Abstract
Fifteen small heat shock protein (sHSP) genes were identified from spruce budworm, Choristoneura fumiferana (L.), an important native forest pest in North America. The transcript levels of each CfHSP were measured under non-stress conditions in all life stages from egg to adult and in five different larval tissues. CfHSP transcript levels showed variation during development, with highest levels in adults and lowest in eggs. Most CfHSP transcripts are highly expressed in larval fat body and Malpighian tubules; two CfHSPs display extremely high expression in the head and epidermis. Upon heat stress, nine CfHSP genes are significantly upregulated, increasing by 50- to 2500-fold depending on developmental stage and tissue type. Upon starvation, eight CfHSPs are upregulated or downregulated, whereas six others retain constant expression. These results suggest that CfHSPs have important and multiple roles in spruce budworm development and in response to heat stress and starvation.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-017-0832-7) contains supplementary material, which is available to authorized users.
Keywords: Spruce budworm, sHSP, Chaperone, Expression profiles, Heat stress, Starvation
Introduction
Small heat shock proteins (sHSPs) are a superfamily of molecular chaperones with a molecular weight range of 12–43 kDa and are characterized by the presence of a conserved α-crystallin domain (ACD) (Franck et al. 2004; Basha et al. 2012). They exhibit ATP-independent, chaperone-like activity by assisting in the correct folding of nascent and stress-accumulated misfolded proteins to prevent irreversible protein aggregation (Basha et al. 2012; King and MacRae 2015).
sHSPs are ubiquitous in both prokaryotes and eukaryotes (Waters and Rioflorido 2007; Aevermann and Waters 2008; Waters 2013), but their structure and function are diverse among the various superfamilies of the stress proteins (Franck et al. 2004). The sHSPs have been implicated in many physiological processes, including cellular stress resistance (Landry et al. 1989), actin and intermediate filament dynamics (Wieske et al. 2001; Quinlan 2002), inhibition of apoptosis (Arrigo 1998), membrane fluidity (Tsvetkova et al. 2002), longevity (Wood et al. 2010), and various diseases (Mackay et al. 2003; Evgrafov et al. 2004).
Insects are highly successful organisms that have a strong ability to adapt to their environment. Insect sHSPs are expressed in a wide array of patterns under normal developmental and stress conditions. A large number of sHSPs have been isolated and studied in insects including members of the Diptera (Drosophila melanogaster, Haass et al. 1990; Morrow et al. 2006), the Lepidoptera (Bombyx mori, Li et al. 2009; Chilo suppressalis, Lu et al. 2014), the Coleoptera (Tribolium castaneum, Mahroof et al. 2005), and the Hymenoptera (Apis cerana, Liu et al. 2012). The expression of an individual sHSP is regulated in a tissue- and developmental stage-specific manner in the absence of stress, suggesting that insect sHSPs play important roles in various developmental processes, as well as metabolic activities and reproduction (Dubrovsky et al. 1996; Huet et al. 1996; Joanisse et al. 1998; Sonoda et al. 2006; Kokolakis et al. 2008; Takahashi et al. 2010). However, much attention has been paid to their functions under stress conditions. sHSPs have important roles in insect adaptation to environmental stresses including heat, cold, dryness, starvation, anoxia, infection, and ultraviolet light (Basha et al. 2012; Zhao and Jones 2012; King and MacRae 2015). Environmental stresses can upregulate or downregulate the expression of sHSPs and lead to enhanced stress tolerance or resistance (Gehring and Wehner 1995; Zhao and Jones 2012; King and MacRae 2015). Insect sHSPs have been suggested to be involved in diapause, a physiological state of reduced metabolism to survive unfavorable environmental conditions (Yocum et al. 1998; Denlinger 2002; Hayward et al. 2005; Rinehart et al. 2006; Li et al. 2007; Rinehart et al. 2007; Zhang et al. 2015), in cold hardening (Qin et al. 2005), in rapid heat hardening (Huang et al. 2007; Liu et al. 2013), and in recovery from cold injury (Colinet et al. 2010a, b).
Phylogenetic and biological analyses suggest that most insect sHSPs have evolved independently among different insect orders (Sakano et al. 2006; Huang et al. 2008; Li et al. 2009; Zhang et al. 2015), implying that species-specific sHSPs may greatly contribute to the adaptability of these insects in diverse environments. Therefore, to understand the mechanisms by which pest insects survive in severe environments, it will be very useful to identify and study the species-specific sHSPs.
Although sHSP genes are important in insect adaptation to environmental extremes, to date, no sHSPs have been studied in the spruce budworm, Choristoneura fumiferana, a destructive native defoliator, whose geographic distribution coincides with that of fir and spruce forests from the east coast of North America to Alaska (Stehr 1967; Régnière et al. 2012). Periodic outbreaks have occurred across tens of millions of square kilometers in eastern North America. Evidence of outbreaks dates back to the sixteenth century and future episodes are anticipated (Royama 1984; Boulanger and Arseneault 2004; Gray 2008). Spruce budworm survives at temperatures of −30 °C in winter (Han and Bauce 1993). In late summer, first-instar larva spin silken hibernacula within which they molt into the second instar and spend nearly 9 months in diapause to survive the cold winter (Royama 1984; Han and Bauce 1993). In addition to the extreme weather, food shortage also has an important negative impact on spruce budworm population and distribution (Frago and Bauce 2014). As a first step to explore potential roles of sHSPs in spruce budworm adaption to environmental stresses, we have identified 15 sHSP genes from the spruce budworm and investigated their expression profiles as well as their response to heat shock and starvation. The results provide some new insights into the roles of sHSPs under normal and stressful conditions.
Materials and methods
Experimental insects
Spruce budworm (C. fumiferana; Lepidoptera: Tortricidae) provided by Insect Production Services, Great Lakes Forestry Centre, Sault Ste. Marie, Canada, were reared on an artificial diet modified from McMorran (1965) and maintained at 22–23 °C, 70% relative humidity, under a 12-h light:dark photoperiod.
Identification of CfHSPs and sequence analysis
To identify the sHSP genes, B. mori and Grapholita molesta sHSP genes (S1 Table 1) from NCBI GenBank were used as query sequences to search against the transcriptome of spruce budworm larvae (unpublished data) by tBLASTn (E-value <1E−0.5). The entire process to construct the spruce budworm transcriptome, including RNA sequencing, de novo assembly, and gene annotation, was as described by Duan et al. (2015).
Sequence alignment and secondary structure prediction
DNA was analyzed using GENETYX software (version 10, Genetyx Corporation) to calculate the theoretical molecular weights and isoelectric points. Conserved protein domains were identified using NCBI BLAST (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). The PROMALS3D Web server was used to predict secondary structures and multiple sequence alignments (http://prodata.swmed.edu/PROMALS3D) (Pei and Grishin 2014).
Phylogenetic analysis
The conserved α-crystallin domains in lepidopteran sHSPs were aligned by MAFFT v7 (Katoh and Standley 2013) with all gaps removed. To remove poorly aligned positions and divergent regions, the GBlocks program (Castresana 2000) was used to select the conserved blocks of the alignment with the default parameters (Table 1). The filtered alignments were subjected to ProtTest 3 (Darriba et al. 2011) to estimate the best amino acid substitution model under Akaike information criterion (AIC). According to the best-fit model, a maximum likelihood tree was constructed using the PhyML v3.0 (Guindon et al. 2010), and the bootstrap values from 100 resamplings were calculated at each node.
Table 1.
Parameters of the identified sHSPs from the spruce budworm, Choristoneura fumiferana
| Name | cDNA (bp) | ORF (aa) | MW (Da) | pI | Accession no. |
|---|---|---|---|---|---|
| CfHsp18.6 | 578 | 163 | 18,576.54 | 10.18 | KX958470 |
| CfHsp19.2 | 731 | 170 | 19,220.49 | 4.86 | KX958477 |
| CfHsp19.6 | 636 | 167 | 19,586.18 | 5.56 | KX958474 |
| CfHsp19.7 | 913 | 177 | 19,733.09 | 4.97 | KX958479 |
| CfHsp20.0 | 779 | 175 | 19,948.2 | 4.86 | KX958478 |
| CfHsp20.2 | 742 | 179 | 20,162.51 | 5.25 | KX958476 |
| CfHsp20.3 | 779 | 180 | 20,328.67 | 5.79 | KX958472 |
| CfHsp21.3 | 1,209 | 186 | 21,304.57 | 5.19 | KX958484 |
| CfHsp21.5a | 718 | 186 | 21,482.13 | 4.85 | KX958475 |
| CfHsp21.5b | 765 | 187 | 21,518.86 | 5.11 | KX958482 |
| CfHsp22.0 | 1,304 | 191 | 21,946.48 | 5.15 | KX958483 |
| CfHsp22.1 | 800 | 190 | 22,094.55 | 6.12 | KX958471 |
| CfHsp23.9 | 1,168 | 209 | 23,862.38 | 5.23 | KX958480 |
| CfHsp24.3 | 1,302 | 223 | 24,343.79 | 4.07 | KX958481 |
| CfHsp28.4 | 873 | 259 | 28,384.73 | 5.28 | KX958473 |
Preparation of samples under non-stress conditions
Day 4 eggs, day 4 sixth-instar larvae (L6D4), day 4 pupae, and newly emerged adults were each homogenized in TRIzol reagent (Thermo Fisher Scientific, USA) and then stored immediately at −20 °C until RNA extraction. Five to ten L6D4 larvae were anesthetized on ice before dissections in which head, epidermis, fat body, midgut, and Malpighian tubules were dissected and rinsed with cold phosphate-buffered saline. The tissues were immediately homogenized in TRIzol reagent and stored at −20 °C until RNA extraction.
