Abstract
We performed a systematic review and meta-analysis to determine whether the use of local antibiotics is a beneficial prophylactic treatment for endophthalmitis in patients treated with anti-VEGF agents. We searched the MEDLINE and EMBASE databases, and the Cochrane Library over the period January 2007 to December 2016. The search terms used included “Endophthalmitis”, “Antibiotic” and “Intravitreal injection”. Studies in which the patients were treated exclusively with intravitreal injections of anti-VEGF were selected. Eight studies fit the inclusion criteria, which included a total of 276,774 injections; 109,178 (39.45%) were associated with the use of antibiotics and 114,821 (60.55%) were not associated with the use of antibiotics. Our meta-analysis indicated a significant risk for endophthalmitis that was 1.70 times greater with the use of antibiotics than that without antibiotics, with a confidence interval of 1.08 to 2.66 (p = 0.02). A meta-regression indicated that the location (operating rooms versus outpatient clinics) of injection did not have a significant effect on the incidence of endophthalmitis. The prophylactic use of antibiotics when administering anti-VEGF intravitreal injections may contribute to a greater incidence of endophthalmitis. This finding, in addition to reducing costs, would eliminate a treatment that has been shown to be unnecessary and even harmful to patients.
Introduction
Intravitreal anti-vascular endothelial growth factor (VEGF) therapy is frequently used as one of the main treatments for many retinal pathologies, including age-related macular degeneration (exudative)1, diabetic macular edema1,2 and retinal vein occlusion3. The use of anti-VEGF agents has increased in recent years and now constitutes one of the most common procedures in ophthalmology. It is projected that this trend will continue for the next few years. Although the risk of injection-associated endophthalmitis is low (approximately 1 in 3000 injections)4, the visual morbidity of this complication is devastating5.
The only preventive measure with some consensus regarding its effectiveness is the application of povidone-iodine to the ocular surface prior to injection, which is the only effective prophylactic measure supported by clinical trials6. Other measures, such as the use of gloves, a blepharostat or masks and the setting where injection was given (operating rooms or outpatient clinics), remain controversial7,8. The use of antibiotics as a method of prophylaxis is part of the normal practice of intraocular procedures. However, recent studies have shown that antibiotics offer no protection against the risk of developing endophthalmitis once anti-VEGF injections have been administered, and, in some cases, the rates of endophthalmitis are actually higher in groups using antibiotics9,10. Although the abandonment of antibiotics before and after intravitreal administration of anti-VEGF appears to be the current trend, the use of antibiotics remains part of the normal procedure for a significant percentage of practitioners11,12.
Currently, no consensus exists regarding the benefits of antibiotic prophylaxis of endophthalmitis after anti-VEGF injections. To clarify this controversy, we present a meta-analysis to evaluate the incidence of endophthalmitis after treatment with anti-VEGF agents associated with 1) use of topical antibiotics and 2) the setting where the injection is performed.
Results
An original search produced 779 possible articles. Removal of duplicates and application of selection criteria reduced the number of articles comparing two groups to eight. As shown in these publications, the proportion of cases of endophthalmitis among the total patients analyzed in each publication was very low (0.035%), with values ranging from 0.012% to 0.100%. In cases of endophthalmitis with and without antibiotic prophylaxis, staphylococcus and streptococcus were the most frequent microorganisms identified in culture-proven endophthalmitis. In six of the studies, the injections were administered in an operating room, and in the two remaining studies, the injections were administered in outpatient clinics. Table 1.
Table 1.
Summary of studies selected for inclusion in the meta-analysis.
| Study | Place | Timing (AB) | Injections | Endoph | Inject. AB | Endoph. AB | Inject. no AB | Endoph. no AB |
|---|---|---|---|---|---|---|---|---|
| Falavarjani et al.33 | 2 | 2 | 5,901 | 6 | 3,975 | 6 | 1,926 | 0 |
| Cheung et al.* 34 | 1 | 2 | 14,960 | 7 | 10,061 | 6 | 4,899 | 1 |
| Fineman et al.8 | 1 | 2 | 10,164 | 3 | 7,415 | 2 | 2,749 | 1 |
| Mason et al.35 | 1 | 2 | 5,233 | 1 | 2,617 | 1 | 2,616 | 0 |
| Park et al.36 | 1 | 1 | 16,186 | 2 | 8,078 | 0 | 8,108 | 2 |
| Falavarjani et al.37 | 2 | 2 | 8,037 | 1 | 2,771 | 1 | 5,266 | 0 |
| Storey et al.38 | 1 | 3 | 147,479 | 52 | 57,654 | 28 | 89,825 | 24 |
| Li et al.* 39 | 1 | 3 | 68,814 | 15 | 16,607 | 4 | 52,207 | 11 |
Codes: Place: 1 = Outpatient clinics, 2 = Operating room; AB: 1 = Pre-injection, 2 = Post-injection, 3 = Pre- and post-injection, 4 = No AB. Abbreviations: Injec.: injections, Endoph.: endophthalmitis, and AB: antibiotic. *Extra information provided by the authors.
