4 mol L−1, squares). Data represent the actual diameters from each group. The S1P alone data are taken from Figure 2. Con, control diameters averaged from the last 5-min incubation period in the absence or presence of CPA but prior to administration of S1P. (b): the same data normalized as a percentage of the control diameter for each group. Values are expressed as mean ± SEM. *P < 0.05 vs. control diameter in the same group.
Effect of the rho/rho kinase pathway on S1P–evoked afferent arteriolar vasoconstriction
The rho/rho kinase pathway also influences afferent arteriolar responses to many vasoactive substances and pressure-dependent vasoconstriction.17, 29–31 To determine the role of the rho/rho kinase pathway in S1P–induced vasoconstriction, we applied the rho kinase inhibitor, Y-27632. Superfusion of Y-27632 increased the baseline afferent arteriolar diameter by 60 ± 12% and significantly attenuated the vasoconstrictor responses to S1P (Fig. 5). Arteriolar diameter averaged 103 ± 1, 101 ± 1, 93 ± 3, 72 ± 9 and 56 ± 10% of the control diameter in response to increasing concentrations of S1P from 10−9 to 10−5 mol L−1, respectively (Fig. 5b, P < 0.05 against S1P alone group).
Figure 5. Effect of the rho kinase inhibitor, Y-27632 on S1P–induced afferent arteriolar vasoconstriction.
(a): afferent arteriolar responses to increasing concentrations of S1P in the absence (diamonds) and presence of the rho kinase inhibitor, Y-27632 (10−5 mol L−1, squares). Data represent the actual diameters from each group. The S1P alone data are taken from Figure 2. Con, control diameters averaged from the last 5-min incubation period in the absence or presence of Y-27632 but prior to administration of S1P. (b): the same data normalized as a percentage of the control diameter for each group. Values are expressed as mean ± SEM. *P < 0.05 vs. control diameter in the same group. †P < 0.05 vs. vasoconstriction with S1P alone at the same concentration.
Effect of combined L-VDCC and the rho kinase pathway inhibition on S1P–evoked afferent arteriolar vasoconstriction
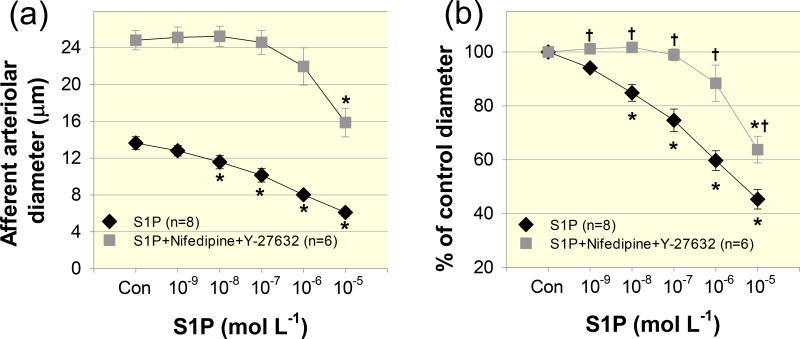
Since neither nifedipine nor Y-27632 completely blocked the S1P–induced vasoconstriction, especially at the high S1P concentration, to determine if inhibition of both pathways could abolish the afferent arteriolar vasoconstrictor responses to S1P, we applied both nifedipine (10−6 mol L−1) and Y-27632 (10−5 mol L−1). Superfusion of both nifedipine and Y-27632 markedly increased afferent arteriolar diameter by 87 ± 8% (P < 0.05) and significantly attenuated S1P–induced vasoconstriction over the entire concentration range of S1P (Figs. 6). The diameter of afferent arterioles averaged 101 ± 0, 102 ± 1, 99 ± 2, 88 ± 7 and 64 ± 5% of the control diameter in response to S1P from 10−9 to 10−5 mol L−1, respectively (Fig. 6b, P < 0.05 against S1P alone group).
Figure 6. Effect of a combination of nifedipine and Y-27632 on S1P–induced afferent arteriolar vasoconstriction.
(a): afferent arteriolar responses to increasing concentrations of S1P in the absence (diamonds) and presence of both nifedipine (10−6 mol L−1) and Y-27632 (10−5 mol L−1, squares) combined. Data represent the actual diameters from each group. The S1P alone data are taken from Figure 2. Con, control diameters averaged from the last 5-min incubation period in the absence or presence of the combination of nifedipine and Y-27632 but prior to administration of S1P. (b): the same data normalized as a percentage of the control diameter for each group. Values are expressed as mean ± SEM. *P < 0.05 vs. control diameter in the same group. †P < 0.05 vs. vasoconstriction with S1P alone at the same concentration.