Heat and starvation stress treatments
In preliminary experiments, L6D4 larvae were incubated in Petri dishes at different temperatures ranging from 23 to 42 °C or at 37 °C for periods of time ranging from 15 min to 2 h. For heat shock treatments, day 4 eggs, L6D4 larvae, day 4 pupae, and newly emerged adults were incubated at 37 °C for 1 h, and then immediately homogenized in TRIzol reagent and stored at −20 °C. Each treatment included an equal number of females and males. Other individuals from the same batches/cohorts were not exposed to heat and were used as controls. To investigate sHSP transcripts in heat-treated tissues, L6D4 larvae were exposed to 37 °C for 1 h and their tissues were then dissected as described in the previous section. Tissues from non-heated larvae were used as controls. Both the treated and control tissues were homogenized in TRIzol reagent and stored at −20 °C.
To investigate the effects of starvation on CfHSP expression, day 1 sixth-instar larvae given an artificial diet under laboratory conditions were controls and larvae in empty plastic cups left for 2 or 5 days were considered starving. Each treatment had four larvae with four independent replications. The treated larvae were homogenized in TRIzol reagent for RNA isolation.
RNA isolation and real-time quantitative polymerase chain reaction
Total RNA was isolated using TRIzol reagent according to the manufacturer’s instructions (Thermo Fisher Scientific, USA). To remove potential genomic DNA contamination, 10–15 μg RNA was treated with DNase I at 37 °C for 15 min and extracted again with TRIzol reagent. The absence of DNA contamination in the RNA samples was confirmed by conducting PCR with RNA template and translation elongation factor-1α (Tef-1α) primers. First-strand complementary DNA (cDNA) samples were generated from 4 μg of total RNA using RNA to cDNA EcoDry Premix (oligo dT) (Clontech Laboratories, Inc.). Reactions were diluted 20-fold using nuclease-free water and subsequently used as template for quantitative PCR. Primers for each selected gene were designed using Primer3 (http://bioinfo.ut.ee/primer3/). Primer sequences and PCR product sizes are provided in Table 2. The specificity of real-time PCR amplification was confirmed by a single peak in melting temperature curve analysis of the real-time PCR amplicon. The real-time quantitative polymerase chain reaction (RT-qPCR) was conducted in 20 μL volumes comprising 10 μL 2× SYBR ExTaq premix (Clontech Laboratories, Inc.), 2 μL cDNA template, and 8 μL gene-specific primers. A Rotor-Gene RG-3000 thermal cycler was used as follows: 40 cycles at 95 °C for 10 s and 65 °C for 20 s. The relative quantities of each transcript were assessed using the 2−∆∆CT method (Livak and Schmittgen 2001). Tef-1α was used to normalize transcript abundance in each sample. The expression levels of each transcript were measured in three or four independent biological samples and at least two technical replicates for each biological sample.
Table 2.
Primers used for quantitative RT-PCR
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Product size (bp) |
|---|---|---|---|
| CfHSP18.6 | GTGTTAAAGCCCGTCCAGAA | CTTCATCTTCCCTGGATTGC | 131 |
| CfHSP19.2 | CGAAGTTACCGGCGATTTTA | ACGCTCGCATTCCAAGTAGA | 119 |
| CfHSP19.6 | CGCTTTTAGCCAGAGATCCA | GCGTCGGAGTAGATTCCTTG | 103 |
| CfHSP19.7 | CGGTGTCGTTAGCTTCAAAT | GCGAACGGTGACAAGAAGTA | 150 |
| CfHSP20.0 | ACTTCGGCTTGGGCATAAC | GAGCCGATGTCTTGAGAAGC | 115 |
| CfHSP20.2 | CTGGCGAGAGATGAAACCAT | CCGACAAGGACAAGTTCCAG | 143 |
| CfHSP20.3 | TGATCCTTTCCGTCCATCTC | AGGTATCCCGCAGGACTTCTA | 126 |
| CfHSP21.3 | CCACATCGTCACAGCATAGC | GTGTACTGGCTGACGTCGAA | 133 |
| CfHSP21.5a | TGTTCAGCATTTTCGTCCAG | CCTTCGGGTAAAGCATATCG | 141 |
| CfHSP21.5b | AGCTTTCTTCTGACGGTGT | CCGTTCCTTCACTCTGATCC | 134 |
| CfHSP22.0 | GTTGCTGGCTTTGACTGTGA | GGAGGACAAACCTGACCAGA | 122 |
| CfHSP22.1 | ACTCAGACTATGGCGCTGCT | TCGAAAATGTCCGTCAAGA | 110 |
| CfHSP23.9 | CGGCTTCCACGAGTACTACC | ACGTCCAAGTTGATCGTGAA | 108 |
| CfHSP24.3 | TACTTGCGGTACTCGCGTTA | AACATGGGGAAACTGAACCA | 133 |
| CfHSP28.4 | ACCGATCCACACGACTGACT | GGAACCGTAGAGGCGAGATA | 136 |
| Tef-1 α | CCGTTTCGAGGAAATCAAGA | AAGGCTCCAGCATGTTGT | 119 |
Data deposition
Nucleic acid and deduced amino acid sequences of the CfHSPs were submitted to the NCBI (http://www.ncbi.nlm.nih.gov/). Each CfHSP was given a name based on the putative protein molecular weight (Table 1).
Statistical analysis
All data are presented as mean ± standard deviation (SD). Differences between treatment groups and the control group were analyzed by one-way ANOVA, followed by Student’s t test.
Results
Identification and sequence characterization of CfHSPs
To identify sHSPs in C. fumiferana, we searched the C. fumiferana transcriptome (unpublished data) using known insect sHSPs as queries. This search identified 15 sHSP genes containing full-length ORFs. The deduced proteins possessed 163 to 259 amino acid residues with derived molecular masses ranging from18.6 to 28.4 kDa. One sHSP was alkaline, having an isoelectric point value of 10.2; the other 14 had acidic pI values ranging from 4.07 to 6.34 (Table 1). Based on the predicted molecular weights, the identified genes were named CfHSP18.6, CfHSP19.2, CfHSP19.6, CfHSP19.7, CfHSP20.0, CfHSP20.2, CfHSP20.3, CfHSP21.3, CfHSP21.5a, CfHSP21.5b, CfHSP22.0, CfHSP22.1, CfHSP23.9, CfHSP24.3, and CfHSP28.4. Previously deposited CfHSP genes (accession numbers AAZ14791 and AAZ14790) display 98.9% identities with CfHSP20.0 and CfHSP21.5b, and thus are likely the same genes.
A multiple alignment and secondary structure analysis revealed that all of the identified CfHSPs possessed the typical conserved α-crystallin domain (ACD) of sHSPs, consisting of approximately 90 amino acids in the central portion of the protein (Fig. 1). Within the ACD, most CfHSPs possessed two α-helixes and seven ß-strands with some exceptions. CfHSP24.3 had only one α-helix and CfHSP21.5b had three α-helixes; CfHSP22.0 and CfHSP20.3 lacked the ß8 sheet, but had an extended α-helix in the domain. A conserved arginine residue in the ß7 region was also identified in 14 of the 15 CfHSPs. The arginine residue has been suggested to be essential for sHSP structural integrity and chaperone-like activity (Kumar et al. 1999; van Montfort et al. 2001). In all animal and most plant sHSPs, regions termed region I and region II are conserved, and play important roles in dimerization and oligomerization (Kim et al. 1998; Fu et al. 2006). These two regions were also identified in the ACD domains. In all of the CfHSPs, ß10 sheets were predicted in the C-terminal extensions. The I/V-X-I/V motifs were conserved in the ß10 sheet in all CfHSPs except CfHSP28.4. These motifs have been suggested to determine the assembly of sHSP dimers into a variety of oligomers (Haslbeck et al. 2005; Poulain et al. 2010; Basha et al. 2012). Apart from the conserved ß10 motifs, both N- and C-terminal regions show a relatively low degree of similarity in sequence composition and length, although there are still some conserved amino acid residues. CfHSP28.4 is the largest sHSP among the 15, owing to the insertion of extra 68 amino acid residues at the N-terminal region (Fig. 1).
