Abstract
African clawed frogs (Xenopus laevis) endure bouts of severe drought in their natural habitats and survive the loss of approximately 30% of total body water due to dehydration. To investigate molecular mechanisms employed by X. laevis during periods of dehydration, the heat shock protein response, a vital component of the cytoprotective stress response, was characterized. Using western immunoblotting and multiplex technology, the protein levels of HSP27, HSP40, HSP60, HSP70, HSC70, and HSP90 were quantified in the liver, skeletal muscle, kidney, lung, and testes from control frogs and those that underwent medium or high dehydration (~16 or ~30% loss of total body water). Dehydration increased HSP27 (1.45–1.65-fold) in the kidneys and lungs, and HSP40 (1.39–2.50-fold) in the liver, testes, and skeletal muscle. HSP60 decreased in response to dehydration (0.43–0.64 of control) in the kidneys and lungs. HSP70 increased in the liver, lungs, and testes (1.39–1.70-fold) during dehydration, but had a dynamic response in the kidneys (levels increased 1.57-fold with medium dehydration, but decreased to 0.56 of control during high dehydration). HSC70 increased in the liver and kidneys (1.20–1.36-fold), but decreased in skeletal muscle (0.27–0.55 of control) during dehydration. Lastly, HSP90 was reduced in the kidney, lung, and skeletal muscle (0.39–0.69 of control) in response to dehydration, but rose in the testes (1.30-fold). Overall, the results suggest a dynamic tissue-specific heat shock protein response to whole body dehydration in X. laevis.
Keywords: Xenopus laevis, Dehydration, Stress response, Chaperone, Cytoprotection
Introduction
The African clawed frog (Xenopus laevis) is an aquatic amphibian that endures bouts of dehydration due to seasonal droughts. These animals can endure approximately 30% loss of total body water due to dehydration, resulting in increased blood viscosity and impaired oxygen delivery, which equates to prolonged periods of ischemia. Physiological studies on dehydrating X. laevis have shown that dehydration results in increased hematocrit, increased resting heart rate, and increased differences in arterial-venous oxygen content (Hillman 1978; Malik and Storey 2009a). In response to dehydration, X. laevis also elevates levels of extracellular ammonia and urea to combat further water loss (Balinsky et al. 1967).
In order to endure arid environmental conditions, dehydration-tolerant vertebrates modulate cell signaling pathways to preserve and stabilize organs, cells, and macromolecules over many weeks (Storey and Storey 2012). When faced with severe dehydration, African clawed frogs activate Nrf2 and Foxo-1 mediated transcription of antioxidant enzymes (Malik and Storey 2009b; Malik and Storey 2011). An increase in GST, AKR, MnSOD, and catalase enzyme levels would promote survival in periods of extreme dehydration and rehydration by helping combat reactive oxygen species during ischemia and reperfusion. Another main cellular response to dehydration reported in X. laevis is the activation of extracellular signal-transduction kinases that activate downstream transcription factors using reversible protein phosphorylation (Malik and Storey 2009a). At the post-transcriptional level, two studies have reported tissue-specific expression of microRNAs in response to dehydration. One study identified differentially expressed microRNAs in the liver, kidney, and ventral skin of dehydrated frogs which are known to regulate the expression of solute carrier protein and mitogen-activated protein kinase mRNA transcripts (Wu et al. 2013). Furthermore, another study revealed dehydration-induced suppression of select microRNAs in the brain of X. laevis, which is characteristic of a known response that promotes the expression of genes involved in neuroprotection and anti-apoptosis (Luu and Storey 2015). Therefore, it is clear that the African clawed frog employs a wide range of physiological and biochemical responses to cope with dehydration stress. However, the roles that heat shock proteins (HSPs) play in response to medium and high levels of dehydration in X. laevis are not yet known.
Heat shock proteins are a highly conserved family of protein chaperones present in X. laevis which are involved in the folding and translocation of cellular proteins under normal conditions, and the inhibition of irreversible protein aggregation and/or misfolding during stress conditions (Heikkila 2010; Storey and Storey 2011). Early developmental studies have documented that hsp27 mRNA was not detectable during Xenopus oogenesis (Heikkila 2010). Although hsp27 mRNA was first reported in early tailbud embryos, stress-induced HSP27 protein expression was not detected until the tadpole stage (Tuttle et al. 2007). This may suggest that HSP27 could be important for enhancing cytoprotection in adult amphibians over the early developmental stages. Furthermore, HSP60, a chaperone found in mitochondria, also appears to play a protective role in modulating and regulating the process of Xenopus limb regeneration (Pearl et al. 2008). HSP70 expression is sensitive to various stressors, such as sodium arsenite, herbimycin A, hydrogen peroxide, cadmium, and ethanol (Wolffe et al. 1984). HSC70 is the constitutively expressed member of the HSP70 family and hsc70 mRNA was shown to be present in a number of adult Xenopus tissues (Ali et al. 1996). Characterization of hsp90 has revealed that it is a highly conserved gene that is constitutively expressed in X. laevis (Heikkila 2010). Lastly, HSP40 is a chaperone protein that has been previously shown to complex with other HSPs to regulate protein folding and cell signaling (Greene et al. 1998; Cajo et al. 2006).
