Abstract
Our previous study shows that an anaerobic intestinal bacterium strain AJ110941P contributes to type 2 diabetes development in mice. Here we phylogenetically and physiologically characterized this unique mouse gut bacterium. The 16S rRNA gene analysis revealed that the strain belongs to the family Lachnospiraceae but shows low sequence similarities ( < 92.5%) to valid species, and rather formed a distinct cluster with uncultured mouse gut bacteria clones. In metagenomic database survey, the 16S sequence of AJ110941P also matched with mouse gut-derived datasets (56% of total datasets) with > 99% similarity, suggesting that AJ110941P-related bacteria mainly reside in mouse digestive tracts. Strain AJ110941P shared common physiological traits (e.g., Gram-positive, anaerobic, mesophilic, and fermentative growth with carbohydrates) with relative species of the Lachnospiraceae. Notably, the biofilm-forming capacity was found in both AJ110941P and relative species. However, AJ110941P possessed far more strong ability to produce biofilm than relative species and formed unique structure of extracellular polymeric substances. Furthermore, AJ110941P cells are markedly long fusiform-shaped rods (9.0–62.5 µm) with multiple flagella that have never been observed in any other Lachnospiraceae members. Based on the phenotypic and phylogenetic features, we propose a new genus and species, Fusimonas intestini gen. nov., sp. nov. for strain AJ110941P (FERM BP-11443).
Introduction
The mammalian digestive tract is one of the largest microbial habitats: more than 1014 cells of microorganisms are present in the entire human gastrointestinal tract1, and anaerobic bacteria are the main constituents of the ecosystems2. Recent extensive studies based on next generation sequencing approach enabled to characterize composition, diversity, and spatial distribution of gut microbial communities3, and suggested that changes in the gut microbiota might be associated with human health and diseases. This attracts a worldwide interest for its potential impact in the field of medical science as well as microbial ecology4.
Culture-independent metagenomic studies revealed that members of the phyla Bacteroidetes and Firmicutes represent the most dominant and prevalent bacterial groups in the human gut ecosystem, and the Firmicutes is in particular the main component accounting for >50% of all the 16S rRNA gene sequences5–7. These intestinal Firmicutes bacteria are known to have an influence on the human health. For instance, several Lactobacillus spp. in the family Lactobacillaceae are widely recognized as beneficial bacteria (lactic acid bacteria) for maintaining the intestinal environment8, whereas some Clostridium spp. in the family Clostridiaceae are known as pathogens causing a bowel inflammation and food poisoning9.
Members of the family Lachnospiraceae constitute the abundant taxa within the phylum Firmicutes in human gut microbiota7. Over half of human intestinal bacteria are not cultivated yet and many of those belong to the family Lachnospiraceae 10. Recently, new bacterial strains representing novel genera in the family Lachnospiraceae were isolated from not only digestive tract but also microbial mat and biogas reactor, i.e., Eisenbergiella tayi strain B086562T11, Fusicatenibacter saccharivorans strain HT03–11T 12, Herbinix hemicellulosilytica strain T3/55T 13, Mobilitalea sibirica strain P3M-3T 14, Murimonas intestini strain SRB-530-5-HT 15 and Anaerobium acetethylicum GluBS11T 16. The family Lachnospiraceae currently consists of 31 genera according to the LPSN database (http://www.bacterio.net/-news.html), Bergey’s Manual of Systematic Bacteriology (second edition), and recent study by Patil et al.16.
Importantly, based on metagenomics, Qin et al. reported that the abundance of Lachnospiraceae bacteria in the gut was positively correlated with type 2 diabetes, one of the major public health concerns17, implying that the Lachnospiraceae might be associated with occurrence of the disease. However, no clear evidence was shown due to the lack of axenic cultures of the possible causing agent. Very recently, we succeeded in isolating a new member of the family Lachnospiraceae, designated strain AJ110941P, from the feces of hyperglycemic obese mouse18, and further demonstrated that the new isolate is obviously involved in development of obesity and diabetes in germ-free (GF) ob/ob mice18. In fact, the intestinal colonization of strain AJ110941P in GF mice induced the typical symptoms such as significant increases in fasting blood glucose levels together with liver and mesenteric adipose tissue weights, and decrease in plasma insulin levels and HOMA-β values18. In this work, we phylogenetically and physiologically characterized the new strain AJ110941P, compared its phenotypic characteristics with other Lachnospiraceae species, and consequently propose the novel genus and species, Fusimonas intestini gen. nov., sp. nov., for this strain.