Fig. 1.
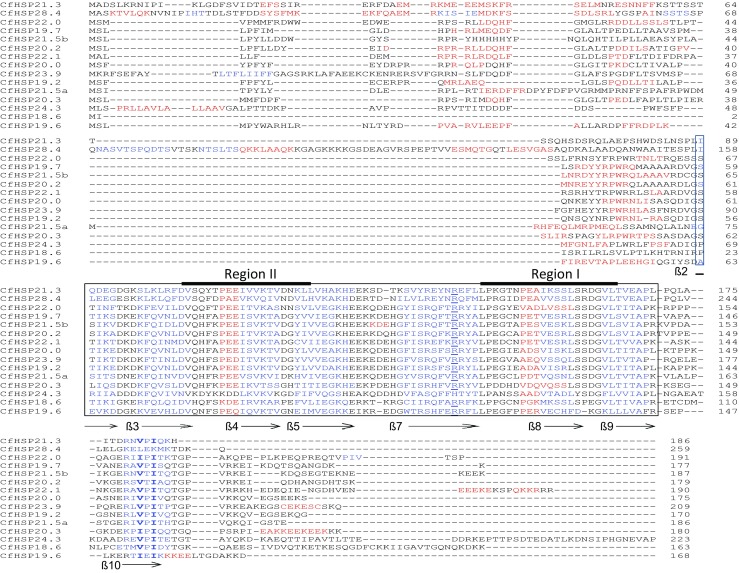
Alignment of 15 CfHSP amino acid sequences, their secondary structures, and domains. The predicted α-crystallin domains are boxed, α-helixes are in red, and ß-pleated sheets are in blue and numbered according to strands in the crystal structures of Triticum aestivum HSP16.9 (van Montfort et al. 2001). The conserved arginines in ß7 are underlined; the conserved V/I-X-I/V motifs in ß10 are in bold. Regions I and II are indicated with a solid black line
Phylogenetic analysis
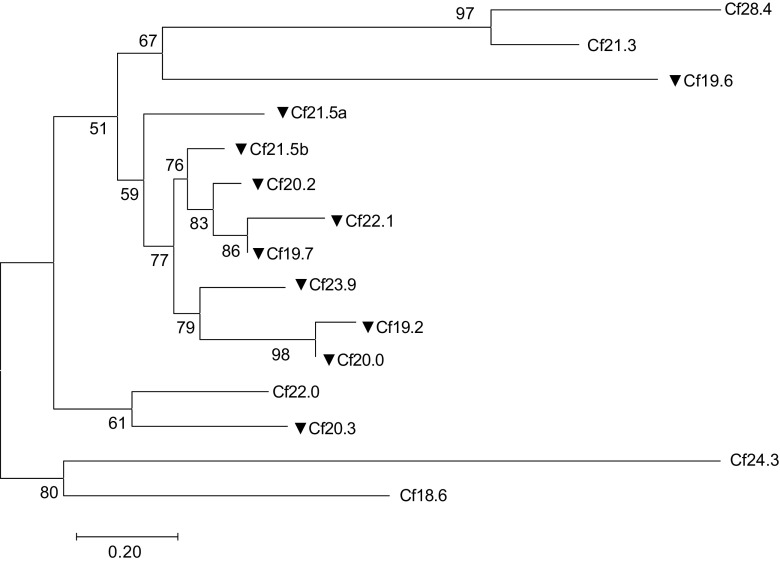
Since accurate alignments are essential for the construction of a reliable phylogenetic tree, the highly variable N- and C-terminal regions were discarded and only the amino acid sequences of the ACD were used for analysis (Fig. 2). The ACD domains in CfHSP and other lepidopteran sHSP were used to perform maximum likelihood analysis using the PhyMLv3.0. The tree indicated that (1) CfHSP28.4 and CfHSP21.3 and CfHSP19.2 and CfHSP20.0 have relatively high sequence similarly, indicating that they are likely from gene duplications, and (2) eight CfHSPs including CfHSP21.5a, CfHSP21.5b, CfHSP20.2, CfHSP22.1, CfHSP19.7, CfHSP23.9, CfHSP19.2, and CfsHSP20 reveled relatively close relationships. The evolutionary relationships of CfHSPs with other lepidopteran sHSPs were also analyzed. The tree indicated that five CfHSPs can be classified into three known lepidopteran sHSPs clusters: CfHSP21.3 and CfHSP28.4 grouped into cluster 1, CfHSP22.0 and CfHSP20.3 grouped into cluster 3, and CfHSP24.3 grouped into cluster 5 (Fig. 3). They are corresponding to BmHSP21.4, BmHSP22.6, and BmHSP 26.6 orthologs (Shen et al. 2011; Chen and Zhang 2015). Five CfHSPs could be assigned to three previously unrecognized lepidopteran sHSP clusters. Cluster 2 consists of CfHSP18.6, PxHSP18.8, BmHSP17.9, and DpHSP17. Cluster 4 contains nine sHSPs, including CfHSP19.7, CfHSP21.5b, and CfHSP20.2. Cluster 6 includes CfHSP19.6, GmHSP19.6, PxHSP19.2b, DpHSP19.3, and BmHSP19.6. The remaining five CfHSPs (CfHSP19.2, CfHSP20.0, CfHSP21.5a, CfHSP22.1, and CfHSP23.9) do not have sufficiently high bootstrap values with other sHSPs, and thus, it does not seem to be appropriate to assign them to any of the clusters. Since the cited lepidopteran sHSPs are from nearly complete genomes and a BLAST search did not reveal other homologs, these five CfHSPs appear to be spruce budworm-specific sHSPs.
Fig. 2.
Phylogenetic tree of CfHSPs. The maximum-likelihood (ML) phylogenetic tree was inferred from the alignment of α-crystallin domain amino acid sequences of the CfHSPs under the best-fit model LG + G, which was estimated by ProTest. The heat-inducible CfHSPs are labeled with black triangles. The bootstrap values from 100 resamplings are shown at each node, and the scale bar represents the average numbers of substitutions per amino acid position. Branches corresponding to partitions reproduced in less than 50% of the bootstrap replicates were collapsed
Fig. 3.