Although HSPs have been extensively characterized in X. laevis in developmental studies, this is the first study to characterize the HSP response to dehydration in adult animals. On par with the dynamic roles in development described above, we hypothesized that HSPs may also play a role in dehydration tolerance and survival in adult African clawed frogs. Using western immunoblotting and Luminex multiplex technology, this study characterizes the expression levels of HSP27, HSP40, HSP60, HSP70, HSC70, and HSP90 in five tissues of adult X. laevis exposed to medium and high levels of dehydration.
Methods
Animals
Adult male African clawed frogs (X. laevis) were purchased from a colony from the University of Toronto and upon delivery, the frogs were acclimatized in tanks of dechlorinated water at 22 ± 1 °C for 3 weeks prior to the start of experiments. Frogs were fed 3–4 pellets of CU Adult Frog diet (PMI Nutrition International) three times/week and water was changed the day after each feeding. Frogs were then randomly divided into groups of control, medium dehydration, and high dehydration conditions, where they were not fed again. For the dehydration experiments, the frogs were weighed and placed into dry containers at 22 ± 1 °C where water was lost through evaporation over time. Animals were weighed at approximately 12 h intervals to determine body water loss due to evaporation. To quantify the extent of dehydration, the percentage of total body water lost was calculated as follows:
where m i, m d, and BWCi are the initial mass, dehydrated mass, and initial body water content of X. laevis frogs, respectively. For the purpose of this study, BWCi of X. laevis frogs was considered to be 0.74 ± 0.02 g H20 per gram body mass as previously determined (Malik and Storey 2009a). Animals in the medium and high dehydration groups were sampled when mean total body water loss reached ~16 and ~30%, respectively. The final mean percentages for total body water loss were 16.43 ± 0.33% SEM for medium dehydration and 31.18 ± 0.83% SEM for high dehydration. All frogs were euthanized swiftly by pithing, and tissues were rapidly dissected, frozen in liquid nitrogen, and stored at −80 °C until use. Four independent biological replicates of the liver, skeletal muscle, kidney, lung, and testes were used for this study.
Animals were cared for in accordance with the guidelines of the Canadian Council on Animal Care and all experimental procedures had the prior approval of the Carleton University Animal Care Committee.
Protein extraction
Protein extracts were prepared as previously described for relative protein quantification with Luminex multiplex technology and western blotting, with few alterations (Logan et al. 2016). Briefly, 50–100 mg of tissue was homogenized 1:3 w:v with a glass homogenizer in Lysis buffer (EMD Millipore, Cat. No. 43-010) combined with various phosphatase and protease inhibitors added immediately before use (1 mM Na3VO4, 10 mM NaF, 10 mM β-glycerophosphate, and 10 μL/mL protease inhibitor cocktail (Cat. No. PIC001, BioShop). After homogenization, the samples were incubated on ice for 30 min with intermittent vortexing every 10 min and then centrifuged according to the manufacturer’s guidelines at 10,000 rpm for 20 min at 4 °C. The supernatant from each sample was collected, and protein concentration was determined using a Bradford protein assay (Biorad, Cat. No. 500-0006). Protein concentrations were standardized for each sample to 10 μg/μL with lysis buffer and a subsequent Bradford assay was done to verify the normalized concentrations.
Multiplex analysis
Relative protein quantification was performed for three heat shock proteins (HSP27, HSP60, and HSP70) using Luminex multiplex analysis following the manufacturer’s instructions (EMD Millipore; Cat. No 48-615MAG). A number of protein amounts (50–300 ng range) were tested initially to assess the limits of detection and the ideal protein amount for quantification to prevent the propensity of signal saturation. For quantification, total soluble protein extracts were diluted with Assay Buffer 1 (EMD Millipore, Cat. No. 43-010) to a final protein amount of 150 ng. Unstimulated HeLa cells as a negative control (Cat. No. 47-205), HS/Ars-treated HeLa cells as a positive control (Cat. No. 47-211), and three blank wells were also run alongside the test samples.
Premixed HSP panel magnetic beads (20×) were sonicated for 15 s, vortexed for 30 s, and diluted to 1× by combining 0.150-mL beads with 2.85 mL of Assay Buffer 1. Prior to use, the diluted beads were further sonicated for 10 s and 25 μL was added to all test wells (including sample wells, blanks, positive and negative controls). Twenty-five microliters of diluted samples (150 ng of protein) and 25 μL of prepared positive and negative controls were added to the appropriate wells, and 25 μL of Assay Buffer 1 was added to the blank wells. The wells were then incubated overnight at 4 °C on an orbital shaker (600–800 rmp) away from light. The next day, the magnetic beads were held with a handheld magnetic plate holder and washed three times with Assay Buffer 1. Then 25 μL/well of diluted detection antibody was added, and wells were sealed and incubated on an orbital shaker (600–800 rpm) for 1 h at room temperature (25 °C). Detection antibody was prepared according to manufacturer’s instructions in which 20× stock of Milliplex Detection antibody was vortexed for 10 s, briefly centrifuged, and diluted to 1× by combining 0.150 mL of detection antibody with 2.85 mL of Assay Buffer 1. Detection antibody was decanted and 25 μL of 1× Streptravidin-Phycoerythrin (SAPE) was subsequently added to each well and incubated with agitation on an orbital shaker (600–800 rpm) for 15 min. 1× SAPE was prepared by combining 30 μL of SAPE with 2.97 mL of Assay Buffer 1. Upon 15-min incubation, without removing SAPE, 25 μL of Amplification Buffer was added to each well, incubated on an orbital shaker at room temperature (25 °C) for 15 min. Post incubation, SAPE + Amplification Buffer was removed and the beads were resuspended in 150 μL of Assay Buffer 1 and analyzed using the Luminex 200 system (Luminex, Austin, TX) using the following parameters: events 50 beads, sample size 100 μL, and gate settings 8000 to 15,000.
