Introduction
Key Teaching Points.
-
•
Cardiac magnetic resonance imaging with upper abdominal slices helps in understanding of the anatomy in order to better guide the pericardial puncture.
-
•
Ipsilateral operator positioning in relation to the direction of the epicardial puncture may make puncturing and mapping uncomfortable for the operator; therefore, contralateral operator positioning may be helpful during epicardial ablation in situs inversus totalis.
-
•
Inverting the image in fluoroscopy “normalizes” the visualization of anatomy in situs inversus totalis, but the operator must pay attention to make movements in a “mirror fashion” because movements seen in fluoroscopy correspond to opposite-order ones.
-
•
Epicardial ventricular tachycardia mapping and ablation can be performed effectively and safely in situs inversus totalis patients.
Cardiac situs is determined by atrial location. In situs inversus, the morphologic right atrium is on the left and the morphologic left atrium is on the right. When the pulmonary anatomy is reversed, the liver and gallbladder are located on the left, whereas the spleen and stomach are located on the right. The remaining internal structures are also a mirror image of the normal anatomy.1 Situs inversus is a rare condition, and when it is associated with dextrocardia it is known as situs inversus totalis.
Sustained ventricular tachycardia (VT) is one of the most common mechanisms of sudden death in chagasic patients. VT circuits have been reported in the epicardial myocardium in up to 70% of chagasic patients,2 and simultaneous epicardial and endocardial mapping and ablation have been successfully performed in these patients.3 It is important for the clinician to have anatomic knowledge for procedural efficacy and safety.
We report our strategy for the management of a patient with situs inversus totalis and chagasic cardiomyopathy with syncope due to sustained VT. Our patient underwent epicardial catheter ablation.
Case report
A 36-year-old woman with Chagas disease was referred to our institution for treatment of recurrent VT and syncope, despite having undergone amiodarone therapy. We performed a 3T cardiac magnetic resonance imaging (MRI) to delineate her scar, followed by electroanatomic mapping with the CARTO 3 system (Biosense Webster, Diamond Bar, CA). An irrigated 3.5-mm catheter (Thermocool SmartTouch, Biosense Webster, Diamond Bar, CA) was utilized to acquire voltage and activation mapping of the epicardial and endocardial ventricular myocardium. Cardiac MRI with upper abdominal slices (Figure 1A and B) was also used to guide the pericardial puncture, which was then performed with a posterior approach. Briefly, a 16G Tuohy needle was inserted in the xyphoid space and directed towards the right shoulder, and a 0.032-mm guide wire was inserted through the needle to confirm entry to the pericardial space (Figure 1C and D). Epicardial substrate mapping was taken in sinus rhythm, outlining the scar with the aid of a deflectable sheath (Agilis, St Jude Medical, St Paul, MN), and high-density mapping of the scar was performed (Figure 2A). We located the late potential (LP) area within the scar by manually adjusting all LPs recorded after the latest surface QRS complex (Figure 2B and C). Additionally, we adjusted the voltage threshold, and an intermediate voltage channel corresponding to the LP area was evidenced (Figure 3D). The phrenic nerve was mapped with high-output pacing and properly tagged. Ventricular endocardial bipolar mapping was then performed using an aortic retrograde approach, showing normal voltage tissue (Figure 3A and B). We performed a coronary artery angiogram in order to avoid coronary injury during ablation and subsequently placed the ablation catheter into the pericardial space close to the LP area (Figure 2E). Activation mapping during inducible VT revealed presystolic potential in this area, and radiofrequency delivery interrupted VT after 7 seconds (Figure 2). We performed substrate modification aimed at eliminating all LPs. After that, high-energy pacing over the scar was taken and new pulses of radiofrequency were applied until no more ventricular tissue was stimulated (Figure 3E). We repeated the programmed ventricular stimulation and VT was no longer inducible. Forty-five days after ablation a cardiac defibrillator was implanted, and during a 4-month follow-up period there was no evidence of sustained VT.
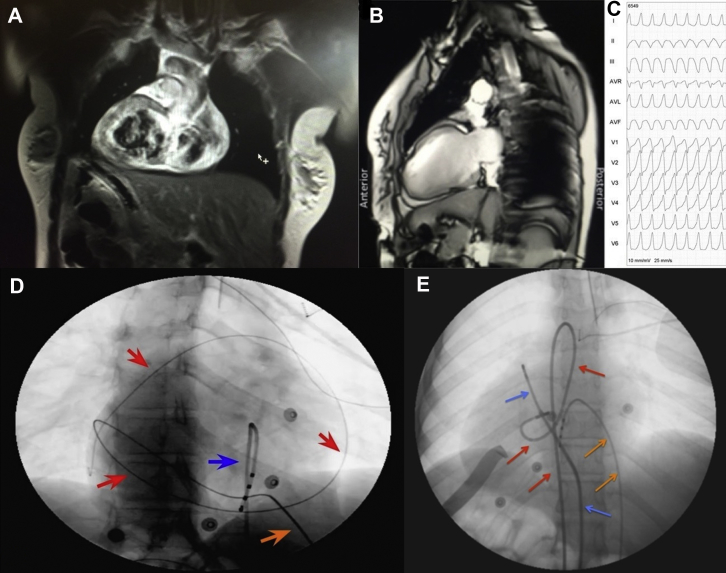
Figure 1.