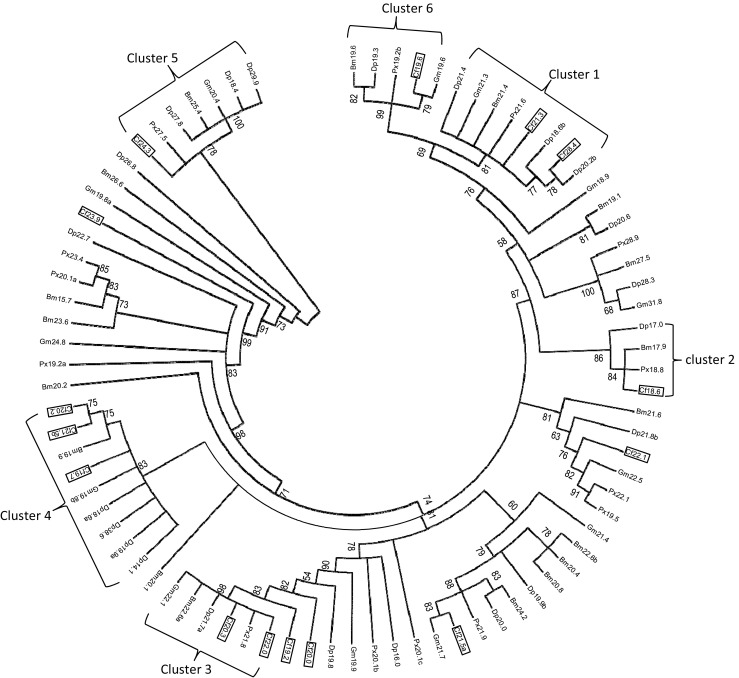
Phylogenetic tree of lepidopteran sHSPs. To construct this phylogenetic tree, 82 lepidopteran sHSP genes were used from the following species: silkworm, B. mori (18 sHSPs); the monarch butterfly, D. plexippus (22 sHSPs); the oriental fruit moth, G. molesta (13 sHSPs); the diamondback moth, P. xylosyella (14 sHSPs); and spruce budworm, C. fumiferana (15 sHSPs). The GenBank accession numbers and ACD domains used for construction of the tree are listed in S1 (Table 1) and S2 (Fig. 1). Only the conserved α-crystallin domain in each sHSP was used in alignment and tree construction. The maximum-likelihood (ML) phylogenetic tree was inferred under the best-fit model LG + G, which was estimated by ProTest. The bootstrap values from 100 resamplings are shown at each node. Branches corresponding to partitions reproduced in less than 50% of the bootstrap replicates were collapsed. Abbreviations of species names: Bm Bombyx mori, Cf Choristoneura fumiferana, Dp Danaus plexippus, Gm Grapholita molesta, Px Plutella xylostella
Stage- and tissue-specific expression profiles of CfHSPs under normal conditions
The expression profiles of CfHSPs at the egg, larval, pupal, and adult stages under normal conditions were determined using RT-qPCR. The transcript levels of individual members are presented as apparent expression levels relative to the internal control gene Tef-1α. Of the 15 identified CfHSP genes, 14 were detected in all life stages. The exception, CfHSP20.3, was not detected from any life stage under non-stress conditions; the only detection was the extremely low expression in pupae and adults after heat shock. Thus, it was excluded from further analysis. Fourteen CfHSPs were detected in four life stages, but the expression levels varied depending on the individual CfHSP and developmental stage (Fig. 4a). Three (CfHSP18.6, CfHSP22.1, and CfHSP 23.9) were constitutively expressed with no significant difference at any life stage; nine (CfHSP19.7, CfHSP20.0, CfHSP20.2, CfHSP21.3, CfHSP21.5a, CfHSP21.5b, CfHSP22.0, CfsHSP24.3, and CfsHSP28.4) were most highly expressed in adults. In contrast, CfHSP19.6 was expressed at a significantly higher level in pupae. In general, CfHSP expression levels from highest to lowest were adult > pupae > larvae > egg, but with some exceptions. For example, CfHSP21.5b and CfHSP24.3 were more highly expressed in larvae than they were in pupae.
Fig. 4.
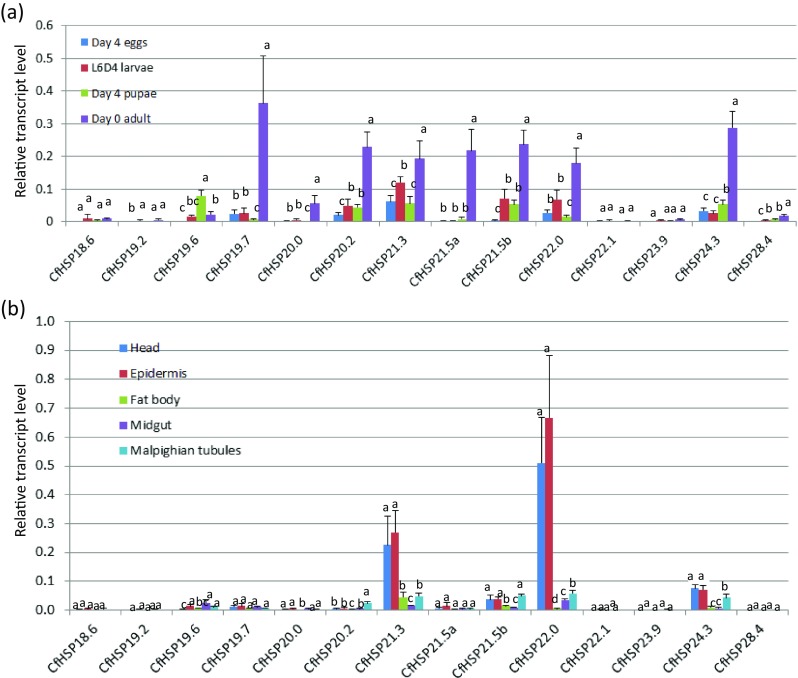
Developmental expression profiles of CfHSP transcripts. a Relative CfHSP transcript levels at different developmental stages. b Relative CfHSP transcript levels in different tissues of day 4 sixth-instar larvae. Values are means ± SD of three to four biological replicates, with two technical replicates each and are expressed as apparent expression levels relative to the control gene, translation elongation factor-1α (Tef-1α). For each gene, different letters above the bars indicate the significant differences compared with the other stages or tissues (P < 0.05)
Head, epidermis, fat body, midgut, and Malpighian tubules were dissected from L6D4 larvae and used to determine tissue-specific expression profiles of CfHSPs. The transcript of each CfHSP was detected in all the larval tissues examined, but the expression levels were gene and tissue dependent (Fig. 4b). The expression of CfHSP21.3 and CfHSP22.0 was extremely high in the head and epidermis; transcript levels were around 50- and 60-fold higher than in other tissues, respectively. Expression of CfHSP24.3 was also higher in head and epidermis than other tissues. Most CfHSPs exhibited high expression in the Malpighian tubules and low expression in the midgut.
Effects of heat stress temperature and time on CfHSP expression
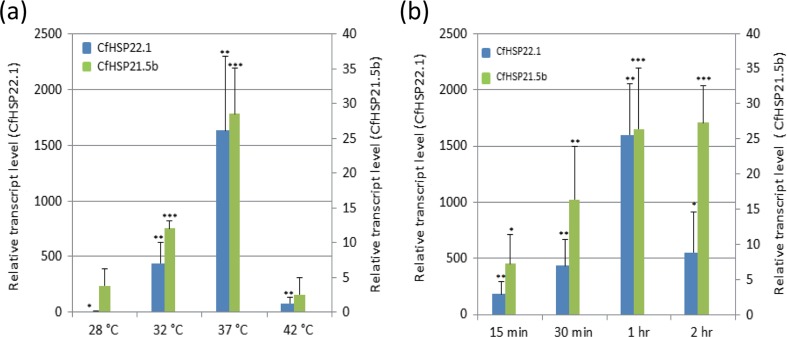
To find appropriate heat stress conditions to examine the CfHSP response, L6D4 larvae were treated at different temperatures (Fig. 5a) or for different lengths of time (Fig. 5b). The relative transcript levels of CfHSP22.1 and CfHSP21.5b (randomly selected) were examined using RT-qPCR. A 5 °C elevation in temperature for 1 h significantly increased CfHSP22.1 expression to approximately 5-fold of the control (Fig. 5a). Expression of CfHSP21.5b was induced by a 5 °C increase in temperature, but not significantly so. A 15-min exposure to 37 °C increased the expression of both CfHSP22.1 and CfHSP21.5b. Treatment at 42 °C for 1 h led to comatose larvae with no increase in the expression of CfHSPs. Thus, overall, treatment at 37 °C for 1 h induced the highest expression of CfHSP22.1 and CfHSP21.5b, and all further heat stress experiments were conducted using these conditions.
Fig. 5.
Effects of heat stress temperature and duration on CfHSP transcript levels. Day 4 sixth-instar larvae were treated at different temperatures for 1 h (a) or at 37 °C for different times (b). Expression levels are represented as relative expression units after normalization to the translation elongation factor-1α (Tef-1α). Values are means ± SD of three independent biological replicates. The expression levels of control samples were set as 1. The asterisks above the bars indicate the significant differences in expression levels by Student’s t test (*P < 0.05, **P < 0.01, and ***P < 0.001). The expression level of CfHSP22.1 at 28 °C was too low to be seen in a, but the statistical significance of the change in transcript level in response to heat stress is shown
Effect of heat stress on the expression of CfHSPs at different life stages
Nine CfHSPs (CfHSP19.2, CfHSP19.6, CfHSP19.7, CfHSP20.0, CfHSP20.2, CfHSP21.5a, CfHSP21.5b, CfHSP22.1, and CfHSP23.9) showed significantly increased expression after heat stress at every developmental stage (Fig. 6a). Interestingly, eight of the heat-inducible CfHSPs displayed evolutionally close relationships (Fig. 2). The increases varied depending on the individual CfHSP gene and developmental stage. Among the nine upregulated CfHSPs, CfHSP22.1 displayed the highest increase after heat stress; transcript levels increased by 400-fold in eggs, 1770-fold in larvae, 1840-fold in pupae, and 1200-fold in adults. Of the heat shock genes, only CfHSP19.7 and CfHSP21.5b exhibited a significantly greater response in adults, and only CfHSP19.6 exhibited a significantly strong response in eggs. In contrast, after heat stress, five genes (CfHSP18.6, CfHSP21.3, CfHSP22.0, CfHSP24.3, and CfHSP28.4) did not show a significant change in expression compared to non-treated controls.
