Abstract
Cyclin-dependent kinase 5 (CDK5) is an unusual CDK whose function has been implicated in protecting the central nervous system (CNS) from oxidative damage. However, there have been few studies of CDK5 in insects. In this study, we identified the AccCDK5 gene from Apis cerana cerana and investigated its role in oxidation resistance. We found that AccCDK5 is highly conserved across species and contains conserved features of the CDK5 family. The results of qPCR analysis indicated that AccCDK5 is highly expressed during the larval and pupal stages and in the adult head and muscle. We further observed that AccCDK5 is induced by several environmental oxidative stresses. Moreover, the overexpression of the AccCDK5 protein in E. coli enhances the resistance of the bacteria to oxidative stress. The activation of CDK5 requires binding to its activator. Therefore, we also identified and cloned cyclin-dependent kinase 5 regulatory subunit 1, which we named AccCDK5r1, from Apis cerana cerana. AccCDK5r1 contains a conserved cell localization targeting domain as well as binding and activation sites for CDK5. Yeast two-hybrid analysis demonstrated the interaction between AccCDK5 and AccCDK5r1. The expression patterns of the two genes were similar after stress treatment. Collectively, these results suggest that AccCDK5 plays a pivotal role in the response to oxidative stresses and that AccCDK5r1 is a potential activator of AccCDK5.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-017-0820-y) contains supplementary material, which is available to authorized users.
Keywords: Cyclin-dependent kinase 5, Cyclin-dependent kinase 5 regulator subunit 1, Oxidative stress, Yeast two-hybrid analysis, Apis cerana cerana
Introduction
The Chinese honeybee (Apis cerana cerana) is the most widely distributed of all native honeybee species in China. Apis cerana cerana has many advantages over Apis mellifera, including the use of sporadic nectariferous plants, a long honey period, and better resistance to cold, mites, and disease (Chen et al. 2015; Radloff et al. 2010; Zhao et al. 2015). Chinese honeybees also play a crucial role in ecological balance and the agricultural industry. However, in recent years, due to the introduction of western honeybees, diseases, pesticide abuse, and environmental pollution, the number of Chinese honeybee colonies has plummeted, and the survival of the species has been seriously threatened (Yang 2005). Numerous stresses could lead to oxidative stress in the habitat of honeybees, including chemical stresses (pesticides, metals, etc.), physical stresses (radiation, temperature, etc.), and physiological stresses (An and Choi 2010; Espín et al. 2014; Kodrík et al. 2015; Meng et al. 2009). Oxidative stress occurs when the equilibrium between reactive oxygen species (ROS) production and antioxidant defense mechanisms is dysregulated (Halliwell 1991). This results in oxidative damage, as manifested by modifications of cellular lipids, proteins, and DNA (Green and Reed 1998; Imlay and Linn 1988; Lushchak 2011).
Cyclin-dependent kinases (CDKs), a family of serine/threonine kinases, form complexes with cyclins, components that are essential for the kinase activity of CDKs (Malumbres et al. 2009). The functions of CDKs in signal integration to regulate gene transcription and cell division have been clearly established (Morgan 1997). Cyclin-dependent kinase 5 (CDK5), an unusual member of the CDK family, was discovered in the 1990s and is known as neuronal CDC-2-like kinase (Hellmich et al. 1992; Lew et al. 1992; Meyerson et al. 1992). CDK5 is a versatile CDK member that regulates many cellular processes, including neuronal migration, actin dynamics, microtubule stability and transport, cell adhesion, axon guidance, synaptic structure and plasticity, and neuronal survival (Dhavan and Tsai 2001). Dysregulation of CDK5 activity is neurotoxic and may lead to disease, including Alzheimer’s disease (AD) and Parkinson’s disease (PD) (Giese 2014; Sun et al. 2008; Zhang et al. 2016).
CDK5 regulatory subunit 1 (CDK5r1), encoding the CDK5 activator p35, plays a crucial role in the kinase activity of CDK5 (Moncini et al. 2016). Activation of CDK5 requires binding to p35, which is a non-cyclin protein that has a short half-life (Tang et al. 1995; Tsai et al. 1994). As an activator of CDK5, p35 contains two domains, including the N-terminal cell localization targeting domain and the CDK5 activation domain. The N-terminal region of p35, known as p10, contains an N-myristoylation consensus sequence (MGXXXS/T) that may be essential for binding to regulatory proteins (Lee et al. 1996) and that functions in targeting CDK5 to the cell membrane (Minegishi et al. 2010). Furthermore, residues 150–200 of p35 bind CDK5, and residues 279–291 are required for the activation of CDK5 in vitro (Poon et al. 1997). Because of the higher expression level of CDK5 relative to p35 in neurons, the kinase activity of CDK5 is mainly determined by the expression level of p35. After treatment with neurotoxins, including hydrogen peroxide and glutamate, p35 is degraded via ubiquitin-dependent and ubiquitin-independent pathways to p25, which has a longer half-life than p35 (Kusakawa et al. 2000; Lee et al. 2000; Takasugi et al. 2016).
Previous studies have illustrated that the role of CDK5 under oxidative stress is complex. Exposure of neurons to oxidative stress activates calpain, a calcium-dependent protease that converts p35 to p25. This conversion results in the overactivation of CDK5 (Nath et al. 2000). Moreover, the increased stability of p25 relative to p35 leads to the inappropriate localization of CDK5 (Patrick et al. 1999). The dysregulation of CDK5 may affect the cellular antioxidant defense system and could lead to increased oxidative stress (Sun et al. 2008). Increased oxidative stress could also cause an increase in ERK activation, and the continuous activation and nuclear localization of ERK could promote neuronal cell death (Dabrowski et al. 2000; Ruffels et al. 2004; Stanciu and DeFranco 2002). Additionally, it has been reported that the H2O2-induced activation of nuclear CDK5 could protect cells from H2O2-induced apoptosis via VRK3 phosphorylation, which could lead to the inhibition of ERK activation (Song et al. 2016).
In this study, we isolated AccCDK5 from A. cerana cerana. We verified AccCDK5 expression patterns at different developmental stages and in several tissues. Additionally, we determined the expression patterns of AccCDK5 in response to several oxidative stressors. We evaluated the antioxidant ability of AccCDK5 overexpressed in E. coli. Due to the peculiar relationship between CDK5 and p35, we cloned AccCDK5r1 and confirmed that AccCDK5r1 is a homolog of CDK5r1 in A. cerana cerana. Moreover, we obtained evidence for the interaction between AccCDK5 and AccCDK5r1. We also determined the expression patterns of AccCDK5r1 after several treatments. Based on our results, we speculate that AccCDK5 plays a crucial role in oxidative stress management and that AccCDK5r1 is an activator of AccCDK5.
Methods
Insects and treatment
In this study, Chinese honeybees (A. cerana cerana) were obtained from the College of Animal Science and Veterinary Medicine, Shandong Agricultural University (Taian, China). Honeybees of various developmental stages, including 4th, 5th, and 6th day instar larvae, white-eyed (Pw), pink-eyed (Pp), and dark-eyed (Pd) pupae, and adult workers (Ad), were obtained. Three samples were analyzed for each stage. The bees were divided into 11 groups, and each group contained 40 randomized individuals. Groups 1–3 were exposed to 4 °C, 42 °C, or UV (254 nm, 30 mJ/cm2), respectively. Group 4 was injected with 2 mM H2O2 (0.5 μL). Groups 5 and 6 were fed with CdCl2 and HgCl2 (6 mg/L was added to the basic adult diet). Groups 7–10 were treated with pesticides (spirodiclofen, acaricide, acetamiprid, imidacloprid, and phoxim), which were diluted to the final concentration (20 μg/mL was added to food), and the sources of the pesticides and their purity are listed in Table S1. The control groups were fed a basic adult diet, which contained water, 70% powdered sugar, and 30% honey from the source colonies. All bees were maintained in an incubator (at a constant temperature of 34 °C with relative humidity at 70%, in a 24-h dark environment), and three honeybees were sampled randomly from each group at appropriate times. Bees were snap-frozen in liquid nitrogen after treatment and were stored at −70 °C.
RNA extraction and cDNA synthesis
Using standard methods for A. mellifera anatomy and dissection as a reference (Carreck et al. 2013), adult workers were dissected into different tissues, including head, epidermis, muscle, midgut, and poison gland. Total RNA was extracted from the samples using RNAiso Plus (TaKaRa, Japan). The concentration and quality of RNA samples were measured using a NanoDrop™ 2000/2000c spectrophotometer (NanoDrop products, Wilmington, DE, 19810, USA), and the RNA samples were stored at −70 °C. Then, the RNA samples (1000 ± 200 ng/μL) were reverse transcribed using 5× All-In-One RT MasterMix (with the AccuRT Genomic DNA Removal Kit) (Applied Biological Materials Inc., Richmond, BC, Canada), which uses oligo dT to prime the reverse transcription. This kit can effectively remove gDNA from RNA samples. Nuclease-free water was used as a negative control in the RT process. The RT procedure was as follows: add the RNA template (up to 2 μg), AccuRT Reaction Mix (4×) (2 μL), and nuclease-free H2O (up to a total volume of 8 μL) to the tube and incubate at 42 °C for 2 min, then add AccuRT Reaction Stopper (5×) (2 μL), 5× All-In-One RT MasterMix (4 μL), and nuclease-free H2O (6 μL). The temperatures and times used for the reaction were 10 min at 25 °C, 15 min (for qPCR) or 50 min (for PCR) at 42 °C, and 5 min at 85 °C. The samples were chilled on ice after the RT process and stored at −20 °C.
