Key Teaching Points.
-
•
KCND3 duplication is a molecular explanation to early repolarization syndrome.
-
•
Routine use of next-generation sequencing (NGS) methods in diagnosis, allowing simultaneous detection of copy number variations (CNV), single-nucleotide variations, and short indels is a real improvement in medical care.
-
•
Before the NGS era, methods to highlight CNV were rarely performed and CNV were often missed. Their causative roles in human disease were underestimated.
Introduction
We report the case of a patient presenting with nonfatal cardiac arrest. Serial electrocardiograms (ECGs) revealed intermittent early repolarization pattern (ERP). Genetic testing demonstrated the presence of a KCND3 duplication in the patient and his 2-year-old daughter, in whom ECG also displayed evident ERP. The genetic basis and mechanisms underlying early repolarization syndrome are discussed.
Case report
A previously healthy 26-year-old man with no familial history of sudden cardiac death was admitted owing to a cardiac arrest that occurred a few hours after playing tennis. Sinus rhythm was restored after 1 external 200-J shock and the patient had an uneventful neurological recovery.
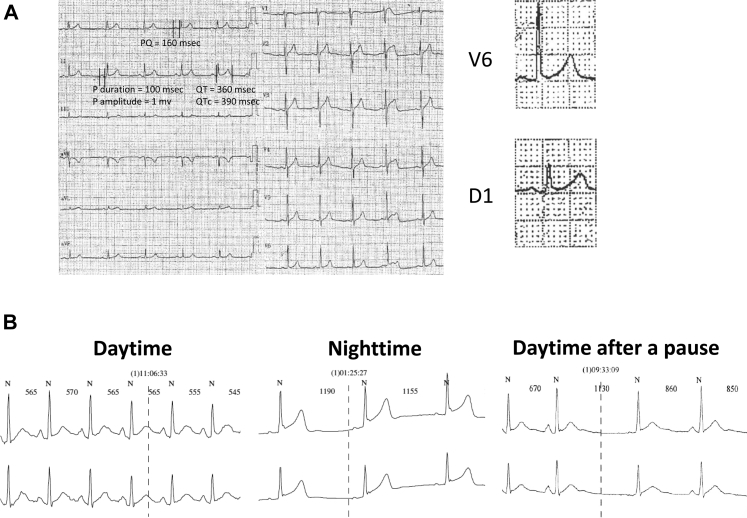
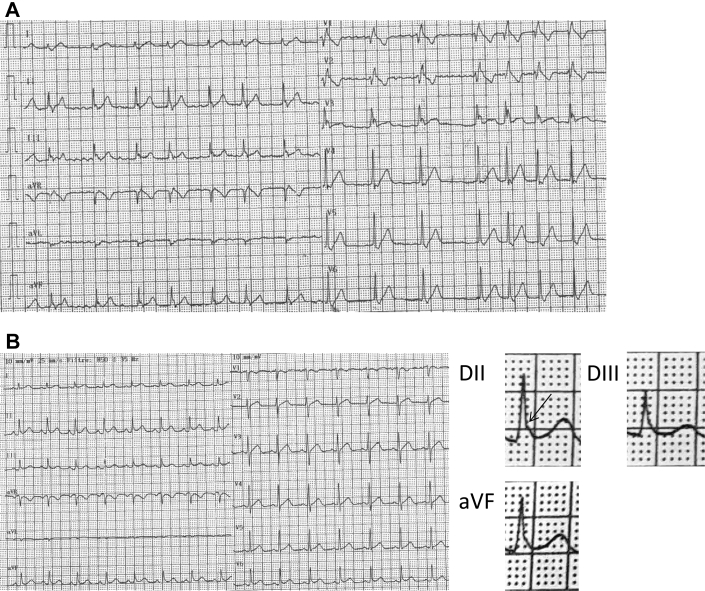
On admission, 12-lead ECG revealed sinus rhythm with 0.8 mm ST-segment elevation in lateral leads (Figure 1A). There was no Brugada ECG pattern after placing leads V1 and V2 at the second intercostal spaces. Twenty-four-hour Holter monitoring showed an increase in the ERP during nighttime with a maximal J-point elevation of 3 mm (Figure 1B). During daytime, J-point elevation did not exceed 2 mm and pause-dependent augmentation of the J wave was either absent or moderate (Figure 1B). Transthoracic echocardiography and cardiac magnetic resonance imaging excluded structural cardiomyopathy. Specifically, cardiac magnetic resonance imaging demonstrated the absence of myocardial fibrosis or right ventricular dysfunction, enlargement, or dyskinesia. Treadmill stress test did not induce ventricular arrhythmias or cardiac ischemia. An implantable