Abstract
The cardiac microvascular reperfusion injury is characterized by the microvascular endothelial cells (CMECs) oxidative damage which is responsible for the progression of cardiac dysfunction. However, few strategies are available to reverse such pathologies. This study aimed to explore the mechanism by which oxidative stress induced CMECs death and the beneficial actions of melatonin on CMECs survival, with a special focused on IP3R-[Ca2+]c/VDAC-[Ca2+]m damage axis and the MAPK/ERK survival signaling. We found that oxidative stress induced by H2O2 significantly activated cAMP response element binding protein (CREB) that enhanced IP3R and VDAC transcription and expression, leading to [Ca2+]c and [Ca2+]m overload. High concentration of [Ca2+]m suppressed ΔΨm, opened mPTP, and released cyt-c into cytoplasm where it activated mitochondria-dependent death pathway. However, melatonin could protect CMECs against oxidative stress injury via stimulation of MAPK/ERK that inactivated CREB and therefore blocked IP3R/VDAC upregulation and [Ca2+]c/[Ca2+]m overload, sustaining mitochondrial structural and function integrity and ultimately blockading mitochondrial-mediated cellular death. In summary, these findings confirmed the mechanisms by which oxidative injury induced CMECs mitochondrial-involved death and provided an attractive and effective way to enhance CMECs survival.
Keywords: Melatonin, Endothelial, IP3R, VDAC, Calcium overload, Apoptosis, Reperfusion injury
Introduction
Acute myocardial infarction (AMI) is featured by a sudden occlusion of epicardial coronary artery, leading to the reduction in blood stream and ischemic damage to the heart. Timely percutaneous coronary intervention (PCI) is a mainstay in the current treatment of AMI through dredging occlusive vessel. However, even after successful reperfusion therapy, at the coronary microvascular level, there remains diminished patency rates or even no-perfusion that known as no-reflow (NR) phenomenon which is associated with reduced clinical improvement and increased 30-day mortality if not adequately treated. Extensive studies have demonstrated that microcirculation oxidative damage is the main factor for the formation of NR, especially that cardiac microvascular endothelial cell (CMEC) injury. Reperfusion is associated with a much larger burst of lethal ROS (even though ROS initially appeared at the stage of hypoxia or ischemia) when compared with the level of ROS at the stage of hypoxia. Admittedly, superfluous ROS induced by reperfusion injury could cause oxidative stress injury through lipid peroxidation and protein oxidative which induce CMEC mitochondrial damage and cellular apoptosis, contributing to the capillaries relaxation dysfunction or obstruction, inflammation, and distal embolization of atheromatous. Thereby, strategies to protect the CMEC against oxidative injury and maintain its normal function may be the crucially adjuvant modality to patients after PCI therapy.
Among oxidative stress-mediated cellular apoptosis, mitochondria are reported to be the transmitter of apoptotic signal through delivering cytochrome c (cyt-c) into cytoplasm (Zhou et al. 2014). However, the upstream trigger of mitochondrial damage is far from clear. Several evidences have established the role of cytoplasmic calcium ([Ca2+]c) overload and the mitochondrial calcium ([Ca2+]m) overload in cell death (Hurst et al. 2016). This paper would explore the detailed role of [Ca2+]c/[Ca2+]m overload in the activation of mitochondrial-dependent cellular apoptosis pathways.
Melatonin (N-acetyl-5-methoxytryptamine) is an endogenous indolamine that has antioxidant, anti-inflammatory, and anti-apoptotic properties (Alonso-Alconada et al. 2013). It reduces perinatal hypoxia-ischemia, improves sleep and comorbid disorders (Laudon and Frydman-Marom 2014), and ameliorates cardiac ischemia/reperfusion injury (Reiter et al. 2016a). However, the beneficial role of melatonin in CMEC apoptosis remains clear. Recently studies have found that MAPK/ERK functions as the classic anti-apoptotic signal (Gaspar et al. 2016) exerting a key role in stem cells survival and growth (Choi 2016; Park et al. 2016). Whether melatonin contributes to CMEC survival via MAPK/ERK pathways remains unknown. In our study, we applied hydrogen peroxide (H2O2), to induce CMECs apoptosis and explored the effect and mechanism of melatonin on the apoptosis of CMEC. The result indicated that exogenous H2O2 could evoke CMECs apoptosis through mitochondrial death pathway by induction of inositol 1,4,5-triphate receptor (IP3R)-mediated [Ca2+]c overload and subsequent voltage-dependent anion channel (VDAC)-mediated [Ca2+]m overload. While melatonin partially abolished such effect of H2O2 via activation of MAPK/ERK pathways, which suppressed IP3R-[Ca2+]c/VDAC/[Ca2+]m apoptotic axis by downregulation of CREB transcription factors, enhancing the resistance of mitochondria and CMECs to oxidative stress.
Materials and methods
The present study was performed in accordance with Declaration of Helsinki and the guidelines of the Ethic Committee of Chinese PLA (People’s Liberty Army) General Hospital, Beijing, China.
CMECs isolation, culture, characterization, and differentiation
CMECs were isolated from neonatal SD rat (aged 5–7 days, weight 12–16 g) hearts by enzyme dissociation method based on our previous study. Briefly, neonatal rats were euthanized by isoflurane and the hearts were removed under sterile conditions. After removing atria, visible connective tissue, right ventricle, and valvular tissue, left ventricle was immersed in 75% ethanol for 20 s to devitalize epicardial and endocardial endothelial cells. The outer one fourth ventricular wall was peeled away. The remaining tissue was digested in 0.5% (w/v) collagenase type I (Gibco, USA) for 20 min and 0.125% (w/v) trypsin (Hyclone, USA) for 10 min at 37 °C in a shaking bath. After addition of Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA), the samples were filtered with 70-nm metal mesh filter followed by centrifugation for 10 min at 400×g and subsequently resuspended in DMEM supplemented with 20% (v/v) fetal bovine serum (FBS) (Hyclone, USA). Then the supernatant were seeded in polystyrene flasks at 37 °C/5% CO2. Cultured cell purity was tested by their morphology, positive immunofluorescence assay of CD31 (Abcam, #ab61910), and uptake of acetylated low-density lipoprotein.
Induction of oxidative stress injury
Oxidative stress apoptosis was induced by H2O2 and serum deprivation. In brief, after cells were washed with PBS, the culture media was replaced with serum-free DMEM supplemented with 0.3 mM H2O2 and then at 37 °C under normoxic conditions for 12 h. For melatonin protection assay, CMECs were pretreated with melatonin (0–10 μm/L) (Han et al. 2016) 12 h before the H2O2-mediated oxidative stress apoptosis.
MTT assay, LDH release, and caspase3 activity
Cell viability was assessed using the 3-(4,5-dimethylthiazohl-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, USA) assay. Briefly, CMECs were seeded in 96-well plates. After treatment with melatonin for 12 h, the cells were incubated with MTT solution (Sigma-Aldrich) at 37 °C for 4 h. Then, the medium was removed, and 100 μL of dimethyl sulfoxide (DMSO) was added to each well. The absorbance was measured at a wavelength of 570 nm. The data are expressed as the ratio of the optical density (OD) value of the treated group to the OD of the control group.
Because the activation of caspase3 represents an essential step in the apoptotic process and LDH release was a consequence of cellular integrity damage during apoptosis process. Thus, caspase3 activity kit and LDH release kit (Beyotime Institute of Biotechnology, China) were used according to the manufacturer’s protocol. The relative caspase3 activity and LDH contents were calculated as the ratio of treated cells to untreated cells. The assay was repeated three times.