Isolation of the AccCDK5 ORF sequence
Recently, genomic sequencing of A. cerana cerana has been completed (Park et al. 2015). To clone the ORF sequence of AccCDK5, the specific primers AccCDK5-5 and AccCDK5-3 (as shown in Table 1) were designed based on the AccCDK5 genomic sequence. The primer design method and PCR protocol introduced by a previous study (Templeton 1992) were used. A 25-μL reaction volume was used in the PCR reaction, which contained 2.5 μL Taq buffer (TransGen Biotech, Beijing, China), 1 μL dNTP Mixture (Sangon Biotech, Shanghai, China), 1 μL of each primer (10 mM), 1 μL complementary DNA (cDNA) template, 0.25 μL Taq DNA Polymerase (TransGen Biotech, Beijing, China), and 18.25 μL double distilled water. The PCR amplification conditions are as shown in Table 2. The PCR product was purified and ligated into the pEASY-T1 simple vector (TransGen Biotech, Beijing, China) and transformed into Trans1-T1 Phage Resistant Chemically Competent Cells (TransGen Biotech, Beijing, China) for sequencing. The sequencing was carried out by Biosune Biotechnology (Shanghai) Co., Ltd. (Shanghai, China), using a 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA) with M13 universal sequencing primers (as shown in Table 1).
Table 1.
Primers used in this study
| Abbreviation | Primer sequence (5′-3′) | Description |
|---|---|---|
| AccCDK5-5 | CGCTAACCACTTTTCATTATTCCGC | cDNA sequence primer, forward |
| AccCDK5-3 | GGTCGTTGCACTACTCGCG | cDNA sequence primer, reverse |
| AccCDK5r1-5 | GCCACCACCACCGCCTCAAC | cDNA sequence primer, forward |
| AccCDK5r1-3 | CGAGGTCGATGCGGAGGGGTC | cDNA sequence primer, reverse |
| AccCDK5-Y-5 | GGATCCATGCAAAAATATGAGAAACTCGAG | Protein expression primer, forward |
| AccCDK5-Y-3 | GTCGACCTGACAACGATCGTTTTTAATGG | Protein expression primer, reverse |
| AccCDK5-F | CGAACGCCGGTAGACCCTTG | qPCR primer, forward |
| AccCDK5-R | CCTTGAGCGGGATGATAAAGTGG | qPCR primer, reverse |
| AccCDK5r1-F | CAACACGCACAACCCGAC | qPCR primer, forward |
| AccCDK5r1-R | GGAAGTCTCTTAAACGGGTGC | qPCR primer, reverse |
| AccCDK5-BD-5 | CATATGATGCAAAAATATGAGAAACTCGAG | pGBKT7 construction primer, forward |
| AccCDK5-BD-3 | GGATCCCTGACAACGATCGTTTTTAATGG | pGBKT7 construction primer, reverse |
| AccCDK5r1-AD-5 | CATATGATGGGTACCGTGTTGTCGTTC | pGADT7 construction primer, forward |
| AccCDK5r1-AD-3 | GAGCTCAGCCGCCTTGGTGGGTAC | pGADT7 construction primer, reverse |
| β-s | AGAATTGATCCACCAATCCA | Standard control primer, forward |
| β-x | GGTACCATGCAGCACATATTATTG | Standard control primer, reverse |
| M13F | TGTAAAACGACGGCCAGT | Universal sequencing primer, forward |
| M3R | CAGGAAACAGCTATGACC | Universal sequencing primer, forward |
Table 2.
PCR amplification conditions
| Primer pair | Amplification conditions |
|---|---|
| AccCDK5-5/AccCDK5-3 | 10 min at 94 °C, 40 s at 94 °C, 40 s at 53 °C, 1 min at 72 °C for 35 cycles, 10 min at 72 °C |
| AccCDK5-Y-5/AccCDK5-Y-3 | 10 min at 94 °C, 40 s at 94 °C, 40 s at 50 °C, 1 min at 72 °C for 35 cycles, 10 min at 72 °C |
| AccCDK5-BD-5/AccCDK5-BD-3 | 10 min at 94 °C, 40 s at 94 °C, 40 s at 50 °C, 1 min at 72 °C for 35 cycles, 10 min at 72 °C |
| AccCDK5r1-5/AccCDK5r1-3 | 10 min at 94 °C, 40 s at 94 °C, 40 s at 55 °C, 90 s at 72 °C for 35 cycles, 10 min at 72 °C |
| AccCDK5r1-AD-5/AccCDK5r1-AD-3 | 10 min at 94 °C, 40 s at 94 °C, 40 s at 50 °C, 70 s at 72 °C for 35 cycles, 10 min at 72 °C |
Bioinformatics and phylogenetic analysis of AccCDK5
Conserved domains of AccCDK5 were analyzed using BLAST by NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Multiple protein sequence alignments were performed using DNAman software 6.0.3 (Lynnon Biosoft Corporation, San Ramon, CA, USA). The theoretical isoelectric point and molecular weight of AccCDK5 were predicted using ExPASy (http://web.expasy.org/compute_pi/). The phylogenetic analysis was conducted using MEGA5.1 software based on the neighbor-joining method.
Real-time quantitative PCR
To determine the expression patterns of AccCDK5, real-time quantitative PCR was performed using the SYBR® PrimeScript™ RT-PCR Kit (TaKaRa, Japan), individual PCR tubes 8-tube strip (clear) (Bio-Rad, Hercules, CA, USA), and the CFX96™ Real-Time System (Bio-Rad, Hercules, CA, USA) with specific primers (AccCDK5-F and AccCDK5-R, shown in Table 1) based on the cDNA sequence as described above. The qPCR primers for AccCDK5 were designed and tested according to previously reported procedures (Bustin et al. 2009; Giulietti et al. 2001). The β-actin gene (GenBank: HM640276) (as shown in Table 1) was selected as a reference gene (Scharlaken et al. 2008) and was used to normalize the variations in RNA extraction yield and efficiencies of reverse transcription and amplification. The efficiency values and correlation coefficients (R 2) of the qPCR primers are listed in Table S2. We also tested and compared additional reference genes (ribosomal protein 49 and tbp-association factor) using Genorm software (versions 3.5) (as shown in supplemental Table S3). A 25-μL reaction volume was used for the qPCR reaction, which contained 9.5 μL double distilled water, 2 μL cDNA template, 0.5 μL of each primer (10 mM), and 12.5 μL SYBR® Premix Ex Taq™. The qPCR protocol was as follows: 95 °C for 30 s, 40 cycles of 95 °C for 5 s, 55 °C for 15 s, and 72 °C for 15 s, and a final melt cycle from 65 to 96 °C. All of the experimental samples were analyzed in triplicate, and the data from the qPCR were analyzed with the Bio-Rad CFX Manager 3.1 (Bio-Rad, Hercules, CA, USA). The relative expression levels of AccCDK5 were calculated using the 2−ΔΔCt comparative CT method (Livak and Schmittgen 2001), and the error bars were calculated by Bio-Rad CFX Manager 3. The mean ± SE from three independent experiments is shown.
The protein expression of AccCDK5 and the antibody preparation
To obtain recombinant AccCDK5 protein, the coding region of AccCDK5 flanked by BamH I and Sal I restriction sites was ligated into the pET-30a(+) vector (Novagen, Darmstadt, Germany). The recombinant plasmid was transformed into E. coli BL21 (DE3) (TransGen Biotech, Beijing, China). A positive clone was cultured in LB medium with 50 μg/mL kanamycin at 37 °C overnight. Then, 100–200 μL of the culture was subcultured into 10 mL of fresh LB medium containing 50 μg/mL kanamycin and incubated at 37 °C for 1–2 h until the optical density at 600 nm reached 0.4–0.6. Expression of recombinant AccCDK5 protein was induced by isopropyl-1-thio-β-d-galactopyranoside (IPTG) (CWbiotech, Beijing, China) at a final concentration of 75 μg/mL at 28 °C for 6–8 h. After induction, the bacterial cells were collected by centrifugation at 13,000 rpm at room temperature for 2 min. SDS-PAGE loading buffer was mixed with the bacteria at a 1:1 ratio, and the mixture was heated at 100 °C for 10 min. Then, the mixed metaprotein was separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The target protein band was excised and macerated with a defined amount of normal saline. Recombinant AccCDK5 protein was injected subcutaneously into white mice to produce antibodies. The mice were injected once a week for 4 weeks. Four days after the last injection, blood from the mice was collected and maintained at 37 °C for 1 h and then 4 °C for 6 h. The serum was collected by centrifugation at 3000 rpm at 4 °C for 15 min, after which the serum was aliquoted and stored at −70 °C.
