Abstract
Salamanders exhibit an extraordinary ability among vertebrates to regenerate complex body parts. However, scarce genomic resources have limited our understanding of regeneration in adult salamanders. Here, we present the ~20 Gb genome and transcriptome of the Iberian ribbed newt Pleurodeles waltl, a tractable species suitable for laboratory research. We find that embryonic stem cell-specific miRNAs mir-93b and mir-427/430/302, as well as Harbinger DNA transposons carrying the Myb-like proto-oncogene have expanded dramatically in the Pleurodeles waltl genome and are co-expressed during limb regeneration. Moreover, we find that a family of salamander methyltransferases is expressed specifically in adult appendages. Using CRISPR/Cas9 technology to perturb transcription factors, we demonstrate that, unlike the axolotl, Pax3 is present and necessary for development and that contrary to mammals, muscle regeneration is normal without functional Pax7 gene. Our data provide a foundation for comparative genomic studies that generate models for the uneven distribution of regenerative capacities among vertebrates.
The Iberian ribbed newt Pleurodeles waltl has a wide spectrum of regeneration abilities. Here, Elewa et al. sequence its ~20 Gb genome and transcriptome to investigate the molecular features underlying its regenerative capacities.
Introduction
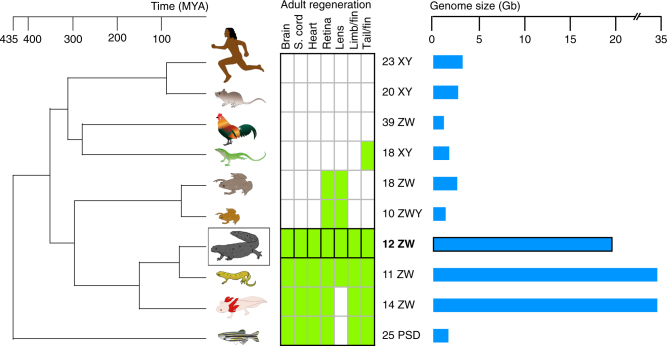
The random manifestation of extensive regeneration capacities in the animal kingdom implies a phylogenetically widespread regeneration potential, which is masked in most species1–5. Among tetrapods, salamanders, such as newts and axolotls, display the largest regenerative repertoire. A newt can rebuild entire limbs, tails, jaws, cardiac muscle, ocular tissues, and restore central nervous system tissues including brain structures6 (Fig. 1). However, it is important to note that major differences exist even among salamanders. In contrast to the paedomorphic axolotl, newts undergo metamorphosis, have a broader regeneration spectrum and mobilize additional cell sources for regeneration of the same body part7. Such interspecies differences among closely related species offer opportunities to reveal valuable information about the evolution of processes that allow or counteract regeneration. Although significant progress has been made to characterize salamander transcriptomes and proteomes8–12, features such as species-specific genes, expansion or contraction of gene families, and the underlying cause of their gigantic genome size remain largely unexplored. In addition, due to their complex and long life cycle, most newt species are cumbersome to breed under laboratory conditions, which has hampered the establishment of genetically modified lines. However, the Iberian ribbed newt Pleurodeles waltl (P. waltl) is easily bred in laboratories and retains the widest known spectrum of regeneration abilities among adult vertebrates13 (Fig. 1; Supplementary Fig. 1a). Here we describe the genome and transcriptome of P. waltl (Methods; Supplementary Methods, Supplementary Fig. 1b, and Supplementary Tables 1–5) as a resource to explore regeneration relevant novelties, and adapt CRISPR/Cas9 technology to perturb key transcription factors involved in regeneration.
Fig. 1.
The Iberian ribbed newt P. waltl is a prime model for adult regeneration. Compared to other research animal models, P. waltl is the most regenerative adult vertebrate amenable to laboratory breeding. Phylogenetic tree from TimeTree66. Regeneration capacities based on6, 13, 67–70. Sex chromosomes from refs. 71–74. Genome sizes from https://www.ncbi.nlm.nih.gov/genome/ (summed from Size (Mb) column)
Results
Sequencing the genome and transcriptome of P. waltl
The diploid genome of P. waltl is organized in 12 chromosome pairs, which have been the subject of classical lampbrush chromosome studies14 (Supplementary Fig. 1c–h). The P. waltl haplotype genome size is ~20 Gb (Supplementary Table 1), making this one of the largest vertebrate genomes sequenced to date. Our genome annotation pipeline identified 14,805 complete protein-coding gene models and we estimate that this set represents 64.8% of P. waltl protein-coding genes (Methods; Supplementary Methods, Supplementary Fig. 2a). The remaining gene content was reconstructed in the de novo transcriptome assembly involving RNAseq data from embryonic, larval, different adult tissues, and limb regeneration stages (Methods; Supplementary Methods, Supplementary Tables 3–5). We estimate that this combined set of gene models and transcripts represents 98.1% of the P. waltl protein-coding repertoire (Supplementary Methods; Supplementary Table 6). To provide a platform for comparative genomic studies, we identified 19,903 orthology groups involving P. waltl protein-coding genes and/or transcripts (Supplementary Methods; Supplementary Fig. 2a, Supplementary Table 1, Supplementary Data 1). Of these orthology groups, 1575 consisted of salamander members only (salamander groups) and 1130 consisted of salamander and Xenopus orthologs only (amphibian groups). The remaining 17,198 groups consisted of salamander and other vertebrate orthologs (human, mouse, chicken, lizard, or zebrafish). Importantly, we did not observe any expansion or loss of non-transposable protein-coding orthologs compared to other vertebrates (more than twofold increase or decrease) (Supplementary Methods, Supplementary Data 1–3).
An expansion of Harbinger elements in the P. waltl genome
A striking feature of the P. waltl genome is the extent and diversity of its repetitive elements. The genome is host to a diverse population of class I and class II transposable elements (Supplementary Table 1). We assembled a repeat library by majority vote k-mer extension15, followed by alignment to the repeat database RepBase16 using Satsuma17, yielding 428 distinct sequences (Methods). Gypsy retrotransposons are the most frequent repetitive elements in P. waltl, followed by the Harbinger transposons, and together account for about two thirds of the genome repetitive content (Supplementary Table 1). A phylogeny of ~1200 Gypsy elements longer than one kilobase indicates continuous expansion of this family (Fig. 2a, b), while Harbinger elements have undergone two distinct evolutionary bursts, with one recent expansion, visible from the distribution of pairwise similarity (Fig. 2a, b; Supplementary Fig. 2b). Harbinger elements are distinct from other transposons in that they carry a Myb-like gene, a proto-oncogene that acts as a transcription factor. While Harbinger elements gave rise to the genes Harbi1 18 and Naif1 19 in the vertebrate lineage, their contribution to vertebrate genome content is extremely rare with the leading example being the genome of coelacanth Latimeria chalumnae (~1 to 4% of the genome)20. Therefore, the Harbinger element expansion we describe in P. waltl is hitherto unprecedented.