Role of ROS on S1P–evoked afferent arteriolar vasoconstriction
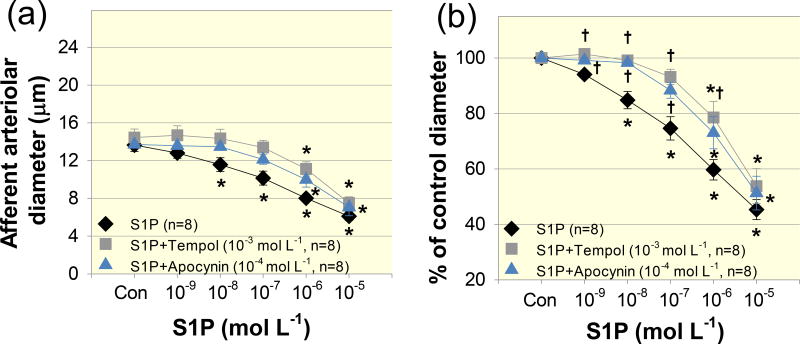
To determine the role of ROS in S1P–induced vasoconstriction of afferent arterioles, we applied the superoxide dismutase mimetic, tempol or the NADPH oxidase inhibitor, apocynin. Baseline arteriolar diameter averaged 14.6 ± 0.8 µm prior to administration of tempol and remained stable over a 15-min tempol incubation period (10−3 mol L−1, P > 0.05), suggesting that tempol did not significantly alter baseline diameter of afferent arterioles. Tempol however, significantly attenuated the vasoconstriction to S1P (Fig. 7). In the presence of tempol, afferent arteriolar diameter averaged 101 ± 1, 99 ± 1, 93 ± 3, 79 ± 6 and 54 ± 6% of the control diameter over S1P concentrations from 10−9 to 10−5 mol L−1, respectively (P < 0.05 against S1P alone group in Fig. 7b). The inhibitory effect of apocynin was similar to the effect of tempol. Arteriolar diameter averaged 99 ± 1, 98 ± 1, 90 ± 3, 74 ± 6 and 51 ± 6 % of the control diameter in response to S1P from 10−9 to 10−5 mol L−1, respectively (P = 0.05 against S1P alone group). These results suggest that ROS participate in S1P–mediated vasoconstriction of afferent arterioles.
Figure 7. Effects of tempol, a reactive oxygen species scavenger and apocynin, the NADPH oxidase inhibitor on S1P–induced afferent arteriolar vasoconstriction.
(a): afferent arteriolar responses to increasing concentrations of S1P in the absence (diamonds) and presence of tempol (10−3 mol L−1, squares) or apocynin (10−4 mol L−1, triangles). Data represent the actual diameters from each group. The S1P alone data are taken from Figure 2. Con, control diameters averaged from the last 5-min incubation period in the absence or presence of tempol or apocynin but prior to administration of S1P. (b): the same data normalized as a percentage of the control diameter for each group. Values are expressed as mean ± SEM. *P < 0.05 vs. control diameter in the same group. †P < 0.05 vs. vasoconstriction with S1P alone at the same concentration.
DISCUSSION
The current study provides evidence supporting a role of L-VDCC and the rho/rho kinase pathway in S1P–mediated afferent arteriolar vasoconstriction in rat kidneys. Consistent with our previous study,11 exogenous S1P evokes potent, concentration-dependent vasoconstriction of afferent arterioles. The vasoconstrictor effect of S1P was largely blocked by the L-VDCC blocker, nifedipine, indicating a prominent role for L-VDCC in S1P–mediated vasoconstriction of afferent arterioles. Similarly, inhibition of the rho kinase pathway with Y-27632 also significantly blunted S1P–induced vasoconstriction, supporting participation of the rho kinase pathway in S1P–mediated afferent arteriolar vasoconstriction. In contrast, depletion of intracellular Ca2+ stores using two chemically distinct Ca2+-ATPase inhibitors, thapsigargin or CPA, showed little effect on S1P–induced vasoconstriction of afferent arterioles, suggesting that mobilization of intracellular Ca2+ stores is not a major contributor for S1P–evoked vasoconstriction of the preglomerular microvasculature. Combined nifedipine and Y-27632 treatment significantly attenuated the S1P–induced vasoconstriction of afferent arterioles over the entire concentration range of S1P tested. Taken together the data provide evidence that both L-VDCC and the rho kinase pathway are involved S1P–induced vasoconstriction of afferent arterioles. We also demonstrate that ROS contributes to S1P–mediated afferent arteriolar vasoconstriction as the superoxide dismutase mimetic, tempol or the NADPH oxidase inhibitor, apocynin, significantly attenuated S1P–mediated vasoconstriction.
Accumulating evidence indicates that S1P is an important regulator of vascular tone and vascular reactivity, especially in resistance vessels.7, 32, 33 The plasma S1P concentration normally ranges from 10−7-10−6 mol L−1 but is generally low in tissues (0.5–75 pmol mg−1).3, 34, 35 In the current studies, we used a well-established technique, the in vitro blood perfused juxtamedullary nephron preparation.11, 36–38 Since kidneys are perfused with reconstituted blood in conditions mimicking human physiological conditions, afferent arterioles exhibit endogenous tone and both myogenic and tubuloglomerular feedback responsiveness (Supporting information, Figures A and B). Therefore, this technique is ideal to address intracellular signaling pathways involved in renal microvascular reactivity. Superfusion of exogenous S1P evoked concentration-dependent vasoconstriction of afferent arterioles in the rat kidneys similar to our previous report.11 S1P caused significant afferent arteriole vasoconstriction at concentrations as low as 10−8 mol L−1, which is in the range of the Kd for S1P receptors.39 Compared to the vasoconstrictor response in non-renal vascular beds such as rat cerebral and mesenteric arteries or rabbit coronary arteries,25, 26, 40, 41 afferent arterioles are more sensitive to S1P. The role of S1P in regulating renal microvascular reactivity is largely unknown. Intravenous infusion of S1P to anesthetized rats decreases renal blood flow9, 11 whereas direct infusion of the S1P1 receptor agonist, FTY-720 into the renal medulla increased medullary blood flow and sodium and water excretion.42 Superfusion of the specific S1P1 receptor agonist SEW2871 evoked modest afferent arteriolar vasoconstriction while pharmacological blockade of S1P2 receptor activation increased afferent arteriolar diameter.11 Global S1P2 receptor knockout mice exhibit a high renal blood flow without changes in systemic blood pressure compared to the wild-type controls.10 These studies suggest that local S1P plays an important role in controlling renal microvascular tone, sodium excretion and water balance, mainly through activation of S1P1 and S1P2 receptors. Since afferent arterioles are the primary renal resistance microvessels controlling renal blood flow and glomerular filtration rate, the potent S1P–dependent vasoconstriction in afferent arterioles implies a fundamental importance of S1P signalling in regulating the renal microcirculation.