Western immunoblotting
Protein extracts prepared as described above were combined with 2× SDS buffer (100 mM Tris-base, 4% w/v SDS, 20% v/v glycerol, 0.2% w/v bromophenol blue, 10% v/v 2-mercaptoethanol, pH 6.8) in a 1:1 v:v ratio, boiled for 10 min, and cooled on ice. Western blotting was performed as previously described (Luu et al. 2015). Briefly, protein samples treated with SDS buffer were loaded onto 8–10% SDS-polyacrylamide gels and resolved at 180 V for 45–90 min using a BioRad Mini-Protean 3 System. Gels were then transferred to 0.45 μM PVDF membranes (EMD Millipore, Cat. No. IPVH00010) by electroblotting at 160 mA for 90 min in transfer buffer (25 mM Tris-base, pH 8.5, 192 mM glycine, 10% v/v methanol). Membranes were washed in TBST (20 mM Tris-base, pH 7.6, 140 mM NaCl, 0.05% v/v Tween-20) for 3 × 5 min, before being blocked with 3–5% w/v powdered milk in TBST for 30 min at room temperature. Membranes were washed again for 3 × 5 min in TBST and probed with primary antibody diluted 1:1000 v/v TBST overnight at 4 °C. Primary antibodies targeting HSP40 (Genscript, Cat. No. AO1241-40), HSC70 (Genetex, Cat. No. GTX111069), and HSP90 (Genetex, Cat. No. GTX109753) were used for this analysis. Membranes were washed again for 3 × 5 min in TBST and probed with HRP-linked anti-rabbit IgG secondary antibody (Bioshop, Cat. No APA007P) diluted 1:4000–8000 v/v TBST for 30–60 min. Membranes were washed once more for 3 × 5 min and visualized by enhanced chemiluminescence (H2O2 and luminol) using a ChemiGenius Bio-Imaging System (Syngene, Frederick, MD). Following exposure, membranes were stained with Coomassie blue (0.25% w/v Coomassie brilliant blue, 7.5% v/v acetic acid, 50% methanol) to visualize all protein bands.
Quantification and statistics
Relative protein levels for HSP27, HSP60, and HSP70 were determined from Luminex multiplex analysis by calculating the blank-subtracted median fluorescence intensity (MFI) for each independent biological replicate within each experimental condition. Quantification of HSP40, HSC70, and HSP90 was performed with western immunoblotting, and subsequent analysis was done with a ChemiGenius Bio-Imaging system (Syngene, Frederick, MD) and the accompanying GeneTools software. Band intensities from immunoblots were standardized against the total amount of protein represented by the intensity of Coomassie blue-stained proteins on the membrane (Wijenayake and Storey 2016). Results from both multiplex and immunoblotting were expressed as mean ± SEM. Statistically significant differences from the control were determined with a one-way ANOVA with Dunnett’s post hoc test, which was performed with SigmaPlot 12.5 (Systat Software Inc., San Jose, CA).
Results
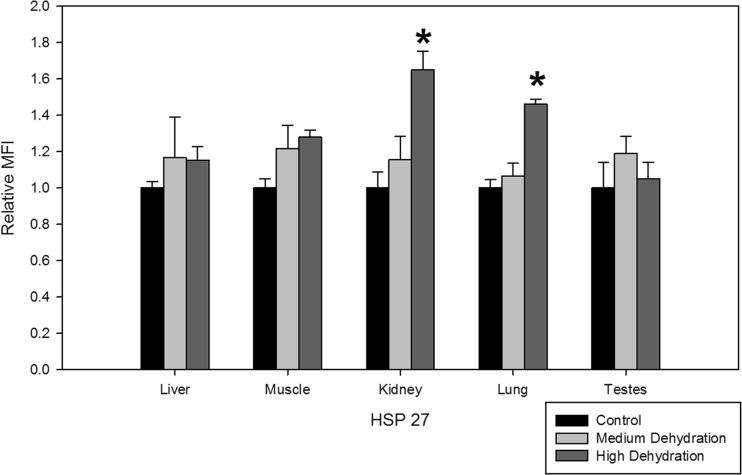
While HSP27 expression did not change during medium dehydration, high dehydration resulted in 1.65 ± 0.10 and 1.46 ± 0.03-fold increase in the kidney and lung tissues, respectively (Fig. 1). Levels of HSP27 in the liver, skeletal muscle, and testes remained unchanged in response to medium and high dehydration.
Fig. 1.