Results and Discussion
Phylogenetic affiliation of strain AJ110941P and its closest relatives
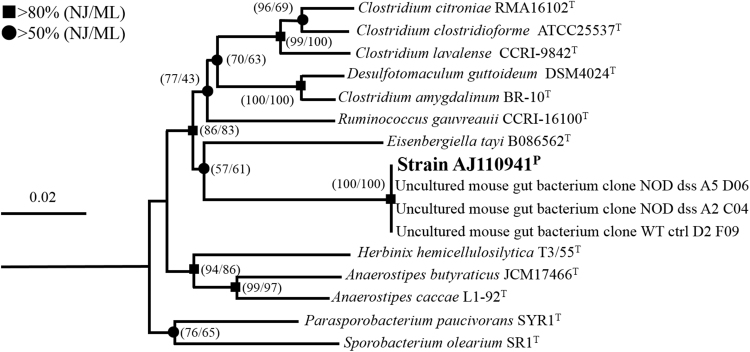
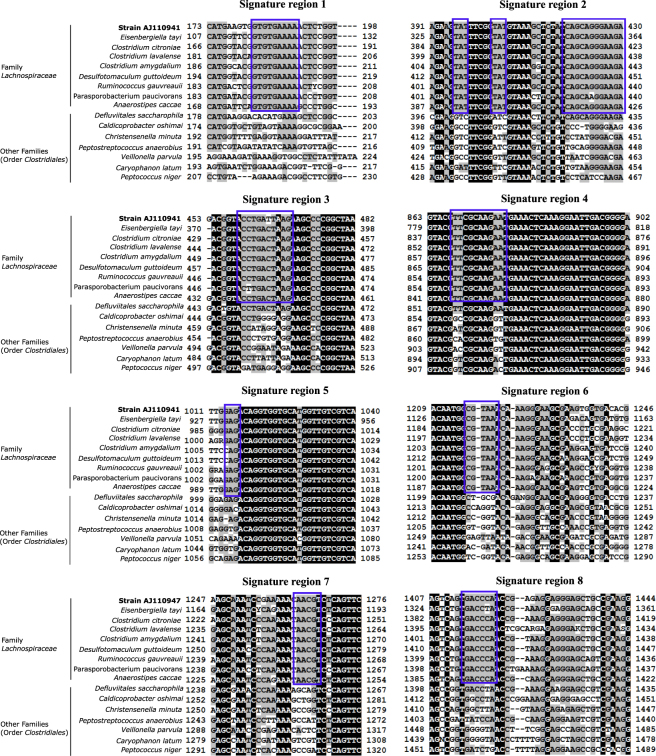
Comparison of 16S rRNA gene sequence of strain AJ110941P with those of validly described species indicated that the strain is moderately related to members of the family Lachnospiraceae with relatively low sequence similarities ( < 92.5%). The most closely related species to strain AJ110941P was Ruminococcus gauvreauii strain CCRI-16110T isolated from the human faecal specimen (92.5% sequence similarity)19. Other close relatives were Clostridium amygdalinum strain BR-10T (92.3%)20, Clostridium citroniae strain RAM16102T (92.1%)21, Eisenbergiella tayi strain B086562T (92.1%)11, Anaerostipes caccae strain L1-92T (92.0%)22, Clostridium lavalense strain CCRI-9842 (92.0%)23, and Desulfotomaculum guttoideum strain DSM 4024 T (92.0%)24. Strain AJ110941P formed a monophyletic cluster with those relatives and was a neighbor of Eisenbergiella tayi strain B086562T (Fig. 1). We further performed multiple alignment of 16S rRNA gene sequences, and found eight signature regions that are highly conserved only among Lachnospiraceae species, but not in other families of the order Clostridiales (Fig. 2). These results suggest that strain AJ110941P is affiliated with the family Lachnospiraceae, and that a new genus should be created for the novel strain because of its low sequence similarities (92.5%) to the close relatives.
Figure 1.
Phylogenetic relationships between strain AJ110941P and the closely related members of the family Lachnospiraceae based on 16S rRNA gene sequences. The phylogenetic tree was constructed by neighbor-joining (NJ) method. The 16S rRNA gene sequence of Peptostreptococcus anaerobius JCM1470T (AB640688) was used as an outgroup. Bootstrap values of >50% and >80% estimated using neighbour-joining (NJ) and maximum-likelihood (ML) methods (1,000 replications) are shown by circle and square at branching points, respectively.
Figure 2.
Alignment of 16S rRNA gene sequences within signature regions able to distinguish between Lachnospiraceae species and other family members. The 16S rRNA genes of Lachnospiraceae species were aligned with corresponding sequences from microorganisms belonging to the order Clostridiales. Identical and similar nucleotides are indicated by black and gray backgrounds, respectively. Signature sequences are boxed in blue.
Comparative 16S rRNA gene sequence analysis revealed that strain AJ110941P showed high 16S rRNA gene sequence similarities (99–100%) to clones retrieved from the mouse digestive tracts: uncultured bacterial clones WT ctrl D2 F09, NOD dss A5 D06 and NOD dss A2 C04 from mice colon tissues25, clones 16saw23-2g06.p1k and 16saw23-1b01.p1k from mice cecal contents26, clone F09 from wild-type mouse’s colon tissue, clones D06 and C04 from colon tissue samples from Nod2 KO mice25, and clones 16saw23-2g06.p1k and 16saw23-1b01.p1k from avirulent pathogen infected mouse26. Based on the IMNGS27 search (metagenome-derived 16S rRNA gene sequence database search), we further found that the 16S rRNA gene sequence of strain AJ110941P matched with 3699 and 826 datasets (with >97% and >99% similarity, respectively), 56% of which were derived from mouse guts (Supplementary Fig. S1). These results suggest that F. intestini and its relatives are dwelling mainly in mouse gastrointestinal tracts.