Relationship between antibiotic therapy and endophthalmitis
To perform this meta-analysis, we selected publications that indicated both the number of anti-VEGF injections associated with the use of antibiotics as well as the number of injections not associated with the use of antibiotics. The studies included in this analysis were characterized by homogeneity regarding the type of healthcare professional that administered the injections (ophthalmologist) and the use of povidone in all cases.
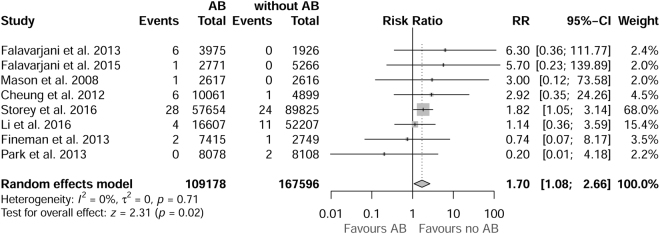
Eight studies were selected, involving a total of 276,774 injections, with 109,178 (39.45%) injections associated with the use of antibiotics and 167,596 (60.55%) injections not associated with the use of antibiotics. The relative risk (RR) of endophthalmitis was 1.70 times greater with the use of antibiotics than without antibiotics, with a confidence interval of 1.08 to 2.66 (the number of cases of endophthalmitis in AB group and non-AB was 48 and 39, respectively). Therefore, the use of prophylactic antibiotics is associated with a higher incidence of endophthalmitis (z = 2.31, p = 0.02). The forest plot (Fig. 1) shows the RR and 95% confidence intervals for each of the studies and the combined RR obtained from the random effects model.
Figure 1.
Forest plot with relative risk (RR) estimates of each study and the combined RR (represented as a rhombus), including the 95% confidence intervals and the weights assigned to each study.
No heterogeneity was observed in the I2 value, which indicates a null-low heterogeneity (I2 = 0% (0%, 31%)), or with the test of heterogeneity (Q (degrees of freedom) = 4.52 (7), p = 0.717). Both the graphical analysis (Figure S1) and the regression test (t = 0.007, p-value = 0.994) indicated the absence of publication bias. With respect to the sensitivity analysis, after excluding the study by Story et al., which had a sample size much greater than the remaining sample, the RR decreased to 1.49 (0.7664, 3.3016), but heterogeneity increased substantially (I2 = 0%–61%). The number of cases of endophthalmitis in AB group and non-AB was 20 and 15 respectively, when Storey et al. was excluded from the analysis.
Meta-regression analysis of the effect of the setting where injection was given
Meta-regression was performed to assess whether the setting where injection was administered (operating room versus outpatient clinics) influenced the incidence of endophthalmitis associated with antibiotic treatment. The analysis results (z = 1.165, p = 0.243) suggest that the setting where the injection was administered had no significant effect on the incidence of endophthalmitis associated with antibiotics.
Moreover, the results of the various meta-regressions showed no significant effect of any of the following variables on the incidence of endophthalmitis associated with antibiotic treatment: (a) the time at which the antibiotic was administered (pre- versus post-injection antibiotics): QM = 2; 42, p = 0.298 and (b) the causative microorganism: QM = 0; 02, p = 0.905.
Discussion
This study presents a meta-analysis that indicates an increased risk of endophthalmitis in patients treated with intravitreal anti-VEGF agents who were also treated with prophylactic topical antibiotics. Our analysis also revealed no significant differences in the risk of developing endophthalmitis for patients who received injections in the operating rooms versus those who received injections in outpatient clinics.