Elevation of intracellular Ca2+ concentration is an important intracellular signalling mechanism utilized in vascular smooth muscle cell contraction. L-VDCC play a prominent role in maintaining renal microvascular tone, the preglomerular microvascular responses to many vasoconstrictors and pressure stimulation.16, 18, 43 In the current study, a wide S1P concentration range was selected to determine the downstream signalling pathways of S1P–mediated vasoconstriction. S1P–induced vasoconstriction was significantly attenuated by inhibition of L-VDCC with nifedipine, consistent with in vivo and in vitro observations.9, 11 Overall, these studies indicate a prominent role for L-VDCC in S1P–mediated vasoconstriction of afferent arterioles, and demonstrate that S1P can regulate renal microvascular tone and hence renal hemodynamics.
In addition to L-VDCC, mobilization of Ca2+ from intracellular stores is another major signalling pathway influencing renal microvascular responses to a variety of vasoactive agents.16, 29, 43 For example, blocking Ca2+ release from intracellular stores with thapsigargin (10−6 mol L−1) or CPA (5×10−5–10−4 mol L−1) attenuated angiotensin II-, norepinephrine- and vasopressin- induced vasoconstriction using the same juxtamedullary nephron preparation,44–46 suggesting that afferent arterioles do respond to agonist-induced mobilization of Ca2+ from intracellular stores. Surprisingly, administration with two structurally different Ca2+-ATPase inhibitors, thapsigargin and CPA at the concentrations that effectively deplete intracellular Ca2+ stores,47, 48 failed to alter the afferent arteriolar response to S1P. Although pre-treatment with thapsigargin led to relaxation of afferent arterioles, S1P–induced afferent arteriolar vasoconstriction was unaltered by thapsigargin. These data suggest that Ca2+ mobilization from intracellular stores is not a major mechanism by which S1P vasoconstricts rat afferent arterioles. This observation differs from what is reported in some non-renal vascular beds such as rat cerebral artery24 or rabbit coronary artery25 where Ca2+ mobilization is important for S1P–mediated vasoconstriction. Possibly the S1P receptor expression patterns are different in those vascular beds with each receptor subtype utilizing different intracellular signalling pathways for vasoconstriction. For example, S1P–induced intracellular Ca2+ mobilization was diminished in embryonic fibroblasts from S1P3 receptor knockout mice but was normal in S1P2 receptor knockout mice.49 This suggests that S1P3 but not S1P2 receptors play a predominant role in S1P–induced intracellular Ca2+ mobilization in mouse embryonic fibroblasts. We previously reported that S1P1 and S1P2 receptors are the major S1P receptors expressed in renal preglomerular microvessels and renal microvascular smooth muscle cells while S1P3 receptors were barely detected.11 This may explain the failure to block S1P–mediated vasoconstriction by inhibition of Ca2+-ATPase activity with either thapsigargin or CPA in the current study. In contrast, S1P3 receptors are the major S1P receptors expressed in rat cerebral arteries.24, 26 Therefore, intracellular Ca2+ mobilization may be a major intracellular signalling pathway mediating S1P–induced vasoconstriction in rat cerebral arteries but not in rat renal arterioles.
Increased Ca2+-sensitivity via the rho/rho kinase pathway could also contribute to afferent arteriolar responses to various agonist- or pressure-mediated vasoconstrictor stimuli,16, 17, 29, 43 and that mechanism has also been implicated in S1P–mediated vasoconstriction in non-renal vascular beds.5, 50, 51 In the current study, inhibition of rho kinase activity with Y-27632 markedly increased baseline diameter of afferent arterioles consistent with previous reports,29, 52 suggesting a role for the rho/rho kinase pathway in regulating resting renal vascular tone. We also found that Y-27632 significantly attenuated afferent arteriole reactivity to S1P. Given that the rho kinase system has been implicated in manipulating the Ca2+ sensitivity of contractile proteins, S1P mediated enhancement of Ca2+ sensitivity could also participate in the afferent arteriole response to S1P. Changes in the vascular rho/rho kinase pathway have been linked to vascular injury or dysfunction associated with cardiovascular disease.13, 53 Therefore, it is important to clarify the underlying mechanisms that contribute to S1P–mediated renal microvascular vasoconstriction.
Since neither nifedipine nor Y-27632 completely abolished the afferent arteriolar vasoconstrictor responses to S1P, we combined both inhibitors to determine if the S1P–induced vasoconstriction could be completely blocked. We found that dual blockade of the L-VDCC and rho/rho kinase pathways significantly attenuated the S1P–induced vasoconstriction over the entire concentration range of S1P tested compared to untreated controls. These observations suggest that activation of L-VDCC or rho kinase activity by exogenous S1P in renal microvasculature may share intracellular signalling pathways rather than act through completely different pathways. It is known that L-VDCC activation-induced vasoconstriction is through the calmodulin-dependent myosin light chain (MLC) kinase activation triggered by Ca2+ influx while the rho/rho kinase-associated vasoconstriction is a result of inhibition of MLC phosphatase via enhanced Ca2+ sensitivity mechanisms.17 A few studies however, suggested that activation of L-VDCC and the rhoA/rho kinase pathway could share some common mechanisms in mediating vasoconstriction.54–58 For example, application of Y-27632 (3×10−6 – 10−5 mol L−1) attenuated vasoconstriction induced by KCl or the L-VDCC agonist, FPL64176, but did not alter the intracellular Ca2+ concentration.54–58 KCl or FPL64176 stimulated rhoA activity but that was inhibited by nifedipine or conditional knockout of Cav1.2 (L-Type) Ca2+ channels,57, 58 suggesting that functional L-VDCC are required for activation of the rhoA/rho kinase pathway in mediating arterial contraction. On the other hand, a study using transgenic mice with cardiac-specific inhibition of rho family proteins showed that L-VDCC currents in ventricular myocytes were regulated by rhoA.59 Therefore, Y-27632 may have direct or indirect inhibitory effects on L-VDCC. However, the combination of Y-27632 and nifedipine caused greater vasodilation than Y-27632 alone (87 ± 8% vs. 60 ± 12%), suggesting that L-type VDCCs are still functional in the presence of Y-27632. Accordingly, there may be some overlap between L-VDCC and the rho kinase pathway in the mechanisms by which S1P vasoconstricts rat afferent arterioles.