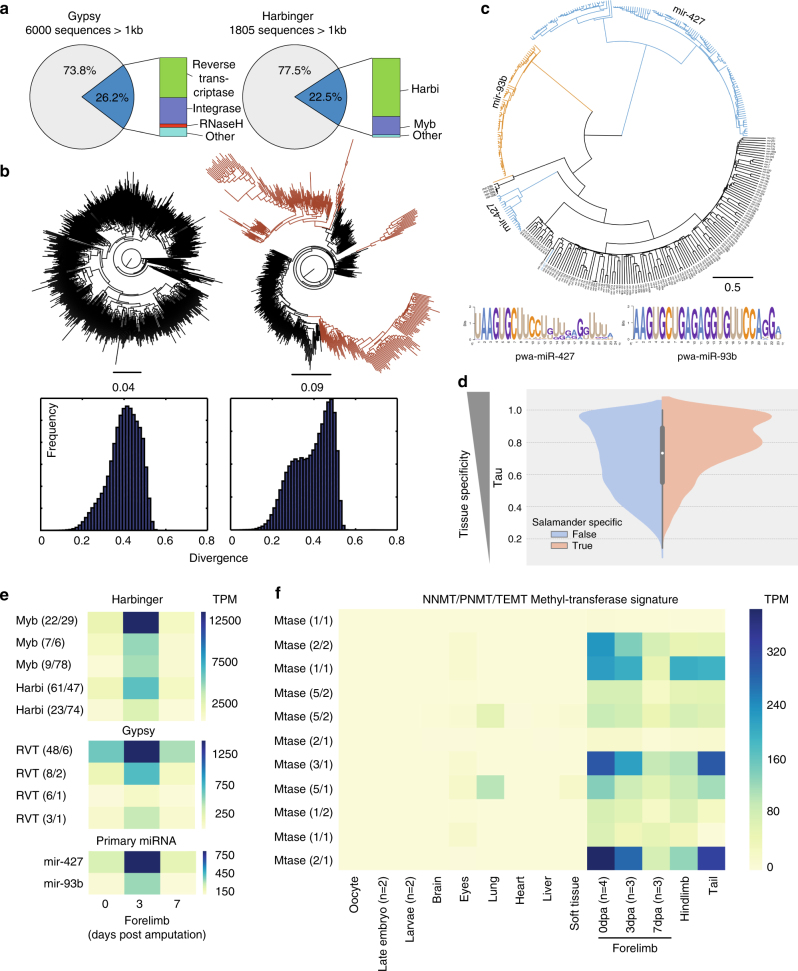
Fig. 2.
Genomic expansion and the expression of Harbinger DNA transposable elements pluripotency miRNAs and salamander-specific methyltransferases during adult limb regeneration in P. waltl. a Portion of Gypsy and Harbinger repeats (>1 kb) retaining a detectable protein domain. b Phylogeny of Gypsy and Harbinger elements. While the tree-based on Gypsy elements indicates a uniform expansion, the tree-based on Harbinger elements shows three branches (brown) resulting from a second distinct burst. The second Harbinger burst also results in a bimodal pairwise genomic distance distribution, compared to a unimodal distribution for the Gypsy elements. c Phylogenic tree of 361 mature P. waltl miRNAs with miR-427 and miR-93b expansion highlighted (top). Sequence logo based on multiple alignment of 155 miR-427 sequences and 66 miR-93b sequences (bottom). Note the embryonic stem cell-specific cell cycle regulating seed AAGUGC within the first eight nucleotides. d Split violin plot showing the tau score distribution of P. waltl genes that have non-salamander vertebrate orthologs (blue) and genes that only have salamander orthologs (beige). e RNAseq quantification of five Harbinger orthology groups encoding Myb or Harbi domain-bearing proteins (top), four Gypsy orthology groups encoding reverse transcriptase domain-bearing proteins (middle) and mir-427 and mir-93b primary transcripts (bottom). In parentheses are the number of transcripts and gene models belonging to each group. The groups chosen were significantly upregulated in the regenerating limb 3 days post amputation (dpa). Differential expression calculated by DESeq2 (Supplementary Table 9). TPM transcripts per million. f Eleven orthology groups of salamander methyltransferase are expressed specifically in appendages of the adult animal. In parentheses are the number of transcripts and gene models belonging to each group
Expansion of embryonic stem cell-specific miRNAs in P. waltl
MicroRNAs (miRNAs) are short non-coding RNAs that regulate post-transcriptional gene expression21–23. A number of miRNAs, including miR-302, are capable of reprogramming mammalian somatic cells into an induced pluripotent state24. We identified 361 miRNA precursors in the P. waltl genome that produce 67 distinct mature miRNAs conserved in Xenopus. In addition, the de novo transcriptome included 202 transcripts bearing conserved miRNA precursors (primary miRNA transcripts) that produce 55 of the 67 genome predicted mature miRNAs (Supplementary Methods, Supplementary Fig. 3, Supplementary Tables 7–8, Supplementary Data 4). Surprisingly, most of the genome predicted precursors were copies of mir-93b25 and mir-42726 (known as mir-430 in zebrafish27 and mir-302 in mammals28) (66 and 155 precursors, respectively) (Fig. 2c). Both mir-93b and mir-427 bear the characteristic embryonic stem cell-specific cell cycle regulating (ESCC) seed (AAGUGC) and are of interest to regeneration studies29 (Fig. 2c). The expansion of mir-427 has been reported in Xenopus (~80 copies)25 and zebrafish (~100 copies)30 and thus predates the Devonian period31. The mir-93b expansion, however, appears to be salamander-specific and we place its occurrence in the Jurassic period or later32.
Harbinger and ESCC miRNA expression during limb regeneration
Given the compelling expansion of ESCC miRNAs and Harbinger transposable elements, we analyzed whether mir-93b, mir-427, Myb, and Harbi domain containing genes are regulated during adult P. waltl limb regeneration (Supplementary Table 9). We mapped total RNAseq reads from three early developmental stages, nine adult (>2 years old) body parts and two limb regeneration stages; 3 and 7 days post amputation (Supplementary Table 3) to 14,805 gene models and 108,713 transcripts belonging to the P. waltl orthology groups in addition to 202 miRNA primary transcripts (Supplementary Methods, Supplementary Data 1,4). We found that mir-93b and mir-427 primary transcripts are upregulated in the regenerating limb 3 days post amputation (p < 0.001) and that their mature miRNAs are detected during limb regeneration (21 and 45 reads per million, respectively) (Fig. 2e; Supplementary Fig. 4, Supplementary Table 9 and Supplementary Data 4). In addition, we found that five orthology groups containing Myb or Harbi domains were upregulated at 3 days post amputation (p < 0.001) (Supplementary Table 9) similar to mir-93b and mir-427 primary transcripts (Fig. 2e). On the contrary, the two vertebrate Harbinger derivative genes Naif1 and Harbi1 were expressed at low levels in all our datasets and showed no regulation during regeneration (Supplementary Table 10). Four Gypsy orthology groups were also upregulated 3 days post amputation (p < 0.01), however, their expression levels were an order of magnitude lower than the Harbingers (Supplementary Table 9). Transposable elements are often domesticated to benefit their hosts and RNA transposable element expression has been described in mammalian pluripotent stem cells33, 34. Our data illustrate that the both RNA and DNA transposons, Gypsy and Harbinger, respectively, in addition to ESCC miRNAs respond to adult salamander injury. The extent to which they co-regulate regeneration awaits further studies.