Western blot analysis
Total protein lysate from adult worker bees that were treated with several stressors (4 °C, H2O2, phoxim, or acaricide) was extracted using a Tissue Protein Extraction Kit (CWbiotech, Beijing, China). The total protein lysate was subjected to SDS-PAGE, and the target band was excised and electrotransferred onto a PVDF membrane (Millipore, Bedford, MA, USA) in a semi-dry transfer apparatus. Three replicate blots were produced from each sample. Western blot analysis was performed according to a previously reported procedure (Meng et al. 2014). The anti-AccCDK5 serum was used as the primary antibody at a 1:1000 (v/v) dilution. Peroxidase-conjugated goat anti-mouse immunoglobulin G (Dingguo, Beijing, China) was used as the secondary antibody at a 1:2000 (v/v) dilution. The development process was performed using the FDbio-Dura ECL Kit (Fudebio, Hangzhou, China). The western blot results were analyzed using Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA).
Disc diffusion assay of recombinant AccCDK5 protein
Disc diffusion assays for cumyl hydroperoxide and HgCl2 were performed using a method modified from Burmeister et al. (2008). The recombinant AccCDK5 protein was overexpressed in E. coli BL21, and empty pET-30a(+) vector-transformed E. coli BL21 cells were used as the control. Approximately 5 × 108 cells were plated on LB-kanamycin agar plates and incubated at 37 °C for 1 h. Then, filter discs (7 mm diameter) soaked in different concentrations of cumyl hydroperoxide or HgCl2 were placed on the surface of the agar. The cells were cultivated at 37 °C for 24 h, after which the inhibition zones around the filter discs were measured. The diameters were measured three times from different angles with a vernier caliper, and three replicate plates were made for every treatment.
The isolation of the ORF sequence of AccCDK5r1 and a yeast two-hybrid analysis
Because CDK5 and p35 have been shown to interact, we used the p35 protein sequence of Homo sapiens (GenBank: CAA56587.1) as a reference to perform a local BLAST (BLAST-2.3.0+) analysis to search for the p35 homolog in A. cerana cerana. From our analysis, an unannotated protein was found, and the specific primers AccCDK5r1–5 and AccCDK5r1–3 (as shown in Table 1) were designed based on this sequence. The PCR product was ligated into a pEASY-T1 vector and transformed into Trans1-T1 Phage Resistant Chemically Competent Cells for sequencing. Bioinformatics and phylogenetic analyses of AccCDK5r1 were performed as described above.
A yeast two-hybrid analysis was performed using The Matchmaker Gold Yeast Two-Hybrid System (Clontech, Dalian, China) after obtaining the sequences of AccCDK5 and AccCDK5r1. The ORF of AccCDK5, flanked by Nde I and BamH I restriction sites, was amplified and subcloned into the pGBKT7 vector. Similarly, the ORF of AccCDK5r1, flanked by Nde I and Sac I restriction sites, was ligated into the pGADT7 vector. To obtain positive clones, the plasmids AccCDK5-BD and AccCDK5r1-AD were co-transformed into the Y2H Gold yeast strain and then cultured on selective SD medium (DDO, SD/-Leu/-Trp and QDO, SD/-Ade/-His/-Leu/-Trp). After 3–5 days, the positive clones were subcultured onto QDO medium, to which X-α-Gal (QDO/X) was added for a second round of selection. In this analysis, pGBKT7-53 and pGADT7-T were used as positive controls, whereas pGBKT7-lam and pGADT7-T were used as negative controls.
Expression patterns of AccCDK5r1 under environmental stress
To determine the expression patterns of AccCDK5r1 in response to environmental stresses, real-time quantitative PCR was performed as described above.
Primers
The primers used in this study are listed in Table 1. All primers were synthesized by Biosune Biotechnology (Shanghai) Co., Ltd. (Shanghai, China).
Data analysis
The results of the gene expression analyses and the diameters of disc diffusion assay are presented as the mean ± SE from three independent experiments. All data were tested for violations of the assumptions for parametric analyses. Significant differences, labeled with different letters, were determined by Duncan’s multiple range tests using the Statistical Analysis System (SAS) version 9.1 software program (SAS Institute, Cary, NC, USA). The same letters indicate that there was no significant difference between the experimental groups, whereas different letters and overlapped letters indicate that there was a significant difference and a non-significant difference between the experimental groups, respectively.
Results
Bioinformatics and phylogenetic analysis of AccCDK5
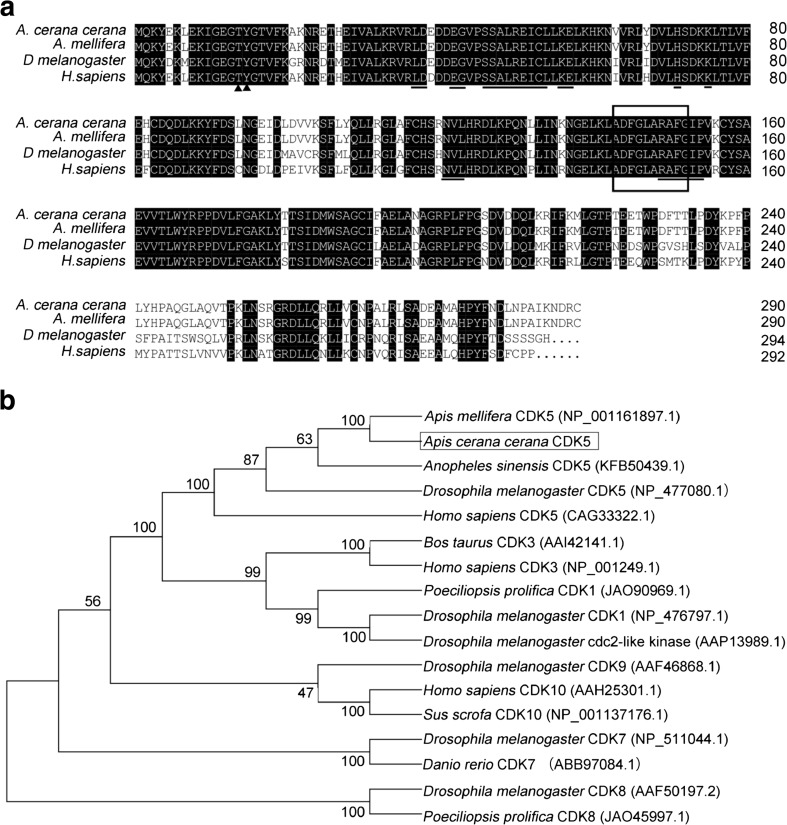
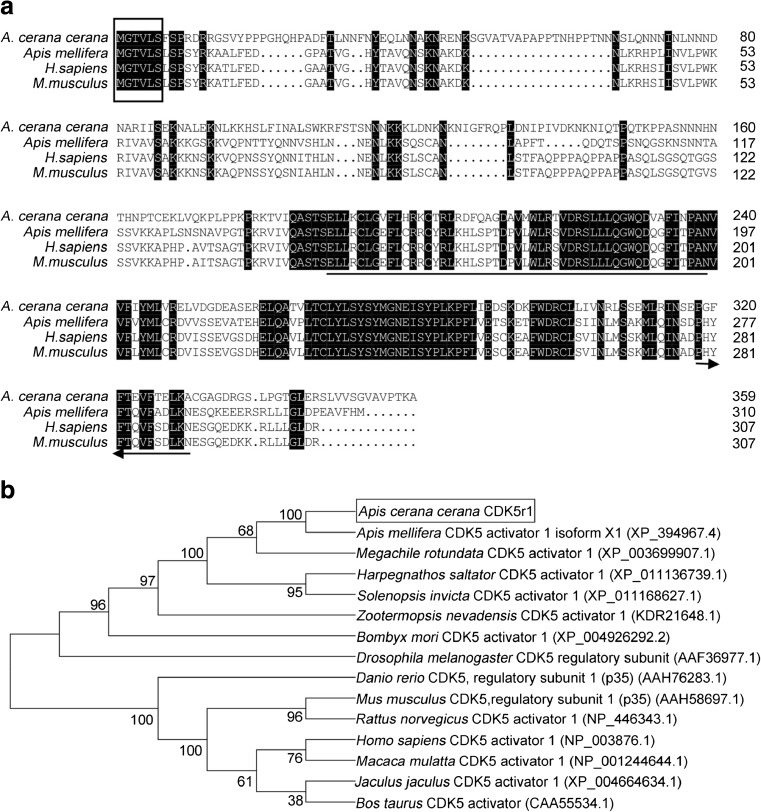
Using reverse-transcription PCR, AccCDK5 was cloned from A. cerana cerana. The open reading frame (ORF) of AccCDK5 is 900 bp and encodes a 299-amino acid polypeptide with a predicted molecular weight of 34.05 kDa and an isoelectric point of 7.6. Multiple sequence alignments of several CDK5s from different species revealed that the putative AccCDK5 has high homology with the other CDK5 genes. AccCDK5 shows high identity with AmCDK5 (A. mellifera, NP_001161897.1), DmCDK5 (Drosophila melanogaster, GI: 17137070), and HsCDK5 (H. sapiens, GI: 30584911). The high degree of homology indicates that CDK5 is conserved across species. As shown in Fig. 1a, the putative AccCDK5 contained the typical features of CDK5, including Thr14 and Tyr15 sites, an activation loop, and binding sites for non-cyclin regulators.
Fig. 1.