We noticed that afferent arterioles still retained a small, but significant vasoconstrictor response to a high pathophysiological concentration of S1P (10−5 mol L−1) during combined nifedipine and Y-27632 treatment. The underlying signalling pathways responsible for the residual vasoconstriction to S1P remain unclear. Several studies indicate that S1P could directly trigger polymerization of actin in different cell types,60–62 but evidence for autopolymerization in vascular smooth muscle cells is currently lacking. Apparently, more studies are required to clarify the role of S1P in regulating renal microvascular function under physiological or pathophysiological conditions.
ROS play an important role in many different physiological and pathological processes.63, 64 Afferent arterioles express NADPH oxidase,65 a major source of ROS within the vasculature.66–68 Consistent with early reports,69, 70 superfusion with tempol or apocynin had little effect on baseline arteriole diameter, suggesting that afferent arterioles are normally not under the influence of substantial oxidative stress. While tempol or apocynin have little effect on baseline arteriolar diameter, both drugs blunted S1P–induced vasoconstriction, suggesting that exogenous S1P–induced vasoconstriction of afferent arterioles involves stimulation of ROS production. This linkage of S1P with ROS accumulation is relevant because excessive increases in ROS production are associated with increased risk of cardiovascular disease. For example, S1P–mediated vasoconstriction is enhanced in isolated perfused kidneys from diabetic rats14 whereas selective deletion of S1P1 receptors in endothelial cells exacerbated ischemia-reperfusion induced kidney injury.71 These observations raise the possibility that aberrant S1P signalling in the preglomerular vasculature could contribute to renal vascular dysfunction under pathological conditions.
In conclusion, the current study reveals that S1P–mediated vasoconstriction involves L-VDCC and the rho/rho kinase pathway in rat afferent arterioles. Mobilization of Ca2+ from intracellular stores is not required for exogenous S1P–mediated vasoconstriction of rat afferent arterioles. This study also demonstrates that ROS contributes to afferent arteriolar vasoconstriction by S1P. Since the afferent arteriole is the dominant resistance element determining glomerular capillary pressure, impaired S1P signalling could negatively impact regulation of glomerular hemodynamics and renal blood flow, and contribute to renal injury under pathological conditions such as ischemia-reperfusion induced acute kidney injury. Better understanding of the functional mechanisms of S1P in renal microvascular regulation could identify important factors contributing to the pathogenesis of renal microvascular dysfunction.
METHODS
Animals
A total of 108 male Sprague-Dawley rats (350 – 400 g, Charles River Laboratories, Raleigh, NC) were used. All rats had free access to standard chow and water and were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Care and Use Committees at the University of Alabama at Birmingham.
The in vitro Blood-perfused Juxtamedullary Nephron Preparation
Studies assessing renal microvascular reactivity were performed in vitro using the blood-perfused juxtamedullary nephron preparation.11, 36–38 Briefly, two rats (kidney donor and blood donor) were used for each experiment. Rats were anesthetized using pentobarbital sodium (50 mg kg−1, ip, Vortech Pharmaceuticals, LTD, Dearborn, MI). The right kidney from the kidney donor was cannulated and perfused with Tyrode’s buffer containing 5.2% bovine serum albumin (BSA, Calbiochem, La Jolla, CA). After completion of the kidney dissection, the 5.2% BSA solution was replaced with the reconstituted blood collected from both the kidney and blood donors (hematocrit of ~33%) as described previously.11, 37 The reconstituted blood was continuously gassed with 95%O2/5%CO2 while renal perfusion pressure was held at 100 mmHg. The inner cortical surface of the kidney was superfused with Tyrode’s buffer containing 1% BSA (37°C). The tested compounds were delivered to the kidney surface via superfusion of 1% BSA in Tyrode’s buffer. The kidney was visualized using a light microscope (40× water-immersion objective, Nikon Eclipse E600FN; Nikon, Tokyo, Japan) and images were recorded on DVD. The inner diameter of arterioles was measured every 12 seconds at a single site using a calibrated image-shearing monitor (Model 908, Vista Electronics, Valencia, CA). The average of all diameter measurements from the final 2-min of each treatment period (5-min intervals) was calculated for the analysis.
Experimental Protocols
The experiments were conducted as follows. An initial equilibration period (> 15 min) was allowed for establishing a steady-state arteriolar diameter. Each protocol began with a 5-min period (baseline diameter) while renal perfusion pressure was held at 100 mmHg for all experiments.
Experiment 1. Effect of exogenous S1P on afferent arteriolar reactivity
The kidney was continuously bathed with 1% BSA superfusate during the initial 5 min period (baseline) and then for 10-min to match the incubation period with pharmacological agents as indicated in each protocol. The averaged diameters from the last 5-min incubation period with 1% BSA or pharmacological agents prior to administration of S1P were referred as the control diameter and were used for normalizing the afferent arteriolar response to S1P as to a percent of the control diameter. Increasing S1P concentrations from 10−9 to 10−5 mol L−1 were superfused directly onto the inner cortical surface for 5-min at each concentration (n = 8). This S1P concentration response, without any intervention, served as the control group.