Fig. 6.
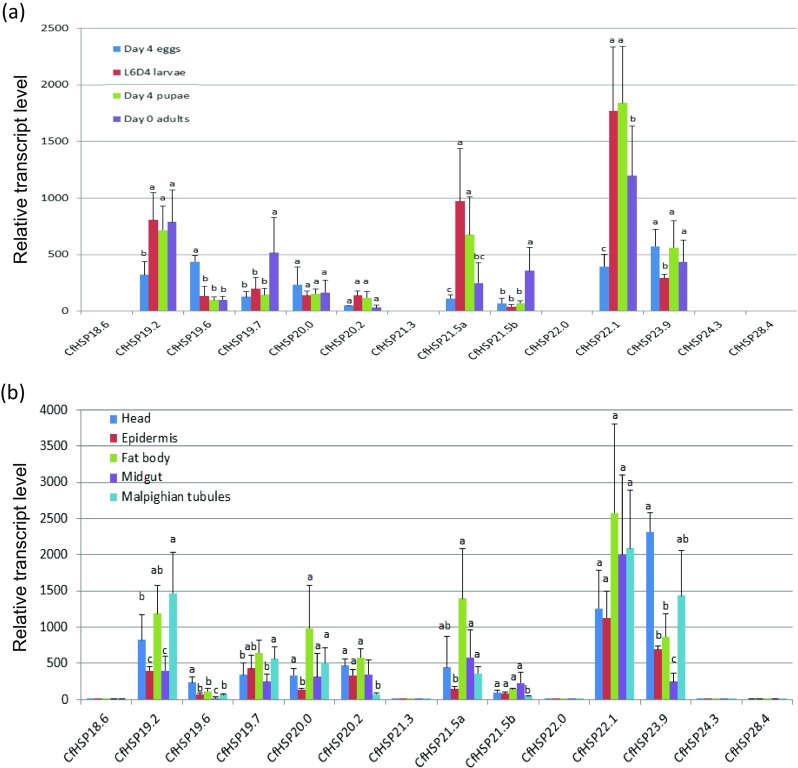
Relative transcript levels of CfHSP in response to heat stress. a Relative expression levels of CfHSP transcripts in whole individuals at different developmental stages after 1-h heat stress of 37 °C. b Relative expression levels of CfHSP transcripts in different L6D4 larval tissues under heat stress. Values are means ± SD of three or four biological replicates and are expressed as relative expression levels between the stress and control groups. Each control group was set as 1. Different letters above the bar for each gene indicate the significant differences in expression levels by Student’s t test
Effect of heat stress on CfHSP expression in different tissues
To determine whether tissues differed in the response of CfHSP genes to heat stress, we examined transcript levels in different tissues after heat stress. After exposure of L6D4 larvae to 37 °C for 1 h, expression levels of the nine heat-inducible CfHSP genes were increased in all of the tested tissues (Fig. 6b). For most CfHSPs, head, fat body, and Malpighian tubules had a greater response to heat stress than did epidermis and midgut. CfHSP23.9 had a greater response in head and Malpighian tubules than in the other tissues, whereas CfHSP20.2 and CfHSP21.5b had weaker responses in Malpighian tubules than in other tissues. These results indicated that the responses of CfHSP genes after heat stress are different in different tissues.
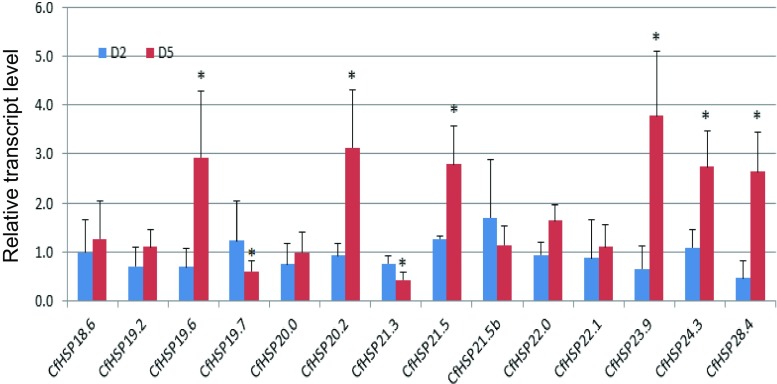
Effects of starvation on CfHSP expression
To investigate the effects of starvation on CfHSP expression, day 1 sixth-instar larvae were fed or starved. The larvae survived without food for 6–8 days at room temperature. Body weight differences between the starved larvae and the controls were about 15–20% on day 2 and 70–80% on day 5. After 2 days of starvation, RT-qPCR revealed no significant differences in the transcript levels of CfHSPs between starved and control larvae (Fig. 7). In contrast, after 5 days of starvation, the increase in transcript levels of six CfHSPs (CfHSP19.6, CfHSP20.2, CfHSP21.5, CfHSP23.9, CfHSP24.3, and CfHSP28.4) were significantly increased (range 2.5- to 3.4-fold). Others, namely, CfHSP19.7 and CfHSP21.3, exhibited significant reduced expression (0.4- to 0.6-fold as compared to the control, respectively). Overall, the transcript levels of eight CfHSPs increased or decreased while that of the other six were unchanged after starvation (Fig. 7).
Fig. 7.
Relative transcript levels of CfHSPs in response to starvation. CfHSP transcripts in larvae starved for two days (D2) or five days (D5). Values are means ± SD of four biologically independent replicates. The expression levels of control samples were set to 1. Statistical significance within genes was determined using the Student’s t test (P < 0.05) and is labeled with an asterisk
Discussion
In this study, 15 sHSPs from C. fumiferana were identified. These genes showed features common to all sHSP families, a conserved α-crystallin domain with six or seven ß-sheets, and non-conserved N- and C-terminal regions (Sun and MacRae 2005; Kriehuber et al. 2010; Basha et al. 2012). These conserved and/or variable regions may be involved in oligomeric formation, substrate binding, and chaperone activity (van Montfort et al. 2001; Giese et al. 2005; Basha et al. 2006; Jaya et al. 2009; Basha et al. 2012). Substrate protein recognition and binding by sHSP are essential for their chaperone functions. The evolutionarily variable N- and C-terminal arms are critical for the ability of sHSPs to bind and protect many different substrate proteins (Jaya et al. 2009; Eyles and Gierasch 2010; Kriehuber et al. 2010; Basha et al. 2012); the diversity of N- or C-terminal primary sequences among the 15 CfHSPs may imply that these genes have diverse, possibly specialized functions among different tissues or organs during developmental stages.
Phylogenetic analysis of the sHSPs in C. fumiferana and four other lepidopteran species revealed that ten CfHSPs have known lepidopteran orthologs (Fig. 3), whereas the remaining five do not, suggesting that they may be spruce budworm specific. A similar pattern of orthology is observed in the sHSP families in other species of Lepidoptera, e.g., B. mori (Li et al. 2009), Spodoptera litura (Shen et al. 2011), and G. molesta (Zhang et al. 2015); approximately one third to half of the identified lepidopteran sHSPs have no known papalogs. Comparing the evolutionary relationship between heat response and the 82 known lepidopteran sHSPs reveals some interesting features; two of six lepidopteran sHSP clusters examined in this (Figs. 2 and 3) and in other studies (Sakano et al. 2006; Chen and Zhang 2015; Zhang et al. 2015) contained members that were heat inducible, whereas those of the other four were not. Among the CfHSPs, eight heat-inducible CfHSPs have relatively close relationships and probably diverged from a common ancestor (Fig. 2). They may greatly contribute to the adaptability of spruce budworm in severe weather conditions, but further research is required to establish this.
All organisms from bacteria to plants to animals have sHSPs. Fu et al. (2006) proposed that animal HSPs originated from the bacterial class A sHSPs by gene transfer from an endosymbiont. Huang et al. (2008) suggested that sHSP genes may have duplicated at an early time in insect evolution and that this was followed more recently by order-specific duplication. In our study, we found that some CfHSP genes are order-specific, whereas others are likely species-specific with no orthologous genes. Nei and Rooney (2005) proposed the birth-and-death evolutionary model, where new genes are created by duplication and some of the duplicated genes remain stable in the genome, whereas others are deleted or become pseudogenes. There is evidence within the CfHSP genes of duplication, of new function, and of lack of function. For example, the CfHSP21.3 and CfHSP28.4 are likely the result of gene duplication (Fig. 2); CfHSP21.3 exhibited extremely high expression in larval epidermis (Fig. 4b), and thus may play a special role in larval growth; and CfHSP20.3 has no expression during development and may be a pseudogene. In general, when genes are subjected to the birth-and-death evolution, environmental conditions play an important role in altering or maintaining their functions. It is reasonable to believe that this is the case for insect sHSP gene evolution as well.