We have also attempted to conduct this study in as rigorous a manner as possible and took extra precautions to control for heterogeneity across studies and publication bias to achieve maximum reliability of the results. The main assumption casting doubt on the validity of the meta-analysis is the lack of significant variations in the procedures for various valued tests. Another factor that adds value to our statistical analysis is the importance of the study by Storey et al. to our conclusions. Increasing the sample size is very important when studying diseases with low incidence such as endophthalmitis
Recent studies have indicated that the use of topical antibiotics could increase resistance to some antibiotics by affecting the conjunctival and nasopharyngeal flora. Moreover, increasing the proportion of resistant bacteria on the ocular surface increases the risk of developing antibiotic-resistant infections that are difficult to treat13. Several possibilities could explain the trend of increasing antibiotic resistance. Patients receiving anti-VEGF intravitreal injection therapy for retinal diseases often require repeated doses for long periods of time. Our data confirm that short courses of topical antibiotics affect the patterns of resistance of periocular flora. Fluoroquinolones are the most commonly used post-injection prophylactic antibiotics in patients due to their broad spectrum and high penetration. Several studies have demonstrated substantial levels of resistance to third- and fourth-generation fluoroquinolones, as well as multi-drug resistance in patients treated with topical antibiotics after multiple intravitreal injections14,15. On the other hand, it would be necessary to take into account the cost saving that may result from the non-instillation of antibiotic eye drops in patients undergoing this type of treatment.
Among other factors that could influence the risk of developing intraocular infection, consideration of the location of treatment administration is essential. A study based on an analysis of 12,249 injections performed by the same surgeon showed significantly lower levels of endophthalmitis when the injection was performed in the operating room instead of outpatient clinics16. By contrast, another study showed that the rate of endophthalmitis after an intravitreal injection was low regardless of whether the procedure was performed in outpatient clinics or in an operating room (0.035 versus 0.065%, respectively)17. The results of our meta-analysis support this finding.
Recently, Dutheil et al.18 presented a similar meta-analysis based on various types of intravitreal injections, including triamcinolone, dexamethasone and perfluoropropane (CF8). The goal of our work was to improve the protocol for the use of anti-VEGF drugs. The inclusion of corticosteroids in an analysis of the rates of endophthalmitis in intravitreal injections may interfere with this objective. Some studies support the idea that the risk of endophthalmitis is not equal among the possible options for intravitreal treatment. Triamcinolone presents a significant increase in the OR of 6.92 for endophthalmitis compared to anti-VEGF agents19. This result is partly explained by the immunosuppression that corticosteroids produce in the ocular microenvironment20.
However, it is necessary to consider of the following limitations in our study: methodological limitations due to a lack of access to patient data-level statistics, sensitivity bias and considering only those manuscripts published in English. All eight studies included in our analysis were retrospective reviews; they were all case-control studies rather than randomized controlled trials due to the fact that the evaluated randomized trials did not met inclusion criteria. Some of case-control studies were carried out in different periods of time, which may have carried changes in hospital prophylaxis procedures; those aspects could not be considered when evaluating the data. Furthermore, none of the studies provided differentiation of positive cultures between groups with/without antibiotic use; therefore, this variable could not be analyzed in our study. However, the data from the eight studies were sufficient to assess the duration of antibiotic treatment and the RR. Therefore, although our findings seem conclusive, these limitations suggest that the results of this study should be interpreted with caution.
We ultimately believe that the decision to use antibiotics in the prophylactic period depends on individual ophthalmologists. Ophthalmologist must analyze the evidence supporting the practice of antibiotic prophylaxis and the evidence contraindicating the use of antibiotics, as the overuse of antibiotics could possibly cause the creation and proliferation of resistant strains and increase drug costs and the likelihood of possible adverse reactions to the drugs administered.
This study included the largest meta-analysis published on this subject to date. The results of our study establish that the prophylactic use of antibiotics for intravitreal anti-VEGF injections is associated with a higher incidence of endophthalmitis. This finding could potentially eliminate an unnecessary intervention that is likely harmful to patients. Additionally, we did not observe any benefits associated with the location at which the administration of the intravitreal injection was performed, such as during a consultation at an outpatient clinic as opposed to during surgery or at another location. These two observations have implications for patient comfort, efficiency and the costs of administering these treatments.
Materials and Methods
The Meta-analysis Of Observational Studies in Epidemiology (MOOSE)21 criteria along with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)22 were used for this review and meta-analysis (Table S1).