Relative protein expression levels of HSP27 in five X. laevis tissues under control, medium dehydration, and high dehydration conditions. Protein levels are represented as median fluorescence intensity (MFI) units obtained from Luminex multiplex analysis. Data are presented in the histogram as relative MFI ± SEM, where n = 4 independent biological replicates. Statistically significant differences compared to the control were determined with a one-way ANOVA, followed by a post hoc Dunnett’s test, and denoted with an asterisk (*p < 0.05)
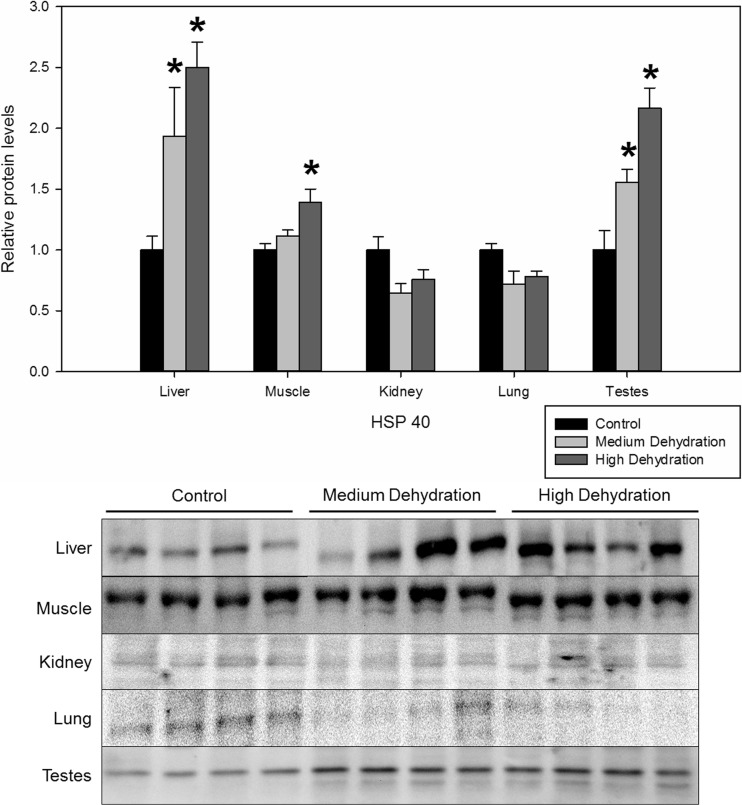
In the liver and testes, HSP40 expression levels increased 1.94 ± 0.40 and 1.56 ± 0.11-fold in response to medium dehydration, respectively (Fig. 2). HSP40 protein levels of the liver, skeletal muscle, and testes increased in response to high dehydration by 2.50 ± 0.21, 1.39 ± 0.11, and 2.17 ± 0.17, respectively, when compared to control levels (Fig. 2). Skeletal muscle HSP40 protein levels did not change with exposure to medium dehydration. The kidney and lung tissues showed limited changes in HSP40 protein levels in response to medium and high dehydration.
Fig. 2.
Relative protein expression levels of HSP40 in five X. laevis tissues under control, medium dehydration, and high dehydration conditions. Protein levels were obtained from western immunoblot signal normalized against protein loaded represented by Coomassie blue-stained proteins on the membrane. Data are presented in the histogram as relative means ± SEM, where n = 4 independent biological replicates. Statistically significant differences compared to the control were determined with a one-way ANOVA, followed by a post hoc Dunnett’s test, and denoted with an asterisk (*p < 0.05)
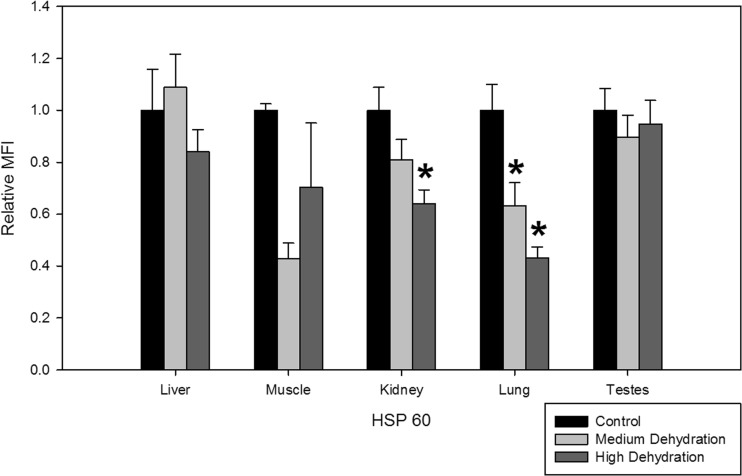
Dehydration resulted in suppression of HSP60 protein levels in the lung and kidney tissues of X. laevis. For lung tissues exposed to medium and high dehydration, HSP60 protein levels decreased to 0.63 ± 0.09 and 0.43 ± 0.04, respectively, compared to control levels (Fig. 3). In the kidney, HSP60 protein levels decreased to 0.64 ± 0.05 with exposure to high dehydration, but did not change with medium dehydration (Fig. 3). Dehydration did not induce any changes in relative HSP60 protein levels in the liver, skeletal muscle, or testes.
Fig. 3.