Morphological, physiological, and biochemical characteristics
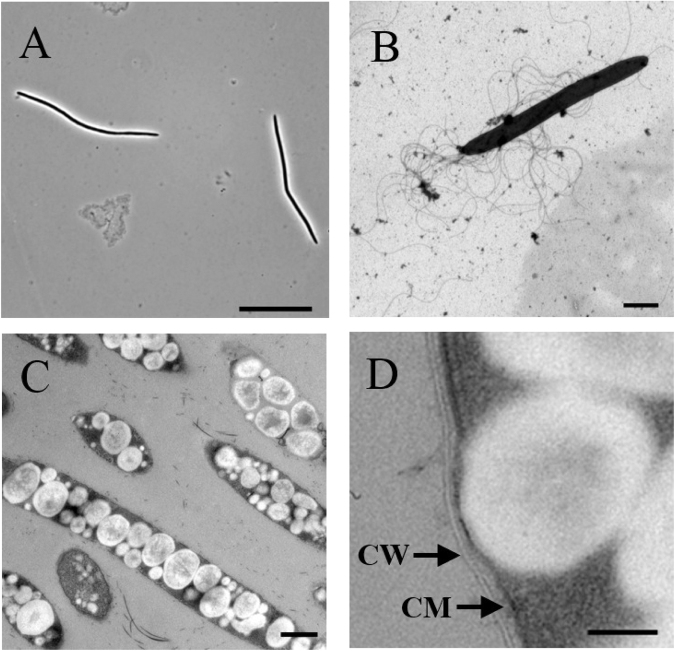
Strain AJ110941P was a strictly anaerobic and heterotrophic bacterium. The temperature range for growth of strain AJ110941P was 15–40 °C (optimum growth at 37 °C). No growth was observed at 10 °C and 45 °C. The strain grew at pH 6.5–8.0, with an optimum at pH 8.0, and no growth occurred at pH 6.0 and 8.5. The strain did not require NaCl for growth and tolerated up to 0.5% (w/v) NaCl. Brown disk-like colonies (0.1–1.4 mm in diameter and <0.2 mm height) were formed in the GAM agar medium after 4 days of incubation at 37 °C. Cells were fusiform-shaped rods, 9.0–62.5 µm in length and 0.55–0.9 µm in width (mean size 25.05 × 0.63 µm), which occurred mainly as single cells (Fig. 3A). Spore formation was not observed. Cells possessed multiple flagella (Fig. 3B), but the motility was not observed. Intracellular polyhydroxyalkanoate (PHA) like compounds were observed (Fig. 3C). Cells were positively stained by Gram-staining and also showed Gram-positive type of cell wall by electron microscopy (Fig. 3D).
Figure 3.
Photomicrographs of strain AJ110941P grown in GAM medium under anaerobic conditions at 37 °C. (A) Phase-contrast photomicrograph. (B) Transmission electron micrograph of negatively stained cells. (C) Transmission electron micrograph of polyhydroxyalkanoate-like compounds accumulated in the cells. (D) Ultrathin section showing the Gram-positive cell wall (CW) and the cytoplasmic membrane (CM). Bars, 20 μm (A), 2 μm (B), 500 nm (C), and 100 nm (D).
The biochemical tests indicated that strain AJ110941P showed positive enzymatic activity for naphthol-AS-BI-phosphohydrolase, α-galactosidase, β-glucuronidase, β-glucosidase, α-glucosidase, α-arabinosidase and N-acetyl-β-D-glucosaminidase, whereas negative reactions were obtained for alkaline phosphatase, esterase, lipase, arylamidase, trypsin, chymotrypsin, acid phosphatase, β-galactosidase, α-mannosidase, α-fucosidase, urease, arginine dihydrolase, glutamate decarboxylase, and protease. Catalase reaction was negative. The main end-products of glucose fermentation were short chain fatty acids such as acetate, butyrate, and lactate, and carbon dioxide. The carbon source utilization test revealed that strain AJ110941P was able to utilize the following substrates (all at 20 mM, unless shown otherwise): glucose, lactose, maltose, raffinose, xylose, sucrose, trehalose (0.1%), cellobiose (0.1%), galactose, xylan (5 g l−1), starch (5 g l−1) and melibiose as a sole carbon source. The following substrates (all at 20 mM, unless shown otherwise) were not utilized: arabinose, rhamnose (0.1%), ribose, mannose, fructose, glycerol (5 mM), cellulose (0.1%), pectin (5 g l−1), soytone (5 g l−1), pyruvate, crotonate (10 mM), tryptone (0.1%), casamino acids (0.1%), yeast extract (0.1%), H2/CO2 (80:20, v/v, head space), inositol (10 g l−1), mannitol (0.1%), melezitose (0.1%) and sorbitol (0.1%). The results suggests that strain AJ110941P prefers monosaccharides and polysaccharides as carbon source.