Restricted expression of salamander methyltransferases
Genes found only in salamanders may offer insight into their unique regenerative abilities. Our annotation pipeline identified 1545 orthology groups that consisted of genes and transcripts in the P. waltl genome and at least one other salamander, but no sequences from the other seven model vertebrates analyzed. We consider these orthology groups to be putatively salamander-specific and refer to them by the protein domain(s) detected in their members. We calculated the tau score35 (an indicator of tissue specificity) for all orthology groups and found that groups with only salamander genes are more tissue specific than groups with orthologs in other vertebrates (Fig. 2d and Methods). Surprisingly, we found eleven salamander orthology groups encoding a NNMT/PNMT/TEMT methyltransferase domain and expressed specifically in adult limbs and tail, four of which were significantly downregulated on the seventh day of regeneration (p < 0.001; Fig. 2f; Supplementary Fig. 2b and Supplementary Table 9). No other salamander orthology groups showed this expression pattern. Together, our data show that P. waltl has evolved genes with tissue-restricted expression profiles and that target methylation may have evolved in a manner consistent with limb regeneration.
Pax3 and Pax7 are present and functional in P. waltl
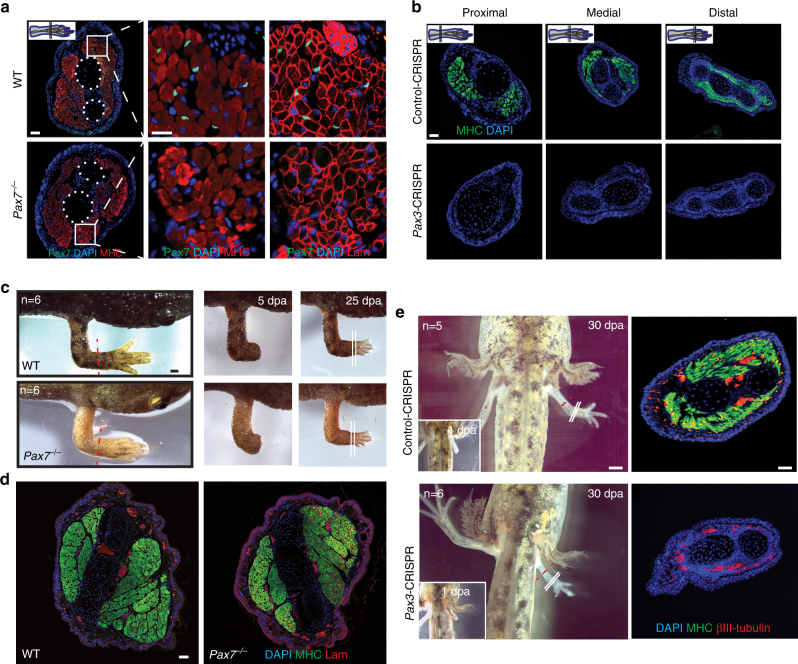
We were intrigued by the absence of Pax3 in the axolotl (Ambystoma mexicanum) transcriptome, genome and previous gene expression studies10, 36–38. Pax3 is a paired-end homeodomain transcription factor required for early development39, 40 and its paralog Pax7 regulates skeletal muscle regeneration7, 41. Manual curation of the Pax gene family and in situ hybridization confirmed the presence of Pax3 and Pax7 in P. waltl (Supplementary Fig. 5a–c). We adapted CRISPR/Cas9 technology to P. waltl and mutated both genes (Methods; Supplementary Fig. 5a,d and Supplementary Table 11). Whereas Pax7 −/− compound heterozygote F1 animals exhibited no apparent deficiencies in muscle development, Pax3 mosaic-mutants died or developed animals with several developmental anomalies, exemplified by muscle-less limbs (Fig. 3a, b; Supplementary Fig. 5e–f and Supplementary Video 1). Next we asked how these mutations affect limb regeneration. Pax7 −/− F1 animals regenerated limbs indistinguishable from wildtype controls, including skeletal muscle and cells in satellite cell position (Fig. 3c, d and Supplementary Fig. 6; Methods). Pax3 mosaic F0 mutants lacking limb skeletal muscle regenerated morphologically normal muscle-less limbs without any other overt phenotypes (Fig. 3e).
Fig. 3.
Newt limb muscle development and regeneration is independent of Pax7. a Pax7 −/− F1 P. waltl have normal limb skeletal muscle. Note the similar myosin heavy chain (MHC) and laminin (Lam) staining in Pax7 −/− and WT animals. Scale bars = 200 µm in the overview and 20 µm in the insert. b Pax3-CRISPR mosaic animals have muscle-less limbs. Immunofluorescent staining for MHC in transverse sections of Pax3-CRISPR and control-CRISPR limbs. Scale bar = 100 µm. c Limb regeneration is not impaired in post-metamorphic Pax7 −/− F1 animals. Scale bar = 1 mm. d Pax7 −/− F1 animals regenerate normal skeletal muscle in the new limb. Note the indistinguishable myosin heavy chain (MHC) and laminin staining in Pax7 −/− and WT animals. Scale bar = 100 µm. e muscle-less limbs in Pax3-CRISPR mosaic animals (stage 45) regenerate muscle-less limbs. Immunofluorescent staining for MHC and the neuronal marker βIII-tubulin in transverse sections. Scale bar = 1 mm in the left panels showing animals and 100 µm in the right panels showing sections. Red dotted lines indicate amputation plane. White lines indicate section plane
Discussion
Salamander genomes are gigantic, ranging in size between 14 and 120 Gb42. While this feature has facilitated genome structure studies using lampbrush chromosomes43, it has delayed sequencing projects and impeded regeneration studies. Lampbrush chromosomes epitomize genome structural plasticity, while salamander regeneration is a prime example of cellular plasticity. Whether these two forms of plasticity are mechanistically related is now a pursuable question.
Reading and editing the P. waltl genome reveals several new features of limb regeneration and genome evolution. Our loss of function data on Pax3 and Pax7 paralogues show that limb regeneration is not dependent on skeletal muscle and that no other tissues give rise to muscle during limb regeneration in P. waltl 44. Furthermore, the expression and developmental functions of Pax3 are conserved between P. waltl and mammals41, which also raises the question whether the lack of Pax3 in the axolotl confers additional functions for Pax7. Mammalian skeletal muscle regeneration is impaired in the absence of Pax7 45 but we have found that Pax7 loss of function does not impair muscle regeneration in P. waltl. In this context, it is important to note that a distinctive process during newt limb regeneration is the reversal of terminal differentiation of skeletal muscle fibers. Myogenic dedifferentiation during limb regeneration produces progenitor cells that build up the new muscle and thus has the potential to compensate for the potential loss of contribution from satellite cells7. However cell tracking studies of satellite cells and their progeny are necessary to resolve this question46.