Relative protein expression levels of HSP60 in five X. laevis tissues under control, medium dehydration, and high dehydration conditions. Other information as in Fig. 1
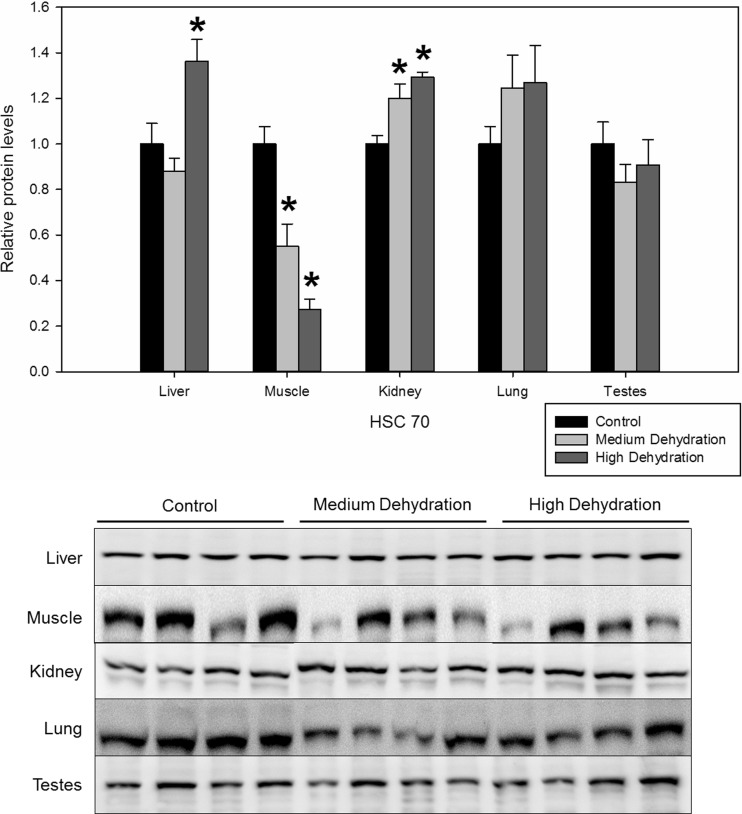
HSC70 was differentially regulated in the liver, skeletal muscle, and kidney. In the liver, HSC70 protein levels increased to 1.36 ± 0.10 with high dehydration exposure (Fig. 4) compared to the control conditions. In skeletal muscle, HSC70 expression was suppressed throughout medium and high dehydration to 0.55 ± 0.10 and 0.27 ± 0.05 of control levels, respectively (Fig. 4). On the contrary, HSC70 increased by 1.20 ± 0.06 and 1.29 ± 0.02-fold compared to control levels in the kidney, with exposure to medium and high dehydration, respectively (Fig. 4). Dehydration did not induce any changes in HSC70 protein levels in the lung or testes.
Fig. 4.
Relative protein expression levels of HSC70 in five X. laevis tissues under control, medium dehydration, and high dehydration conditions. Other information as in Fig. 2
In the liver, kidney, lung, and testes, dehydration induced differential HSP70 protein expression (Fig. 5). The liver and lung HSP70 significantly increased by 1.70 ± 0.13 and 1.51 ± 0.15-fold of control levels with exposure to high dehydration (Fig. 5). In the testes, HSP70 increased by 1.39 ± 0.06 during medium dehydration and remained elevated during high dehydration with a 1.48 ± 0.11-fold increase over relative control levels. Kidney HSP70 levels increased during medium dehydration by 1.57 ± 0.08-fold, but decreased to 0.56 ± 0.08 of control levels with exposure to high dehydration (Fig. 5). Protein levels of skeletal muscle HSP70 did not change with in response to dehydration exposure.
Fig. 5.
Relative protein expression levels of HSP70 in five X. laevis tissues under control, medium dehydration, and high dehydration conditions. Other information as in Fig. 1
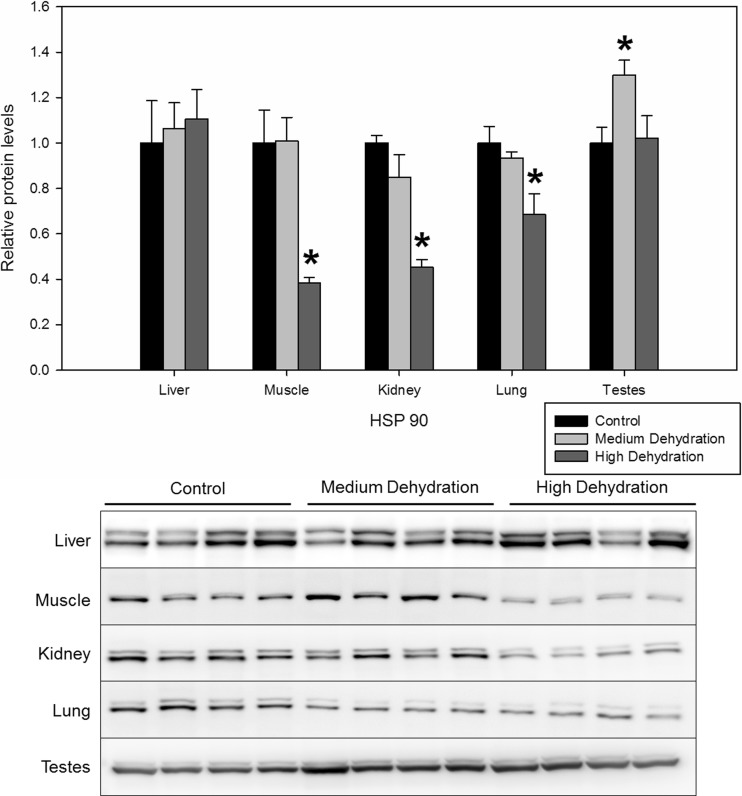
Lastly, both forms of HSP90 (HSP90α and HSP90β) were detected by immunoblotting and were quantified together to assess total HSP90 levels. In the skeletal muscle, kidney, and lung tissues, HSP90 protein levels remained unchanged with medium dehydration, but decreased with high dehydration exposure to 0.39 ± 0.02, 0.45 ± 0.03, and 0.68 ± 0.09 of control levels, respectively (Fig. 6). In the testes, while there were no changes in HSP90 protein levels between control and high dehydration conditions, a 1.30 ± 0.0-fold increase was observed with exposure to medium dehydration (Fig. 6). There were no dehydration-induced changes in HSP90 protein levels in the liver.