Effects of melatonin on the proliferation of CMECs
Cell proliferation was evaluated via cell counting kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology, China). For CCK-8 test, CMECs were plated onto 96-well plates (1 × 103 cells/well) with melatonin (0–10 μm/L) in a triplicate pattern. Experiments were performed from 1 to 7 days after plating by the addition of 100 μL of fresh medium in 10 μL of the CCK-8 solution for another 2 h at 37 °C. The OD at 570 nm was measured. The assay was repeated three times.
Immunofluorescence staining
IP3R, VDAC, and caspase3 expression was tested by immunofluorescence staining. In brief, cells were collected and fixed with 4% paraformaldehyde for 10 min, then permeabilized via 0.5% Triton X-100 for 10 min, and blocked with 10% goat serum albumin (Invitrogen, USA) for 1 h at room temperature. Subsequently, samples were incubated with primary antibodies overnight at 4 °C, then washed with PBS three times, and incubated with secondary antibody for 45 min at room temperature. At last, 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, USA) was added for 5 min and cells were analyzed under a fluorescent microscope.
Western blot analysis
Protein was collected after cells were treated. After centrifugation at 14,000×g for 10 min at 4 °C, the supernatants were collected and quantified with BCA protein assay (Beyotime Institute of Biotechnology, China). Equal amounts of proteins were loaded on 8–15% SDS-PAGE gels and then transferred to PVDF membranes (Sigma). The membranes were incubated with 5% nonfat milk for 2 h at room temperature followed with primary antibody/β-actin (1:2000), caspase3, IP3R, VDAC, ERK, pERK, and CREB purchased from Cell Signaling Technology. After being washed in TBST for 30 min, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 45–60 min at room temperature (Santa Cruz Biotechnology). Bands were visualized by enhanced chemiluminescence (ECL) reagent (Beyotime Institute of Biotechnology, China) after the membranes were washed with TBST.
Mitochondrial membrane potential and mPTP opening
The mitochondrial transmembrane potential was analyzed by Mitochondrial Membrane Potential Detection Kit (JC-1) according to the manufacturer’s instructions. Briefly, 2.5 g/mL JC-1 was added into the culture for 30 min at 37 °C. After being washed with binding buffer, the cells were stained with DAPI and then analyzed under a fluorescent microscope. The opening of the mPTP was visualized as a rapid dissipation of tetramethylrhodamine ethyl ester fluorescence according to our previous study.
RNAi knockdown
The small interfering RNA (siRNA) targeting IP3R and VDAC was purchased from Santa Cruz Biotechnology. For the RNAi knockdown, cells were seeded in the plates containing medium without antibiotics for 24 h before transfection. The siRNAs were transfected into the cells by Lipofectamine 2000 (Invitrogen) in serum-free Opti-MEM (Invitrogen). The expression levels of proteins in transfected cells were determined by western blot analysis. The cells transfected after 96 h were harvested and used for further analysis.
[Ca2+]c and [Ca2+]m detection
The contents of [Ca2+]c was imaged with Fluo-2 (Molecular Probes). Samples were then directly examined by confocal microscopy using the ×40 1.42 NA oil immersion objective. For quantification of the concentrations change of [Ca2+]c, flow cytometry was used. For analysis of [Ca2+]m, Rhod-2 (Molecular Probes) was used and the images were captured by confocal microscopy. Fluorescence intensity of Furo-2 and Rhod-2 was measured by excitation wavelengths of 340 and 550 nm, and emission wavelengths of 500 and 570 nm, respectively. Data (F/F0) were obtained by dividing fluorescence intensity (F) by (F0) at resting level (t = 0) which was normalized by control groups.
Statistical analysis
Data are expressed as mean + SD. Comparisons between two groups were measured using Student’s t test. Differences among groups were detected by one-way ANOVA. A value of P < 0.05 was considered as significantly different.
Results
Characterization of cultured CMECs
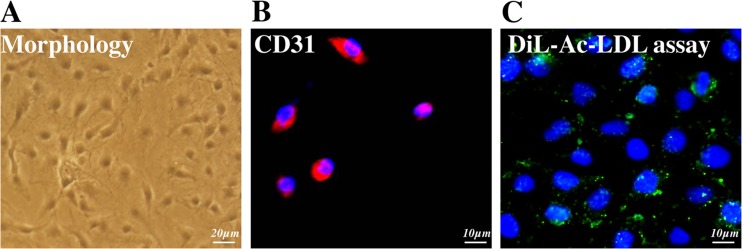
CMECs cultured in medium, as indicated in Fig. 1a, demonstrated a spindle shape and cobblestone-like morphology. Immunofluorescence was used to characterize the surface marker of CMECs, and the result presented in Fig. 1b, c suggested that the CMECs expressed CD31 which is the main endothelia marker. Meanwhile, DiL-Ac-LDL phagocytic test indicated that the isolated cells could phagocytize DiL-Ac-LDL. Together, the above assay suggested the harvested cells in our experiments were CMECs.
Fig. 1.
Characterization of CMECs. a Isolated ADMSCs displayed f a spindle shape and cobblestone-like morphology. b, c Immunofluorescence results showed that CMECs were uniformly positive for CD31 and could absorb DiL-Ac-LDL. Bar = 50 μm
The effects of melatonin on CMECs viability and proliferation
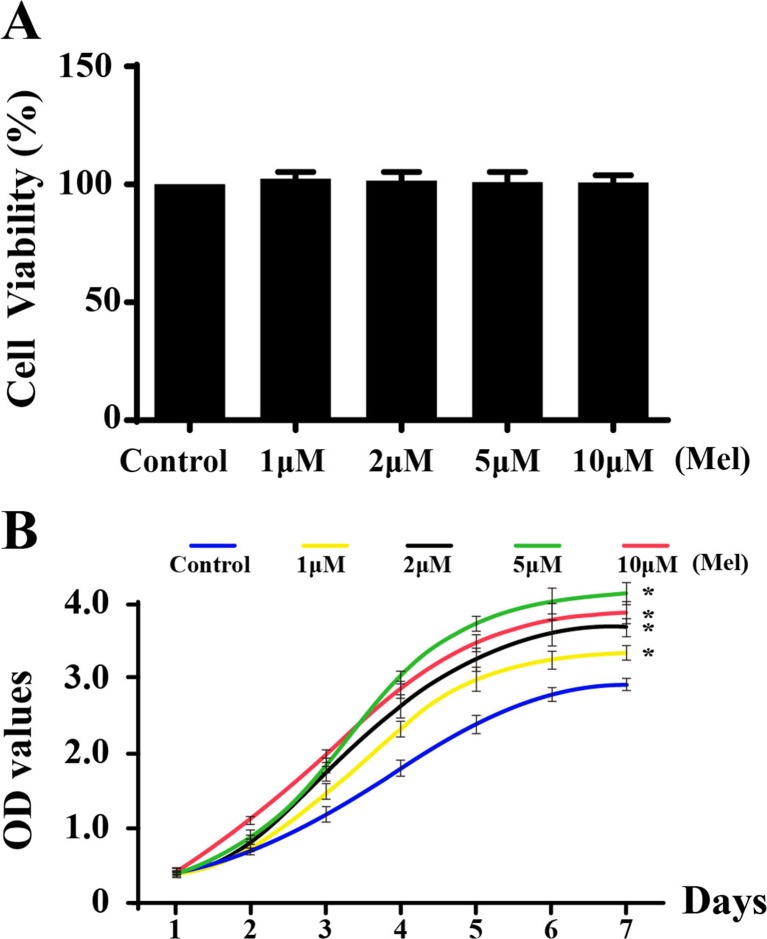
First, MTT was used to explore the effect of melatonin on CMECs viability. As shown in Fig. 2a, at the concentrations used, melatonin (0–10 μm/L) incubation for 24 h had little impact on cell viability compared with normal cell, indicating that melatonin had no toxic effect on CMECs. Furthermore, we used CCK-8 assay to assess the growth kinetics of CMECs under the treatment of melatonin. As shown in Fig. 2b, the growth ability of the CMECs improved gradually with the increase in the melatonin concentrations. However, during the first 24 h, there was no substantial difference between the groups.