The genomic expansion and expression of ESCC miRNAs and Myb domain-bearing Harbingers, along with limb-specific expression of putatively salamander-specific methyltransferases present new possibilities to uncover mechanisms of adult vertebrate regeneration using P. waltl as a model organism. For example, the multitude of mir-427 copies in fish and amphibian genomes in addition to that of mir-93b in newts may provide an opportunity for individual copies to evolve and acquire expression rights and co-regulate a regenerative response after injury. In X. laevis and zebrafish, miR-427/430 helps clear of cache of maternally supplied RNA when embryos transition to rely on their own gene expression47, 48. One intriguing possibility is that miR-427 performs a similar function in P. waltl during limb regeneration. In this model, miR-427 clears the cytoplasmic cache of mRNAs, thereby erasing a cell’s “working memory” and allowing dedifferentiation to occur, while the “deep memory” of the cell’s identity and function would not be lost by a permanent inactivation of gene expression.
Methods
Genomic DNA isolation
High molecular weight genomic DNA was purified from a single larva after discarding the digestive system. The larva represents the first generation of our P.waltl colony, established from fertilized eggs produced in a laboratory colony located in Madrid, Spain. The larva is therefore the fourth/fifth generation of laboratory-bred P. waltl, originally obtained with permission from a wild population in Doñana National Park (Spain) for research purposes by Agustin Gonzalez. The body was segmented into five pieces and each piece was placed in 1.5 mL centrifuge tube. A total of 700 μL digestion buffer (50 mM Tris-HCl pH 8.0, 100 mM EDTA, 100 mM NaCl, 1% SDS) was added to each sample. 50 μL 10 mg mL−1 Proteinase K was added to the sample and incubated 55–60 °C overnight with mixing. Another 50 μL of 10 mg mL−1 Proteinase K was added in the morning and the samples incubated for 4 more hours. Next, 20 μL 10 mg mL−1 RNase A (DNase-free) was added to the sample and incubated at 37 °C for 1–2 h. The solution was transferred to a pre-spun (1500 × g for 1–2 min) phase lock gel (PLG) 2 mL Heavy tube. 0.5 mL phenol-chloroform-isoamyl alcohol (25:24:1) was added to the sample in the PLG 2 mL tube and mixed well by repeated inversion. Next, tubes were centrifuged at full speed for 5 min in a microcentrifuge. The resultant aqueous phase was transferred to a fresh pre-spun PLG 2 mL Heavy tube. A total of 0.5 mL chloroform-isoamyl alcohol (CI, 24:1) was added to the sample in the PLG 2 mL tube and mixed well by repeated inversion. Tubes were centrifuged at full speed for 5 min in a microcentrifuge. The resultant aqueous phase was transferred to a fresh microcentrifuge tube. 100% isopropanol was added and mixed thoroughly by repeated inversion. High molecular weight DNA was recovered using a heat-sealed glass micropipette tip and transferred to a 1.5 mL microcentrifuge tube containing 70% ethanol. The glass tip with DNA was then dipped into 95% ethanol twice. DNA was allowed to dry partially before being pushed off the tip into a microcentrifuge tube containing 400 μL TE and resolubilized overnight. DNA quality was assessed using DropSense and gel electrophoresis.
Karyotyping
Cells were arrested in metaphase using classical procedures, standardized for P. waltl 49: stage 20 embryos (n = 6) were placed in saline solution (30 mM NaCl, 0.34 mM KCl, 0.9 mM CaCl2) containing 0.5% colchicine. After 18 h at 21 °C, the embryos were fixed with a mix of 50% acetic acid/ 50% EtOH for 3–5 min, washed in distilled water and transferred to 50% acetic acid, were they were dissociated mechanically with a Pasteur glass pipette. Cells were then dropped on Superfrost slides, let dry, and stained with DAPI for confocal analysis. For Supplementary Figure 1, a Z-stack confocal projection was made with an interval of 0.57 μm between planes, and the twelve pairs of chromosomes were identified and distributed in three groups according to ref. 50. For an easy interpretation of the reader, colors were given applying color filters to individually selected chromosomes using Adobe Photoshop. The full extent of the chromosomes in the Z-projection was then selected, filled with color, and organized to provide the karyotype.
Lampbrush chromosome analyses
Ovarian biopsies were performed on adult females of P. waltl that were anesthetized in 0.15% MS222 (Amino-benzoic Acid Ethyl, Fluka). Stage IV–VI oocytes were selected and maintained in MBS buffer (Modified Barth’s solution) at 18 °C. Germinal vesicles were manually isolated from the oocytes and dissected in 75 mmol L−1 KCl, 25 mmol L−1 NaCl, 0.01 mmol L−1 MgCl2 and 0.01 mmol L−1 CaCl2, pH7.2, and LBCs were prepared as previously described51. Nuclear spread preparations were centrifuged at 300 × g for 10 min, and at 3000 × g for 30 min at 4 °C. Standard transmitted light were performed at the ImagoSeine core facility (member of the IBiSA and the France-BioImaging (ANR-10-INBS-04) infrastructure) using a wide field Leica microscope with phase contrast plan Apo oil objectives: ×40 (NA = 1.3), ×63, ×100 (NA = 1.4). Images were captured using a CoolSnap HQ, Photometrics camera driven by the software Metamorph6 (Universal imaging). Series of 11 confocal Z-planes (0.7 µm distance) were collected. Pixel size of the images were 0.1625 μm, 0.103 μm, and 0.065 μm for the ×40, ×63, and ×100 objectives, respectively.
Genome size calculation
Nuclear content was measured using Vicia faba nuclei as reference (2C DNA content = 26.9 pg)52 (Supplementary Fig. 1). All samples were prepared according to52 and stained with 4ʹ,6-diamidino-2-phenylindole (DAPI) and measured using a BD Influx™ by Becton Dickinson flow cytometer. The lasers used for detection and excitation were 488 nm and 355 nm, respectively. From the mean peak positions of P. waltl (20,255) and V. faba (13,746) the 2 C DNA content was calculated as 39.6 pg using the equation:
P.waltl 2 C value = V.faba 2 C value (reference) × ((P.waltl 2 C mean peak position)/(V.faba 2 C mean peak position))
Based on the equation:
haploid genome size = (0.978 × 2 C value)/2, the haploid P. waltl genome size was calculated to be 19.38 Gb (Supplementary Fig. 1).
Our estimates agree with Licht and Lowcock (2 C value = 42.7 pg)53, Morescalchi and Olmo (2 C value = 39 pg)54 and are lower than Lizana and collegues (2 C value = 48.25 pg)55.
Short read genome sequencing
Libraries for Illumina sequencing were generated and sequenced at SciLifeLabs (Stockholm). Two Illumina TruSeq PCR-free libraries (insert size 180 bp) were prepared and paired-end 2 × 125 bp sequencing was done on HiSeq2500 (HiSeq Control Software 2.2.58/RTA 1.18.64) with a setup in High Output mode (Supplementary Table 2).