Experiment 2. Role of L-VDCC in S1P–evoked afferent arteriolar reactivity
A contribution of L-VDCC in S1P–mediated afferent arteriolar vasoconstriction was previously assessed using the selective L-VDCC blockers, nifedipine or diltiazem on a single concentration of S1P (10−7 mol L−1).11 In the current study, we determined the S1P concentration-response relationship in the presence of nifedipine to determine the role of L-VDCC on afferent arteriolar reactivity over a wide S1P concentration range from 10−9 to 10−5 mol L−1. Briefly, following a 5-min baseline period, the superfusate solution (1% BSA in Tyrode’s buffer) was switched to a similar solution containing nifedipine (10−6 mol L−1, n = 6) for 10-min before the S1P concentration-response was determined in the presence of nifedipine. This concentration of nifedipine is the minimum concentration required to block the KCl-induced depolarization.11, 72
Experiment 3. Role of intracellular Ca2+ mobilization in S1P–evoked afferent arteriolar vasoconstriction
Intracellular Ca2+ stores were depleted using the selective Ca2+-ATPase inhibitors thapsigargin or cyclopiazonic acid (CPA). A protocol similar to the nifedipine experiments was performed. Briefly, after a 5-min baseline period, the kidney was superfused with thapsigargin (10−6 mol L−1) for 10-min and then the S1P concentration-response (10−9 – 10−5 mol L−1) was determined in the presence of thapsigargin (n = 6). In a separate set of experiments (n = 6), thapsigargin was replaced by CPA (10−4 mol L−1) and the S1P concentration-response was determined in the presence of CPA to confirm the role of intracellular Ca2+ mobilization in S1P–induced vasoconstriction of afferent arterioles.
Experiment 4. Role of the rho/rho kinase pathway in S1P–evoked afferent arteriolar vasoconstriction
Increased Ca2+-sensitivity mechanism via activation of the rho/rho kinase pathway contributes to afferent arteriolar responses to many vasoconstrictors.17, 29–31 To determine involvement of the rho/rho kinase pathway in S1P–mediated arteriolar vasoconstriction, the rho kinase inhibitor, Y-27632 was applied. The protocol was similar to the nifedipine experiments except nifedipine was replaced by Y-27632. Briefly, after a 5-min baseline period, the kidney was superfused with Y-27632 (10−5 mol L−1) for 10-min and then the S1P concentration-response (10−9 – 10−5 mol L−1) was determined in the presence of Y-27632 (n = 6).
Experiment 5. Effect of combined inhibition of L-VDCC and the rho/rho kinase pathway in S1P–evoked afferent arteriolar vasoconstriction
Since neither nifedipine nor Y-27632 alone completely blocked the vasoconstrictor effect of S1P, we examined the concentration response of S1P in the presence of both L-VDCC blocker, nifedipine and the rho kinase inhibitor, Y-27632. The protocol was similar to the nifedipine experiments except that nifedipine and Y-27632 were used together. Briefly, after a 5-min baseline period, the kidney was exposed to the superfusate containing nifedipine (10−6 mol L−1) and Y-27632 (10−5 mol L−1) for 10-min and the S1P concentration-response (10−9 – 10−5 mol L−1) was determined in the continued presence of nifedipine and Y-27632 (n = 6).
Experiment 6. Contribution of ROS in S1P–evoked afferent arteriolar vasoconstriction
S1P is linked to an increase in ROS generation in non-renal arteries6, 27, 28 but the role of ROS in S1P–mediated afferent arteriolar vasoconstriction is unknown. We assessed afferent arteriolar responses to increasing concentrations of S1P during administration of the membrane-permeable superoxide dismutase mimetic, tempol or the NADPH oxidase inhibitor, apocynin. Briefly, after a 5-min baseline period, the kidney was superfused with tempol (10−3 mol L−1, n=8) or apocynin (10−4 mol L−1, n=8) for 15-min and then the S1P concentration-response (10−9 – 10−5 mol L−1) was determined in the presence of tempol. Since afferent arteriolar diameters remained fairly stable over a 30-min superfusion with 1% BSA 11, the S1P alone group was still used as the control group.
Drug Preparation
S1P (ENZO Life Sciences, Inc., Farmingdale, NY) was dissolved in methanol as described previously 11. Stock solutions of nifedipine, thapsigargin or apocynin were freshly prepared in ethanol. CPA was dissolved in dimethyl sulfoxide to get a stock solution of 10−1 mol L−1. Y-27623 was freshly prepared in H2O to get a stock solution of 5×10−3 mol L−1. The concentrations of methanol, ethanol or dimethyl sulfoxide used for dissolving drugs were previously demonstrated to have no effects in this preparation.11, 73 All stock solutions were further diluted with 1% BSA superfusate to get the desired concentrations before experiments began.
Statistical Analysis
All data are expressed as means ± SEM. One-way analysis of variance for repeated measures was used for within-group analyses followed by post-hoc analyses with Dunnett’s multiple range test. To compare the overall effect of each drug, we added the percentage diameter values across all doses for each rat, which is an approximation of the area under the curve on logarithmic scale of concentration (dose). Dunnett’s test was used to test the 7 treatment groups against the control group, S1P alone group, using R package “multcomp”. For treatment groups that are significantly different from the control group, we conducted a one-tailed t test against the control group at each dose for reduced effect of S1P. Bonferroni correction was used to adjust for the multiple testing. Significance level was set at 0.05.
Supplementary Material
Acknowledgments
This study was supported by a National Scientist Development Grant (10SDG3770010) and a Grant-in-Aid (15GRNT25240015) from the American Heart Association to ZG, and by grants from NIH (DK044628, HL074167, HL098135 and HL095499) to EWI. We acknowledge support from the UAB-UCSD O’Brien Core Center for Acute Kidney Injury Research (NIH P30-DK079337) for this project.