cardioverter-defibrillator (Ilesto VR DX, Biotronik, Berlin, Germany) was implanted. One year after implantable cardioverter-defibrillator implantation, during nighttime, the patient experienced an electrical storm with 3 ventricular fibrillation episodes converted to sinus rhythm after 3 consecutive 31-J shocks. The initial ECG demonstrated atrial fibrillation and complete right bundle branch block with a heart rate of 88 beats/minute and an ERP with descending ST segment in inferior leads and lead V3 (Figure 2A). A few hours after the electrical storm, ECG showed sinus rhythm with narrow QRS complexes and J-point elevation of 2.5 mm in inferior leads with minimal (<1 mm) ST elevation (Figure 2B). Thereafter, hydroquinidine hydrochloride was started at a daily dose of 600 mg and no ventricular arrhythmias recurred over 18-month follow-up.
Figure 1.
A: After his first out-of-hospital cardiac arrest, the patient's electrocardiogram showed sinus rhythm and minimal J ST-segment elevation in lateral leads. B: Twenty-four-hour Holter recording demonstrated augmentation of the early repolarization pattern during sleep. During daytime, after a pause, there was no augmentation of the early repolarization pattern.
Figure 2.
A: Electrocardiogram performed a couple of hours after an electrical storm revealed atrial fibrillation, complete right bundle branch block, and J-point elevation with descending ST segment in inferior and V3 leads. Note that the T wave is positive in lead V3. B: A few hours after hospital admission, atrial fibrillation converted spontaneously to sinus rhythm and early repolarization pattern was evident only in inferolateral leads.
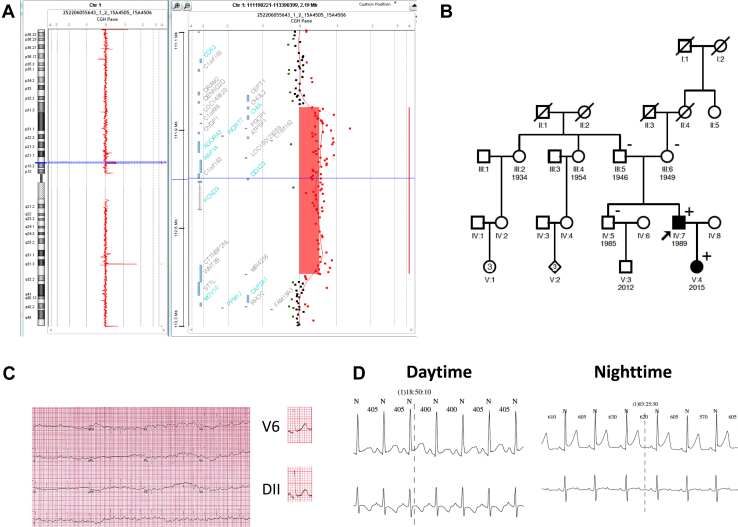
His asymptomatic 2-year-old daughter's ECG demonstrated 1 mm J-point elevation in the inferolateral leads (Figure 3A). Ambulatory ECG monitoring showed that during nighttime early repolarization increased to 2.5 mm (Figure 3B). Electrocardiograms of his parents and his 31-year-old brother did not show an ERP. Phenotypic characteristics of the patient and his first-degree relatives are summarized in Table S1.