Chemotaxonomic characteristics
Whole-cell fatty acid compositions, the major respiratory quinones, and G + C content of strain AJ110941P were determined. The cellular fatty acid profiles were as follows: C18:1 cis9 (44.3%), C16:0 (22.5%), C18:1 trans9 or cis6 (11.4%), C14:0 (9.8%), C18:0 (8.5%), C17:1 or cycloC17:0 (3.5%). The major respiratory quinone was MK-5 (H4). The G + C content of genomic DNA was 41.1 mol%.
Biofilm formation in strain AJ110941P and relative species
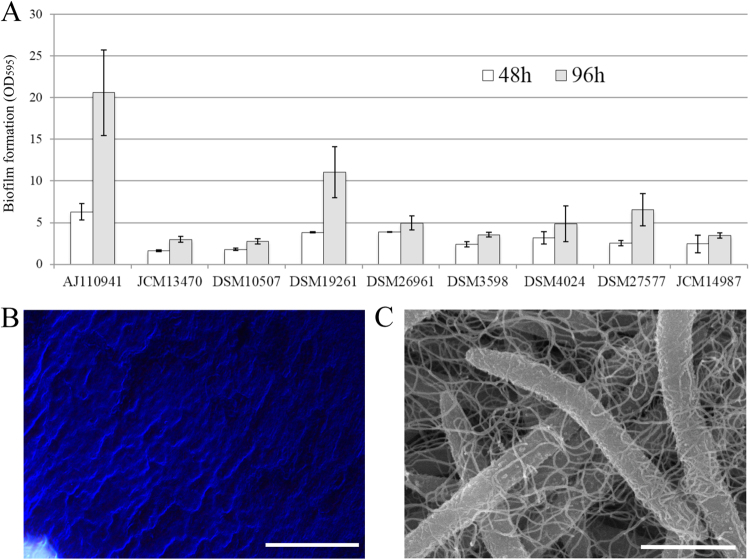
Biofilm-forming capacity of strain AJ110941P was determined by crystal violet (CV) method, and was compared with those of other Lachnospiraceae species. Strain AJ110941P produced the highest amount of biofilm among the all Lachnospiraceae species tested (Fig. 4A). The microscopic analysis further showed that the CV-stained biofilm of strain AJ110941P was rugged and polymerically-coated structure (Fig. 4B). Intriguingly, we also observed that strain AJ110941P notably produced large amounts of extracellular polymeric substances (EPS) by scanning electron microscopy (Fig. 4C). Considering that EPS is widely known to play an important role in bacterial adhesion, both EPS-producing and biofilm-forming capacities of strain AJ110941P might be associated with colonization in the mouse gastrointestinal tracts, though further investigation is required to verify this.
Figure 4.
Comparison of biofilm forming-capacity among Lachnospiraceae strains. (A) Quantification of surface-attached biofilms of the Lachnospiraceae species grown in GAM medium at 37 °C for 48 h (white bars) and 96 h (gray bars), respectively. Biofilms were stained with crystal violet and quantified by measuring at 595 nm. The data represents the average of three biological replicates and the standard deviation is indicated by vertical bars. (B) Phase-contrast photomicrograph of biofilm-like aggregates of strain AJ110941P, after staining with crystal violet. (C) Scanning electron micrograph (SEM) of strain AJ110941P grown in GAM medium at 37 °C for 48 h. Bars, 20 μm (B) and 1.5 μm (C).
Morphological and phenotypic comparisons among strain AJ110941P and its close relatives
Morphological and phenotypic characteristics of AJ110941P were compared with those of the close relatives within the family Lachonospiraceae showing more than 92% 16S rRNA gene sequence similarity to the novel strain (Table 1). The novel strain and the close relatives share several common physiological traits: it is a Gram-positive, mesophilic, anaerobic, and fermentative bacterium with relatively low G + C content. However, strain AJ110941P possesses some unique features that differentiate it from other closely related species. One of the most distinctive features is its cell morphology. Strain AJ110941P shows fusiform-shaped cells with obviously long length (9.0–62.5 μm), while all the other relatives are rod11,21, oval20 or coccus-shaped cells19 (0.5–10.0 μm in length). This new isolate also possesses multiple flagella (Fig. 3B), whereas other close relatives have no or only one terminal flagellum. Strain AJ110941P contains C18:1 cis9 as the most abundant cellular fatty acid, but other relatives have C16:0. Furthermore, it can be distinguished from the other close relatives by its organic substrate utilization pattern. For example, lactose and raffinose can be utilized by only strain AJ110941P, whereas other substrates (e.g., glucose, maltose and sucrose) are commonly utilized. Besides, strain AJ110941P produced large amounts of EPS, and showed the highest biofilm-forming capacity among Lachnospiraceae species tested in the present study (Fig. 4). By these clear distinctive phenotypic features and low 16S rRNA gene sequence similarities of less than 92.5%, strain AJ110941P can be convincingly distinguished from the related genera within the family Lachonospiraceae. On the basis of its morphological, physiological, chemotaxonomic and phylogenetic properties, we propose the name Fusimonas intestini gen. nov., sp. nov. for the mice gut associated strain AJ110941P.