Magnetic resonance images (MRI) and pericardial puncture. A: Frontal axis and B: lateral views of a cardiac MRI display the situs inversus, dextrocardia, apical left ventricle pointing to the right, and the inverted position of the liver and its relation to the heart. C: Electrocardiogram shows the morphology of ventricular tachycardia recorded with right precordial lead position. D: A 45° right anterior oblique view showing a guidewire in the pericardial space encircling the cardiac silhouette (red arrows) through a 16G Tuohy needle (orange arrow). A quadripolar catheter (blue arrow) is positioned in the apex of the right ventricle as a guide for puncturing. E: A 0° anterior-posterior view displaying the catheters positioned in the epicardial space (blue arrows: ablation catheter in the epicardial space through the epicardial sheath), a quadripolar catheter in the right ventricle through the inferior vena cava (orange arrows), and a second ablation catheter in the left ventricular outflow tract through the aortic root (red arrows). The precordial electrocardiogram leads are also positioned in the right aspect (V1 to V5).
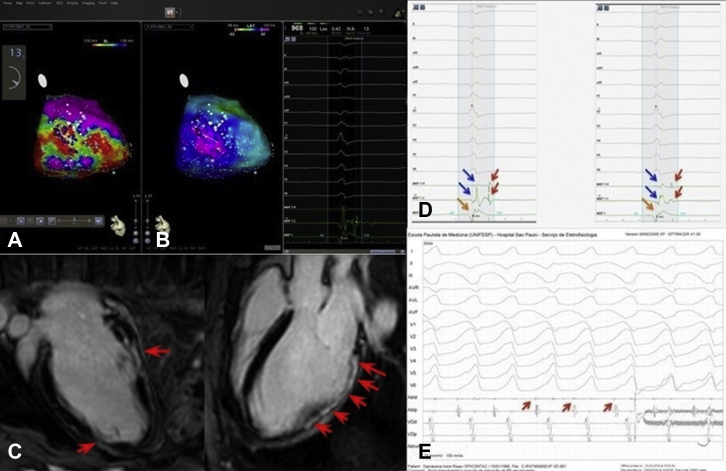
Figure 2.
Delineation of epicardial arrhythmogenic substrate. A: A low-voltage area below 1.5 mV is mapped in the posterolateral region of the left ventricle. B: Late potential area is demonstrated by activation mapping, as described in the text. C: A modified 3-chamber magnetic resonance imaging view of the left ventricle shows extensive epimesocardial scar in delayed enhancement (red arrows) that is related to a bipolar mapping viewed in A. D: Two examples of late potential (red arrows) recorded with the mapping catheter (MAP 1-2 and 3-4) placed within the patient's scar. Blue arrows are the far-field signal, and orange arrows point to the unipolar signal (MAP 1). E: Presystolic potential is recorded in the mapping catheter before the radiofrequency delivery (red arrow) that terminated the ventricular tachycardia.
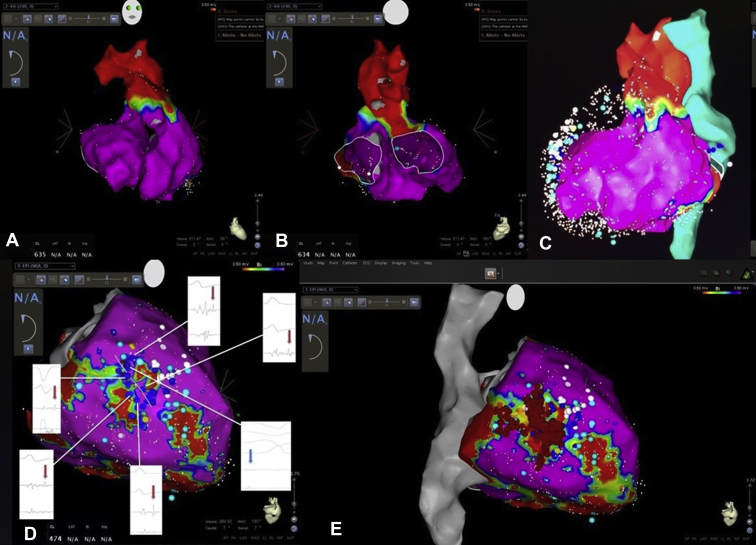
Figure 3.