Characterization of cyclin-dependent kinase 5 (CDK5) from various species. a Multiple amino acid sequence alignments of AccCDK5 protein sequences with other CDK5 proteins, AmCDK5, DmCDK5, and HsCDK5. Thr14 and Tyr15 are marked by black triangles. The activation loop (T-loop) is boxed. The binding sites of non-cyclin regulators of CDK5 are marked by horizontal lines. b Phylogenetic analysis of CDK5 from several species. AccCDK5 is boxed
As shown in Fig. 1b, AccCDK5 has a close evolutionary relationship with AmCDK5 from Apis mellifera, which is in agreement with the multiple amino acid sequence alignments of CDK5.
Developmental and tissue-specific expression patterns of AccCDK5
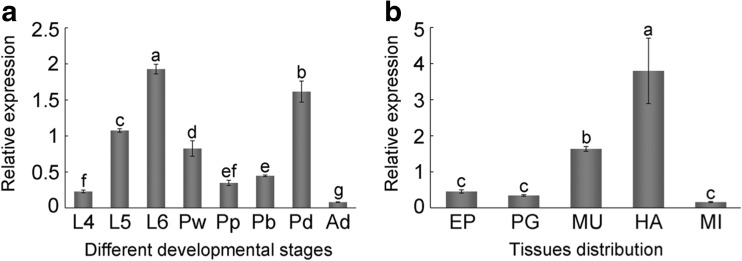
To investigate the expression patterns of AccCDK5 at different developmental stages and in several tissues, qPCR was used. We found that there were significant differences (P < 0.001, F = 273.76) among different developmental stages in the relative expression of AccCDK5. As shown in Fig. 2a specifically, AccCDK5 was highly expressed during the larval and pupal stages. During the larval stage, the relative expression of AccCDK5 increased from L4 to L6 and was highest at L6. During the pupal stage, AccCDK5 expression levels in dark-eyed pupae were higher than in the other pupae. The AccCDK5 expression level during the adult stage was lower than in the other stages. We then analyzed AccCDK5 expression in different tissues and found that the expression level was higher in head and muscle tissue than in the other three tissues (Fig. 2b, P < 0.001, F = 41.87). The expression pattern was consistent with our expectations given the functions of AccCDK5.
Fig. 2.
The relative expression of AccCDK5 in different developmental stages and tissues. a Different developmental stages: larval (L1–L5, from the first to fifth instars), pupal (Pw, white-eyed pupae; Pp, pink-eyed pupae; Pb, brown-eyed pupae; and Pd, dark-eyed pupae), and adult workers (Ad). b Tissue distribution: EP (epidermis), PG (poison gland), MU (muscle), HA (head), and MI (midgut). The error bars represent the mean ± SE from three independent experiments. The letters above the columns represent significant differences (P < 0.001) based on Duncan’s multiple range tests
Expression patterns of AccCDK5 under environmental stresses
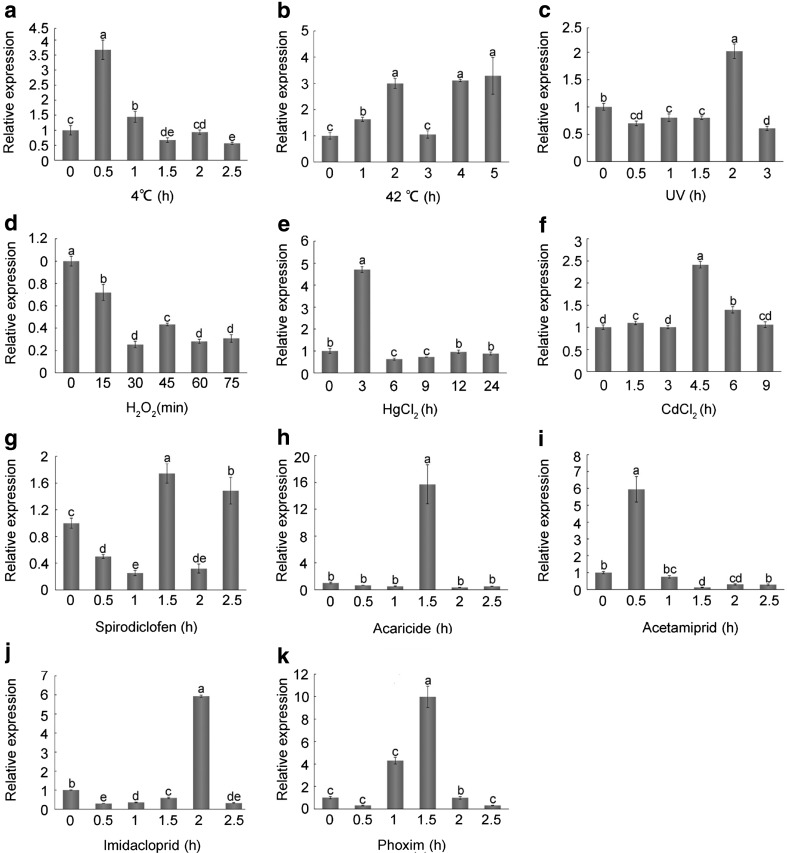
To investigate the expression patterns of AccCDK5 under environmental stresses, adult workers were subjected to ultraviolet light (UV), H2O2, 4 °C, 42 °C, heavy metals (HgCl2, CdCl2), or pesticides (spirodiclofen, acaricide, acetamiprid, imidacloprid, and phoxim) treatments. As shown in Fig. 3, the relative expression of AccCDK5 was upregulated in the majority of the treatments except the H2O2 treatment. Data analysis demonstrated that there were significant differences (P < 0.001) between samples from treated bees and the controls, and the F statistics are listed in Table S4. As shown in Fig. 3a, b, the relative expression level of AccCDK5 was increased by 3.7-fold after 0.5 h of exposure to 4 °C and 3.2-fold after 5 h of exposure to 42 °C. After UV treatment, the relative expression levels of AccCDK5 increased by 2-fold at 2.5 h (Fig. 3c). The H2O2 treatment caused a nearly 80% decrease in the relative expression of AccCDK5 at 30 min (Fig. 3d). The relative expression levels of AccCDK5 increased by 4.7- and 2.4-fold at 3 and 4.5 h after HgCl2 and CdCl2 treatments, respectively, and then decreased back to the basal levels (Fig. 3e, f). Spirodiclofen treatment changed the messenger RNA (mRNA) levels of AccCDK5 only slightly, with a 1.7-fold increase at 1.5 h (Fig. 3g). By contrast, acaricide, acetamiprid, imidacloprid, and phoxim treatments increased the relative expression levels of AccCDK5 by 15.7-, 6-, 5.9-, and 10-fold, respectively, at different time points (Fig. 3h–k).
Fig. 3.
Expression profiles of AccCDK5 under environmental stress. Total RNA was extracted from Apis cerana cerana treated with various stresses at the indicated time; the treatments include a 4 °C, b 42 °C, c UV, d H2O2, e HgCl2, f CdCl2, g spirodiclofen, h acaricide, i acetamiprid, j imidacloprid, and k phoxim. β-Actin was used as an internal control. The error bars represent the mean ± SE from three independent experiments. The letters above the columns represent significant differences (P < 0.001) based on Duncan’s multiple range tests
Protein expression levels of AccCDK5 under environmental stresses
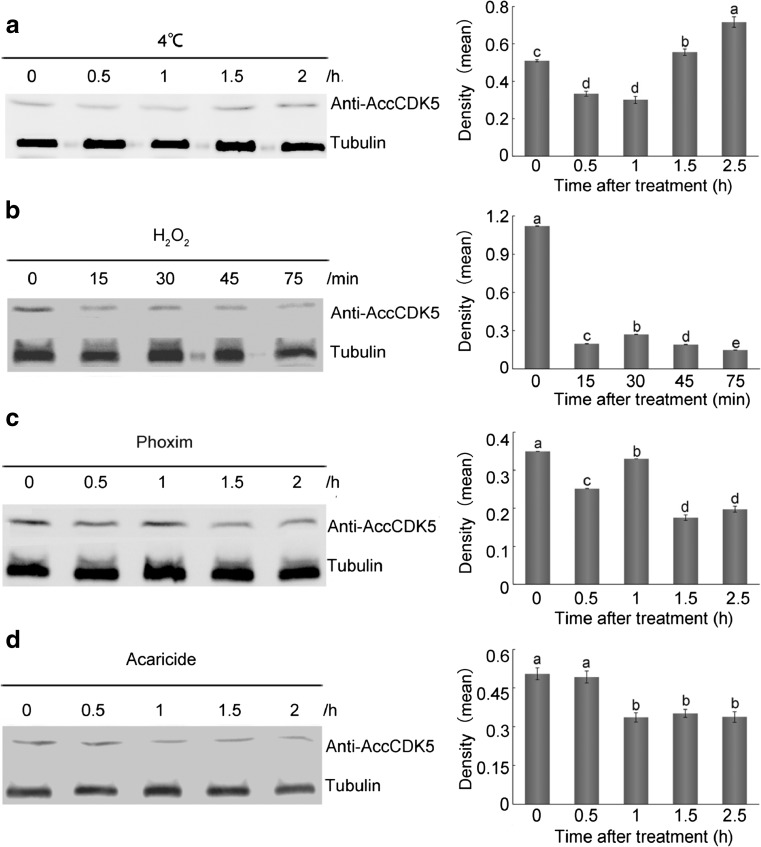
To study the expression patterns of AccCDK5 in response to environmental stresses, we performed western blot analysis. Total protein lysates from A. cerana cerana treated with 4 °C, H2O2, phoxim, or acaricide were probed using anti-AccCDK5 antibody. The analysis of the densities (as shown in supplemental Tables S5–S8 and Fig. S1) indicated that the amounts of protein from each sample were approximately equal. As shown in Fig. 4a (P < 0.001, F = 69.82) and supplemental Table S3, the levels of AccCDK5 expression were reduced after exposure to 4 °C for 0.5 and 1 h and induced after 2 and 2.5 h. The expression of AccCDK5 was downregulated after H2O2 treatment (Fig. 4b, P < 0.001, F = 67,379.2, as well as supplemental Table S4). As shown in Fig. 4c, d and supplemental Tables S5 and S6, the levels of expression of AccCDK5 were reduced slightly after phoxim (P < 0.001, F = 270.43) and acaricide (P = 0.003, F = 15.71) treatment.