Fig. 6.
Relative protein expression levels of HSP90 in five X. laevis tissues under control, medium dehydration, and high dehydration conditions. Other information as in Fig. 2
Discussion
HSP70 and HSC70 expression in response to dehydration was highly tissue-specific and perhaps plays a role in osmoregulation, since HSPs have been shown to play an important role in the trafficking and expression of sodium ion channels (Goldfarb et al. 2006). In one study, overexpression of human hsc70 in Xenopus oocytes was shown to decrease the function and expression of sodium channels (Goldfarb et al. 2006). However, if the expression was accompanied by a moderate expression of hsp70, sodium ion channels retained function and increased in expression (Goldfarb et al. 2006). This suggests that hsc70 and hsp70 levels are tightly controlled to regulate sodium ion channels, a potential mechanism that African clawed frogs may use in osmoregulation during prolonged dehydration. In the present study, dehydration induced a suppression of HSC70 expression in the skeletal muscle, and an increase in HSP70 protein levels in the liver, lung, and kidney. This tissue-specific differential expression of HSP70 and HSC70 may represent a chaperone protein-mediated regulation of sodium ion channel expression and function. As such, regulation of HSP70 and HSC70 may potentially play critical roles in X. laevis dehydration tolerance. In addition to targeting sodium transport channels, HSPs facilitate kidney-specific osmoregulation. An increase in kidney HSC70 during both stages of X. laevis dehydration may contribute to osmoregulation by promoting aquaporin-2 (AQP2) function. AQP2 is a protein channel found in the collecting ducts of the kidneys, which functions to resorb water from the kidneys back into the bloodstream (Brown 2003). Previous work has shown that vasopressin induces a co-localization of HSP70 and AQP2 on the apical membrane of principal cells in rat kidney collecting ducts (Lu et al. 2007). Although knocking down HSC70 still results in AQP2 accumulation on membranes, protein channel function becomes impaired, which exemplifies the importance of this chaperone protein in maintaining AQP2 structure and kidney function in dehydrated X. laevis (Lu et al. 2007). The results may suggest that increases in kidney HSC70 protein levels are part of the cellular response to impede body water loss in X. laevis by elevating AQP2 protein activity. Although the present study demonstrates that HSP70 and HSC70 are regulated in a way that would promote osmoregulation and water retention in X. laevis, further studies are warranted to characterize the regulation of ion channels and aquaporins.
In the present study, the relative protein levels of HSP40 significantly increased during dehydration in the liver, muscle, and testes. HSP40 is a co-chaperone that closely interacts with HSP70 to fold, assemble, and translocate proteins across membranes (Kampinga and Craig 2010). HSP40 assists HSP70 by binding and transferring substrate targets to HSP70, in addition to assisting HSP70 with ATP hydrolysis required for chaperone function (Greene et al. 1998; Suh et al. 1998; Cajo et al. 2006). The dehydration-induced upregulation of HSP40 observed in this study may suggest that co-chaperones may be produced to assist other HSPs (such as HSP70) during periods of dehydration stress exposure in X. laevis.
Another HSP that showed a robust response to dehydration exposure was HSP27, which increased in the lungs and kidneys. Throughout dehydration, these two organs are particularly susceptible to stress in anurans, as the act of breathing accelerates dehydration, whereas the kidneys deal with the burden of osmoregulation. HSP27 has been shown to play a pro-survival role during cellular stresses by inhibiting the formation of apoptosomes. In this scenario, HSP27 inhibits apoptosome formation by directly interacting with cytochrome c and caspase-3 (Garrido et al. 2000; Concannon et al. 2001). An increase in HSP27 in these two tissues may suggest that not only are protein turnover rates reduced through general chaperone activity, but cell death signaling is also suppressed in order to maintain cellular homeostasis. Correspondingly, a previous study has shown a decrease in microRNAs that target anti-apoptotic proteins and thereby facilitate a pro-survival response in the brain of X. laevis during dehydration (Luu and Storey 2015).