Substrate mapping and ablation. A: Anteroposterior view and B: posteroanterior (PA) view of the right and left ventricular endocardium, displaying normal tissue voltage. C: Left anterior oblique view of endocardial voltage mapping, including the pulmonary artery, aortic root (in red), and inferior and superior vena cava (in green), displaying a normal myocardial voltage. The epicardial surface is shadowed by a mesh tool added to white and blue floating dots. D: PA view of the epicardium with a voltage threshold reduced to 0.5-0.6 mV, revealing an intermediate voltage channel within the scar that showed late potential (LP), shown in dark blue dots in the voltage mapping. The near-field electrogram is indicated with red arrows in detail. White dots indicate phrenic nerve capture during high-energy output pacing. E: PA view of ablation points (red dots) in the channel within the patient's scar. After interruption of ventricular tachycardia during radiofrequency delivery, all LP signals were eliminated. After that, high-output stimulation was performed, and additional lesions were delivered in regions where any QRS was captured. The superior and inferior vena cava are represented in gray, and the aortic root and pulmonary artery are represented in red.
Discussion
Epicardial ablation of VT in a patient with situs inversus totalis is challenging for several reasons: (1) it is difficult to localize clinical VT on surface electrocardiogram because, as in this patient, electrocardiogram surface electrodes are usually placed in the left thorax, making it difficult for the clinician to understand VT depolarization wavefronts; (2) the anatomy must be well known before epicardial puncture in order to avoid injury to the abdominal viscera, especially the liver; (3) manipulating catheter ablation using the electroanatomic system as a guide requires attention, because movements must be performed in the opposite direction of what would normally be done; and (4) ipsilateral operator positioning in relation to the direction of the epicardial puncture may make puncturing and mapping uncomfortable for the operator; therefore, contralateral operator positioning may be helpful. Image inversion may attenuate difficulties related to anatomy distortions when using fluoroscopy, but imaging inversion is not available in the CARTO system. In our case inverting fluoroscopy images facilitated the anatomic understanding of the heart, but manipulating the catheter required attention because the movements seen in fluoroscopy corresponded to opposite-order ones. Pericardial puncture was previously planned based on cardiac MRI with upper abdominal slices and was guided by fluoroscopy and by using a quadripolar catheter in the right ventricular apex as a landmark. In the initial stage of the puncture the Touhy needle was positioned so as to tangentiate the posterior portion of the rib edge and then, after 2 cm of insertion, directed posterior toward the right shoulder. We believe that this strategy reduced the risk of injury to the internal thoracic artery (shallow initial puncture) and allowed a posterior access to the epicardial space (area of interest for ablation). As the posterior wall of the left ventricle was the region of interest, an aortic retrograde approach was chosen for mapping the endocardial surface, since this technique allows a better mapping of this region. As discussed earlier, inverting the image in fluoroscopy “normalized” the visualization of anatomy, but the operator had to pay attention because movements were displayed in a “mirror fashion” in fluoroscopy. A cardiac defibrillator was implanted 45 days after ablation because this patient was enrolled in a study protocol that required an MRI 45 days after the procedure.
Conclusion
Despite unusual patient anatomy and arrangement of catheters, epicardial VT mapping and ablation could be performed effectively and safely.
Footnotes
This work was supported by Biosense Webster and FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo).
References
- 1.Winer-Muram H.T. Adult presentation of heterotaxic syndromes and related complexes. J Thorac Imaging. 1995;10:43–57. doi: 10.1097/00005382-199501010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Sosa E., Scanavacca M., D'Avila A., Piccioni J., Sanchez O., Velarde J.L., Silva M., Reolão B. Endocardial and epicardial ablation guided by nonsurgical transthoracic epicardial mapping to treat recurrent ventricular tachycardia. J Cardiovasc Electrophysiol. 1998;9:229–239. doi: 10.1111/j.1540-8167.1998.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 3.Henz B.D., Do Nascimento T.A., Dietrich C. de O., Dalegrave C., Hernandes V., Mesas C.E., Leite L.R., Cirenza C., Asirvatham S.J., de Paola A.A. Simultaneous epicardial and endocardial substrate mapping and radiofrequency catheter ablation as first-line treatment for ventricular tachycardia and frequent ICD shocks in chronic chagasic cardiomyopathy. J Interv Card Electrophysiol. 2009;26:195–205. doi: 10.1007/s10840-009-9433-4. [DOI] [PubMed] [Google Scholar]