Table 1.
Phenotypic characteristics of strain AJ110941P and its related members in the family Lachnospiraceae.
| Characteristics | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Isolation source | Feces (Mouse) | Feces (Human) | UASB reactor | Clinical sample (Human) | Blood (Human) |
| Cell shape | Fusiform | Coccus | Oval/Rod | Rod | Rod |
| Cell size (μm) | 9.0–62.5×0.55–0.9 | 0.5–1.0 | 0.5–10.0×0.5–1.0 | 2.0–5.0×0.8–1.1 | 3.4–7.3×0.4–0.7 |
| Motility | − | − | + | nd | − |
| Flagella | +(multiple) | nd* | +(one flagellum) | nd | − |
| Spore formation | − | nd | + | + | − |
| Biofilm formation (48h) | 6.28 | 2.47 | nd | 3.84 | 3.88 |
| (OD595) (96h) | 20.58 | 3.46 | nd | 11.45 | 4.96 |
| DNA G+C (mol%) | 41.1 | nd | 32 | nd | 46.0 |
| Growth temperature (°C) | 15–40 | 35–37 | 20–60 | 37 | 15–45 |
| Growth pH | 6.5–8.0 | nd | 6.5–8.0 | nd | nd |
| Major fatty acid | C18:1 cis9 | C16:0 | nd | nd | C16:0 |
| Catalase reaction | − | − | − | nd | + |
| Utilization of: | |||||
| Arabinose | − | − | + | nd | − |
| Cellobiose | + | − | + | − | − |
| Fructose | − | + | + | nd | − |
| Galactose | + | + | − | nd | − |
| Glucose | + | + | + | + | − |
| Glycerol | − | − | + | nd | − |
| Inositol | − | + | + | nd | − |
| Lactose | + | − | − | − | − |
| Maltose | + | − | + | + | − |
| Mannitol | − | + | + | − | − |
| Mannose | − | − | − | + | − |
| Melezitose | − | − | nd | − | − |
| Melibiose | + | − | + | nd | nd |
| Raffinose | + | − | nd | − | − |
| Rhamnose | − | − | − | + | − |
| Ribose | − | + | + | nd | − |
| Starch | + | − | + | − | − |
| Sorbitol | − | + | nd | − | − |
| Sucrose | + | + | + | + | − |
| Trehalose | + | − | nd | + | − |
| Xylose | + | − | + | + | − |
Description of Fusimonas gen. nov
Fusimonas (Fu.si.mo′nas. L. n. fusus spindle; L. fem. n. monas a unit; N.L. fem. n. Fusimonas a spindle-shaped bacterium (unit)).
Cells are Gram-positive, non-motile, non-spore-forming and long fusiform-shaped. The strain is mesophilic and strictly anaerobic. The DNA G + C content is 41.1 mol%. The main fatty acids are C18:1 cis9, C16:0, and C18:1 trans9 or cis6. The major respiratory quinone is MK-5 (H4). The type species of the genus is Fusimanas intestini.
Description of Fusimonas intestini sp. nov
Fusimonas intestini (in.tes.ti′ni. L. gen. n. intestini of the gut).
The species displays the following characteristics in addition to those given in the genus description. Cells are long fusiform-shaped, approximately 9.0–62.5 µm in length and 0.55–0.9 µm in width. Growth occurs at 15–40 °C (optimum temperature at 37 °C), at pH 6.5–8.0 (optimum growth at pH 8.0), and at 0.3–0.5% (w/v) NaCl. In GAM agar, colonies (0.1–1.4 mm in diameter and <0.2 mm height) are brown-color and disk like form. The strain is catalase-negative. Based on testing with the API ZYM, API ID32A and API 20 A system, naphthol-AS-BI-phosphohydrolase, α-galactosidase, β-glucuronidase, β-glucosidase, α-glucosidase, α-arabinosidase and N-acetyl-β-D-glucosaminidase are detected. No activity is found for alkaline phosphatase, esterase, lipase, arylamidase, trypsin, chymotrypsin, acid phosphatase, β-galactosidase, α-mannosidase, α-fucosidase, urease, arginine dihydrolase, glutamate decarboxylase, and protease. The following substrates serve as a sole carbon source for the isolate: cellobiose, glucose, lactose, maltose, raffinose, xylose, sucrose, trehalose, galactose, xylan, starch and melibiose. Arabinose, rhamnose, ribose, mannose, fructose, glycerol, cellulose, pectin, soytone, pyruvate, crotonate, tryptone, casamino acids, yeast extract, H2/CO2, inositol, mannitol, melezitose and sorbitol do not support growth. The end-products of glucose metabolism are common short chain fatty acids and CO2. The cellular fatty acids profile includes C18:1 cis9 (44.3%), C16:0 (22.5%), C18:1 trans9 or cis6 (11.4%), C14:0 (9.8%), C18:0 (8.5%), C17:1 or cycloC17:0 (3.5%).
The type strain is AJ110941P isolated from the feces of five weeks-old hyperglycemic obesity model mouse. Strain AJ110941P has been deposited in the International Patent Organism Depositary (IPOD), National Institute of Technology and Evaluation (NITE) as a patent strain (FEMR BP-11443).