Fig. 2.
Effect of melatonin on cell viability and proliferation. a Melatonin had no cytotoxic effect on CMECs. b The growth curve of CMECs under different concentrations of melatonin. *P < 0.05 vs. control group
Oxidative stress injury induced IP3R-dependent [Ca2+]c overload that contributed to CMECs apoptosis
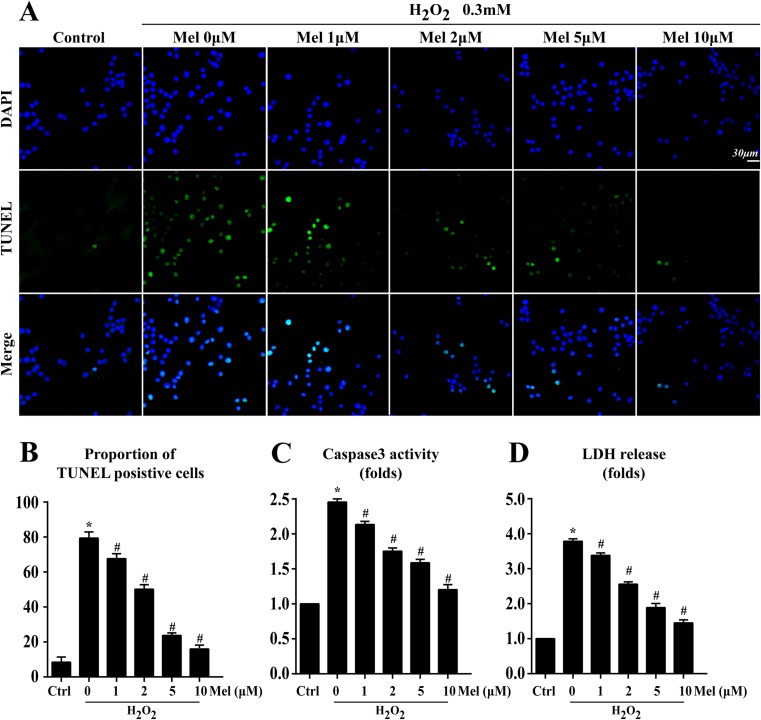
First, according to our previous study (Zhou et al. 2014), 0.3 mM H2O2 for 12 h was used to induce cell oxidative injury and apoptosis. Next, since no significant difference was observed in the cell numbers of CMECs with melatonin for the first 24 h or not, melatonin (0–10 μm/L) treatment for 12 h was used to exclude the effect of proliferation on the oxidative stress experiments. Meanwhile, TUNEL assay was performed to identify the apoptotic rate of CMECs under H2O2-mediated oxidative stress and the anti-apoptosis role of melatonin in CMECs under H2O2. The result (Fig. 3a, b) revealed that H2O2 increased oxidative apoptosis levels in CMECs because approximately 78.64 ± 4.35% of cells were TUNEL+, which was notably inhibited by melatonin in a concentration-dependent manner as evidenced by lower percentage of TUNEL+ cells (melatonin 1 μm/L, 68.26 ± 5.41%; 2 μm/L, 56.34 ± 3.21%; 5 μm/L, 28.42 ± 5.17%; 10 μm/L, 18.33 ± 4.36%, p < 0.05 vs H2O2 Fig. 3a), indicating that melatonin could block the lethal impact of H2O2 on CMECs. Furthermore, two parallel experiments were conducted via LDH release and caspase3 activity (Fig. 3c, d) to further provide the beneficial action of melatonin on CMECs apoptosis. Similar results were obtained that more LDH release and increased caspase3 activity appeared in H2O2 group, and however, these changes were blocked by melatonin treatment. Because the minimum anti-apoptotic effect of melatonin was 10 μm/L, and thereby, 10 μm/L of melatonin was used for the following experiments.
Fig. 3.
Melatonin protected CMECs against oxidative stress injury. a, b Cell death was determined by TUNEL assay. a Annexin V/PI assay. b The change of caspase activity under different doses of melatonin. c The LDH release assay indicated that melatonin could reduce CMECs oxidative stress damage. Blue are cell nuclei; *P < 0.05 vs. control group, #P < 0.05 vs. H2O2 group
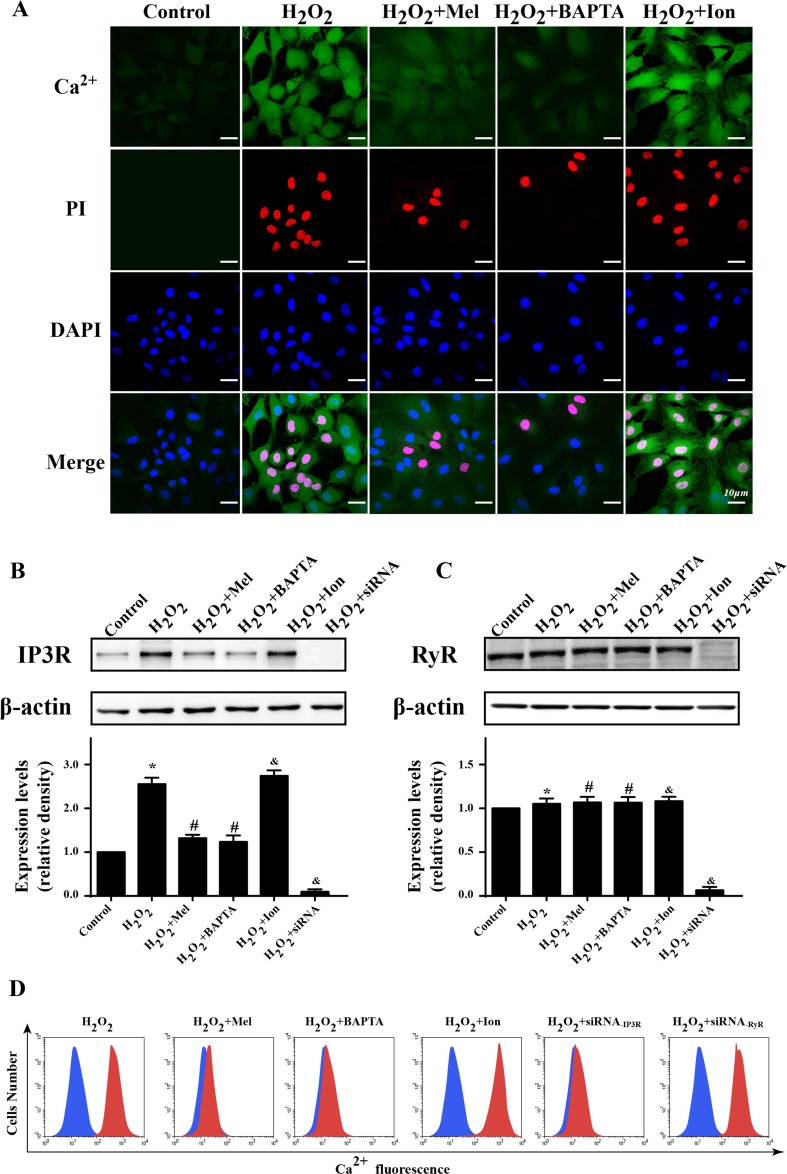
Several reports have suggested that [Ca2+]c overload was the main reason for cellular apoptosis under oxidative stress injury (Zhang et al. 2016). To establish the role of [Ca2+]c overload in H2O2-mediated cellular death, we used co-immunofluorescence of [Ca2+]c and PI. As shown in Fig. 4a, H2O2 markedly elevated the fluorescence of [Ca2+]c which was coupled with increased PI+ cells. Furthermore, removal of cytoplasmic free Ca2+ by calcium chelator BAPTA both reduced the [Ca2+]c contents and the number of PI+ cells, suggesting that H2O2 was the trigger of [Ca2+]c overload which evoked CMECs death. Regarding melatonin, it could reduce both of [Ca2+]c and PI fluorescence. However, these beneficial effects were canceled by ionomycin, a calcium agonist, which weaken the inhibitory actions of melatonin on [Ca2+]c and PI fluorescence. These data illustrated that melatonin could protect CMESs against H2O2-induced oxidative stress injury via suppression of [Ca2+]c overload.