Search methods used to identify studies
We searched MEDLINE, EMBASE and the Cochrane Database of Systematic Reviews from January 2007 (starting date of treatment injecting intravitreal anti-VEGFs) to December 2016. The terms used included “Endophthalmitis”, “Antibiotic” and “Intravitreal injection”. Different combinations of terms/descriptors were used to ensure the inclusion of a greater number of studies. For details of the search strategy, please see the supplementary materials (Table S2).
Selection criteria for studies
During these investigations, specific predetermined selection criteria were applied to individually assess the eligibility of the studies. The inclusion criteria were as follows: (1) studies published in English, (2) studies that produced original data on the problem and (3) studies examining the incidence, prevalence or risk of post-injection endophthalmitis associated with prophylactic topical antibiotics administered before or after injections of anti-VEGF (ranibizumab, bevacizumab and aflibercept) and prophylactic administration of antibiotics (independent variables).
The exclusion criteria were as follows: (1) studies in which patients were treated with intravitreal anti-VEGF injections in combination with another therapeutic strategy (for example, photodynamic therapy) and (2) studies involving intravitreal triamcinolone injections or another type of steroid used as a main therapeutic agent or combined with other anti-VEGF agents, which is justified because the delimitation only includes a single class of agents, inhibitors of VEGF. We attempted to specifically exclude intravitreal corticosteroids among articles that met the criteria of homogeneity with the rest of the meta-analyses. This process was performed by collaboration of the authors. Table S3 details the PICOS (population, interventions, comparators, outcomes and study.
Study selection, data collection and risk of bias assessment
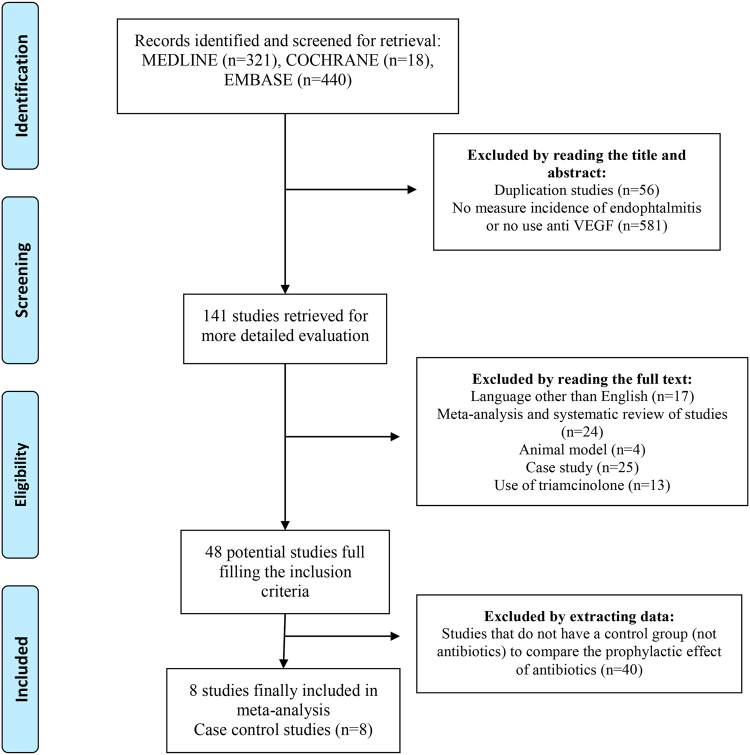
One author (MB) conducted all literature searches and collated the abstracts. Two authors (MB and RM) separately reviewed the abstracts and determined the suitability of the articles for inclusion in the study based on the selection criteria. Any disagreement was resolved through discussions with a third reviewer (AP). In addition, reference lists of all publications meeting the inclusion criteria were manually searched to identify any further studies not identified via electronic searches. The search strategy is described in Fig. 2. To assess the methodological quality of the studies, we used the Strengthening the Reporting of Observational Studies in Epidemiology Statement (STROBE) checklist for observational studies23.
Figure 2.
Identification and selection of studies for the meta-analysis.
Publication bias was assessed graphically using a funnel plot and a Galbraith plot24 and via regression tests for funnel plot asymmetry25. Identification of publications that could influence the outcome of the study was conducted with the leave-one-out sensitivity analysis26.
For quality assessment analysis, the following items were evaluated in each of the publications: (1) Research question explicitly defined, 2) Sample size, 3) Characteristics and definition of study population, 4) Specification of inclusion and exclusion criteria, 5) Good definition and assessment (accurate and reliable methods), 6) Statistical analysis, 7) Completeness of reporting, 8) Study design, 9) External validity of results 10) conflict of interest in the conduct of the study.