Footnotes
DISCLOSURES
None.
References
- 1.Maceyka M, Milstien S, Spiegel S. Sphingosine-1-phosphate: the Swiss army knife of sphingolipid signaling. J Lipid Res. 2009;50(Suppl):S272–276. doi: 10.1194/jlr.R800065-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proia RL, Hla T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest. 2015;125:1379–1387. doi: 10.1172/JCI76369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochim Biophys Acta. 2008;1780:606–611. doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolz SS, Vogel L, Sollinger D, Derwand R, Boer C, Pitson SM, Spiegel S, Pohl U. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 2003;108:342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
- 6.Keller M, Lidington D, Vogel L, Peter BF, Sohn HY, Pagano PJ, Pitson S, Spiegel S, Pohl U, Bolz SS. Sphingosine kinase functionally links elevated transmural pressure and increased reactive oxygen species formation in resistance arteries. Faseb J. 2006;20:702–704. doi: 10.1096/fj.05-4075fje. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi J, Michel T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc Res. 2009;82:212–220. doi: 10.1093/cvr/cvp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochim Biophys Acta. 2008;1781:477–482. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bischoff A, Finger J, Michel MC. Nifedipine inhibits sphinogosine-1-phosphate-induced renovascular contraction in vitro and in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:179–182. doi: 10.1007/s002100100446. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz JN, Arend LJ, Robitz R, Paul RJ, MacLennan AJ. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R440–446. doi: 10.1152/ajpregu.00085.2006. [DOI] [PubMed] [Google Scholar]
- 11.Guan Z, Singletary ST, Cook AK, Hobbs JL, Pollock JS, Inscho EW. Sphingosine-1-phosphate evokes unique segment-specific vasoconstriction of the renal microvasculature. J Am Soc Nephrol. 2014;25:1774–1785. doi: 10.1681/ASN.2013060656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692–696. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yogi A, Callera GE, Aranha AB, Antunes TT, Graham D, McBride M, Dominiczak A, Touyz RM. Sphingosine-1-phosphate-induced inflammation involves receptor tyrosine kinase transactivation in vascular cells: upregulation in hypertension. Hypertension. 2011;57:809–818. doi: 10.1161/HYPERTENSIONAHA.110.162719. [DOI] [PubMed] [Google Scholar]
- 14.Bautista-Perez R, Arellano A, Franco M, Osorio H, Coronel I. Sphingosine-1-phosphate induced vasoconstriction is increased in the isolated perfused kidneys of diabetic rats. Diabetes Res Clin Pract. 2011;94:e8–11. doi: 10.1016/j.diabres.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Winkler MS, Nierhaus A, Holzmann M, Mudersbach E, Bauer A, Robbe L, Zahrte C, Geffken M, Peine S, Schwedhelm E, Daum G, Kluge S, Zoellner C. Decreased serum concentrations of sphingosine-1-phosphate in sepsis. Crit Care. 2015;19:372. doi: 10.1186/s13054-015-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navar LG, Arendshorst WJ, Pallone TL, Inscho EW, Imig JD, Bell PD. The Renal Microcirculation. In: Tuma RFD, Wa LK, editors. Compr Physiol. San Diego: Elsevier; 2011. pp. 550–684. [Google Scholar]
- 17.Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95:405–511. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inscho EW. Mysteries of Renal Autoregulation. Hypertension. 2009;53:299–306. doi: 10.1161/HYPERTENSIONAHA.108.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imig JD. Afferent arteriolar reactivity to angiotensin II is enhanced during the early phase of angiotensin II hypertension. Am J Hypertens. 2000;13:810–818. doi: 10.1016/s0895-7061(00)00264-8. [DOI] [PubMed] [Google Scholar]
- 20.Carmines PK. The renal vascular response to diabetes. Curr Opin Nephrol Hypertens. 2010;19:85–90. doi: 10.1097/MNH.0b013e32833240fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan Z, Fuller BS, Yamamoto T, Cook AK, Pollock JS, Inscho EW. Pentosan polysulfate treatment preserves renal autoregulation in Ang II-infused hypertensive rats via normalization of P2X1 receptor activation. Am J Physiol Renal Physiol. 2010;298:F1276–1284. doi: 10.1152/ajprenal.00743.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inscho EW, Cook AK, Andrea N, Clarke Zhang S, Guan Z. P2X1 receptor-mediated vasoconstriction of afferent arterioles in Ang II-infused hypertensive rats fed a high salt diet. Hypertension. 2011;57:780–787. doi: 10.1161/HYPERTENSIONAHA.110.168955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan Z, Giddens MI, Osmond DA, Cook AK, Hobbs JL, Zhang S, Yamamoto T, Pollock JS, Pollock DM, Inscho EW. Immunosuppression preserves renal autoregulatory function and microvascular P2X1 receptor reactivity in ANG II-hypertensive rats. Am J Physiol Renal Physiol. 2013;304:F801–807. doi: 10.1152/ajprenal.00286.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coussin F, Scott RH, Wise A, Nixon GF. Comparison of sphingosine 1-phosphate-induced intracellular signaling pathways in vascular smooth muscles: Differential role in vasoconstriction. Circ Res. 2002;91:151–157. doi: 10.1161/01.res.0000028150.51130.36. [DOI] [PubMed] [Google Scholar]
- 25.Choi SK, Ahn DS, Lee YH. Comparison of contractile mechanisms of sphingosylphosphorylcholine and sphingosine-1-phosphate in rabbit coronary artery. Cardiovasc Res. 2009;82:324–332. doi: 10.1093/cvr/cvp054. [DOI] [PubMed] [Google Scholar]