Fig. 4.
Western blot analysis of AccCDK5 changes after a 4 °C, b H2O2, c phoxim, and d acaricide treatment. AccCDK5 protein was immunoblotted with anti-AccCDK5. The signal for the binding reaction was visualized with HRP substrate. The error bars represent the mean ± SE from three independent experiments. The letters above the columns represent significant differences based on Duncan’s multiple range tests
Characterization of recombinant AccCDK5 protein
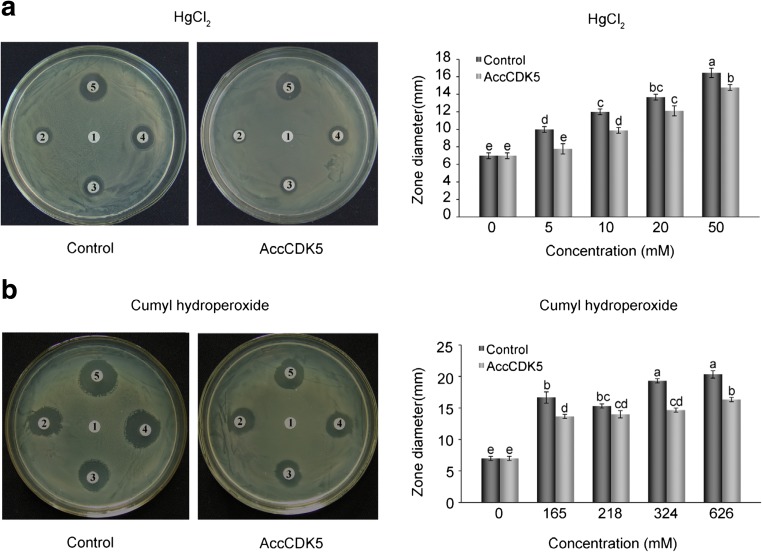
Next, we determined the protective effects of recombinant AccCDK5 protein using the disc diffusion method. Our results show that the inhibition zones around the filters soaked with HgCl2 or cumyl hydroperoxide were smaller in diameter on the plates containing E. coli overexpressing AccCDK5 than on the control plates (as shown in Fig. 5 and supplemental Tables S9 and S10). Analysis of the diameter of the inhibition zones revealed that there were significant differences between E. coli that overexpressed AccCDK5 and the controls at the same concentration of HgCl2 (P < 0.001, F = 71.15) or cumyl hydroperoxide (P < 0.001, F = 94.35).
Fig. 5.
Disc diffusion assays. Disc diffusion assays using E. coli overexpressing AccCDK5. AccCDK5 was overexpressed in E. coli, and bacteria transformed with pET-30a(+) were used as negative controls. Filter discs soaked with different concentrations of HgCl2 or cumyl hydroperoxide were placed on the agar plates. The killing zones around the filters were measured after overnight exposure. The error bars represent the mean ± SE from three independent experiments. The letters above the columns represent significant differences (P < 0.001) based on Duncan’s multiple range tests
Cloning and sequence analysis of AccCDK5r1
Therefore, to understand the functional mechanism of AccCDK5, we isolated AccCDK5r1, the homolog of mammalian p35. The ORF of AccCDK5r1 is 1083 bp, and it encodes a 360-amino acid protein. The predicted molecular mass of AccCDK5r1 is 40.42 kDa, whereas its predicted isoelectric point is 9.47. As shown in Fig. 6, multiple sequence alignments suggested that AccCDK5r1 has a high amino acid identity in the region of the N-myristoylation consensus sequence (MGXXXS/T) and the binding and activation site with cyclin-dependent kinase 5 regulator 1 from H. sapiens, M. musculus, and Danio rerio. Next, phylogenetic analysis results show that AccCDK5r1 has a close evolutionary relationship with cyclin-dependent kinase 5 activator 1 from A. mellifera.
Fig. 6.
Characterization of cyclin-dependent kinase 5 regulator 1 (CDK5r1) from different species. a Multiple amino acid sequence alignments of Apis cerana cerana CDK5 regulatory subunit 1 (AccCDK5r1), Danio rerio CDK5 regulatory subunit 1 (p35) (AAH76283.1), Homo sapiens CDK5 activator 1 (NP_003876.1), and Mus musculus CDK5 regulatory subunit 1 (p35) (AAH58697.1). The N-myristoylation consensus sequence (MGXXXS/T) is boxed. CDK5 binding sites are marked by horizontal lines. CDK5 activation sites are marked by arrows. b Phylogenetic analysis of CDK5r1 from several species. AccCDK5r1 is boxed
Yeast two-hybrid analysis
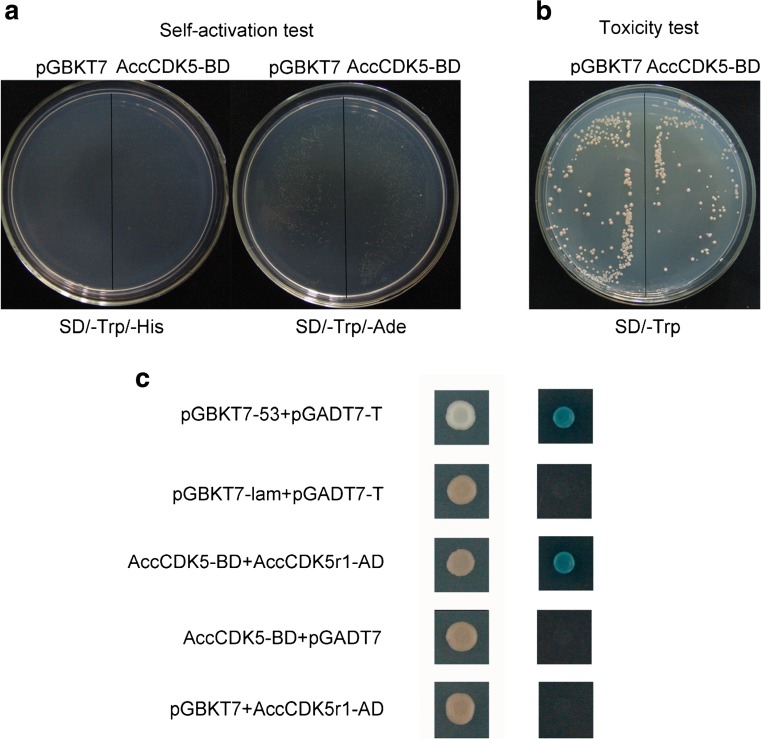
To investigate the interaction between AccCDK5 and AccCDK5r1, a yeast two-hybrid analysis was performed. As shown in Fig. 7a, cells transformed with the pGBKT7 vector or the AccCDK5-BD plasmid do not grow on SD Medium-His and SD Medium-Ade, which precludes the self-activation of the AccCDK5-BD plasmid. As shown in Fig. 7b, cells transformed with the pGBKT7 vector or the AccCDK5-BD plasmid grew to similar extents, which suggests that AccCDK5-BD is a non-toxic protein. Next, AccCDK5-BD and AccCDK5r1-AD were co-transformed into the Y2H Gold yeast strain. Cells were able to grow on SD Medium-Leu/-Trp plates, which indicates that the plasmids were transformed successfully. The indicated strain was inoculated on plates of SD Medium-Ade-/His-/Leu-/Trp, and cells transformed with AccCDK5-BD and AccCDK5r1-AD grew on SD Medium-Ade/-His/-Leu/-Trp. The positive, interacting clones grew on the SD Medium-Ade/-His/-Leu/-Trp with X-α-gal, which confirmed the interaction between AccCDK5 and AccCDK5r1 (Fig. 7c).
Fig. 7.