These ten items were evaluated for each of the individual studies, assigning a three-level code (1: poor reporting/quality, 2: acceptable, 3: good). The quality score for each of the papers included in the analysis was between acceptable and good quality/reporting.
Data synthesis and analysis
The primary outcome measure was the relative risk (RR) of endophthalmitis. The random effects model with restricted maximum likelihood estimation (Restricted Maximum Likelihood, REML) was used27. The existence of statistical heterogeneity between studies was evaluated using the I2 index28,29.
A subgroup analysis was also performed to assess the effect of certain covariates on the RR of endophthalmitis. These analyses were conducted using meta-regression30 with mixed model effects27.
All analyses were performed using the metafor31 library in R software (R Core Team, 2016)32.
Precis
The use of antibiotics in the prophylaxis of endophthalmitis when administering intravitreal injections of anti-VEGF agents may be harmful. Our meta-regression analysis also indicated that outcomes do not vary with respect to the setting where injection was given (outpatient clinics or operating rooms) of antibiotic administration.
Electronic supplementary material
Acknowledgements
We thank the authors who collaborated in the compilation of this article by providing additional information. We also thank the statistical center Biostatech (Universidad de Santiago de Compostela, Spain) for providing the averages for the preparation of this work.
Author Contributions
Acquisition of data: M.B., R.M. and A.P. Analysis and interpretation of data: M.P. and M.B. Drafting of manuscript: M.B. and F.G.U. Critical revision: M.F. and M.B.-T. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18412-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fong AH, Lai TY. Long-term effectiveness of ranibizumab for age-related macular degeneration and diabetic macular edema. Clinical interventions in aging. 2013;8:467–483. doi: 10.2147/CIA.S36811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elman MJ, et al. Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122:375–381. doi: 10.1016/j.ophtha.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Y, Qu Y. Comparison of intravitreal bevacizumab with intravitreal triamcinolone acetonide for treatment of cystoid macular edema secondary to retinal vein occlusion: a Meta-analysis. International journal of ophthalmology. 2015;8:1234–1239. doi: 10.3980/j.issn.2222-3959.2015.06.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz SG, Flynn HW., Jr. Endophthalmitis Associated with Intravitreal Anti-Vascular Endothelial Growth Factor Injections. Current ophthalmology reports. 2014;2:1–5. doi: 10.1007/s40135-013-0033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arevalo JF, Jap A, Chee SP, Zeballos DG. Endogenous endophthalmitis in the developing world. International ophthalmology clinics. 2010;50:173–187. doi: 10.1097/IIO.0b013e3181d26dfc. [DOI] [PubMed] [Google Scholar]
- 6.Avery RL, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina. 2014;34(Suppl 12):S1–S18. doi: 10.1097/IAE.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 7.Schimel AM, Scott IU, Flynn HW., Jr. Endophthalmitis after intravitreal injections: should the use of face masks be the standard of care? Archives of ophthalmology. 2011;129:1607–1609. doi: 10.1001/archophthalmol.2011.370. [DOI] [PubMed] [Google Scholar]
- 8.Fineman MS, Hsu J, Spirn MJ, Kaiser RS. Bimanual assisted eyelid retraction technique for intravitreal injections. Retina. 2013;33:1968–1970. doi: 10.1097/IAE.0b013e318287da92. [DOI] [PubMed] [Google Scholar]
- 9.Bhavsar AR, et al. Update on risk of endophthalmitis after intravitreal drug injections and potential impact of elimination of topical antibiotics. Archives of ophthalmology. 2012;130:809–810. doi: 10.1001/archophthalmol.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storey P, et al. The role of topical antibiotic prophylaxis to prevent endophthalmitis after intravitreal injection. Ophthalmology. 2014;121:283–289. doi: 10.1016/j.ophtha.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 11.Samia-Aly E, Cassels-Brown A, Morris DS, Stancliffe R, Somner JE. A survey of UK practice patterns in the delivery of intravitreal injections. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians. 2015;35:450–454. doi: 10.1111/opo.12217. [DOI] [PubMed] [Google Scholar]