Fig. 4.
Melatonin reduce CMECs death through alleviation of IP3R-dependent [Ca2+]c overload. a The co-immunofluorescence of [Ca2+]c and PI. The higher [Ca2+]c was indicative of more PI+ death cell. BAPTA, a calcium chelator that reduce the levels of intracellular Ca2+. Ion, ionomycin, a calcium agonist that induced [Ca2+]c overload. b, c The expression of IP3R and RyR. Oxidative mainly increased IP3R expression that was blocked by melatonin. d Flow cytometry was used to quantitatively detect the change of [Ca2+]c under melatonin treatment. Meanwhile, siRNA knockdown of IP3R could alleviate the contents of [Ca2+]c in response to oxidative stress. *P < 0.05 vs. control group, #P < 0.05 vs. H2O2 group, and P < 0.05 vs. H2O2 + melatonin group
Because the change of [Ca2+]c was regulated by endoplasmic reticulum (ER) calcium channel, especially RyR and IP3R, which were found to be the primary damage signals under H2O2-induced oxidative stress injury (Haorah et al. 2007; Zhang et al. 2016). Through western blots, our study found that H2O2 could enhance the expression of IP3R but not RyR (Fig. 4b, c). However, melatonin could significantly repress IP3R upregulation. To further provide the evidence for IP3R and RyR in [Ca2+]c overload, we used siRNA to knockdown their expression and observed the role of them in [Ca2+]c fluctuation. The results in Fig. 4d demonstrated that knockdown of IP3R but not RyR could reduce the concentration of [Ca2+]c, which brought the similar results when compared with melatonin group. Furthermore, activation of IP3R but not RyR had the ability to elevate [Ca2+]c under melatonin, which significantly canceled the protective effect of melatonin on [Ca2+]c overload. Altogether, these data suggested that H2O2 stimulated [Ca2+]c overload via upregulation of IP3R expression which was inhibited by melatonin.
IP3R-dependent [Ca2+]c overload activated VDAC-mediated [Ca2+]m overload that caused mitochondrial structural and functional destroy
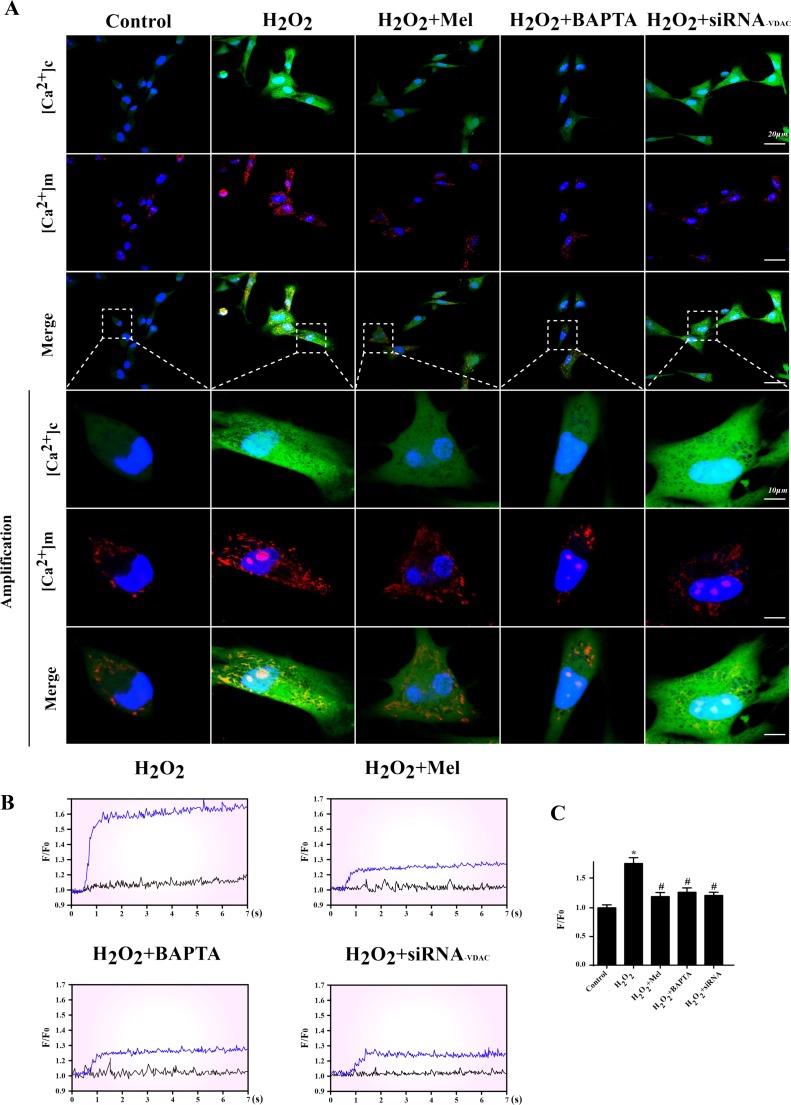
As the second calcium store in cell, mitochondria could absorb the excessive cytoplasm calcium in response to [Ca2+]c overload, resulting into [Ca2+]m overload. Among these, VDAC has been reported to be the primary channels for cytoplasmic free Ca2+ flow into mitochondria (Fernandez-Sanz et al. 2014; Monaco et al. 2015). Therefore, we hypothesized that [Ca2+]m overload was secondary to [Ca2+]c elevation via VDAC. Firstly, we used [Ca2+]c and [Ca2+]m probes to observe the change of Ca2+ concentration. Under H2O2, higher [Ca2+]c concentration was paralleled to the content of [Ca2+]m (Fig. 5a). However, removal of [Ca2+]c could significantly lead to the decrease in [Ca2+]m under H2O2. Furthermore, inhibition of VDAC had no effects on [Ca2+]c concentration but was able to inhibit the [Ca2+]m overload, suggesting that VDAC was the key passageway for [Ca2+]c inflow into mitochondria. Melatonin treatment could both reduce [Ca2+]c and [Ca2+]m overload. Next, calcium map was used to quantitatively analyze the change of [Ca2+]m when knockdown of VDAC. The data indicated in Fig. 5b demonstrated that the increase of [Ca2+]m influence under H2O2 was blocked by VDAC knockdown or melatonin treatment. These results hinted that the upregulation of VDAC was the main reason for H2O2-induced [Ca2+]m overload while melatonin held the capacity of suppressing VDAC expression, and therefore, alleviating the [Ca2+]m.
Fig. 5.