In the present study, complex expression profiles of 14 CfHSPs were discovered through detailed investigation of the spruce budworm at six life stages and in five larval tissues under controlled conditions (Fig. 4). Similar results have been observed in B. mori (Li et al. 2009), S. litura (Shen et al. 2011), C. suppressalis (Lu et al. 2014), and Plutella xylostella (Chen and Zhang 2015), suggesting that insect sHSPs have diverse biological functions under developmental conditions and individual sHSP genes may have special roles in different species and tissues. In general, the highest and lowest levels of CfHSP expression occurred in adults and fertilized eggs, respectively (Fig. 4a). A similar pattern occurs in S. litura (Shen et al. 2011) and P. xylostella (Chen and Zhang 2015). It may be that highly expressed sHSPs are involved in adult-specific physiological events, such as reproduction. In addition, spruce budworm adults, compared to eggs, can survive exposure to more highly fluctuating temperatures in the field (Royama 1984). Adults need protection against both high and low temperatures, and the highly expressed CfHSPs may provide the ability to deal with temperature extremes. Further experiments are necessary to demonstrate the roles of CfHSPs in C. fumiferana.
Two CfHSPs, CfHSP21.3 and CfHSP22.0, are very highly expressed in the head and epidermis under non-stressed conditions. In G. molesta, GmHSP21.3 is highly expressed in the head (Zhang et al. 2015). GmHSP21.3 and CfHSP21.3 are closely related; both are classified within the cluster 1 (Fig. 3). Interestingly, CfHSP22.0 shares 89% amino acid identity with CsHSP21.7a, which is highly expressed in the head and epidermis of C. suppressalis (Lu et al. 2014). Although corroborative evidence from more species is necessary, these results suggest that the head and epidermis specifically expressed sHSPs may be evolutionarily conserved in Lepidoptera and may have special yet-to-be-determined roles in these tissues.
During the spruce budworm life cycle, extreme temperatures and food shortages are two of the most important factors that negatively affect distribution and outbreaks (Gray 2008; Frago and Bauce 2014). In the present study, we found nine CfHSPs that are heat inducible. Most of these may well be spruce budworm specific, since they are without known orthologs (Figs. 2, 3, and 6). We investigated the heat stress response of five larval tissues and found that the response to heat stress was tissue-dependent. The fat body and Malpighian tubules were the most sensitive tissues to heat stress (Fig. 6b), which is also the case in silkworm (Chandrakanth et al. 2015). The fat body is involved in many metabolic roles. Like an “invertebrate liver,” it stores and releases energy and synthesizes numerous biological substances essential for normal physiological function in insects. The Malpighian tubules excrete water, waste, and maintain osmotic balance. The highly expressed CfHSPs in the fat body and Malpighian tubules may be related to one or more of these processes. Shen et al. (2011) suggest that sHSPs may be involved in the catabolism of toxic metabolites or reabsorption of water in the Malpighian tubules. However, the function of the highly expressed sHSPs in Malpighian tubules is still not clear.
In addition, eight CfHSPs are upregulated or downregulated by starvation (Fig. 7). Five of the starvation-controlled CfHSPs are also heat inducible, suggesting that the expression of these genes is controlled by either elevated temperature or starvation or both. Although the correlation between CfHSP expression levels and stress resistance is currently unknown, a number of studies suggest that the highly expressed sHSP proteins are associated with thermotolerance (Gehring and Wehner 1995; Kim et al. 1998; Nakamoto et al. 2000; Zhao and Jones 2012). Therefore, it is tempting to speculate that the heat- or starvation-response CfHSP genes play important roles in spruce budworm adaptation to unfavorable environmental conditions. However, as pointed out by Sun and MacRae (2005), the relationship between the expression of sHSP and organismal thermotolerance is unclear, because modification of sHSP expression only sometimes impacts thermotolerance. Moreover, an increase of sHSPs at the transcript level is not always correlated with higher protein levels under heat stress (Zhao and Jones 2012). Therefore, further research is necessary to clarify how the heat stress-sensitive CfHSPs contribute to spruce budworm adaptation to unfavorable environmental conditions.
The correlation between CfHSP gene expression levels at control and heat stress conditions were weak. Among the seven CfHSP genes highly expressed at the adult stage under normal condition, five (CfHSP19.7, CfHSP20.0, CfHSP20.2, CfHSP21.5a, and CfHSP21.5b) were heat inducible and two (CfHSP22.0 and CfHSP24.3) were not (Figs. 4a and 6). Two CfHSPs (CfHSP21.3 and CfHSP22.0) highly expressed in the epidermis and head are also not heat inducible (Figs. 4b and 6). These phenomena are likely common in insect sHSPs such as G. molesta (Zhang et al. 2015). The different expression profiles in normal and stress conditions imply that different factors may be involved in the control of CfHSP gene transcription and that individual CfHSPs may play distinct roles in normal and stress conditions. Insect sHSPs play a role in a wide range of biological functions: embryogenesis (Haass et al. 1990; Michaud and Tanguay 2003), diapause (King and MacRae 2015), oogenesis and spermatogenesis (Economou et al. 2017), and development and differentiation (Shen et al. 2011). However, because a large number of sHSPs are species-specific, their biological functions may vary from one species to another. Further detailed studies are necessary to demonstrate sHSP roles in stress and non-stress conditions.
Food shortage is a common situation in nature for insects, and little is known about the strategies used to overcome it (Frago and Bauce 2014). In this study, we found that eight CfHSP genes are upregulated or downregulated by starvation. Many studies have demonstrated that starvation or crowding can regulate the expression of sHSPs. Wang et al. (2007) reported that crowding or starvation induces the expression of many sHSPs in the migratory locust, Locusta migratoria. Chapuis et al. (2011) reported similar results in the Australian plague locust, Chortoicetes terminifera, and the endoparasitoid wasp, Pteromalus puparum. Chen and Zhang (2015) noted that in the diamondback moth, P. xylostella, four sHSPs are downregulated. Drosophila HSP26, HSP27, and HSP70 are upregulated after starvation (Wang et al. 2004). Scharf et al. (2016) noted that in T. castaneum, starvation leads to impaired cold tolerance. It has been hypothesized that sHSPs may be involved in water and/or ion balance and energy consumption (Wang et al. 2007; Chapuis et al. 2011; Chen and Zhang 2015; Scharf et al. 2016), but further detailed experiments are necessary to examine how sHSPs are involved in these events.
It is thought that certain insect heat shock proteins and associated cochaperones interact with substrate proteins to form structural networks that serve as a first line of defense against cell damage by playing important roles in maintaining cellular homeostasis, protein synthesis, stress tolerance, and diapause (Jaya et al. 2009; King and MacRae 2015). However, the whole picture is still not clear. Studies investigating how HSPs interact with other chaperones and their client proteins will help us better understand how they contribute to insect survival during diapause and other times of increased stress.
In summary, natural populations of spruce budworm are constantly exposed to changing and non-optimal environmental conditions. Here, we identified 15 CfHSP genes and analyzed their expression profiles under normal conditions and under heat stress or starvation in the laboratory. The gene expression profiles suggest that CfHSPs may have important roles in development and contribute to the response to extreme environments. Since the field environment is very different from controlled conditions in the laboratory, it will be interesting to investigate how the daily and seasonal changes in temperatures affect CfHSP expression in wild populations and the relationship between CfHSP expression and thermal tolerance. A greater understanding of these processes may be useful in predicting the distribution and outbreaks of this and other pest insects.
Electronic supplementary material
List of the sHSPs from four lepidopteran species used in the construction of the phylogenetic tree. (XLSX 14 kb)
The conserved α-crystallin domain sequences (ACD) used in phylogenetic tree construction. (DOCX 25 kb)
Acknowledgements
We greatly thank William Fick for his editorial assistance, two anonymous reviewers for their constructive comments, and Insect Production Services at Great Lake Forestry Centre for providing the insect materials. This work was supported by grants from the Genomics Research and Development Initiative (GRDI) and Canadian Forest Service, Natural Resources Canada.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-017-0832-7) contains supplementary material, which is available to authorized users.