Genome assembly strategy
Illumina paired-end reads from two 180 bp insert size genomic libraries were assembled into contigs using Abyss (28) using a k = 71. Illumina paired-end RNAseq reads from representative body sections of P. waltl were assembled into transcripts using Trans-Abyss (29) (k = 69) with the aim to reconstruct only longer, complete transcripts rather than isoforms or short RNAs. Trans-Abyss transcripts were then used to scaffold the genomic Abyss-contigs in a third assembly aimed at improving the reconstruction of gene content. The final assembly—denominated Pw_v4.2—was 19.66 Gb in size and included ~66 million contigs and 106,895 scaffolds (Table 1). Approximately 45% of the genome assembly was captured in contigs ranging between 1 kb and 10 kb in size and ~6% (1.2 Gb) were assembled in contigs and scaffolds longer than 10 kb, the longest being 438.8 kb in size (Supplementary Fig. 1b).
Repeat library assembly
We assembled a repeat library by majority vote k-mer (k = 25) extension algorithm15 to the P. waltl genome assembly Pw_v4.2, resulting in a set of 428 consensus repeat sequences larger than 1000 nt. Out of these, 349 were identified as known repeats through nucleotide alignments against RepBase (version 21.10 downloaded 10/23/2016)16 using Satsuma (https://sourceforge.net/projects/satsuma/ last update 2016-12-14)17 and selecting the longest alignments respectively if multiple overlapping hits were reported.
Phylogeny of Gypsy and Harbinger elements
We computed pairwise genomic distances using Satsuma for 6000 randomly selected Gypsy and Harbinger elements longer than 1000 nucleotides, requiring alignments to cover the same region in each element. This resulted in 625 Gypsy and 567 Harbinger elements, for which we built phylogeny using the neighbor joining method implemented in phylip56, and rooting the tree on midpoint. For bootstrapping, the longest 44 and 50 sequences for Gyspy and Harbinger elements, respectively, were aligned using MUSCLE57, from which Maximum Likelihood trees were built using PhyML58, 59. Bootstrap values were estimates from 100 trials.
Transcriptome RNA preparation and sequencing
25 indexed Illumina TruSeq Stranded total RNA Zero-Gold libraries (mean insert size 150 bp) from 20 P. waltl body parts and regeneration stages were combined into four pools and sequenced on 4 lanes (one pool per lane) on Illumina HiSeq High Output mode v4, PE 2 × 125 bp at the Science for Life Laboratory, Stockholm (Supplementary Table 3). In all cases, RNA was isolated using Trizol after grinding the sample with a mortar and pestle in liquid nitrogen and precipitated overnight in isopropanol. RNA integrity was assessed using Agilent Bioanalyzer. Samples with RIN ≥ 8.0 were used for library preparation.
Genome annotation generation of gene models with MAKER
MAKER was used in a two-step approach to identify gene models in the P. waltl genome.
MAKER Tier 1: The clustered assembled transcripts, the Augustus hinted gene models and the protein sequences from uniref90 were used as input evidence to build the annotation with MAKER.
MAKER Tier 2: Nucleotide gene models from the MAKER Tier 1 annotation were used as input for the second round of gene annotation, instead of the Augustus hinted gene models.
In total, 79,916 gene models were identified using this approach. 19,915 gene models aligned with a transcript in the de novo assembled transcriptome (percent identity > 98%, alignment length > 99 bp).
Genome editing
Cas9 mRNA and gRNA were synthesized using mMESSAGE T7 ULTRA Transcription Kit (Invitrogen) and MEGAshortscript T7 Transcription Kit (Invitrogen), respectively. Genomic DNA was extracted with Qiagen DNAeasy kit. The PCR products were cloned into TOPO cloning vector (Invitrogen). Individual clones were sequenced with T7 primer (Supplementary Table 9).
Single-cell fertilized eggs were obtained by either natural or induced breeding and injected according to previously published protocols with modifications60, 61. Briefly, 500 pg Cas9 RNA and 100 pg gRNA were mixed into 5 nl and injected into freshly laid single-cell-stage embryos. The animals were raised according to60. The screening of F0 animals was done by both genotyping (see above) and phenotype characterization, including pigmentation analysis and immunohistochemistry of limbs/tails, according to39, 40. The Pax7 −/− and Tyr −/− F1 animals were produced by crossing between adult F0 animals. The larvae or post-metamorphic newts were anaesthetized within 0.01% or 0.1% ethyl-p-aminobenzoate (benzocaine; Sigma) prior to imaging and tissue collection. Newts utilized for this study were processed according to Swedish Board of Agriculture animal ethical regulations (N429/12).
In situ hybridization and immunohistochemistry
The PCR fragments of Pax3 and Pax7 were cloned into TOPO cloning vectors. The digoxigenin-labeled antisense RNA probes were synthesized using T7 RNA polymerase (Roche). Whole-mount in situ hybridizations were performed by using albino Tyr −/− F1 embryos according to62. In situ hybridizations on 10 µm transverse sections were carried out by fixing sections in 4% formaldehyde for 15 min, treating with 0.2 N HCL for 12 min, washing, and then incubating in acetylation buffer (0.1 M trietholamine and 0.25% acetic anhydride) for 10 min. Next, the slides were rinsed in RNase free water and permeabilized with a solution of 1 μg mL−1 Proteinase K (Roche) and 2 mM CaCl2 for 15 min at 37 °C. The slides were incubated in prehybridization buffer (50% deionized form of amide, 5 × saline sodium citrate (SSC), 5 × Denhardt’s solution, 250 μg mL−1 yeast RNA, 500 μg mL−1 herring sperm single-stranded DNA (ssDNA); Sigma) for 2 h at room temperature before being incubated in hybridization solution (50 ng probe, 50% deionized form of amide, 5 × SSC, 5 × Denhardt’s solution, 250 μg mL−1 yeast RNA, 500 μg mL−1 herring sperm ssDNA) for 16 h at 60 °C. The slides were then washed with 0.2 × SSC buffer containing 0.05% tween 20 at 70 °C for 3 × 163. Finally, BM purple was used to visualize the signal. For immunostainings, sections were blocked with 10% donkey serum in 0.1% Triton-X for 30 min at room temperature. Sections were incubated with a relevant primary antibody overnight at 4 C and with secondary antibodies for 1 h at room temperature. Antibodies were diluted in blocking buffer and sections were mounted in mounting medium (DakoCytomation) containing 5 mg mL−1 1 4,6-diamidino-2-phenylindole (Sigma)64.
Cryosectioning and in situ hybridization for miRNAs
Limb samples were collected at the indicated timepoints and fixed overnight at 4 °C, in freshly made 4% PFA in PBS. After fixation, samples were washed three times in PBS and PBST, equilibrated in 30% sucrose in PBS, embedded in Tissue Tek (Sakura, Torrance, CA, USA) and frozen for cryosectioning. Frozen tissues were sectioned into 20 μm-thick longitudinal sections.