IP3R-dependent [Ca2+]c overload activated VDAC-mediated [Ca2+]m overload. a The co-immunofluorescence of [Ca2+]c and [Ca2+]m. Higher [Ca2+]c was associated with [Ca2+] through VDAC because siRNA knockdown of VDAC could significantly abate the increase in [Ca2+]m under oxidative stress. b, c The [Ca2+]m map via confocal microscopy by Rhod-2. Fluorescence intensity of Rhod-2 was measured by excitation wavelengths of 550 nm and emission wavelengths of 570 nm, respectively. Data (F/F0) were obtained by dividing fluorescence intensity (F) by (F0) at resting level (t = 0) which was normalized by control groups. *P < 0.05 vs. control group, #P < 0.05 vs. H2O2 group
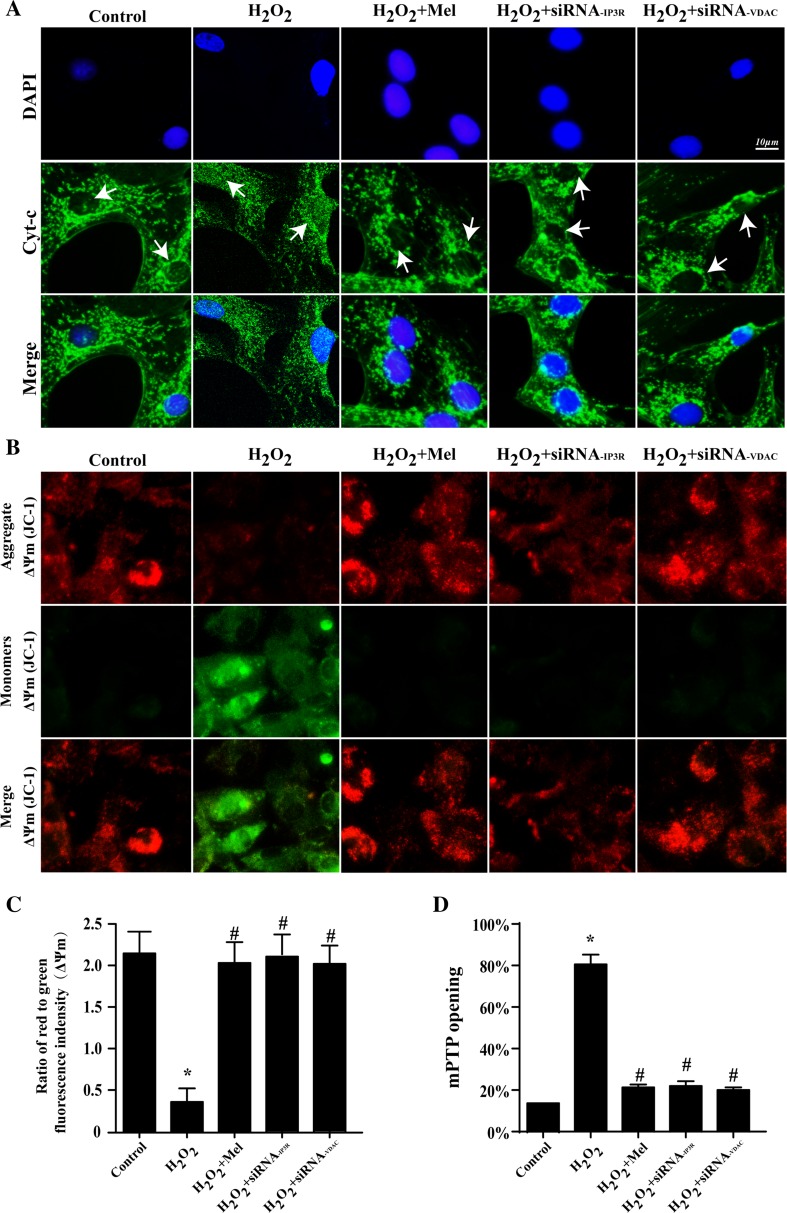
Previous studies have found that mitochondria were the center of H2O2-mediated oxidative stress injury via releasing cyt-c (Zhou et al. 2014, 2015). We therefore guessed that [Ca2+]m overload could be the pathologic mechanisms for cyt-c releasing. As the result displayed in Fig. 6a, H2O2 caused more cyt-c leakage out of mitochondria with evidenced by more cyt-c dispersed into cytoplasm and even into nuclear. However, melatonin could partially repress the cyt-c diffusion. Importantly, knockdown of VDAC under H2O2 restored the punctiform of cyt-c and blocked cyt-c leakage into nuclear. These data suggested that IP3R-[Ca2+]c elevation and VDAC-[Ca2+]m overload were responsible for H2O2-induced cyt-c release. Furthermore, the cyt-c release could be the consequence of the destruction of mitochondrial structural and function. The damage of mitochondrial membrane potential (ΔΨm) and the opening of mitochondrial permeability transition pore (mPTP) were prerequisite for cyt-c release (Kagan et al. 2004). The results in Fig. 6b, d indicated that IP3R-[Ca2+]c elevation and VDAC-[Ca2+]m overload also aggregated mPTP opening and dissipated ΔΨm when compared with control group. However, melatonin had the ability to maintain ΔΨm and limit mPTP opening.
Fig. 6.
[Ca2+]m overload aggregated mitochondrial damage and triggered the mitochondria-related cell death pathways. a The re-location of cyt-c from mitochondria into cytoplasm suggesting the activation of mitochondria-related cell death pathways. b, c The change of mitochondrial membrane potential (ΔΨm). d Melatonin could reduce the mPTP opening. P < 0.05 vs. control group, #P < 0.05 vs. H2O2 group
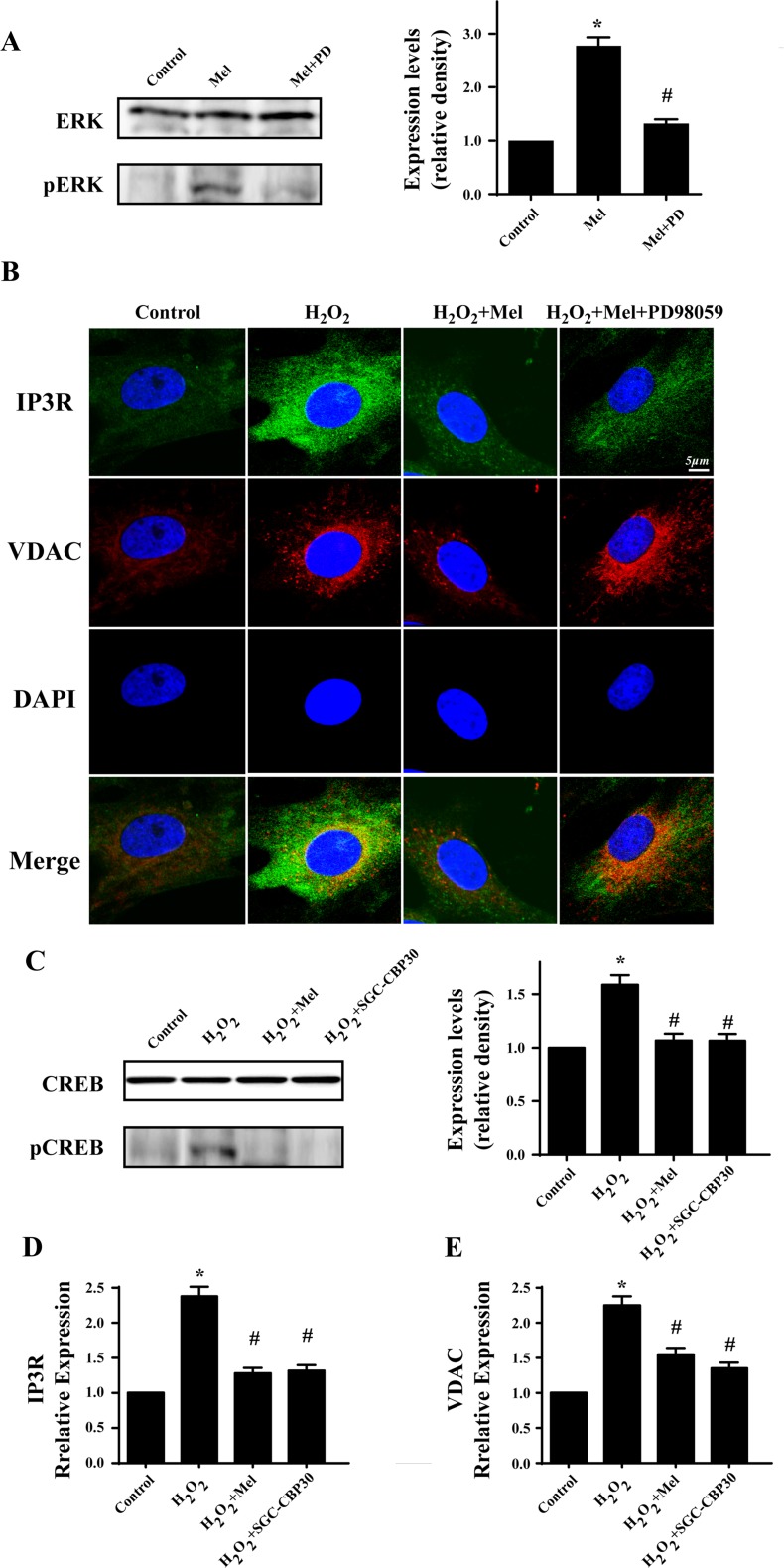
MAPK/ERK was activated by melatonin and contributed to the protective effects of melatonin on CMECs under H2O2
MAPK/ERK is the classical anti-apoptotic pathway and exerts proliferation promotion effect on many types of cell under different conditions (Kim and Choi 2015; Sun et al. 2015a, b). Melatonin has been found to be the activator of MAPK/ERK. Therefore, we speculated that the beneficial effect of melatonin may be due to its activation of MAPK/ERK (Hill et al. 2013). The result suggested that melatonin could induce more pERK expression which was inhibited by ERK inhibitor PD98059 (Fig. 7a). Furthermore, to provide further evidence for the role of MAPK/ERK in melatonin-mediated CMECs survival, we used IP3R/VDAC co-immunofluorescence with PD98059. The result of co-immunofluorescence of IP3R/VDAC, as indicated in Fig. 7b, exhibited that melatonin modified IP3R/VDAC expression via MAPK/ERK. In fact, MAPK/ERK could control the phosphorylation of cAMP response element binding protein (CREB), a transcription factors (TFs) that functions as the gene switch in nuclear to upregulate and/or downregulate gene transcription (Moosavi et al. 2016). In the present paper, H2O2 increased p-CREB which was blocked by melatonin (Fig. 7c). Furthermore, the changes of IP3R and VDAC were in accordance with the alteration of CREB. However, inhibitor of CREB by SGC-CBP30 could abate the H2O2-mediated IP3R/VDAC upregulation (Fig. 7d, e). These results suggested that H2O2 signaled IP3R-[Ca2+]c/VDAC-[Ca2+]m axis via activation of CREB which was inhibited by melatonin through MAPK/ERK. The MAPK family includes ERK, JNK, and p38, the major signal transduction molecules regulated by growth factors, cytokines, and stress (Chang and Karin 2001).
Fig. 7.