Yeast two-hybrid analysis of AccCDK5 and AccCDK5r1. a Self-activation test for AccCDK5-BD and b toxicity test for AccCDK5-BD. c AccCDK5-BD and AccCDK5r1-AD fusion constructs were co-transformed into the Y2H Gold yeast strain and grown on DDO and QDO SD media. The positive clones were confirmed on QDO/X SD media
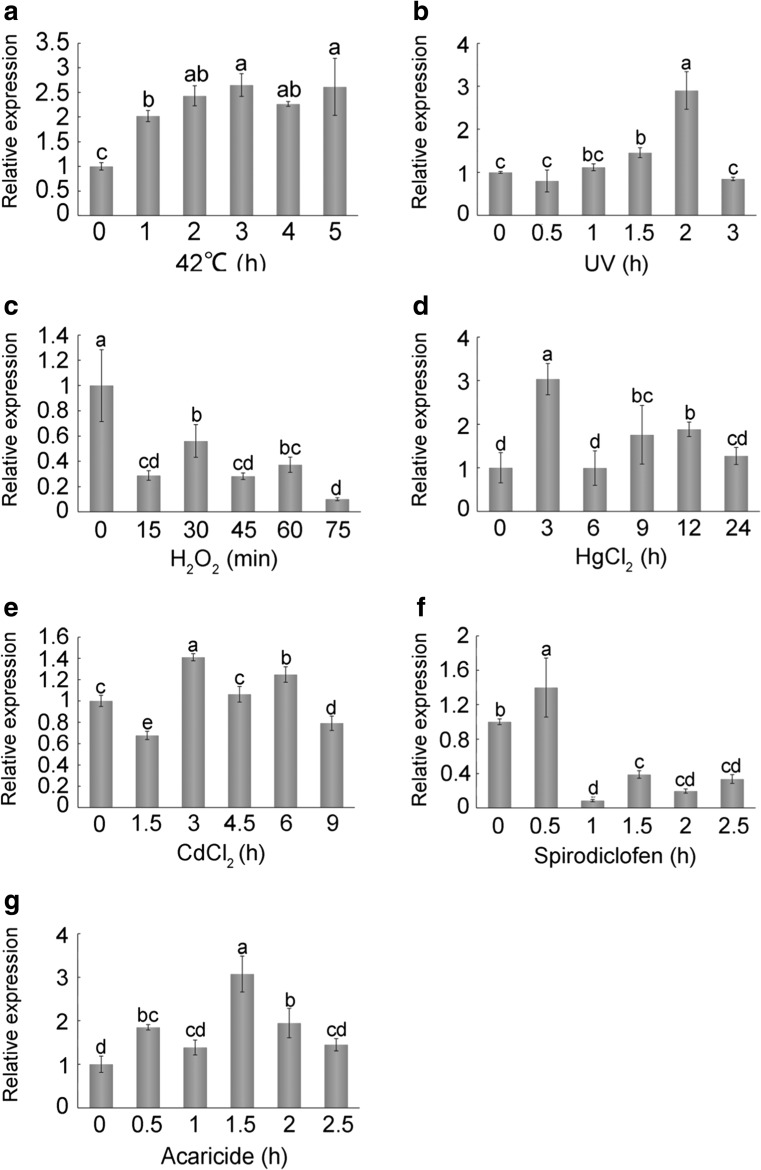
Expression profiles of AccCDK5r1 under environmental stress
To explore the relationship between AccCDK5 and AccCDK5r1, we analyzed the expression patterns of AccCDK5r1 after several stress treatments. Expression of AccCDK5r1 was induced after stress treatments (Fig. 8). Notably, the expression patterns of AccCDK5r1 were approximately consistent with those of AccCDK5 after the same treatment.
Fig. 8.
Expression profiles of AccCDK5r1 under environmental stress. Total RNA was extracted from Apis cerana cerana treated with various stresses for the indicated amounts of time. The treatments included a 42 °C, b UV, c H2O2, d HgCl2, e CdCl2, f spirodiclofen, and g acaricide. β-Actin was used as an internal control. The error bars represent the mean ± SE from three independent experiments. The letters above the columns represent significant differences (P < 0.001) based on Duncan’s multiple range tests
Discussion
CDK5 is an atypical member of the CDK family. In the early 1990s, CDK5 was first discovered in bovine brain tissue (Lew et al. 1992), and since then, great advancements have been made in determining the function and the mechanism of this gene. Activators of CDK5 as well as the activation mechanism of CDK5 and the cellular processes that require CDK5 are distinctly different from those of the other CDK family members (Cheung and Ip 2007). The proper localization and regulation of CDK5 is crucial for its functions, and the absence of CDK5 is lethal (Trunova and Giniger 2012). However, the majority of research on CDK5 has focused on humans and other mammals. Few CDK5 studies in insects have been systematically performed.
In this study, we isolated AccCDK5 from A. cerana cerana. The sequence analysis of AccCDK5 revealed that there was high identity with other CDK5s from different species and that AccCDK5 contained conserved features of the CDK5 family. These two observations indicated that CDK5 is conserved across species. Phylogenetic analysis showed that AccCDK5 belonged to the CDK5 group and had a close evolutionary relationship with AmCDK5. In summary, we infer that AccCDK5 is a member of the CDK5 family and has functions similar to those of other reported CDK5s.
It has previously been reported that CDK5 kinase activity is indispensable for neurite outgrowth during the course of neuronal differentiation (Nikolic et al. 1996). The central nervous system mass increases exponentially up to 6 days after hatching in D. melanogaster (Power 1952). Adult-specific head sensory structures form during the larval and pupal stages in D. melanogaster and A. mellifera, and mushroom bodies develop abundantly during the larval stage (Farris et al. 1999). Moreover, CDK5 is involved in the regulation of remodeling of mushroom body neurons in D. melanogaster (Smith-Trunova et al. 2015). The specific expression pattern of AccCDK5 in different developmental stages may reflect unique demands in the development processes and nervous system formation. CDK5 is ubiquitously expressed throughout the organism, but the highest kinase activity of CDK5 is detected in postmitotic neurons (Cheung and Ip 2007). Tissue-specific expression analysis showed that AccCDK5 is expressed in different tissues but is most highly expressed in the head and muscle, the main locations of the nervous system in bees (Carreck et al. 2013).
There are numerous stresses in the habitat of honeybees, including unsuitable temperatures, UV, H2O2, heavy metals, and pesticides, which can induce ROS production and eventually lead to oxidative stress (Lushchak 2011). However, the expression patterns of CDK5 under environmental stresses have not been studied extensively in insects. Previous studies demonstrated that downregulation of nestin caused by oxidative stress in neuronal precursor cells led to activation of CDK5 (Sahlgren et al. 2006), and that CDK5 modified p53 posttranslationally and increased p53 stability in response to H2O2 (Lee et al. 2008). In our study, AccCDK5 was induced by the exposure of honeybees to 42 °C, 4 °C, H2O2, UV, HgCl2, CdCl2, and several pesticides.
The normal temperature of a hive is between 33 and 36 °C (Tautz et al. 2003). High and low temperatures can lead to oxidative stress (Bagnyukova et al. 2007; Malek et al. 2004). Heat shock and cold stress can induce the phosphorylation of tau, a substrate of CDK5, which is associated with increases in CDK5 (Lau et al. 2002). In our study, AccCDK5 transcript levels increased quickly after exposure to 42 and 4 °C, suggesting that AccCDK5 is involved in protecting honeybees from ROS damage caused by dramatic temperature fluctuations. UV radiation is absorbed by DNA bases and eventually causes DNA damage and increased ROS production (Gomez-Mendoza et al. 2016; Kottuparambil et al. 2012). In addition, CDK5 has been reported to be a mediator of the response to DNA damage (Zhu et al. 2011). After UV exposure, AccCDK5 was induced. This effect suggests that AccCDK5 is related to the DNA damage and ROS response after UV treatment.
Cadmium can induce oxidative stress and, consequently, neuronal death pathways (Antoniali 2014). MeHg-induced hyperphosphorylation of tau can lead to neuropathological changes in the mouse brain (Fujimura et al. 2009). In our study, AccCDK5 was induced by the heavy metals HgCl2 and CdCl2. As mentioned above, phosphorylation of tau is associated with increases in CDK5. HgCl2 and CdCl2 treatment may lead to hyperphosphorylation of tau and oxidative stress in the nervous system, thus resulting in increases in AccCDK5. The strong oxidizing agent H2O2 also causes oxidative damage. Although the transcription and translation levels of AccCDK5 were downregulated, the results of the disc diffusion assay revealed that overexpression of AccCDK5 enhances the resistance of bacteria to oxidative stress, indicating that AccCDK5 may be involved in the response to oxidative stress.
One of the main features of pesticide toxicity is the induction of oxidative stress (Asghari et al. 2016). Several other studies also showed an increase in oxidative stress after exposure to pesticides, and chronic systemic pesticide exposure results in features of Parkinson’s disease, which is connected to the dysregulation of CDK5 (Betarbet et al. 2000; Piner et al. 2007; Thomaz et al. 2009). Our results revealed that the expression level of AccCDK5 was upregulated after pesticide treatments. These results suggest that the ingestion of food containing pesticides may lead to oxidative stress and the dysregulation of AccCDK5. We also detected the downregulation of AccCDK5 at the protein level after acaricide and phoxim treatments, a result that was different from the mRNA expression data. The upregulation of AccCDK5 at the transcriptional level may be a compensatory mechanism to account for decreased protein levels. For different genes, the protein-per-mRNA ratio is different; additionally, the ratio might change after different treatments. The square of Pearson’s correlation coefficient (R 2) between the mRNA and protein concentrations averages 0.09 to 0.46 (Abreu et al. 2009).
Furthermore, the stability of proteins is essential to their structure and function (Becktel and Schellman 1987). After exposure to stress, the stability of the AccCDK5 protein may decrease and eventually lead to a decrease in AccCDK5 protein levels. The different concentrations, intensities, and mechanism of the stresses (such as heat shock and cold stress, UV, heavy metal, and pesticides) may lead to diverse responses.