Contrary to other HSPs, dehydration caused HSP90 and HSP60 protein levels to decrease in the skeletal muscle, kidney, and lung tissues. Dehydration-induced suppression in HSP90 may suggest that a general HSP response is activated during dehydration. This is because HSP90 is known to be highly expressed during periods of non-stress (Heikkila 2010) to bind and inhibit the activity of heat shock factor 1 (HSF-1), the vital transcription factor that is involved in transcription of hsp genes (Zou et al. 1998). This function is specific to HSP90 as it was shown that reductions in HSP70, HSC70, or HSP27 levels did not dramatically activate HSF-1 (Zou et al. 1998). This suggests that HSP90 expression may be downregulated in the muscle, kidney, and lungs during dehydration to facilitate HSF-1 transcriptional activity. However, it should be noted that changes in relative HSP protein levels, particularly the constitutively expressed members, may also be affected by other factors such as modifications to the proteasome degradation system that may occur during dehydration. HSP90 protein levels increased in the testes of X. laevis in response to dehydration. One study has characterized a testis-specific mechanism for HSP90 in the binding, stabilization, and activation of testis-specific serine/threonine kinases (TSSKs) (Jha et al. 2013). The TSSK family is a group of post-meiotic kinases expressed in spermatids and is crucial for proper spermiogenesis (Xu et al. 2008). Downregulating HSP90 function has been shown to prevent TSSK4 and TSSK6 catalytic activity and increase TSSK ubiquitination and proteasomal degradation (Jha et al. 2013). Therefore, the observed upregulation of HSP90 may represent a testis-specific response to dehydration, but given that the roles of TSSKs are far from fully understood, more studies are warranted to further elucidate interaction between HSP90 and TSSKs in the testes.
Lastly, the mitochondrial chaperone, HSP60, decreased in expression in the kidney and lungs in response to dehydration. Although there are contradictory studies which disagree on the role of HSP60, some studies suggest a pro-survival role (Ghosh et al. 2008; Cappello et al. 2008), whereas others have shown that HSP60 mediates a pro-apoptotic mechanism by cleaving and activating pro-caspase 3 (Samali et al. 1999; Xanthoudakis et al. 1999). If the latter is the case, suppression in HSP60 in the kidney and lungs is consistent with increases in HSP27 in these tissues to mediate a general pro-survival response.
In summary, the present study showed that six HSPs were differentially regulated in five different tissues of X. laevis under medium and high dehydration conditions. Changes in protein levels of HSP70 and HSC70 suggest that they may be responding to dehydration by regulating ion channels and aquaporin proteins, which are important in osmoregulation. Co-chaperones such as HSP40 that were increased in response to dehydration may be assisting other HSPs in substrate binding and ATP hydrolysis. An increase in HSP27 and a decrease in HSP60 may mutually target crucial proteins in apoptosis and promote a pro-survival response in the kidneys and lungs. Lastly, HSP90 downregulation may facilitate HSF-1-induced gene expression in multiple tissues, with the exception of the testes, where HSP90 may play a unique role in regulating testis-specific kinase activity. The results suggest that HSPs may play an important role in X. laevis dehydration response, but additional studies are warranted to understand how key targets in osmoregulation and anti-apoptosis are regulated in these dehydration-tolerant animals.
Acknowledgements
We thank Jan Storey for the editorial review of this article. This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant (#6793) to KBS. BEL holds a NSERC Canada Graduate Research Scholarship, SW holds a postgraduate Queen Elizabeth II Graduate Scholarship in Science and Technology, and KBS holds the Canada Research Chair in Molecular Physiology.
Author contributions
BEL and SW are co-first authors. BEL conceived, designed, performed the experiments, analyzed the data, and wrote the paper. SW conceived, designed, performed the experiments, analyzed the data, and wrote the paper. AIM conceived and performed some of the experiments. KBS contributed reagents, materials, and wrote the paper.
References
- Ali A, Salter-Cid L, Flajnik MF, Heikkila JJ. Isolation and characterization of a cDNA encoding a Xenopus 70-kDa heat shock cognate protein, Hsc70.I. Comp Biochem Physiol B Biochem Mol Biol. 1996;113:681–687. doi: 10.1016/0305-0491(95)02081-0. [DOI] [PubMed] [Google Scholar]
- Balinsky JB, Choritz EL, Coe CG, van der Schans GS. Amino acid metabolism and urea synthesis in naturally aestivating Xenopus laevis. Comp Biochem Physiol. 1967;22:59–68. doi: 10.1016/0010-406X(67)90166-1. [DOI] [PubMed] [Google Scholar]
- Brown D. The ins and outs of aquaporin-2 trafficking. Am J Physiol Ren Physiol. 2003;284:F893–F901. doi: 10.1152/ajprenal.00387.2002. [DOI] [PubMed] [Google Scholar]
- Cajo GC, Horne BE, Kelley WL, et al. The role of the DIF motif of the DnaJ (Hsp40) co-chaperone in the regulation of the DnaK (Hsp70) chaperone cycle. J Biol Chem. 2006;281:12436–12444. doi: 10.1074/jbc.M511192200. [DOI] [PubMed] [Google Scholar]
- Cappello F, Conway de Macario E, Marasà L, et al. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol Ther. 2008;7:801–809. doi: 10.4161/cbt.7.6.6281. [DOI] [PubMed] [Google Scholar]