Figure 3.
A: Array comparative genomic hybridization analysis (Agilent 180k microarray) showing the 1.23-Mb duplication in chromosomal region 1p13.3p13.2 (chr 1: 111,772,409_113,005,539 bp (hg19)) in proband's DNA compared to 2 control DNAs, in a mirror view. Left panel: blue line indicating the 12p11.21 position of the deletion on the chromosome whole view. Right panel: detailed view of the duplicated region. B: Familial pedigree of the patient with a neo-duplication in the Kv4.3 α subunit of the human cardiac fast transient outward K+ channel (KCND3). The patient's 2-year-old daughter also carries the duplication of KCND3. (+) represents gene-positive patients and black-filled symbol represents phenotype-positive patients. C: Twelve-lead electrocardiogram of the patient's daughter showing mild early repolarization pattern. D: Twenty-four-hour Holter monitoring showed augmentation of early repolarization pattern during nighttime compared to daytime.
To identify a molecular explanation for the early repolarization syndrome observed in the family, genomic DNA samples were tested by next-generation sequencing (NGS) using a custom design based on a SeqCap EZ Solution-Based Enrichment strategy (Roche NimbleGen, Madison, WI), as previously described.1 The panel was designed to identify disease-causing mutations in 48 arrhythmia syndrome–causing genes (Table S2). Target regions included coding exons (with a 30-bp padding) and 5′ and 3′ untranslated regions. Identified putative mutations were further verified using either Sanger sequencing for single-nucleotide variations and short indels, or array comparative genomic hybridization methodology or quantitative polymerase chain reaction (PCR) for copy number variation (CNV). Among genomic variants identified for the proband, only a KCND3 duplication could be considered a disease-causing mutation in the tested genes. According to American College of Medical Genetics and Genomics and Association of Medical Pathologists guidelines, no additional pathogenic or likely pathogenic gene variants were detected.2 This CNV was further confirmed by array comparative genomic hybridization and quantitative PCR. The molecular analysis led to detection of a 1.23-Mb duplication in chromosomal region 1p13.3p13.2 (chr 1: 111,772,409_113,005,539 bp (hg19); Figure 3C). This region contains pseudogenes, noncoding RNA genes, and also 12 protein-coding genes referenced in the Online Mendelian Inheritance in Man (OMIM) catalog. Among these 12 genes, only KCND3 duplication seems clinically relevant. Further segregation analysis was performed to definitely validate its pathogenicity in the proband and in his family. The presence of this duplication was tested on all available family members. As shown on the pedigree (Figure 3D), neither of the proband's parents carried the duplication. Only the patient's daughter carries this de novo CNV. The paternity was confirmed by microsatellite analysis using the AmpFℓSTR Identifiler PCR Amplification Kit (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions (data not shown).
Discussion
A total KCND3 duplication was identified in a patient with nonfatal cardiac arrest and an intermittent ECG ERP.
ERP is an electrocardiographic abnormality defined by an elevation of the QRS-ST junction of at least 0.1 mV from baseline in 2 contiguous leads, predisposing to sudden cardiac death.3 In our patient early repolarization was intermittent, making the diagnosis difficult. Even if 24-hour Holter showed ERP, a definite diagnosis based on 12-lead ECG was made only after an electrical storm occurred. Such dynamicity on the ERP has already been reported and should prompt clinicians to repeat ECG and perform 24-hour Holter ECG in order to detect intermittent elevation of the J point.4 As J-point elevation was present in inferior leads and in lead V3, we considered the possibility of an overlap syndrome between early repolarization and Brugada syndrome.5 However, in lead V3 there was no ST-segment elevation and the T wave was positive, pleading against Brugada syndrome. An ajmaline challenge would have been useful, since it has opposite effects on Brugada syndrome and early repolarization ECG patterns.6 Given the risk of provoking ventricular arrhythmias, the patient declined the test.