Melatonin signaled MAPK/ERK pathways to inactivate CREB transcription promoter which reduces the expression of IP3R and VDAC under H2O2. a The activation of melatonin on MAPK/ERK. b MAPK/ERK was responsible for the inhibitory role of melatonin on IP3R and VDAC. c Melatonin could reduce the expression of CREB and its activation. SGC-CBP30, the specific inhibitor of CREB pathways. d, e The transcription promoter CREB was the downstream of ERK, which was activated by oxidative stress and contributed to the elevation of IP3R and VDAC. Melatonin impaired the activation of CREB and therefore reduced the IP3R and VDAC. *P < 0.05 vs. control group, #P < 0.05 vs. H2O2 group
Discussion
Although PCI therapy for MI could open the obstructed major blood vessels, but it also caused the ischemia-reperfusion (I/R) injury to the cardiac cells due to the excessive production of ROS which perturbed the intracellular redox balance that contributed to cell dysfunction and programmed cell death. Several studies have found the increased ischemia or inflammation induced oxidative stress injury that evoked cellular death via mitochondrial-dependent apoptosis pathways (Potier et al. 2007; Zhou et al. 2014). Among these processes, mitochondria released cyt-c into cytoplasm in response to excessive oxidative stress (Zhou et al. 2017a). However, the upstream signal of mitochondrial damage remains unclear. In this paper, we found that calcium overload, especially mitochondrial calcium overload, was responsible for mitochondria damage and cyt-c release. Under H2O2, oxidative stress could activate CREB that enhanced the IP3R and VDAC gene expression. As the result of increased IP3R, more Ca2+ released into cytoplasm and caused [Ca2+]c overload. Next, VDAC contributed to excessive [Ca2+]c flow into mitochondria, leading to [Ca2+]m overload. High [Ca2+]m was associated with cyt-c leakage because inhibition of [Ca2+]m overload could limit cyt-c releasing into cytoplasm. Moreover, [Ca2+]m overload was also linked with ΔΨm collapse and mPTP opening which were the necessary step for subsequent cyt-c detachment from mitochondria. Thus, we established the role of IP3R-[Ca2+]c/VDAC-[Ca2+]m axis in initiating mitochondrial damage. Among such pathologic process, Ca2+ was the critical messenger that transmitted from ER to mitochondria, delivering oxidative injury signal to mitochondria, the cellular apoptosis executor. In fact, in normal cells, the free [Ca2+]c is regulated by the balance of Ca2+ pump in ER (Peters and Piper 2007). IP3R functions as the critical Ca2+ release proteins, which has been reported to be involved into oxidative damage (Ivanova et al. 2014; Shah et al. 2015; Vervloessem et al. 2015). And these studies argue that oxidative stress could directly activate the IP3R that promotes Ca2+ release from ER into cytoplasm (Grimm 2012; Haorah et al. 2007). However, in our studies, we found that IP3R was modified at gene level by oxidative stress via CREB. CREB is a cellular transcription factor, which binds to certain DNA sequences called cAMP response elements (CRE) and increases/decreases the transcription of the target genes (Chapple et al. 2013; Saggioro 2011). Our study provided evidences that oxidative stress activated CREB that significantly enhanced the IP3R/VDAC transcription and proteins expression. Higher IP3R was the primary causes for [Ca2+]c overload. Additionally, as the second calcium store, mitochondria could absorb excessive cytoplasmic Ca2+ in response to the elevation of [Ca2+]c. The VDAC was the key channel for [Ca2+]c inflow into mitochondria, which was upregulated by oxidative stress via CREB. Through elevation of [Ca2+]c and increased VDAC expression, there was a steep rise in [Ca2+]m that reduced ΔΨm and opened mPT, finally contributing to cyt-c leakage into cytoplasm to initiate mitochondrial-dependent death pathways. Several studies have explored the mechanism by which [Ca2+]m induced mitochondrial structural and function damage, especially cyt-c release. These process could be involved in the mitochondrial fission (Cosentino and Garcia-Saez 2014; Orlova et al. 2015) and cardiolipin oxidation (Givvimani et al. 2015; Hough et al. 2014). Although mitochondria are at the center of oxidative stress, mitochondrial-dependent death pathways are not the only way for cellular damage. Yet, there are other apoptotic routes including caspase8-dependent death-receptor pathway (Thorburn 2004) and caspase12-involved ER (endoplasmic reticulum stress pathway) (Shore et al. 2011) that are likely to participate in the cell death along with the progress in oxidative stress injury. However, whether these death signals have a role in CMECs apoptosis and whether Ca2+ is the connector of these signals remains unclear.