- Concannon CG, Orrenius S, Samali A. Hsp27 inhibits cytochrome c-mediated caspase activation by sequestering both pro-caspase-3 and cytochrome c. Gene Expr. 2001;9:195–201. doi: 10.3727/000000001783992605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Bruey J-M, Ducasse C, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. J Biol Chem. 2008;283:5188–5194. doi: 10.1074/jbc.M705904200. [DOI] [PubMed] [Google Scholar]
- Goldfarb SB, Kashlan OB, Watkins JN, et al. Differential effects of Hsc70 and Hsp70 on the intracellular trafficking and functional expression of epithelial sodium channels. Proc Natl Acad Sci U S A. 2006;103:5817–5822. doi: 10.1073/pnas.0507903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene MK, Maskos K, Landry SJ. Role of the J-domain in the cooperation of Hsp40 with Hsp70. Proc Natl Acad Sci U S A. 1998;95:6108–6113. doi: 10.1073/pnas.95.11.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila JJ. Heat shock protein gene expression and function in amphibian model systems. Comp Biochem Physiol A Mol Integr Physiol. 2010;156:19–33. doi: 10.1016/j.cbpa.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Hillman SS. The roles of oxygen delivery and electrolyte levels in the dehydrational death of Xenopus laevis. J Comp Physiol B. 1978;128:169–175. doi: 10.1007/BF00689481. [DOI] [Google Scholar]
- Jha KN, Coleman AR, Wong L, et al. Heat shock protein 90 functions to stabilize and activate the testis-specific serine/threonine kinases, a family of kinases essential for male fertility. J Biol Chem. 2013;288:16308–16320. doi: 10.1074/jbc.M112.400978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SM, Luu BE, Storey KB. Turn down genes for WAT? Activation of anti-apoptosis pathways protects white adipose tissue in metabolically depressed thirteen-lined ground squirrels. Mol Cell Biochem. 2016;416:47–62. doi: 10.1007/s11010-016-2695-0. [DOI] [PubMed] [Google Scholar]
- Lu HAJ, Sun TX, Matsuzaki T, et al. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem. 2007;282:28721–28732. doi: 10.1074/jbc.M611101200. [DOI] [PubMed] [Google Scholar]
- Luu BE, Storey KB. Dehydration triggers differential microRNA expression in Xenopus laevis brain. Gene. 2015;573:64–69. doi: 10.1016/j.gene.2015.07.027. [DOI] [PubMed] [Google Scholar]
- Luu BE, Tessier SN, Duford DL, Storey KB. The regulation of troponins I, C and ANP by GATA4 and Nkx2-5 in heart of hibernating thirteen-lined ground squirrels, Ictidomys tridecemlineatus. PLoS One. 2015;10:e0117747. doi: 10.1371/journal.pone.0117747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AI, Storey KB (2009a) Activation of extracellular signal-regulated kinases during dehydration in the African clawed frog, Xenopus laevis [DOI] [PubMed]
- Malik AI, Storey KB. Activation of antioxidant defense during dehydration stress in the African clawed frog. Gene. 2009;442:99–107. doi: 10.1016/j.gene.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Malik AI, Storey KB. Transcriptional regulation of antioxidant enzymes by FoxO1 under dehydration stress. Gene. 2011;485:114–119. doi: 10.1016/j.gene.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Pearl EJ, Barker D, Day RC, Beck CW. Identification of genes associated with regenerative success of Xenopus laevis hindlimbs. BMC Dev Biol. 2008;8:66. doi: 10.1186/1471-213X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Cai J, Zhivotovsky B, et al. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of Jurkat cells. EMBO J. 1999;18:2040–2048. doi: 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey KB, Storey JM. Heat shock proteins and hypometabolism: adaptive strategy for proteome preservation. Res Rep Biol. 2011;2:57–68. doi: 10.2147/RRB.S13351. [DOI] [Google Scholar]
- Storey KB, Storey JM. Aestivation: signaling and hypometabolism. J Exp Biol. 2012;215:1425–1433. doi: 10.1242/jeb.054403. [DOI] [PubMed] [Google Scholar]
- Suh WC, Burkholder WF, Lu CZ, et al. Interaction of the Hsp70 molecular chaperone, DnaK, with its cochaperone DnaJ. Proc Natl Acad Sci U S A. 1998;95:15223–15228. doi: 10.1073/pnas.95.26.15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle AM, Gauley J, Chan N, Heikkila JJ. Analysis of the expression and function of the small heat shock protein gene, hsp27, in Xenopus laevis embryos. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:112–121. doi: 10.1016/j.cbpa.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Wijenayake S, Storey KB. The role of DNA methylation during anoxia tolerance in a freshwater turtle (Trachemys scripta elegans) J Comp Physiol B. 2016;186:333–342. doi: 10.1007/s00360-016-0960-x. [DOI] [PubMed] [Google Scholar]
- Wolffe AP, Glover JF, Tata JR. Culture shock. Synthesis of heat-shock-like proteins in fresh primary cell cultures. Exp Cell Res. 1984;154:581–590. doi: 10.1016/0014-4827(84)90182-4. [DOI] [PubMed] [Google Scholar]
- Wu CW, Biggar KK, Storey KB. Dehydration mediated microRNA response in the African clawed frog Xenopus laevis. Gene. 2013;529:269–275. doi: 10.1016/j.gene.2013.07.064. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S, Roy S, Rasper D, et al. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 1999;18:2049–2056. doi: 10.1093/emboj/18.8.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Hao Z, Jha KN, et al. Targeted deletion of Tssk1 and 2 causes male infertility due to haploinsufficiency. Dev Biol. 2008;319:211–222. doi: 10.1016/j.ydbio.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, et al. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/S0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]