- 26.Salomone S, Yoshimura S, Reuter U, Foley M, Thomas SS, Moskowitz MA, Waeber C. S1P3 receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. Eur J Pharmacol. 2003;469:125–134. doi: 10.1016/s0014-2999(03)01731-x. [DOI] [PubMed] [Google Scholar]
- 27.Lidington D, Schubert R, Bolz SS. Capitalizing on diversity: an integrative approach towards the multiplicity of cellular mechanisms underlying myogenic responsiveness. Cardiovasc Res. 2013;97:404–412. doi: 10.1093/cvr/cvs345. [DOI] [PubMed] [Google Scholar]
- 28.Hui S, Levy AS, Slack DL, Burnstein MJ, Errett L, Bonneau D, Latter D, Rotstein OD, Bolz SS, Lidington D, Voigtlaender-Bolz J. Sphingosine-1-phosphate signaling regulates myogenic responsiveness in human resistance arteries. PLoS One. 2015;10:e0138142. doi: 10.1371/journal.pone.0138142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inscho EW, Cook AK, Webb RC, Jin LM. Rho-kinase inhibition reduces pressure-mediated autoregulatory adjustments in afferent arteriolar diameter. Am J Physiol Renal Physiol. 2009;296:F590–597. doi: 10.1152/ajprenal.90703.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyata K, Satou R, Shao W, Prieto MC, Urushihara M, Kobori H, Navar LG. ROCK/NF-kappaB axis-dependent augmentation of angiotensinogen by angiotensin II in primary-cultured preglomerular vascular smooth muscle cells. Am J Physiol Renal Physiol. 2014;306:F608–618. doi: 10.1152/ajprenal.00464.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeya K, Wang X, Kathol I, Loutzenhiser K, Loutzenhiser R, Walsh MP. Endothelin-1, but not angiotensin II, induces afferent arteriolar myosin diphosphorylation as a potential contributor to prolonged vasoconstriction. Kidney Int. 2015;87:370–381. doi: 10.1038/ki.2014.284. [DOI] [PubMed] [Google Scholar]
- 32.Watterson KR, Ratz PH, Spiegel S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell Signal. 2005;17:289–298. doi: 10.1016/j.cellsig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Skoura A, Hla T. Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc Res. 2009;82:221–228. doi: 10.1093/cvr/cvp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 35.Takabe K, Paugh SW, Milstien S, Spiegel S. "Inside-out" signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casellas D, Carmines PK, Navar LG. Microvascular reactivity of in vitro blood perfused juxtamedullary nephrons from rats. Kidney Int. 1985;28:752–759. doi: 10.1038/ki.1985.194. [DOI] [PubMed] [Google Scholar]
- 37.Inscho EW, Carmines PK, Navar LG. Juxtamedullary afferent arteriolar responses to P1 and P2 purinergic stimulation. Hypertension. 1991;17:1033–1037. doi: 10.1161/01.hyp.17.6.1033. [DOI] [PubMed] [Google Scholar]
- 38.Guan Z, Pollock JS, Cook AK, Hobbs JL, Inscho EW. Effect of epithelial sodium channel blockade on the myogenic response of rat juxtamedullary afferent arterioles. Hypertension. 2009;54:1062–1069. doi: 10.1161/HYPERTENSIONAHA.109.137992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 40.Bischoff A, Czyborra P, Fetscher C, Meyer Zu Heringdorf D, Jakobs KH, Michel MC. Sphingosine-1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br J Pharmacol. 2000;130:1871–1877. doi: 10.1038/sj.bjp.0703515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, Waeber C. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol. 2008;153:140–147. doi: 10.1038/sj.bjp.0707581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Q, Xia M, Wang Z, Li PL, Li N. A novel lipid natriuretic factor in the renal medulla: sphingosine-1-phosphate. Am J Physiol Renal Physiol. 2011;301:F35–41. doi: 10.1152/ajprenal.00014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salomonsson M, Sorensen CM, Arendshorst WJ, Steendahl J, Holstein-Rathlou NH. Calcium handling in afferent arterioles. Acta Physiol Scand. 2004;181:421–429. doi: 10.1111/j.1365-201X.2004.01314.x. [DOI] [PubMed] [Google Scholar]
- 44.Inscho EW, Imig JD, Cook AK. Afferent and efferent arteriolar vasoconstriction to angiotensin II and norepinephrine involves release of Ca2+ from intracellular stores. Hypertension. 1997;29:222–227. doi: 10.1161/01.hyp.29.1.222. [DOI] [PubMed] [Google Scholar]
- 45.Imig JD, Cook AK, Inscho EW. Postglomerular vasoconstriction to angiotensin II and norepinephrine depends on intracellular calcium release. Gen Pharmacol. 2000;34:409–415. doi: 10.1016/s0306-3623(01)00078-7. [DOI] [PubMed] [Google Scholar]
- 46.Fallet RW, Ikenaga H, Bast JP, Carmines PK. Relative contributions of Ca2+ mobilization and influx in renal arteriolar contractile responses to arginine vasopressin. Am J Physiol Renal Physiol. 2005;288:F545–551. doi: 10.1152/ajprenal.00150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mason MJ, Garcia-Rodriguez C, Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991;266:20856–20862. [PubMed] [Google Scholar]
- 48.Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- 49.Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, Yang AH, Zhang G, Brown JH, Chun J. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J Biol Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 50.Zhou H, Murthy KS. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am J Physiol Cell Physiol. 2004;286:C1130–1138. doi: 10.1152/ajpcell.00429.2003. [DOI] [PubMed] [Google Scholar]