Previous studies have demonstrated that most substrates mediate their interaction with CDK5 through p35 and that the regulation of the p35 mRNA level is a major determinant of CDK5 activity. The p35 protein may act as both an activator and an adaptor for CDK5, and this protein plays an indispensable role in CDK5 function (Lim et al. 2003; Ross et al. 2002). CDK5 and p35 form a complex that participates in cellular processes (Büchner et al. 2015). Therefore, we cloned the gene from A. cerana cerana that codes for p35 and named it AccCDK5r1.
The deduced dCdk5a protein, the D. melanogaster protein homologous to p35, is much longer than p35 (Ma and Haddad 1999). In our study, the molecular weight of AccCDK5r1 is greater than 35 kDa, but the high homology of the N-myristoylation consensus sequence (MGXXXS/T) and the CDK5 binding and activation domains, which are required for CDK5 activation, suggests that AccCDK5r1 may play the role of CDK5 activator and adaptor in A. cerana cerana (Amin et al. 2002). Phylogenetic analysis revealed that AccCDK5r1 has a closer evolutionary relationship with the corresponding proteins in insects than in other species. Based on the sequence and phylogenetic analyses, we propose that AccCDK5r1 in A. cerana cerana is the homolog of CDK5r1. Yeast two-hybrid analysis indicates that AccCDK5r1 interacts with AccCDK5, which supports our hypothesis.
The activity of CDK5 is primarily determined by the available amount of CDK5 activator (Hisanaga and Endo 2010; Hisanaga and Saito 2003). In this study, the expression patterns of AccCDK5 and AccCDK5r1 were similar, which indicates a correlation between AccCDK5 and AccCDK5r1. Notably, AccCDK5r1 was downregulated after H2O2 treatment, and a similar mechanism may be responsible for the downregulation of AccCDK5. These results provide further evidence that AccCDK5r1 is the homolog of CDK5r1 in A. cerana cerana.
Apis cerana cerana, like other insects, encounters multiple environmental stresses. In this study, we employed bioinformatics, phylogenetic analyses, expression profiling, and interaction studies to elucidate the relationship between AccCDK5 and AccCDK5r1. We identified and characterized a potential physiological function of AccCDK5. The results revealed that AccCDK5 and AccCDK5r1 play a role in the response to oxidative stresses. Our work forms the basis for future studies of AccCDK5 and its activator AccCDK5r1.
Electronic supplementary material
(DOCX 153 kb)
Acknowledgements
This work was financially supported by the fund earmarked for the China Agriculture Research System (No. CARS-45), the National Natural Science Foundation of China (No. 31572470), Shandong Province Modern Agricultural Technology System Innovation Team Special Fund (SDAIT-24-04), and Shandong Province Agriculture Fine Varieties Breeding Projects (2014–2016).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-017-0820-y) contains supplementary material, which is available to authorized users.
Contributor Information
Baohua Xu, Phone: +86-538-8245679, Email: bhxu@sdau.edu.cn.
Xingqi Guo, Phone: +86-538-8245679, Email: xqguo@sdau.edu.cn.
References
- Abreu RDS, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol BioSyst. 2009;5:1512–1526. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin ND, Albers W, Pant HC. Cyclin-dependent kinase 5 (cdk5) activation requires interaction with three domains of p35. J Neurosci Res. 2002;67:354–362. doi: 10.1002/jnr.10116. [DOI] [PubMed] [Google Scholar]
- An MI, Choi CY. Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: effects on hemolymph and biochemical parameters. Comp Biochem Physiol B: Biochem Mol Biol. 2010;155:34–42. doi: 10.1016/j.cbpb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Antoniali G (2014) Molecular mechanisms of neurodegeneration involving the effect of environmental pollutants on dna repair enzymes. Mol Signal Regul Glial Cells:44–56
- Asghari MH, Moloudizargari M, Bahadar H, Abdollahi M (2016) A review of the protective effect of melatonin in pesticide-induced toxicity. Expert Opinion on Drug Metabolism & Toxicology:1–10 doi:10.1080/17425255.2016.1214712 [DOI] [PubMed]
- Bagnyukova TV, Danyliv SI, Zin’ko OS, Lushchak VI. Heat shock induces oxidative stress in rotan Perccottus glenii tissues. J Therm Biol. 2007;32:255–260. doi: 10.1016/j.jtherbio.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Becktel WJ, Schellman JA. Protein stability curves. Biopolymers. 1987;26:1859–1877. doi: 10.1002/bip.360261104. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Büchner A, Krumova P, Ganesan S, Bähr M, Eckermann K, Weishaupt JH. Sumoylation of p35 modulates p35/cyclin-dependent kinase (Cdk) 5 complex activity. NeuroMolecular Med. 2015;17:12–23. doi: 10.1007/s12017-014-8336-4. [DOI] [PubMed] [Google Scholar]
- Burmeister C, Luersen K, Heinick A, Hussein A, Domagalski M, Walter RD, Liebau E. Oxidative stress in Caenorhabditis elegans: protective effects of the Omega class glutathione transferase (GSTO-1) FASEB J. 2008;22:343–354. doi: 10.1096/fj.06-7426com. [DOI] [PubMed] [Google Scholar]
- Bustin SA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Carreck NL, et al. Standard methods for Apis mellifera anatomy and dissection. J Apic Res. 2013;52:1–40. doi: 10.3896/IBRA.1.52.4.03. [DOI] [Google Scholar]
- Chen YW, Wang CH, An J, Kaikuang H. Susceptibility of the Asian honey bee,Apis cerana, to American foulbrood,Paenibacillus larvae larvae. J Apic Res. 2015;39:169–175. doi: 10.1080/00218839.2000.11101038. [DOI] [Google Scholar]
- Cheung ZH, Ip NY. The roles of cyclin-dependent kinase 5 in dendrite and synapse development. Biotechnol J. 2007;2:949–957. doi: 10.1002/biot.200700056. [DOI] [PubMed] [Google Scholar]
- Dabrowski A, Boguslowicz C, Dabrowska M, Tribillo I, Gabryelewicz A. Reactive oxygen species activate mitogen-activated protein kinases in pancreatic acinar cells. Pancreas. 2000;21:376–384. doi: 10.1097/00006676-200011000-00008. [DOI] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Espín S, Martínez-López E, Jiménez P, María-Mojica P, García-Fernández AJ. Effects of heavy metals on biomarkers for oxidative stress in Griffon vulture (Gyps fulvus) Environ Res. 2014;129:59–68. doi: 10.1016/j.envres.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Farris S, Robinson G, Davis R, Fahrbach S. Larval and pupal development of the mushroom bodies in the honey bee, Apis mellifera. J Comp Neurol. 1999;414:97–113. doi: 10.1002/(SICI)1096-9861(19991108)414:1<97::AID-CNE8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Usuki F, Sawada M, Takashima A. Methylmercury induces neuropathological changes with tau hyperphosphorylation mainly through the activation of the c-jun-N-terminal kinase pathway in the cerebral cortex, but not in the hippocampus of the mouse brain. Neurotoxicology. 2009;30:1000–1007. doi: 10.1016/j.neuro.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Giese KP. Generation of the Cdk5 activator p25 is a memory mechanism that is affected in early Alzheimer’s disease. Front Mol Neurosci. 2014;7:36. doi: 10.3389/fnmol.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- Gomez-Mendoza M, Banyasz A, Douki T, Markovitsi D, Ravanat JL (2016) Direct oxidative damage of naked DNA generated upon absorption of UV radiation by nucleobases. J Phys Chem Lett. doi:10.1021/acs.jpclett.6b01781 [DOI] [PubMed]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med. 1991;91:S14–S22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- Hellmich MR, Pant HC, Wada E, Battey JF. Neuronal cdc2-like kinase a cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci U S A. 1992;89:10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga S, Endo R. Regulation and role of cyclin-dependent kinase activity in neuronal survival and death. J Neurochem. 2010;115:1309–1321. doi: 10.1111/j.1471-4159.2010.07050.x. [DOI] [PubMed] [Google Scholar]
- Hisanaga S, Saito T. The regulation of cyclin-dependent kinase 5 activity through the metabolism of p35 or p39 Cdk5 activator. Neurosignals. 2003;12:221–229. doi: 10.1159/000074624. [DOI] [PubMed] [Google Scholar]
- Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Kodrík D, Bednářová A, Zemanová M, Krishnan N. Hormonal regulation of response to oxidative stress in insects—an update. Int J Mol Sci. 2015;16:25788–25816. doi: 10.3390/ijms161025788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottuparambil S, Shin W, Brown MT, Han T. UV-B affects photosynthesis, ROS production and motility of the freshwater flagellate, Euglena agilis Carter. Aquat Toxicol. 2012;122-123:206–213. doi: 10.1016/j.aquatox.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Kusakawa G, Saito T, Onuki R, Ishiguro K, Kishimoto T, Hisanaga S. Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem. 2000;275:17166–17172. doi: 10.1074/jbc.M907757199. [DOI] [PubMed] [Google Scholar]