- 12.Xing L, Dorrepaal SJ, Gale J. Survey of intravitreal injection techniques and treatment protocols among retina specialists in Canada. Canadian journal of ophthalmology. Journal canadien d’ophtalmologie. 2014;49:261–266. doi: 10.1016/j.jcjo.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Hsu J, Gerstenblith AT, Garg SJ, Vander JF. Conjunctival flora antibiotic resistance patterns after serial intravitreal injections without postinjection topical antibiotics. American journal of ophthalmology. 2014;157:514–518 e511. doi: 10.1016/j.ajo.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Milder E, Vander J, Shah C, Garg S. Changes in antibiotic resistance patterns of conjunctival flora due to repeated use of topical antibiotics after intravitreal injection. Ophthalmology. 2012;119:1420–1424. doi: 10.1016/j.ophtha.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, et al. Antibiotic resistance of conjunctiva and nasopharynx evaluation study: a prospective study of patients undergoing intravitreal injections. Ophthalmology. 2010;117:2372–2378. doi: 10.1016/j.ophtha.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Abell RG, Kerr NM, Allen P, Vote BJ. Intravitreal injections: is there benefit for a theatre setting? The British journal of ophthalmology. 2012;96:1474–1478. doi: 10.1136/bjophthalmol-2012-302030. [DOI] [PubMed] [Google Scholar]
- 17.Tabandeh H, et al. Endophthalmitis associated with intravitreal injections: office-based setting and operating room setting. Retina. 2014;34:18–23. doi: 10.1097/IAE.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 18.Benoist d’Azy C, Pereira B, Naughton G, Chiambaretta F, Dutheil F. Antibioprophylaxis in Prevention of Endophthalmitis in Intravitreal Injection: A Systematic Review and Meta-Analysis. PloS one. 2016;11:e0156431. doi: 10.1371/journal.pone.0156431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanderBeek BL, Bonaffini SG, Ma L. The Association between Intravitreal Steroids and Post-Injection Endophthalmitis Rates. Ophthalmology. 2015;122:2311–2315 e2311. doi: 10.1016/j.ophtha.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucher RS, et al. Effect of intravitreal triamcinolone acetonide on susceptibility to experimental bacterial endophthalmitis and subsequent response to treatment. Archives of ophthalmology. 2005;123:649–653. doi: 10.1001/archopht.123.5.649. [DOI] [PubMed] [Google Scholar]
- 21.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 24.Galbraith RF. Some Applications of Radial Plots. Journal of the American Statistical Association. 1994;89:1232–1242. doi: 10.1080/01621459.1994.10476864. [DOI] [Google Scholar]
- 25.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in medicine. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 26.Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Research synthesis methods. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 27.Raudenbush, S. W. The handbook of research synthesis and meta-analysis. (eds. Cooper, H. et al.). Ch. 16, pp. 295–315 (New York: Russell Sage Foundation, 2009).
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Statistics in medicine. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 31.Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. 2010 36, 48, 10.18637/jss.v036.i03 (2010).
- 32.Team, R. C. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2015. http.www.R-project.org (2016).
- 33.Falavarjani KG, et al. Incidence of acute endophthalmitis after intravitreal bevacizumab injection in a single clinical center. Retina. 2013;33:971–974. doi: 10.1097/IAE.0b013e31826f0675. [DOI] [PubMed] [Google Scholar]
- 34.Cheung CS, et al. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology. 2012;119:1609–1614. doi: 10.1016/j.ophtha.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Mason JO, 3rd, et al. Incidence of acute onset endophthalmitis following intravitreal bevacizumab (Avastin) injection. Retina. 2008;28:564–567. doi: 10.1097/IAE.0b013e3181633fee. [DOI] [PubMed] [Google Scholar]
- 36.Park Y, Kim KS, Park YH. Acute endophthalmitis after intravitreal injection and preventive effect of preoperative topical antibiotics. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2013;29:900–905. doi: 10.1089/jop.2013.0052. [DOI] [PubMed] [Google Scholar]
- 37.Falavarjani KG, et al. Endophthalmitis after resident-performed intravitreal bevacizumab injection. Canadian journal of ophthalmology. Journal canadien d’ophtalmologie. 2015;50:33–36. doi: 10.1016/j.jcjo.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Storey P, et al. The effect of prophylactic topical antibiotics on bacterial resistance patterns in endophthalmitis following intravitreal injection. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2016;254:235–242. doi: 10.1007/s00417-015-3035-x. [DOI] [PubMed] [Google Scholar]
- 39.Li AL, et al. Endophthalmitis after intravitreal injection: role of prophylactic topical ophthalmic antibiotics. Retina. 2016;36:1349–1356. doi: 10.1097/IAE.0000000000000901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.