Because ventricular fibrillation occurred after exercise and during nighttime, vagal tone appears to have facilitated ventricular arrhythmia in the present patient.7 It has been proposed that vagally induced bradycardia, rather that vagal tone per se, modulates arrhythmia risk in early repolarization.4 Yet, 24-hour Holter analysis showed an increase in ERP during nighttime but not after cardiac pauses that occurred during daytime (Figure 1B). Furthermore, ERP was not increased after a long diastolic interval while the patient's ECG displayed atrial fibrillation (Figure 2A). This strongly suggests that vagal tone, rather than heart rate per se, modulates early repolarization. The significance of atrial fibrillation incidence after an electrical storm is unclear. At first, we considered that an internal shock induced atrial fibrillation. However, it remains possible that the KCND3 duplication promoted atrial fibrillation. Indeed, the resulting increase in Ito current in atrial myocytes might induce dispersion of repolarization, which, in association with high vagal tone, might promote atrial fibrillation.
Despite advances in molecular, cellular, and genetic understanding, the pathophysiologic mechanisms underlying ERP are not yet fully understood. Experimentally the inscription of the J wave is caused by transmural differences in the early phases of the cardiac action potential and factors that increase the net repolarizing current increase the magnitude of the J-point elevation. To date, 7 genes were reported as associated with early repolarization syndrome: KCNJ8 encoding for the pore-forming subunit of the IK-ATP channel, the cardiac L-type calcium channels (CACNA1C, CACNB2, and CACNA2D1), genes encoding for sodium channel (SCN5A and SCN10A), and ABCC9 encoding for the binding cassette transporter of IK-ATP (SUR2A).8 The voltage-gated cardiac fast transient outward K+ current (ItoF) plays a predominant role in determining the initial repolarization phase of the action potential in cardiac myocytes.9 The cardiac channel that underlies ItoF is composed by 2 different subunits: the α subunit, so called “Kv4.3,” encoded by the KCND3 gene; and the β subunit, so called “Kv channel-interacting protein 2” or KChIP2, encoded by the KCNIP2 gene in 4:4 complexes.10 Gain-of-function mutations in the KCND3 potassium channel were previously reported in Brugada syndrome patients and loss-of-function mutations were associated with early-onset cerebellar ataxia, intellectual disability, oral apraxia, and epilepsy. Moreover, in a meta-analysis of genome-wide association studies of the ERP, an interesting locus on chromosome 1 intronic to the KCND3 gene was found.11 Finally, mutations in this gene are rarely associated with long QT syndrome, in which late phase of repolarization is crucial.12 According to these comments, for the first time our study suggests the association between KCND3 duplication and early repolarization syndrome.
Experimental evidence has demonstrated that transmural gradient in Ito participates in J-wave inscription.13 The role of Ito in J-point syndrome pathophysiology is further supported by the effectiveness of the Ito-blocker quinine to prevent ventricular arrhythmia episodes in patients with ERP or Brugada syndrome.14 We did not perform experiments to study the changes in Ito current resulting from KCND3 duplication, but it seems to us reasonable to speculate that the KCND3 duplication would probably result in an increase in transmural dispersion of Ito, therefore facilitating ventricular arrhythmias that occurred in our patient. The causative role of the KCND3 duplication in promoting the ERP is further supported by the available segregation analysis that shows that the patient's daughter carrying the KCND3 duplication manifests early repolarization ECG pattern (Figure 3).