References
- Aevermann BD, Waters ER. A comparative genomic analysis of the small heat shock proteins in Caenorhabditis elegans and briggsae. Genetica. 2008;133:307–319. doi: 10.1007/s10709-007-9215-9. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- Basha E, Friedrich KL, Vierling E. The N-terminal arm of small heat shock proteins is important for both chaperone activity and substrate specificity. J Bio Chem. 2006;281:39943–39952. doi: 10.1074/jbc.M607677200. [DOI] [PubMed] [Google Scholar]
- Basha E, O’Neill H, Vierling E. Small heat shock proteins and α-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci. 2012;37:106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger Y, Arseneault D. Spruce budworm outbreaks in eastern Quebec over the last 450 years. Can J For Res. 2004;34:1035–1043. doi: 10.1139/x03-269. [DOI] [Google Scholar]
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Chandrakanth N, Ponnuvel KM, Moorthy SM, Sasibhushan S, Sivaprasad V. Analysis of transcripts of heat shock protein genes in silkworm, Bombyx mori (Lepidoptera: Bombycidae) Eur J Entomol. 2015;112:676–687. [Google Scholar]
- Chapuis MP, Simpson SJ, Blondin L, Sword GA. Taxa-specific heat shock proteins are over-expressed with crowding in the Australian plague locust. J Insect Physiol. 2011;57:1562–1567. doi: 10.1016/j.jinsphys.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Chen XE, Zhang YL. Identification of multiple small heat-shock protein genes in Plutella xylostella (L.) and their expression profiles in response to abiotic stresses. Cell Stress Chaperones. 2015;20:23–35. doi: 10.1007/s12192-014-0522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet H, Lee SF, Hoffmann A. Knocking down expression of Hsp22 and Hsp23 by RNA interference affects recovery from chill coma in Drosophila melanogaster. J Exp Biol. 2010;213:4146–4150. doi: 10.1242/jeb.051003. [DOI] [PubMed] [Google Scholar]
- Colinet H, Lee SF, Hoffmann A. Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster. FEBS J. 2010;277:174–185. doi: 10.1111/j.1742-4658.2009.07470.x. [DOI] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger DL. Regulation of diapause. Annu Rev Entoml. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- Duan J, Ladd T, Doucet D, Cusson M, Mittapalli O, Krell PJ, Quan G. Transcriptome analysis of the emerald ash borer (EAB), Agrilus planipennis: de novo assembly, functional annotation and comparative analysis. PLoS One. 2015;10:e0134824. doi: 10.1371/journal.pone.0134824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky EB, Dretzen G, Berger EM. The broad-complex gene is a tissue-specific modulator of the ecdysone response of the Drosophila hsp23 gene. Mol Cell Biol. 1996;16:6542–6552. doi: 10.1128/MCB.16.11.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou K, Kotsiliti E, Mintzas AC. Stage and cell-specific expression and intracellular localization of the small heat shock protein Hsp27 during oogenesis and spermatogenesis in the Mediterranean fruit fly, Ceratitis capitata. J Insect Physiol. 2017;96:64–72. doi: 10.1016/j.jinsphys.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N, Van Impe K, Fedotov V, Dadali E. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36:602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- Eyles SJ, Gierasch LM. Nature’s molecular sponges: small heat shock proteins grow into their chaperone roles. Proc Natl Acad Sci U S A. 2010;16:2727–2728. doi: 10.1073/pnas.0915160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frago E, Bauce É. Life-history consequences of chronic nutritional stress in an outbreaking insect defoliator. PLoS One. 2014;9:e88039. doi: 10.1371/journal.pone.0088039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck E, Madsen O, van Rheede T, Ricard G, Huynen MA, de Jong WW. Evolutionary diversity of vertebrate small heat shock proteins. J Mol Evol. 2004;59:792–805. doi: 10.1007/s00239-004-0013-z. [DOI] [PubMed] [Google Scholar]
- Fu X, Jiao W, Chang Z. Phylogenetic and biochemical studies reveal a potential evolutionary origin of small heat shock proteins of animals from bacterial class A. J Mol Evol. 2006;62:257–266. doi: 10.1007/s00239-005-0076-5. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Wehner R. Heat shock protein synthesis and thermotolerance in Cataglyphis, an ant from the Sahara desert. Proc Natl Acad Sci U S A. 1995;92:2994–2998. doi: 10.1073/pnas.92.7.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KC, Basha E, Catague BY, Vierling E. Evidence for an essential function of the N terminus of a small heat shock protein in vivo, independent of in vitro chaperone activity. Proc Natl Acad Sci U S A. 2005;102:18896–18901. doi: 10.1073/pnas.0506169103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DR. The relationship between climate and outbreak characteristics of the spruce budworm in eastern Canada. Clim Chang. 2008;87:361–383. doi: 10.1007/s10584-007-9317-5. [DOI] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Haass CH, Klein UL, Kloetzel PM. Developmental expression of Drosophila melanogaster small heat-shock proteins. J Cell Sci. 1990;96:413–418. doi: 10.1242/jcs.96.3.413. [DOI] [PubMed] [Google Scholar]
- Han EN, Bauce E. Physiological changes and cold hardiness of spruce budworm larvae, Choristoneura fumiferana (Clem.), during pre-diapause and diapause development under laboratory conditions. Can Ent. 1993;125:1043–1053. doi: 10.4039/Ent1251043-6. [DOI] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Hayward SA, Pavlides SC, Tammariello SP, Rinehart JP, Denlinger DL. Temporal expression patterns of diapause-associated genes in flesh fly pupae from the onset of diapause through post-diapause quiescence. J Insect Physiol. 2005;51:631–640. doi: 10.1016/j.jinsphys.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Huang LH, Chen B, Kang L. Impact of mild temperature hardening on thermotolerance, fecundity, and Hsp gene expression in Liriomyza huidobrensis. J Insect Physiol. 2007;53:1199–1205. doi: 10.1016/j.jinsphys.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Huang LH, Wang HS, Kang L. Different evolutionary lineages of large and small heat shock proteins in eukaryotes. Cell Res. 2008;18:1074–1076. doi: 10.1038/cr.2008.283. [DOI] [PubMed] [Google Scholar]
- Huet F, Lage JD, Ruiz C, Richards G. The role of ecdysone in the induction and maintenance of hsp27 transcripts during larval and prepupal development of Drosophila. Dev Genes Evol. 1996;206:326–332. doi: 10.1007/s004270050059. [DOI] [PubMed] [Google Scholar]
- Jaya N, Garcia V, Vierling E. Substrate binding site flexibility of the small heat shock protein molecular chaperones. Proc Natl Acad Sci U S A. 2009;106:15604–15609. doi: 10.1073/pnas.0902177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanisse DR, Michaud S, Inaguma Y, Tanguay RM. Small heat shock proteins of Drosophila: developmental expression and functions. J Biosci. 1998;23:369–376. doi: 10.1007/BF02936130. [DOI] [Google Scholar]
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- King AM, MacRae TH. Insect heat shock proteins during stress and diapause. Annu Rev Entomol. 2015;60:59–75. doi: 10.1146/annurev-ento-011613-162107. [DOI] [PubMed] [Google Scholar]
- Kokolakis G, Tatari M, Zacharopoulou A, Mintzas AC. The hsp27 gene of the Mediterranean fruit fly, Ceratitis capitata: structural characterization, regulation and developmental expression. Insect Mol Biol. 2008;17:699–710. doi: 10.1111/j.1365-2583.2008.00840.x. [DOI] [PubMed] [Google Scholar]
- Kriehuber T, Rattei T, Weinmaier T, Bepperling A, Haslbeck M, Buchner J. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010;24:3633–3642. doi: 10.1096/fj.10-156992. [DOI] [PubMed] [Google Scholar]
- Kumar LS, Ramakrishna T, Rao CM. Structural and functional consequences of the mutation of a conserved arginine residue in αA and αB crystallins. J Bio Chem. 1999;274:24137–24141. doi: 10.1074/jbc.274.34.24137. [DOI] [PubMed] [Google Scholar]