Tissue sections were dehydrated through a MeOH/PBST series (25, 50, and 75%), followed by MeOH for 10 min and rehydrated through the opposite series of MeOH. The sections were then permeabilized with 2 μg mL−1 proteinase K (Thermo Scientific), inactivated with glycine solution (2 mg mL−1 in PBST) and washed in PBST. Before hybridization, the slides were incubated with hybridization buffer (50% formamide, 5× SSC, 50 µg mL−1 heparin, 0.2% Tween-20, and 100 μg mL−1 yeast tRNA (Sigma)) for 1 h at RT. Digoxygenin-labeled locked nucleic acid (LNA) probes against the mature form of miRs-93b and 427 were custom-made and ordered from Exiqon (Denmark). The probes were diluted in hybridization buffer (50 nM) and denatured for 5 min at 95 °C. Hybridization was carried overnight at 53 °C. The next day proceeded with washes in 5× SSC for 10 min and 0.2× SSC for 1 h at hybridization temperature. Then, slides were incubated in blocking buffer (10% sheep serum and 2% bovine serum albumin in PBST) at RT for 30 mins. Alkaline phosphatase associated anti-digoxigenin antibody (Roche) was diluted 1:1000 in blocking buffer, and slides were kept in the antibody solution at RT for 90 min. After washing the antibody with PBST thrice, sections were incubated with freshly prepared alkaline phosphatase (AP) buffer (100 mM Tris-HCl, pH 9.5, 50 mM MgCl2, 100 mM NaCl, 0.2% Tween-20 in water) three times for 5 min. Finally, BM Purple (Roche) was used to stain the sections, in the dark, until development of a blue/purple color. Slides were rinsed with PBST several times, and a drop of 0.5 M EDTA was added to stop the reaction. Slides were mounted with 80% glycerol and imaged using a Olympus Microscope (Center Valley, PA, USA) at ×10 magnification.
Probe Sequences:
miR-93b: 5ʹ-TCATGGAACACCTCTCAGCACTT-3ʹ
miR-427: 5ʹ-AACCTCAACAGGAAGCACTTA-3ʹ
scramble: 5ʹ-GTGTAACACGTCTATACGCCCA-3ʹ
Small RNA sequencing
Libraries from small RNAs were prepared using TruSeq Small RNA preparation kit. A total of 1 μg of total RNA from 10 samples were processed according to manufacturer instructions. Libraries were sequenced on Illumina Miseq (Rapid mode), single read 1 × 50 bp.
microRNA annotation
miRNAs were named based on the Xenopus miRNA sharing the same seed.
microRNA quantification
miRNA expression levels were quantified using miRDeep2 (quantifier.pl). To identify miRNAs associated with regeneration. Since our small-RNA dataset was intended for discovery, not differential expression analysis we did not have replicates for each source. To mitigate the lack of replicates in pursuit of miRNAs associated with regeneration, we divided the miRNA expression data into three groups: adult tissue (brain, eyes, heart, liver, lung), regenerating limb (limb 3 dpa, limb 7 dpa); and embryo (larvae, late embryo). Then we tested whether there was significant miRNA up or downregulation between these groups based on a Gauss–Laplace distribution, as implemented in moose265 (http://grabherr.github.io/moose2/).
The top 10 miRNAs upregulated in the regenerating group compared to the adult group (ranked by Bonferroni-corrected e-value) were also the top 10 upregulated in the embryonic group compared to the adult group.
Gene expression tissue specificity
We used the tau score to quantify tissue specificity of salamander-specific orthology groups compared to orthology groups with members from other vertebrates. A tau score represents the average ratio of a gene’s expression between each tissue and the tissue with the highest expression of that gene. Note that “tissue” often refers to a body part that is a composite of tissues (i.e., heart, brain, etc). A gene with equal expression across tissues will yield an average ratio of 1. In order to make the tau score intuitive (a higher score corresponds to higher tissue specificity), the average ratio is subtracted from 1. Therefore, a gene with equal expression across tissues will have a tau score of 1–1 = 0. Conversely, if a gene is expressed in one tissue and not expressed in the others, the average ratio will approach zero and after subtracting from 1, the tau score will approach 1. Therefore, a gene is more tissue specific the closer its tau score is to 1.
Data availability
Datasets are deposited under BioProject PRJNA353981 and BioSample accession SAMN07571895, Runs SRR6001098-SRR6001140. Transcriptome and genome assemblies are available upon request.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
We thank Elly Tanaka and colleagues for sharing the axolotl genome assembly prior to publication. We acknowledge continuous support from members of the Frisén Lab (KI/CMB), Science for Life Laboratory, the National Genomics Infrastructure (NGI) the Swedish National Infrastructure for Computing (SNIC) and Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) for providing assistance in massive parallel sequencing and computational infrastructure (projects b2015079, b2015188, b2016417, SNIC 2016/1-20 and SNIC 2016/1-251). We thank Agustin Gonzalez and his lab (UCM, Madrid) for facilitating the embryos from which our initial colony was established. Mihaela Martis (BILS – SciLifeLab) is acknowledged for assistance concerning transcriptome assembly, Sarantis Giatrellis (CMB – KI) for assistance measuring nuclear DNA content and Karen Echeverri for facilitating miRNA in situ hybridization experiments. We thank Marc Friedlander for advice regarding miRNA prediction. A.E. is supported by NIH Ruth Kirschstein postdoctoral fellowship F32GM117806. This work was supported by grants from the National Natural Science Foundation of China (31771617) to H.W., the European Research Council, the Swedish Research Council, Wenner-Gren Foundation and Cancerfonden to A.S.
Author contributions
A.E. conceived the study, assembled and annotated the P. waltl transcriptome, predicted miRNAs in the genome and transcriptome, merged the N. viridescens transcriptomes, performed orthology group analysis and wrote the manuscript. H.W. performed the Pax3 and Pax7 in situ hybridization, gene editing, genotype and phenotype analysis and wrote the corresponding part of the manuscript. C.T.-L. assembled the P. waltl genome, annotated its protein-coding genes and edited the manuscript. A.J. established the P. waltl colony and breeding program, helped in the design of the sampling strategy, performed karyotyping from primary cell cultures, CRISPR/Cas9 egg injections, phenotype characterization, breeding, and assisted with figure preparation for the manuscript. M.P.-M. prepared, acquired and analyzed lampbrush chromosome images. Z.Y. preformed nuclear DNA quantification. Y.A .assisted with gene expression analysis. N.Z. performed parts of the transposable element analysis. G.B. performed miRNA in situ hybridization. A.K. and L.S.H. performed in situ hybridization. I.A. and R.S. contributed to P. waltl transcriptome assembly. M.G. designed the transposable element analysis, assembled repeat libraries, and identified repeat content, transposable element distribution and wrote the manuscript. B.A. participated in the planning of the study and supervised the genome sequencing and analysis and edited the manuscript. A.S. conceived the study, supervised all aspects of the project and wrote the manuscript.
Competing interests
The authors declare no competing financial interests.
Footnotes
Ahmed Elewa, Heng Wang and Carlos Talavera-López contributed equally to this work.
Electronic supplementary material
Supplementary Information accompanies this paper at doi:10.1038/s41467-017-01964-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ahmed Elewa, Email: ahmed.elewa@ki.se.