- 51.Lim M, Choi SK, Cho YE, Yeon SI, Kim EC, Ahn DS, Lee YH. The role of sphingosine kinase 1/sphingosine-1-phosphate pathway in the myogenic tone of posterior cerebral arteries. PLoS One. 2012;7:e35177. doi: 10.1371/journal.pone.0035177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura A, Hayashi K, Ozawa Y, Fujiwara K, Okubo K, Kanda T, Wakino S, Saruta T. Vessel- and vasoconstrictor-dependent role of rho/rho-kinase in renal microvascular tone. J Vasc Res. 2003;40:244–251. doi: 10.1159/000071888. [DOI] [PubMed] [Google Scholar]
- 53.Hartmann S, Ridley AJ, Lutz S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front Pharmacol. 2015;6:276. doi: 10.3389/fphar.2015.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003;93:548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- 55.Ghisdal P, Vandenberg G, Morel N. Rho-dependent kinase is involved in agonist-activated calcium entry in rat arteries. J Physiol. 2003;551:855–867. doi: 10.1113/jphysiol.2003.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villalba N, Stankevicius E, Simonsen U, Prieto D. Rho kinase is involved in Ca2+ entry of rat penile small arteries. Am J Physiol Heart Circ Physiol. 2008;294:H1923–1932. doi: 10.1152/ajpheart.01221.2007. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez-Tenorio M, Porras-Gonzalez C, Castellano A, Del Valle-Rodriguez A, Lopez-Barneo J, Urena J. Metabotropic regulation of RhoA/Rho-associated kinase by L-type Ca2+ channels: new mechanism for depolarization-evoked mammalian arterial contraction. Circ Res. 2011;108:1348–1357. doi: 10.1161/CIRCRESAHA.111.240127. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Tenorio M, Porras-Gonzalez C, Castellano A, Lopez-Barneo J, Urena J. Tonic arterial contraction mediated by L-type Ca2+ channels requires sustained Ca2+ influx, G protein-associated Ca2+ release, and RhoA/ROCK activation. Eur J Pharmacol. 2012;697:88–96. doi: 10.1016/j.ejphar.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 59.Yatani A, Irie K, Otani T, Abdellatif M, Wei L. RhoA GTPase regulates L-type Ca2+ currents in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2005;288:H650–659. doi: 10.1152/ajpheart.00268.2004. [DOI] [PubMed] [Google Scholar]
- 60.Idzko M, Panther E, Corinti S, Morelli A, Ferrari D, Herouy Y, Dichmann S, Mockenhaupt M, Gebicke-Haerter P, Di Virgilio F, Girolomoni G, Norgauer J. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. Faseb J. 2002;16:625–627. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- 61.Chowdhury I, Chaqour B. Regulation of connective tissue growth factor (CTGF/CCN2) gene transcription mRNA stability in smooth muscle cells Involvement of RhoA GTPase and p38 MAP kinase and sensitivity to actin dynamics. Eur J Biochem. 2004;271:4436–4450. doi: 10.1111/j.1432-1033.2004.04382.x. [DOI] [PubMed] [Google Scholar]
- 62.Donati C, Bruni P. Sphingosine 1-phosphate regulates cytoskeleton dynamics: implications in its biological response. Biochim Biophys Acta. 2006;1758:2037–2048. doi: 10.1016/j.bbamem.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 63.Montezano AC, Touyz RM. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid Redox Signal. 2014;20:164–182. doi: 10.1089/ars.2013.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Araujo M, Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal. 2014;20:74–101. doi: 10.1089/ars.2013.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilcox CS. Redox regulation of the afferent arteriole and tubuloglomerular feedback. Acta Physiol Scand. 2003;179:217–223. doi: 10.1046/j.0001-6772.2003.01205.x. [DOI] [PubMed] [Google Scholar]
- 66.Sharma K, Cook A, Smith M, Valancius C, Inscho EW. TGF-beta impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol. 2005;288:F1069–1077. doi: 10.1152/ajprenal.00345.2004. [DOI] [PubMed] [Google Scholar]
- 67.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tain YL, Muller V, Szabo A, Dikalova A, Griendling K, Baylis C. Lack of long-term protective effect of antioxidant/anti-inflammatory therapy in transplant-induced ischemia/reperfusion injury. Am J Nephrol. 2006;26:213–217. doi: 10.1159/000093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ichihara A, Hayashi M, Hirota N, Saruta T. Superoxide inhibits neuronal nitric oxide synthase influences on afferent arterioles in spontaneously hypertensive rats. Hypertension. 2001;37:630–634. doi: 10.1161/01.hyp.37.2.630. [DOI] [PubMed] [Google Scholar]
- 70.Fellner RC, Cook AK, O’Connor PM, Zhang S, Pollock DM, Inscho EW. High-salt diet blunts renal autoregulation by a reactive oxygen species-dependent mechanism. Am J Physiol Renal Physiol. 2014;307:F33–40. doi: 10.1152/ajprenal.00040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ham A, Kim M, Kim JY, Brown KM, Fruttiger M, D’Agati VD, Lee HT. Selective deletion of the endothelial sphingosine-1-phosphate 1 receptor exacerbates kidney ischemia-reperfusion injury. Kidney Int. 2014;85:807–823. doi: 10.1038/ki.2013.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inscho EW, Ohishi K, Cook AK, Belott TP, Navar LG. Calcium activation mechanisms in the renal microvascular response to extracellular ATP. Am J Physiol Renal Physiol. 1995;268:F876–884. doi: 10.1152/ajprenal.1995.268.5.F876. [DOI] [PubMed] [Google Scholar]
- 73.Inscho EW, Cook AK, Mui V, Imig JD. Calcium mobilization contributes to pressure-mediated afferent arteriolar vasoconstriction. Hypertension. 1998;31:421–428. doi: 10.1161/01.hyp.31.1.421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.