This study highlights the medical benefit of genetic exploration pipelines using NGS that are able to detect both point mutations and CNV with a unique workflow. Before the NGS era, methods used to study the human genome and to detect mutations in patients, such as Sanger sequencing or screening methods (such as dHPLC or High Resolution Melting analysis), were not able to detect CNV. Alternative tedious and expensive methods, such as multiplex ligation-dependent probe amplification assay or quantitative PCR, were needed to highlight them. Because of their cost, such methods were not routinely performed in diagnostic laboratories, and CNV were often missed. Routine use of NGS methods in diagnosis, allowing simultaneous detection of CNV, single-nucleotide variations, and short indels, is a real improvement in medical care and may increase the percentage of patients with a positive molecular diagnosis. Finally, we believe that NGS methods in association with segregation studies will allow identification of novel genetic variants responsible for arrhythmic syndromes.
Conclusion
This is the first case of KCND3 duplication in a patient with early repolarization syndrome. This finding reinforces the role of Ito in the physiopathology of malignant early repolarization syndrome.
Acknowledgments
Drs Samuel Chauveau and Alexandre Janin contributed equally to this article.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2017.08.003.
Contributor Information
Philippe Chevalier, Email: philippe.chevalier@chu-lyon.fr.
Gilles Millat, Email: gilles.millat@chu-lyon.fr.
Appendix. Supplementary data
References
- 1.Chanavat V., Janin A., Millat G. A fast and cost-effective molecular diagnostic tool for genetic diseases involved in sudden cardiac death. Clin Chim Acta. 2016;453:80–85. doi: 10.1016/j.cca.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med Off J Am Coll Med Genet. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haïssaguerre M., Derval N., Sacher F. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 4.Aizawa Y., Sato A., Watanabe H. Dynamicity of the J-wave in idiopathic ventricular fibrillation with a special reference to pause-dependent augmentation of the J-wave. J Am Coll Cardiol. 2012;59:1948–1953. doi: 10.1016/j.jacc.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Kawata H., Morita H., Yamada Y. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: a novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm. 2013;10:1161–1168. doi: 10.1016/j.hrthm.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Roten L., Derval N., Sacher F. Ajmaline attenuates electrocardiogram characteristics of inferolateral early repolarization. Heart Rhythm. 2012;9:232–239. doi: 10.1016/j.hrthm.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Aizawa Y., Sato M., Ohno S., Horie M., Takatsuki S., Fukuda K., Chinushi M., Usui T., Aonuma K., Hosaka Y., Haïssaguerre M., Aizawa Y. Circadian pattern of fibrillatory events in non-Brugada-type idiopathic ventricular fibrillation with a focus on J waves. Heart Rhythm. 2014;11:2261–2266. doi: 10.1016/j.hrthm.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Antzelevitch C., Yan G.-X., Ackerman M.J. J-wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. J Arrhythm. 2016;32:315–339. doi: 10.1016/j.joa.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenstein J.L., Wu R., Po S., Tomaselli G.F., Winslow R.L. Role of the calcium-independent transient outward current I(to1) in shaping action potential morphology and duration. Circ Res. 2000;87:1026–1033. doi: 10.1161/01.res.87.11.1026. [DOI] [PubMed] [Google Scholar]
- 10.Deschênes I., DiSilvestre D., Juang G.J., Wu R.C., An W.F., Tomaselli G.F. Regulation of Kv4.3 current by KChIP2 splice variants: a component of native cardiac I(to)? Circulation. 2002;106:423–429. doi: 10.1161/01.cir.0000025417.65658.b6. [DOI] [PubMed] [Google Scholar]
- 11.Sinner M.F., Porthan K., Noseworthy P.A. A meta-analysis of genome-wide association studies of the electrocardiographic early repolarization pattern. Heart Rhythm. 2012;9:1627–1634. doi: 10.1016/j.hrthm.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delpón E., Cordeiro J.M., Núñez L. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008;1:209–218. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan G.X., Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 14.Haïssaguerre M., Sacher F., Nogami A. Characteristics of recurrent ventricular fibrillation associated with inferolateral early repolarization role of drug therapy. J Am Coll Cardiol. 2009;53:612–619. doi: 10.1016/j.jacc.2008.10.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.