András Simon, Email: andras.simon@ki.se.
References
- 1.Tanaka EM. The molecular and cellular choreography of appendage regeneration. Cell. 2016;165:1598–1608. doi: 10.1016/j.cell.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 2.Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013;29:611–620. doi: 10.1016/j.tig.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galliot B, Chera S. The Hydra model: disclosing an apoptosis-driven generator of Wnt-based regeneration. Trends Cell Biol. 2010;20:514–523. doi: 10.1016/j.tcb.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Roberts-Galbraith RH, Newmark PA. On the organ trail: insights into organ regeneration in the planarian. Curr. Opin. Genet. Dev. 2015;32:37–46. doi: 10.1016/j.gde.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Brockes JP. Amphibian limb regeneration: rebuilding a complex structure. Science. 1997;276:81–87. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- 6.Simon A, Tanaka EM. Limb regeneration. Wiley interdisciplinary Rev. Dev. Biol. 2013;2:291–300. doi: 10.1002/wdev.73. [DOI] [PubMed] [Google Scholar]
- 7.Sandoval-Guzman T, et al. Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell. 2014;14:174–187. doi: 10.1016/j.stem.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Looso M, et al. A de novo assembly of the newt transcriptome combined with proteomic validation identifies new protein families expressed during tissue regeneration. Genome Biol. 2013;14:R16. doi: 10.1186/gb-2013-14-2-r16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdullayev I, Kirkham M, Bjorklund AK, Simon A, Sandberg R. A reference transcriptome and inferred proteome for the salamander Notophthalmus viridescens. Exp. Cell. Res. 2013;319:1187–1197. doi: 10.1016/j.yexcr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Bryant DM, et al. A tissue-mapped axolotl de novo transcriptome enables identification of limb regeneration factors. Cell Rep. 2017;18:762–776. doi: 10.1016/j.celrep.2016.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Che R, Sun Y, Wang R, Xu T. Transcriptomic analysis of endangered Chinese salamander: identification of immune, sex and reproduction-related genes and genetic markers. PLoS ONE. 2014;9:e87940. doi: 10.1371/journal.pone.0087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, et al. A transcriptome for the study of early processes of retinal regeneration in the adult newt, Cynops pyrrhogaster. PLoS ONE. 2014;9:e109831. doi: 10.1371/journal.pone.0109831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi T, et al. Molecular genetic system for regenerative studies using newts. Dev. Growth Differ. 2013;55:229–236. doi: 10.1111/dgd.12019. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix J. Etude descriptive des chromosomes en écouvillon dans le genre pleurodeles (Amphibien, Urodèle) Ann. Embryol. Morphogen. 1968;1:179–202. [Google Scholar]
- 15.Li R, et al. ReAS: recovery of ancestral sequences for transposable elements from the unassembled reads of a whole genome shotgun. PLoS Comput. Biol. 2005;1:e43. doi: 10.1371/journal.pcbi.0010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao W, Kojima KK, Kohany O. Repbase Update, a database of repetitive elements in eukaryotic genomes. Mobile DNA. 2015;6:11. doi: 10.1186/s13100-015-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabherr MG, et al. Genome-wide synteny through highly sensitive sequence alignment: Satsuma. Bioinformatics. 2010;26:1145–1151. doi: 10.1093/bioinformatics/btq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapitonov VV, Jurka J. Harbinger transposons and an ancient HARBI1 gene derived from a transposase. DNA Cell Biol. 2004;23:311–324. doi: 10.1089/104454904323090949. [DOI] [PubMed] [Google Scholar]
- 19.Sinzelle L, et al. Transposition of a reconstructed Harbinger element in human cells and functional homology with two transposon-derived cellular genes. Proc. Natl Acad. Sci. USA. 2008;105:4715–4720. doi: 10.1073/pnas.0707746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JJ, Sumiyama K, Amemiya CT. A living fossil in the genome of a living fossil: Harbinger transposons in the coelacanth genome. Mol. Biol. Evol. 2012;29:985–993. doi: 10.1093/molbev/msr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 22.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 23.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anokye-Danso F, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang GQ, Maxwell ES. Xenopus microRNA genes are predominantly located within introns and are differentially expressed in adult frog tissues via post-transcriptional regulation. Genome Res. 2008;18:104–112. doi: 10.1101/gr.6539108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe T, et al. Stage-specific expression of microRNAs during Xenopus development. FEBS Lett. 2005;579:318–324. doi: 10.1016/j.febslet.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 27.Giraldez AJ, et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308:833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 28.Suh MR, et al. Human embryonic stem cells express a unique set of microRNAs. Dev. Biol. 2004;270:488–498. doi: 10.1016/j.ydbio.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Greve TS, Judson RL, Blelloch R. microRNA control of mouse and human pluripotent stem cell behavior. Annu. Rev. Cell. Dev. Biol. 2013;29:213–239. doi: 10.1146/annurev-cellbio-101512-122343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen PY, et al. The developmental miRNA profiles of zebrafish as determined by small RNA cloning. Genes Dev. 2005;19:1288–1293. doi: 10.1101/gad.1310605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shubin NH, Daeschler EB, Coates MI. The early evolution of the tetrapod humerus. Science. 2004;304:90–93. doi: 10.1126/science.1094295. [DOI] [PubMed] [Google Scholar]
- 32.Frobisch NB, Shubin NH. Salamander limb development: integrating genes, morphology, and fossils. Dev. Dyn. 2011;240:1087–1099. doi: 10.1002/dvdy.22629. [DOI] [PubMed] [Google Scholar]
- 33.Friedli M, Trono D. The developmental control of transposable elements and the evolution of higher species. Annu. Rev. Cell Dev. Biol. 2015;31:429–451. doi: 10.1146/annurev-cellbio-100814-125514. [DOI] [PubMed] [Google Scholar]
- 34.Friedli M, et al. Loss of transcriptional control over endogenous retroelements during reprogramming to pluripotency. Genome Res. 2014;24:1251–1259. doi: 10.1101/gr.172809.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanai I, et al. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics. 2005;21:650–659. doi: 10.1093/bioinformatics/bti042. [DOI] [PubMed] [Google Scholar]
- 36.Baddar NW, Woodcock MR, Khatri S, Kump DK, Voss SR. Sal-Site: research resources for the Mexican axolotl. Methods Mol. Biol. 2015;1290:321–336. doi: 10.1007/978-1-4939-2495-0_25. [DOI] [PubMed] [Google Scholar]