Melatonin is an indolamine produced by the pineal gland (Dominguez-Rodriguez et al. 2017) and it can exert a potent antioxidant effect (Reiter et al. 2016b). Its free radical scavenger properties have been used to advantage in different organ transplants in animal experiments (Chen et al. 2016; Reiter et al. 2016a). Several concentrations and administration pathways have been tested and melatonin has shown encouraging beneficial results in many transplants of organs such as the liver (Xu et al. 2017), lungs (Yu et al. 2017b), heart (Reiter et al. 2016a), pancreas (Majidinia et al. 2017), and kidneys (Lin et al. 2016). Evidences from several studies have suggested that melatonin reduced excessive oxidative stress and enhance cellular survival (Back et al. 2016; Mayo et al. 2017). In the present studies, we demonstrated that melatonin could suppress [Ca2+]c/[Ca2+]m overload, maintain ΔΨm, reduce mPTP opening, block cyt-c leakage, and stop mitochondrial-dependent cellular death. Notably, it is known to us that it would be more beneficial that treatment after rather than before I/R reduces myocardial apoptosis. And the different effects of melatonin on pre- and post-conditioning are different. Recent studies have found that pre-treatment of melatonin could activate the AMPK pathways to suppress mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis, preventing cardiac microvascular against reperfusion injury (Zhou et al. 2017b). Considering the AMPK is reduced in response to reperfusion, therefore, the pre-treatment of melatonin is used to restore the balance of AMPK pathways. Besides, other studies have found that post-treatment of melatonin attenuated post-MI injury via upregulation of Tom70 that stops the cycle of mitochondrial impairment and ROS generation (Pei et al. 2017). Furthermore, the post-treatment of melatonin could rescue Trx system by reducing Txnip expression via Notch1/Hes1/Akt signaling in a membrane receptor-dependent manner (Yu et al. 2017a). These data indicate that the role of melatonin pre-treatment is to activate the defensive pathways against reperfusion injury. While the post-treatment of melatonin is more likely to stop the damage signal. These could be the different mechanisms of melatonin pre- and post-conditioning in cardiac I/R injury. Furthermore, in the present study, we demonstrated that the beneficial effect of melatonin was owing to its activation on MAPK/ERK which inactivated the CREB and weakened IP3R/VDAC transcription and expression. The MAPK family, especially ERK was the classical anti-apoptotic pathways. Notably, ERK is phosphorylated activation and subsequently migrate into the nucleus where it regulates the activity of several transcription promoters related to cellular survival under oxidative stress (Kim and Choi 2015). In the present study, MAPK/ERK negatively regulated CREB activation and therefore reduced cytoplasmic and mitochondrial calcium pump, reversing the balance of [Ca2+]c/[Ca2+]m. Thus, we proved that melatonin played a pivotal role in preventing CMECs from oxidative stress-induced death via downregulation of IP3R-[Ca2+]c/VDAC-[Ca2+]m axis, exhibiting melatonin could be used as an adjuvant to strengthen the resistance of CMECs against oxidative injury. Recent studies have identified melatonin played a key role in cardiopotection including reduction in infarction size, improvement in LVEF, and reversion of cardiac remodeling (Herrera et al. 2015; Hu et al. 2016; Sirtori et al. 2015; Yiallourou et al. 2016). In particular, our study broadens the potential application of melatonin by demonstrating the beneficial effect of melatonin on CMECs oxidative injury. These data altogether hint that melatonin would be a new therapeutic tool for the injured heart by improving cardiac function and enhancing the resistance of microvascular to reperfusion injury.
In conclusion, we illustrate the mechanism by which oxidative stress induced mitochondrial oxidative injury in CMECs. Calcium functions as the transmitter that elevated by IP3R and permeates widely into mitochondria via VDAC, causing mitochondria calcium overload which is responsible for the collapse of mitochondrial structure and function. While, melatonin could stimulate MAPK/ERK that inactive CREB and downregulate IP3R/VDAC expression, contributing to the [Ca2+]c/[Ca2+]m homeostasis and stopping mitochondrial-dependent death pathways. Our study demonstrates the potential therapeutic target that may ameliorate the survival of CMEC and microvascular after I/R injury.
Acknowledgments
This study was supported by grants from the National 863 high technology R&D Program (No. 2011AA020101), National Natural Science Foundation of China (No: 81270186, 81102079), and Key Project of Natural Science Foundation of China (No: 81030002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Hao Zhou, Email: zhouhao301@outlook.com.
Yundai Chen, Email: cyundai@vip.163.com.
References
- Alonso-Alconada D, Alvarez A, Arteaga O, Martinez-Ibarguen A, Hilario E. Neuroprotective effect of melatonin: a novel therapy against perinatal hypoxia-ischemia. Int J Mol Sci. 2013;14:9379–9395. doi: 10.3390/ijms14059379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back K, Tan DX, Reiter RJ. Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J Pineal Res. 2016;61:426–437. doi: 10.1111/jpi.12364. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chapple SJ, Cheng X, Mann GE. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol. 2013;1:319–331. doi: 10.1016/j.redox.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, et al. Melatonin pretreatment enhances the therapeutic effects of exogenous mitochondria against hepatic ischemia-reperfusion injury in rats through suppression of mitochondrial permeability transition. J Pineal Res. 2016;61:52–68. doi: 10.1111/jpi.12326. [DOI] [PubMed] [Google Scholar]
- Choi YH. The cytoprotective effect of isorhamnetin against oxidative stress is mediated by the upregulation of the Nrf2-dependent HO-1 expression in C2C12 myoblasts through scavenging reactive oxygen species and ERK inactivation. Gen Physiol Biophys. 2016;35:145–154. doi: 10.4149/gpb_2015034. [DOI] [PubMed] [Google Scholar]
- Cosentino K, Garcia-Saez AJ. Mitochondrial alterations in apoptosis. Chem Phys Lipids. 2014;181:62–75. doi: 10.1016/j.chemphyslip.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A et al (2017) Effect of intravenous and intracoronary melatonin as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: results of the melatonin adjunct in the acute myocardial infarction treated with angioplasty trial. J Pineal Res:62. doi:10.1111/jpi.12374 [DOI] [PubMed]
- Fernandez-Sanz C, et al. Defective sarcoplasmic reticulum-mitochondria calcium exchange in aged mouse myocardium. Cell Death Dis. 2014;5:e1573. doi: 10.1038/cddis.2014.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar R et al (2016) The cytoprotective effect of biglycan core protein involves toll-like receptor 4 signaling in cardiomyocytes. J Mol Cell Cardiol. doi:10.1016/j.yjmcc.2016.08.006 [DOI] [PubMed]