- Landry J, Chrétien P, Lambert H, Hickey E, Weber LA. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AQ, Popova-Butler A, Dean DH, Denlinger DL. Proteomics of the flesh fly brain reveals an abundance of upregulated heat shock proteins during pupal diapause. J Insect Physiol. 2007;53:385–391. doi: 10.1016/j.jinsphys.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Li ZW, Li X, Yu QY, Xiang ZH, Kishino H, Zhang Z. The small heat shock protein (sHSP) genes in the silkworm, Bombyx mori, and comparative analysis with other insect sHSP genes. BMC Evol Biol. 2009;9:1–14. doi: 10.1186/1471-2148-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QN, Zhu BJ, Dai LS, Fu WW, Lin KZ, Liu CL. Overexpression of small heat shock protein 21 protects the Chinese oak silkworm Antheraea pernyi against thermal stress. J Insect Physiol. 2013;59:848–854. doi: 10.1016/j.jinsphys.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xi D, Kang M, Guo X, Xu B. Molecular cloning and characterization of Hsp27. 6: the first reported small heat shock protein from Apis cerana cerana. Cell Stress Chaperones. 2012;17:539–551. doi: 10.1007/s12192-012-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu MX, Hua J, Cui YD, Du YZ. Five small heat shock protein genes from Chilo suppressalis: characteristics of gene, genomic organization, structural analysis, and transcription profiles. Cell Stress Chaperones. 2014;19:91–104. doi: 10.1007/s12192-013-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alpha A-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- Mahroof R, Zhu KY, Subramanyam B. Changes in expression of heat shock proteins in Tribolium castaneum (Coleoptera: Tenebrionidae) in relation to developmental stage, exposure time, and temperature. Ann Entomol Soc Am. 2005;98:100–107. doi: 10.1603/0013-8746(2005)098[0100:CIEOHS]2.0.CO;2. [DOI] [Google Scholar]
- McMorran A. A synthetic diet for the spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae) Can Entomol. 1965;97:58–62. doi: 10.4039/Ent9758-1. [DOI] [Google Scholar]
- Michaud S, Tanguay RM. Expression of the Hsp23 chaperone during Drosophila embryogenesis: association to distinct neural and glial lineages. BMC Dev Boil. 2003;14:9. doi: 10.1186/1471-213X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G, Heikkila JJ, Tanguay RM. Differences in the chaperone-like activities of the four main small heat shock proteins of Drosophila melanogaster. Cell Stress Chaperones. 2006;11:51–60. doi: 10.1379/CSC-166.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto H, Suzuki N, Roy SK. Constitutive expression of a small heat-shock protein confers cellular thermotolerance and thermal protection to the photosynthetic apparatus in Cyanobacteria. FEBS Lett. 2000;483:169–174. doi: 10.1016/S0014-5793(00)02097-4. [DOI] [PubMed] [Google Scholar]
- Nei M, Rooney AP. Concerted and birth-and-death evolution of multigene families. Annu Rev Genet. 2005;39:121–152. doi: 10.1146/annurev.genet.39.073003.112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J, Grishin NV. PROMALS3D: multiple protein sequence alignment enhanced with evolutionary and three-dimensional structural information. Methods Mol Biol. 2014;1079:263–271. doi: 10.1007/978-1-62703-646-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain P, Gelly JC, Flatters D. Detection and architecture of small heat shock protein monomers. PLoS One. 2010;5:e9990. doi: 10.1371/journal.pone.0009990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Neal SJ, Robertson RM, Westwood JT, Walker VK. Cold hardening and transcriptional change in Drosophila melanogaster. Insect Mol Biol. 2005;14:607–613. doi: 10.1111/j.1365-2583.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- Quinlan R. Cytoskeletal competence requires protein chaperones. Pro Mol Subcell Biol. 2002;28:219–234. doi: 10.1007/978-3-642-56348-5_12. [DOI] [PubMed] [Google Scholar]
- Régnière J, St-Amant R, Duval P. Predicting insect distributions under climate change from physiological responses: spruce budworm as an example. Biol Invasions. 2012;14:1571–1586. doi: 10.1007/s10530-010-9918-1. [DOI] [Google Scholar]
- Rinehart JP, Hayward SA, Elnitsky MA, Sandro LH, Lee RE, Denlinger DL. Continuous up-regulation of heat shock proteins in larvae, but not adults, of a polar insect. Proc Natl Acad Sci U S A. 2006;103:14223–14227. doi: 10.1073/pnas.0606840103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart JP, Li A, Yocum GD, Robich RM, Hayward SA, Denlinger DL. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc Natl Acad Sci U S A. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royama TO. Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol Monogr. 1984;54:429–462. doi: 10.2307/1942595. [DOI] [Google Scholar]
- Sakano D, Li B, Xia Q, Yamamoto K, Fujii H, Aso Y. Genes encoding small heat shock proteins of the silkworm, Bombyx mori. Biosci Biotechnol Biochem. 2006;70:2443–2450. doi: 10.1271/bbb.60176. [DOI] [PubMed] [Google Scholar]
- Scharf I, Wexler Y, MacMillan HA, Presman S, Simson E, Rosenstein S. The negative effect of starvation and the positive effect of mild thermal stress on thermal tolerance of the red flour beetle, Tribolium castaneum. Sci Nature. 2016;103:1–10. doi: 10.1007/s00114-016-1344-5. [DOI] [PubMed] [Google Scholar]
- Shen Y, Gu J, Huang LH, Zheng SC, Liu L, Xu WH, Feng QL, Kang L. Cloning and expression analysis of six small heat shock protein genes in the common cutworm, Spodoptera litura. J Insect Physiol. 2011;57:908–914. doi: 10.1016/j.jinsphys.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Ashfaq M, Tsumuki H. Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70, and hsp19.5) from the diamondback moth, Plutella xylostella (L.) and their expression in relation to developmental stage and temperature. Arch Insect Biochem Physiol. 2006;62:80–90. doi: 10.1002/arch.20124. [DOI] [PubMed] [Google Scholar]
- Stehr GW. On coniferophagous species of Choristoneura (Lepidoptera: Tortricidae) in North America: II. Geographic distribution in accordance with forest regions. Can Entomol. 1967;99:456–463. doi: 10.4039/Ent99456-5. [DOI] [Google Scholar]
- Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cell Mol Life Sci. 2005;62:2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi KH, Rako L, Takano-Shimizu T, Hoffmann AA, Lee SF. Effects of small Hsp genes on developmental stability and microenvironmental canalization. BMC Evol Biol. 2010;10:1–11. doi: 10.1186/1471-2148-10-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkova NM, Horváth I, Török Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, Vígh L. Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci U S A. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Montfort RLM, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat Struct Mol Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- Wang HD, Kazemi-Esfarjani P, Benzer S. Multiple-stress analysis for isolation of Drosophila longevity genes. Proc Natl Acad Sci U S A. 2004;101:12610–12615. doi: 10.1073/pnas.0404648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Wang XH, Zhou CS, Huang LH, Zhang SF, Guo W, Kang L. cDNA cloning of heat shock proteins and their expression in the two phases of the migratory locust. Insect Mol Biol. 2007;16:207–219. doi: 10.1111/j.1365-2583.2006.00715.x. [DOI] [PubMed] [Google Scholar]
- Waters ER, Rioflorido I. Evolutionary analysis of the small heat shock proteins in five complete algal genomes. J Mol Evol. 2007;65:162–174. doi: 10.1007/s00239-006-0223-7. [DOI] [PubMed] [Google Scholar]
- Waters ER. The evolution, function, structure, and expression of the plant sHSPs. J Exp Bot. 2013;64:391–403. doi: 10.1093/jxb/ers355. [DOI] [PubMed] [Google Scholar]
- Wieske M, Benndorf R, Behlke J, Dölling R, Grelle G, Bielka H, Lutsch G. Defined sequence segments of the small heat shock proteins HSP25 andαB-crystallin inhibit actin polymerization. FEBS J. 2001;268:2083–2090. doi: 10.1046/j.1432-1327.2001.02082.x. [DOI] [PubMed] [Google Scholar]
- Wood KL, Voss OH, Huang Q, Parihar A, Mehta N, Batra S, Doseff AI. The small heat shock protein 27 is a key regulator of CD8+ CD57+ lymphocyte survival. J Immunol. 2010;184:5582–5588. doi: 10.4049/jimmunol.0902953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum GD, Joplin KH, Denlinger DL. Upregulation of a 23 kDa small heat shock protein transcript diapause in the flesh fly, Sarcophaga crassipalpis. Insect Bioch Mol Biol. 1998;28:677–682. doi: 10.1016/S0965-1748(98)00046-0. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zheng J, Peng Y, Liu X, Hoffmann AA, Ma CS. Stress responses of small heat shock protein genes in Lepidoptera point to limited conservation of function across phylogeny. PLoS One. 2015;10:e0132700. doi: 10.1371/journal.pone.0132700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Jones WA. Expression of heat shock protein genes in insect stress responses. Invertebrate Surviv J. 2012;90:93–101. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of the sHSPs from four lepidopteran species used in the construction of the phylogenetic tree. (XLSX 14 kb)
The conserved α-crystallin domain sequences (ACD) used in phylogenetic tree construction. (DOCX 25 kb)