- Lau LF, Seymour PA, Sanner MA, Schachter JB. Cdk5 as a drug target for the treatment of Alzheimer’s disease. J Mol Neurosci. 2002;19:267–273. doi: 10.1385/JMN:19:3:267. [DOI] [PubMed] [Google Scholar]
- Lee KY, Rosales JL, Tang D, Wang JH. Interaction of cyclin-dependent kinase 5 (Cdk5) and neuronal Cdk5 activator in bovine brain. J Biol Chem. 1996;271:1538–1543. doi: 10.1074/jbc.271.3.1538. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jeong MW, Kim W, Choi YH, Kim KT. Cooperative roles of c-Abl and Cdk5 in regulation of p53 in response to oxidative stress. J Biol Chem. 2008;283:19826–19835. doi: 10.1074/jbc.M706201200. [DOI] [PubMed] [Google Scholar]
- Lew J, Beaudette K, Litwin CM, Wang JH. Purification and characterization of a novel proline-directed protein kinase from bovine brain. J Biol Chem. 1992;267:13383–13390. [PubMed] [Google Scholar]
- Lim ACB, Qu D, Qi RZ. Protein-protein interactions in Cdk5 regulation and function. Neurosignals. 2003;12:230–238. doi: 10.1159/000074625. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lushchak VI. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol. 2011;101:13–30. doi: 10.1016/j.aquatox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Ma E, Haddad G. A Drosophila CDK5alpha-like molecule and its possible role in response to O2 deprivation. Biochem Biophys Res Commun. 1999;261:459–463. doi: 10.1006/bbrc.1999.1069. [DOI] [PubMed] [Google Scholar]
- Malek RL, Sajadi H, Abraham J, Grundy MA, Gerhard GS. The effects of temperature reduction on gene expression and oxidative stress in skeletal muscle from adult zebrafish. Comp Biochem Physiol Toxicol Pharmacol Cbp. 2004;138:363–373. doi: 10.1016/j.cca.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Malumbres M, et al. Cyclin-dependent kinases: a family portrait. Nat Cell Biol. 2009;11:1275–1276. doi: 10.1038/ncb1109-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng JY, Zhang CY, Zhu F, Wang XP, Lei CL. Ultraviolet light-induced oxidative stress: effects on antioxidant response of Helicoverpa armigera adults. J Insect Physiol. 2009;55:588–592. doi: 10.1016/j.jinsphys.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Meng F, Zhang Y, Liu F, Guo X, Xu B. Characterization and mutational analysis of omega-class GST (GSTO1) from Apis cerana cerana, a gene involved in response to oxidative stress. PLoS One. 2014;9:e93100. doi: 10.1371/journal.pone.0093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson M, et al. A family of human cdc2-related protein kinase. EMBO J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi S, Asada A, Miyauchi S, Fuchigami T, Saito T, Hisanaga S. Membrane association facilitates degradation and cleavage of the cyclin-dependent kinase 5 activators p35 and p39. Biochemistry. 2010;49:5482–5493. doi: 10.1021/bi100631f. [DOI] [PubMed] [Google Scholar]
- Moncini S, et al. Functional characterization of CDK5 and CDK5R1 mutations identified in patients with non-syndromic intellectual disability. J Hum Genet. 2016;61:283–293. doi: 10.1038/jhg.2015.144. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Nath R, Davis M, Probert AW, Kupina NC, Ren X, Schielke GP, Wang KK. Processing of cdk5 activator p35 to its truncated form (p25) by calpain in acutely injured neuronal cells. Biochem Biophys Res Commun. 2000;274:16–21. doi: 10.1006/bbrc.2000.3070. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Dudek H, Kwon Y, Ramos Y, Tsai L. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- Park D, et al. Uncovering the novel characteristics of Asian honey bee, Apis cerana, by whole genome sequencing. BMC Genomics. 2015;16:1. doi: 10.1186/1471-2164-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Piner P, Sevgiler Y, Uner N. In vivo effects of fenthion on oxidative processes by the modulation of glutathione metabolism in the brain of Oreochromis niloticus. Environ Toxicol. 2007;22:605–612. doi: 10.1002/tox.20286. [DOI] [PubMed] [Google Scholar]
- Poon RY, Lew J, Hunter T. Identification of functional domains in the neuronal Cdk5 activator protein. J Biol Chem. 1997;272:5703–5708. doi: 10.1074/jbc.272.9.5703. [DOI] [PubMed] [Google Scholar]
- Power ME. A quantitative study of the growth of the central nerve system of a holometabolous insect, Drosophila melanogaster. J Morphol. 1952;91:389–411. doi: 10.1002/jmor.1050910302. [DOI] [Google Scholar]
- Radloff SE, et al. Population structure and classification of Apis cerana. Apidologie. 2010;41:589–601. doi: 10.1051/apido/2010008. [DOI] [Google Scholar]
- Ross S, Tienhaara A, Lee MS, Tsai LH, Gill G. GC box-binding transcription factors control the neuronal specific transcription of the cyclin-dependent kinase 5 regulator p35. J Biol Chem. 2002;277:4455–4464. doi: 10.1074/jbc.M110771200. [DOI] [PubMed] [Google Scholar]
- Ruffels J, Griffin M, Dickenson JM. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: role of ERK1/2 in H2O2-induced cell death. Eur J Pharmacol. 2004;483:163–173. doi: 10.1016/j.ejphar.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Sahlgren CM, Pallari HM, He T, Chou YH, Goldman RD, Eriksson JE (2006) A nestin scaffold links Cdk5/35 signaling to oxidant-induced cell death. Embo Journal 25:4808-4819. doi: 10.1038/sj.emboj.7601366 [DOI] [PMC free article] [PubMed]
- Scharlaken B, de Graaf DC, Goossens K, Brunain M, Peelman LJ, Jacobs FJ. Reference gene selection for insect expression studies using quantitative real-time PCR: the head of the honeybee, Apis mellifera, after a bacterial challenge. J Insect Sci. 2008;8:1–10. doi: 10.1673/031.008.3301. [DOI] [Google Scholar]
- Smith-Trunova S, Prithviraj R, Spurrier J, Kuzina I, Gu Q, Giniger E. Cdk5 regulates developmental remodeling of mushroom body neurons in Drosophila. Dev Dyn. 2015;224:1550–1563. doi: 10.1002/dvdy.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, et al. Stress-induced nuclear translocation of CDK5 suppresses neuronal death by downregulating ERK activation via VRK3 phosphorylation. Sci Rep. 2016;6:28634. doi: 10.1038/srep28634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu M, DeFranco DB. Prolonged nuclear retention of activated extracellular signal-regulated protein kinase promotes cell death generated by oxidative toxicity or proteasome inhibition in a neuronal cell line. J Biol Chem. 2002;277:4010–4017. doi: 10.1074/jbc.M104479200. [DOI] [PubMed] [Google Scholar]
- Sun KH, de Pablo Y, Vincent F, Shah K. Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J Neurochem. 2008;107:265–278. doi: 10.1111/j.1471-4159.2008.05616.x. [DOI] [PubMed] [Google Scholar]
- Takasugi T, Minegishi S, Asada A, Saito T, Kawahara H, Hisanaga S. Two degradation pathways of the p35 Cdk5 (cyclin-dependent kinase) activation subunit, dependent and independent of ubiquitination. J Biol Chem. 2016;291:4649–4657. doi: 10.1074/jbc.M115.692871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, et al. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J Biol Chem. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- Tautz J, Maier S, Groh C, Rossler W, Brockmann A. Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc Natl Acad Sci. 2003;100:7343–7347. doi: 10.1073/pnas.1232346100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton NS. The polymerase chain reaction. History, methods, and applications. Diagn Mol Pathol. 1992;1:58–72. doi: 10.1097/00019606-199203000-00008. [DOI] [PubMed] [Google Scholar]
- Thomaz JM, Martins ND, Monteiro DA, Rantin FT, Kalinin AL. Cardio-respiratory function and oxidative stress biomarkers in Nile tilapia exposed to the organophosphate insecticide trichlorfon (NEGUVON®) Ecotoxicol Environ Saf. 2009;72:1413–1424. doi: 10.1016/j.ecoenv.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Trunova S, Giniger E. Absence of the Cdk5 activator p35 causes adult-onset neurodegeneration in the central brain of Drosophila. Dis Model Mech. 2012;5:210–219. doi: 10.1242/dmm.008847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Yang G. Harm of introducing the western honeybee Apis mellifera L. to the Chinese honeybee Apis cerana F. and its ecological impact. Acta Entomol Sin. 2005;48:401–406. [Google Scholar]
- Zhang P, et al. Cdk5-dependent activation of neuronal inflammasomes in Parkinson’s disease. Mov Disord. 2016;31:366–376. doi: 10.1002/mds.26488. [DOI] [PubMed] [Google Scholar]
- Zhao HX, Zeng XN, Liang Q, Zhang XF, Huang WZ, Chen HS, Luo YX. Study of the obp5 gene in Apis mellifera ligustica and Apis cerana cerana. Genet Mol Res. 2015;14:6482–6494. doi: 10.4238/2015.June.12.1. [DOI] [PubMed] [Google Scholar]
- Zhu J, Li W, Mao Z. Cdk5: mediator of neuronal development, death and the response to DNA damage. Mech Ageing Dev. 2011;132:389–394. doi: 10.1016/j.mad.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 153 kb)