Methods
Cultivation of strain AJ110941P
Strain AJ110941P (P = patent strain) was isolated from the feces of five weeks-old db/db mouse (obesity model mouse) as described previously18. In brief, the cultivation and isolation were performed using Eggerth-Gagnon medium (pH 7.7) with headspace gas of H2/N2/CO2 (5:90:5, v/v/v) at 37 °C under anaerobic conditions28. Since strain AJ110941P was also able to grow in Gifu anaerobic medium (GAM, Nissui Pharmaceutical Co. Ltd, Tokyo, Japan) with headspace gas of N2/CO2 (80:20, v/v), liquid and solid (containing 1.6% agar) GAM media were used for further morphological and physiological characterizations.
Morphological, physiological and biochemical analyses
Cells of strain AJ110941P were observed by phase-contrast microscopy (PROVIS, Olympus, Tokyo, Japan), transmission electron microscopy (H-7000, Hitachi, Tokyo, Japan), and scanning electron microscopy (S-4500, Hitachi) as described previously29,30. Gram staining was performed using a Gram-stain kit (Wako, Tokyo, Japan) according to the manufacturer’s instructions, and stained-cells were observed by microscopy. Temperature range for growth were investigated on GAM liquid cultures incubated at 5, 10, 15, 20, 25, 30, 35, 37, 40, 45, and 50 °C, respectively. The pH ranges were tested at pH 5.0, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, and 10.0. The NaCl concentration range for growth was determined at 0.3, 0.5, 1.0, 1.5, 2.0, and 2.5%. The biochemical features were characterized using API ZYM, API ID32A and API 20 A (BioMerieux, SA, France) as described previously31. Sole carbon source utilization test was performed using the basal medium supplemented with one of 32 different carbon sources as described previously32. The basal medium contained (per liter): KH2PO4, 0.136 g; NaHCO3, 2.52 g; NH4Cl, 0.535 g; MgCl2.6H2H, 0.204 g; CaCl2.2H2O, 0.147 g; Na2S.9H2O, 0.3 g; cysteine·HCl, 0.3 g; trace elements solution32, 1 ml; vitamin solution32, 1 ml; and resazurin solution (1 ml; 1 mg ml−1). All cultures were incubated anaerobically at 37 °C. Growth and substrate utilization were determined by monitoring increase in OD600. Catalase activity was determined using a 3% hydrogen peroxide solution. Main end-products of glucose metabolism were determined by using LC20 HPLC system with a Shim-pack SPR-H column (Shimadzu, Tokyo, Japan) and GC-2014 gas chromatography (Shimadzu) after 8 days of cultivation.
Chemotaxonomic analyses
Chemotaxonomic analyses of strain AJ110941P were performed according to the previously described methods33. Briefly, the genomic DNA G + C content and major respiratory quinones were analyzed using LC10 HPLC system with a Shim-pack CLC-ODS (Shimadzu) and Zorbax SB-C18 (Agilent Technologies Palo Alto, CA, USA), respectively. Cellular fatty acid compositions were determined using a GCMS-QP2010 system (Shimadzu).
Phylogenetic analysis based on 16S rRNA gene
Nearly complete 16S rRNA gene sequence (1469 nt, GenBank accession no. AB861470) of strain AJ110941P was previously determined18. Comparative 16S rRNA gene sequence analysis was performed using BLAST program against the nucleotide collection (nr/nt) database at NCBI. Multiple alignments of the 16S rRNA gene sequences of strain AJ110941P were performed with its closest relatives within family Lachnospiraceae using CLUSTAL W program. The phylogenetic tree was constructed by neighbor-joining method. The percentage nucleotide similarity was calculated by using p-distance available in MEGA software34. Bootstrap values were estimated using neighbour-joining and maximum-likelihood methods (each 1,000 replications).
Biofilm formation assay
We determined the biofilm formation activity for strain AJ110941P and other eight close relatives (type strains) of the family Lachnospiraceae; Anaerostipes caccae JCM 13470 T 22; Blautia hydrogenotrophica DSM 10507 T 35; Clostridium citroniae DSM 19261 T 21; Desulfotomaculum guttoideum DSM 4024 T 24; Eisenbergiella tayi DSM 26961 T 11; Eubacterium fissicatena DSM 3598 T 36; Murimonas intestini DSM 27577 T 15; Ruminococcus gauvreauii JCM 14987 T19. Biofilm formation assay was performed by crystal violet method as described previous study37. In brief, full-grown cultures of strain AJ110941P and other relative spices were inoculated to fresh GAM broth (1% inoculation) in 96-well polystyrene tissue culture plates (Becton Dickinson Labware, Franklin Lakes, NJ, USA) in anaerobic glove box. After static incubation for 48 h and 96 h at 37 °C under anaerobic condition, 20 μl of 1% crystal violet solution (solved in 33% acetic acid; Wako, Osaka, Japan) was added. After static incubation for 30 min at room temperature, all crystal violet solutions were removed and each wells was carefully washed twice with sterilized distilled water. The crystal violet stained substances were solved in 95% ethanol, and measured the absorbance of the crystal violet solution on a SPARK 10 M multimode microplate reader (TECAN, Männedorf, Switzerland) at 595 nm. For microscopic observation, biofilm-like aggregate of strain AJ110941P grown in GAM medium for 96 h was transferred to the glass surface (Matsunami, Osaka, Japan), dried, washed with sterilized distilled water, and stained with crystal violet solution. Phase-contrast image was collected by using an Olympus PROVIS microscope.