- 37.Keinath MC, et al. Initial characterization of the large genome of the salamander Ambystoma mexicanum using shotgun and laser capture chromosome sequencing. Sci. Rep. 2015;5:16413. doi: 10.1038/srep16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knapp D, et al. Comparative transcriptional profiling of the axolotl limb identifies a tripartite regeneration-specific gene program. PLoS ONE. 2013;8:e61352. doi: 10.1371/journal.pone.0061352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epstein JA. Pax3 and vertebrate development. Methods Mol. Biol. 2000;137:459–470. doi: 10.1385/1-59259-066-7:459. [DOI] [PubMed] [Google Scholar]
- 40.Joven A, Morona R, Gonzalez A, Moreno N. Spatiotemporal patterns of Pax3, Pax6, and Pax7 expression in the developing brain of a urodele amphibian, Pleurodeles waltl. J. Comp. Neurol. 2013;521:3913–3953. doi: 10.1002/cne.23276. [DOI] [PubMed] [Google Scholar]
- 41.Buckingham M, Relaix F. PAX3 and PAX7 as upstream regulators of myogenesis. Semin. Cell Dev. Biol. 2015;44:115–125. doi: 10.1016/j.semcdb.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Sun C, et al. LTR retrotransposons contribute to genomic gigantism in plethodontid salamanders. Genome Biol. Evol. 2012;4:168–183. doi: 10.1093/gbe/evr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan GT. Lampbrush chromosomes and associated bodies: new insights into principles of nuclear structure and function. Chromosome Res. 2002;10:177–200. doi: 10.1023/A:1015227020652. [DOI] [PubMed] [Google Scholar]
- 44.Dunis DA, Namenwirth M. The role of grafted skin in the regeneration of x-irradiated axolotl limbs. Dev. Biol. 1977;56:97–109. doi: 10.1016/0012-1606(77)90157-9. [DOI] [PubMed] [Google Scholar]
- 45.Kuang S, Chargé SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J. Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Simon A. Skeletal muscle dedifferentiation during salamander limb regeneration. Curr. Opin. Genet. Dev. 2016;40:108–112. doi: 10.1016/j.gde.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Giraldez AJ, et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 48.Lund E, Liu M, Hartley RS, Sheets MD, Dahlberg JE. Deadenylation of maternal mRNAs mediated by miR-427 in Xenopus laevis embryos. RNA. 2009;15:2351–2363. doi: 10.1261/rna.1882009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaylet A. [Change in the karyotype by a homozygous pericentric inversion in the newt Pleurodeles waltlii Michahelles] Chromosoma. 1971;35:288–299. doi: 10.1007/BF00326279. [DOI] [PubMed] [Google Scholar]
- 50.Gallien CL. [The karyotype of the urodele Pleurodeles poireti Gervais. Comparative study of karyotypes in the genus Pleurodeles]. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D. 1966;262:122–125. [PubMed] [Google Scholar]
- 51.Penrad-Mobayed M, Kanhoush R, Perrin C. Tips and tricks for preparing lampbrush chromosome spreads from Xenopus tropicalis oocytes. Methods. 2010;51:37–44. doi: 10.1016/j.ymeth.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Dolezel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007;2:2233–2244. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- 53.Licht LE, Lowcock LA. Genome size and metabolic-rate in salamanders. Comp. Biochem. Phys. B. 1991;100:83–92. doi: 10.1016/0305-0491(91)90089-V. [DOI] [Google Scholar]
- 54.Morescalchi A, Olmo E. Sirenids - family of polyploid urodeles. Experientia. 1974;30:491–492. doi: 10.1007/BF01926310. [DOI] [PubMed] [Google Scholar]
- 55.Lizana M, et al. Determination of cellular DNA content of Iberian salamanders by flow cytometry. Amphibia-Reptilia. 2000;21:411–418. doi: 10.1163/156853800300059304. [DOI] [Google Scholar]
- 56.Felsenstein J. Mathematics vs. evolution: mathematical evolutionary theory. Science. 1989;246:941–942. doi: 10.1126/science.246.4932.941. [DOI] [PubMed] [Google Scholar]
- 57.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst. Biol. 2006;55:539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- 59.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 60.Joven A, Kirkham M, Simon A. Husbandry of Spanish ribbed newts (Pleurodeles waltl) Methods Mol. Biol. 2015;1290:47–70. doi: 10.1007/978-1-4939-2495-0_4. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi T, Takeuchi T. Gene manipulation for regenerative studies using the Iberian ribbed newt, Pleurodeles waltl. Methods Mol. Biol. 2015;1290:297–305. doi: 10.1007/978-1-4939-2495-0_23. [DOI] [PubMed] [Google Scholar]
- 62.Monsoro-Burq, A. H. A rapid protocol for whole-mount in situ hybridization on Xenopus embryos. CSH Prot. 2007, pdbprot4809, 10.1101/pdb.prot4809 (2007). [DOI] [PubMed]
- 63.Kirkham M, Hameed LS, Berg DA, Wang H, Simon A. Progenitor cell dynamics in the Newt Telencephalon during homeostasis and neuronal regeneration. Stem cell reports. 2014;2:507–519. doi: 10.1016/j.stemcr.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H, et al. Turning terminally differentiated skeletal muscle cells into regenerative progenitors. Nat. Commun. 2015;6:7916. doi: 10.1038/ncomms8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berghoff BA, Karlsson T, Källman T, Wagner EGH, Grabherr MG. RNA-sequence data normalization through in silico prediction of reference genes: the bacterial response to DNA damage as case study. BioData Min. 2017;10:30. doi: 10.1186/s13040-017-0150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar S, Stecher G, Suleski M, Hedges SB. TimeTree: a resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 2017;34:1812–1819. doi: 10.1093/molbev/msx116. [DOI] [PubMed] [Google Scholar]
- 67.Miyake A, Araki M. Retinal stem/progenitor cells in the ciliary marginal zone complete retinal regeneration: a study of retinal regeneration in a novel animal model. Dev. Neurobiol. 2014;74:739–756. doi: 10.1002/dneu.22169. [DOI] [PubMed] [Google Scholar]
- 68.Yoshii C, Ueda Y, Okamoto M, Araki M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev. Biol. 2007;303:45–56. doi: 10.1016/j.ydbio.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 69.Lozito TP, Tuan RS. Lizard tail skeletal regeneration combines aspects of fracture healing and blastema-based regeneration. Development. 2016;143:2946–2957. doi: 10.1242/dev.129585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berg DA, et al. Efficient regeneration by activation of neurogenesis in homeostatically quiescent regions of the adult vertebrate brain. Development. 2010;137:4127–4134. doi: 10.1242/dev.055541. [DOI] [PubMed] [Google Scholar]
- 71.Roco Aacute, et al. Coexistence of Y, W, and Z sex chromosomes in Xenopus tropicalis. Proc. Natl Acad. Sci. USA. 2015;112:E4752–E4761. doi: 10.1073/pnas.1505291112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Session AM, et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature. 2016;538:336–343. doi: 10.1038/nature19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sessions SK. Evolutionary cytogenetics in salamanders. Chromosome Res. 2008;16:183–201. doi: 10.1007/s10577-007-1205-3. [DOI] [PubMed] [Google Scholar]
- 74.Liew WC, Orbán L. Zebrafish sex: a complicated affair. Brief Funct. Genomics. 2014;13:172–187. doi: 10.1093/bfgp/elt041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Datasets are deposited under BioProject PRJNA353981 and BioSample accession SAMN07571895, Runs SRR6001098-SRR6001140. Transcriptome and genome assemblies are available upon request.