- Givvimani S, Pushpakumar SB, Metreveli N, Veeranki S, Kundu S, Tyagi SC. Role of mitochondrial fission and fusion in cardiomyocyte contractility. Int J Cardiol. 2015;187:325–333. doi: 10.1016/j.ijcard.2015.03.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S. The ER-mitochondria interface: the social network of cell death. Biochim Biophys Acta. 2012;1823:327–334. doi: 10.1016/j.bbamcr.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Han D, et al. Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. J Pineal Res. 2016;60:178–192. doi: 10.1111/jpi.12299. [DOI] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Gorantla S, Zheng J, Persidsky Y. Alcohol-induced blood-brain barrier dysfunction is mediated via inositol 1,4,5-triphosphate receptor (IP3R)-gated intracellular calcium release. J Neurochem. 2007;100:324–336. doi: 10.1111/j.1471-4159.2006.04245.x. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Farias JG, Ebensperger G, Reyes RV, Llanos AJ, Castillo RL. Pharmacological approaches in either intermittent or permanent hypoxia: a tale of two exposures. Pharmacol Res. 2015;101:94–101. doi: 10.1016/j.phrs.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Hill SM, Cheng C, Yuan L, Mao L, Jockers R, Dauchy B, Blask DE. Age-related decline in melatonin and its MT1 receptor are associated with decreased sensitivity to melatonin and enhanced mammary tumor growth. Curr Aging Sci. 2013;6:125–133. doi: 10.2174/1874609811306010016. [DOI] [PubMed] [Google Scholar]
- Hough MA, Silkstone G, Worrall JA, Wilson MT. NO binding to the proapoptotic cytochrome c-cardiolipin complex. Vitam Horm. 2014;96:193–209. doi: 10.1016/B978-0-12-800254-4.00008-8. [DOI] [PubMed] [Google Scholar]
- Hu W, et al. Melatonin: the dawning of a treatment for fibrosis? J Pineal Res. 2016;60:121–131. doi: 10.1111/jpi.12302. [DOI] [PubMed] [Google Scholar]
- Hurst S, Hoek J, Sheu SS (2016) Mitochondrial Ca2+ and regulation of the permeability transition pore. J Bioenerg Biomembr. doi:10.1007/s10863-016-9672-x [DOI] [PMC free article] [PubMed]
- Ivanova H, Vervliet T, Missiaen L, Parys JB, De Smedt H, Bultynck G. Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim Biophys Acta. 2014;1843:2164–2183. doi: 10.1016/j.bbamcr.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Kagan VE, et al. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic Biol Med. 2004;37:1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. 2015;89:867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- Laudon M, Frydman-Marom A. Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int J Mol Sci. 2014;15:15924–15950. doi: 10.3390/ijms150915924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YW, et al. Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-kappaB DNA-binding activity. J Pineal Res. 2016;60:277–290. doi: 10.1111/jpi.12308. [DOI] [PubMed] [Google Scholar]
- Majidinia M, Sadeghpour A, Mehrzadi S, Reiter RJ, Khatami N, Yousefi B (2017) Melatonin: a pleiotropic molecule that modulates DNA damage response and repair pathways. J Pineal Res. doi:10.1111/jpi.12416 [DOI] [PubMed]
- Mayo JC, Sainz RM, Gonzalez Menendez P, Cepas V, Tan DX, Reiter RJ (2017) Melatonin and sirtuins: a “not-so unexpected” relationship. J Pineal Res 62. doi:10.1111/jpi.12391 [DOI] [PubMed]
- Monaco G, et al. The BH4 domain of anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the voltage-dependent anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria. J Biol Chem. 2015;290:9150–9161. doi: 10.1074/jbc.M114.622514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosavi F, Hosseini R, Saso L, Firuzi O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des Devel Ther. 2016;10:23–42. doi: 10.2147/DDDT.S96936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova DD, Tribulovich VG, Garabadzhiu AV, Barlev NA, Martin S. The role of mitochondrial dynamics in cell death. Tsitologiia. 2015;57:184–191. [PubMed] [Google Scholar]
- Park BK, Gonzales EL, Yang SM, Bang M, Choi CS, Shin CY. Effects of triclosan on neural stem cell viability and survival. Biomol Ther (Seoul) 2016;24:99–107. doi: 10.4062/biomolther.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei HF et al (2017) Melatonin attenuates postmyocardial infarction injury via increasing Tom70 expression. J Pineal Res 62. doi:10.1111/jpi.12371 [DOI] [PubMed]
- Peters SC, Piper HM. Reoxygenation-induced Ca2+ rise is mediated via Ca2+ influx and Ca2+ release from the endoplasmic reticulum in cardiac endothelial cells. Cardiovasc Res. 2007;73:164–171. doi: 10.1016/j.cardiores.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007;13:1325–1331. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016a) Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. doi:10.1111/jpi.12360 [DOI] [PubMed]
- Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- Saggioro D. Anti-apoptotic effect of tax: an NF-kappaB path or a CREB way? Viruses. 2011;3:1001–1014. doi: 10.3390/v3071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SZ, Zhao D, Khan SH, Yang L. Regulatory mechanisms of endoplasmic reticulum resident IP3 receptors. J Mol Neurosci. 2015;56:938–948. doi: 10.1007/s12031-015-0551-4. [DOI] [PubMed] [Google Scholar]
- Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol. 2011;23:143–149. doi: 10.1016/j.ceb.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirtori CR, Arnoldi A, Cicero AF. Nutraceuticals for blood pressure control. Ann Med. 2015;47:447–456. doi: 10.3109/07853890.2015.1078905. [DOI] [PubMed] [Google Scholar]
- Sun K, Fan J, Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B. 2015;5:8–24. doi: 10.1016/j.apsb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Vervloessem T, Yule DI, Bultynck G, Parys JB. The type 2 inositol 1,4,5-trisphosphate receptor, emerging functions for an intriguing Ca(2)(+)-release channel. Biochim Biophys Acta. 2015;1853:1992–2005. doi: 10.1016/j.bbamcr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P et al (2017) Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res 62. doi:10.1111/jpi.12399 [DOI] [PubMed]
- Yiallourou SR, Wallace EM, Miller SL, Horne RS. Effects of intrauterine growth restriction on sleep and the cardiovascular system: the use of melatonin as a potential therapy? Sleep Med Rev. 2016;26:64–73. doi: 10.1016/j.smrv.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Yu L et al (2017a) Melatonin rescues cardiac thioredoxin system during ischemia-reperfusion injury in acute hyperglycemic state by restoring Notch1/Hes1/Akt signaling in a membrane receptor-dependent manner. J Pineal Res 62. doi:10.1111/jpi.12375 [DOI] [PubMed]
- Yu S et al (2017b) Melatonin regulates PARP1 to control the senescence-associated secretory phenotype (SASP) in human fetal lung fibroblast cells. J Pineal Res. doi:10.1111/jpi.12405 [DOI] [PubMed]
- Zhang Y, et al. Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic Biol Med. 2016;95:278–292. doi: 10.1016/j.freeradbiomed.2016.03.035. [DOI] [PubMed] [Google Scholar]
- Zhou H et al. (2017a) Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening J Am Heart Assoc 6. doi:10.1161/JAHA.116.005328 [DOI] [PMC free article] [PubMed]
- Zhou H, et al. Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci Rep. 2015;5:12898. doi: 10.1038/srep12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, et al. Exendin-4 protects adipose-derived mesenchymal stem cells from apoptosis induced by hydrogen peroxide through the PI3K/Akt-Sfrp2 pathways. Free Radic Biol Med. 2014;77:363–375. doi: 10.1016/j.freeradbiomed.2014.09.033. [DOI] [PubMed] [Google Scholar]
- Zhou H et al (2017b) Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res. doi:10.1111/jpi.12413 [DOI] [PMC free article] [PubMed]