Metagenomic database search of 16S rRNA gene sequence of strain AJ110941P
The potential habitability of strain AJ110941P was investigated using IMNGS web platform (https://www.imngs.org/)27, which is the biggest and most detailed 16S rRNA gene amplicon datasets available to date. The current size of the IMNGS database includes 88,579 of 16S rRNA gene amplicon datasets from 96 different environments27. In the present study, we used the 16S rRNA gene sequence of strain AJ110941P (AB861470) as a query sequence, and selected the all 16S rRNA sequences available from the NCBI sequence reads archive (SRA) with 97% sequence similarity threshold. The results of metagenomic database search were categorized based on the level of sequence similarities by BLAST search above 99 and 97% similarities, respectively.
Electronic supplementary material
Acknowledgements
We acknowledge Aya Akiba and Eri Hara for phenotypic analysis and determination of the DNA G + C content, isoprenoid quinone, and cellular fatty acids. We thank Ranko Nishi and Mizuki Kobayashi for their research assistance.
Author Contributions
H.T. conceived and designed the experiments. H.K., X-Y.M., and H.T performed experiments and analyzed data. K.K., H.K., H.T and Y.K. wrote the manuscript. All co-authors contributed to the discussion of the results obtained in this study, and reviewed and edited the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Hiroyuki Kusada and Keishi Kameyama contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-18122-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 3.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridaura VK, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello EK, et al. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank DN, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter J. Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl Environ Microbiol. 2008;74:4985–4996. doi: 10.1128/AEM.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Issa M, Ananthakrishnan AN, Binion DG. Clostridium difficile and inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1432–1442. doi: 10.1002/ibd.20500. [DOI] [PubMed] [Google Scholar]
- 10.Goodman AL, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci USA. 2011;108:6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amir I, Bouvet P, Legeay C, Gophna U, Weinberger A. Eisenbergiella tayi gen. nov., sp. nov., isolated from human blood. Int J Syst Evol Microbiol. 2014;64:907–914. doi: 10.1099/ijs.0.057331-0. [DOI] [PubMed] [Google Scholar]
- 12.Takada T, Kurakawa T, Tsuji H, Nomoto K. Fusicatenibacter saccharivorans gen. nov., sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2013;63:3691–3696. doi: 10.1099/ijs.0.045823-0. [DOI] [PubMed] [Google Scholar]
- 13.Koeck, D. E. et al. Herbinix hemicellulosilytica, gen. nov., sp. nov., a thermophilic cellulose-degrading bacterium isolated from a thermophilic biogas reactor. Int J Syst Evol Microbiol (2015). [DOI] [PubMed]
- 14.Podosokorskaya OA, et al. Mobilitalea sibirica gen. nov., sp. nov., a halotolerant polysaccharide-degrading bacterium from western siberia, Russia. Int J Syst Evol Microbiol. 2014;64:2657–2661. doi: 10.1099/ijs.0.057109-0. [DOI] [PubMed] [Google Scholar]
- 15.Klaring K, et al. Murimonas intestini gen. nov., sp. nov., an acetate-producing bacterium of the family Lachnospiraceae isolated from the mouse gut. Int J Syst Evol Microbiol. 2015;65:870–878. doi: 10.1099/ijs.0.000030. [DOI] [PubMed] [Google Scholar]
- 16.Patil Y, Junghare M, Pester M, Muller N, Schink B. Anaerobium acetethylicum gen. nov., sp. nov., a strictly anaerobic, gluconate-fermenting bacterium isolated from a methanogenic bioreactor. Int J Syst Evol Microbiol. 2015;65:3289–3296. doi: 10.1099/ijsem.0.000410. [DOI] [PubMed] [Google Scholar]
- 17.Qin J, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 18.Kameyama K, Itoh K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014;29:427–430. doi: 10.1264/jsme2.ME14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domingo MC, et al. Ruminococcus gauvreauii sp. nov., a glycopeptide-resistant species isolated from a human faecal specimen. Int J Syst Evol Microbiol. 2008;58:1393–1397. doi: 10.1099/ijs.0.65259-0. [DOI] [PubMed] [Google Scholar]
- 20.Parshina SN, et al. Soehngenia saccharolytica gen. nov., sp. nov. and Clostridium amygdalinum sp. nov., two novel anaerobic, benzaldehyde-converting bacteria. Int J Syst Evol Microbiol. 2003;53:1791–1799. doi: 10.1099/ijs.0.02668-0. [DOI] [PubMed] [Google Scholar]
- 21.Warren YA, Tyrrell KL, Citron DM, Goldstein EJ. Clostridium aldenense sp. nov. and Clostridium citroniae sp. nov. isolated from human clinical infections. J Clin Microbiol. 2006;44:2416–2422. doi: 10.1128/JCM.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwiertz A, et al. Anaerostipes caccae gen. nov., sp. nov., a new saccharolytic, acetate-utilising, butyrate-producing bacterium from human faeces. Syst Appl Microbiol. 2002;25:46–51. doi: 10.1078/0723-2020-00096. [DOI] [PubMed] [Google Scholar]
- 23.Domingo MC, et al. Clostridium lavalense sp. nov., a glycopeptide-resistant species isolated from human faeces. Int J Syst Evol Microbiol. 2009;59:498–503. doi: 10.1099/ijs.0.001958-0. [DOI] [PubMed] [Google Scholar]
- 24.Stackebrandt E, et al. Phylogenetic analysis of the genus Desulfotomaculum: evidence for the misclassification of Desulfotomaculum guttoideum and description of Desulfotomaculum orientis as Desulfosporosinus orientis gen. nov., comb. nov. Int J Syst Bacteriol. 1997;47:1134–1139. doi: 10.1099/00207713-47-4-1134. [DOI] [PubMed] [Google Scholar]
- 25.Smith P, et al. Host genetics and environmental factors regulate ecological succession of the mouse colon tissue-associated microbiota. PLoS One. 2012;7:e30273. doi: 10.1371/journal.pone.0030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stecher B, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagkouvardos I, et al. IMNGS: A comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci Rep. 2016;6:33721. doi: 10.1038/srep33721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh K, Mitsuoka T. Characterization of clostridia isolated from faeces of limited flora mice and their effect on caecal size when associated with germ-free mice. Lab Anim. 1985;19:111–118. doi: 10.1258/002367785780942589. [DOI] [PubMed] [Google Scholar]
- 29.Tamaki H, et al. Armatimonas rosea gen. nov., sp. nov., of a novel bacterial phylum, Armatimonadetes phyl. nov., formally called the candidate phylum OP10. Int J Syst Evol Microbiol. 2011;61:1442–1447. doi: 10.1099/ijs.0.025643-0. [DOI] [PubMed] [Google Scholar]
- 30.Sekiguchi Y, Kamagata Y, Nakamura K, Ohashi A, Harada H. Fluorescence in situ hybridization using 16S rRNA-targeted oligonucleotides reveals localization of methanogens and selected uncultured bacteria in mesophilic and thermophilic sludge granules. Appl Environ Microbiol. 1999;65:1280–1288. doi: 10.1128/aem.65.3.1280-1288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamaki H, et al. Flavobacterium limicola sp. nov., a psychrophilic, organic-polymer-degrading bacterium isolated from freshwater sediments. Int J Syst Evol Microbiol. 2003;53:519–526. doi: 10.1099/ijs.0.02369-0. [DOI] [PubMed] [Google Scholar]
- 32.Sekiguchi Y, Kamagata Y, Nakamura K, Ohashi A, Harada H. Syntrophothermus lipocalidus gen. nov., sp. nov., a novel thermophilic, syntrophic, fatty-acid-oxidizing anaerobe which utilizes isobutyrate. Int J Syst Evol Microbiol. 2000;50:771–779. doi: 10.1099/00207713-50-2-771. [DOI] [PubMed] [Google Scholar]
- 33.Hanada S, Takaichi S, Matsuura K, Nakamura K. Roseiflexus castenholzii gen. nov., sp. nov., a thermophilic, filamentous, photosynthetic bacterium that lacks chlorosomes. Int J Syst Evol Microbiol. 2002;52:187–193. doi: 10.1099/00207713-52-1-187. [DOI] [PubMed] [Google Scholar]
- 34.Tamura K, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C, Finegold SM, Song Y, Lawson PA. Reclassification of Clostridium coccoides, Ruminococcus hansenii, Ruminococcus hydrogenotrophicus, Ruminococcus luti, Ruminococcus productus and Ruminococcus schinkii as Blautia coccoides gen. nov., comb. nov., Blautia hansenii comb. nov., Blautia hydrogenotrophica comb. nov., Blautia luti comb. nov., Blautia producta comb. nov., Blautia schinkii comb. nov. and description of Blautia wexlerae sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2008;58:1896–1902. doi: 10.1099/ijs.0.65208-0. [DOI] [PubMed] [Google Scholar]
- 36.Taylor MM. Eubacterium fissicatena sp.nov. isolated from the alimentary tract of the goat. J Gen Microbiol. 1972;71:457–463. doi: 10.1099/00221287-71-3-457. [DOI] [PubMed] [Google Scholar]
- 37.Kusada, H., Hanada, S., Kamagata, Y. & Kimura, N. The effects of N-acylhomoserine lactones, beta-lactam antibiotics and adenosine on biofilm formation in the multi-beta-lactam antibiotic-resistant bacterium Acidovorax sp. strain MR-S7. J Biosci Bioeng 118, 14–19, 10.1016/j.jbiosc.2013.12.012 (2014). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.