Abstract
Background
Melatonin therapy shows positive effects on neuroprotective factor brain-derived neurotrophic factor (BDNF) expression and neuronal apoptosis in neonatal hemolytic hyperbilirubinemia. We hypothesized that melatonin promotes BDNF expression and anti-apoptotic effects in neonatal hemolytic hyperbilirubinemia through a phospholipase (PLC)-mediated mechanism.
Material/Methods
A phenylhydrazine hydrochloride (PHZ)-induced neonatal hemolytic hyperbilirubinemia model was constructed in neonatal rats. Four experimental groups – a control group (n=30), a PHZ group (n=30), a PHZ + melatonin group (n=30), and a PHZ + melatonin+U73122 (a PLC inhibitor) group (n=30) – were constructed. Trunk blood was assayed for serum hemoglobin, hematocrit, total and direct bilirubin, BDNF, S100B, and tau protein levels. Brain tissue levels of neuronal apoptosis, BDNF expression, PLC activity, IP3 content, phospho- and total Ca2+/calmodulin-dependent protein kinase type IV (CaMKIV) expression, and phospho- and total cAMP response element binding protein (CREB) expression were also assayed.
Results
PHZ-induced hemolytic hyperbilirubinemia was validated by significantly decreased serum hemoglobin and hematocrit as well as significantly increased total and direct serum bilirubin (p<0.05). Neonatal bilirubin-induced neurotoxicity was validated by significantly decreased serum BDNF, brain BDNF, and serum S100B, along with significantly increased serum tau protein (p<0.05). PHZ-induced hemolytic hyperbilirubinemia significantly decreased serum BDNF, brain BDNF, and PLC/IP3/Ca2+ pathway activation while increasing neuronal apoptosis levels (p<0.05), all of which were partially rescued by melatonin therapy (p<0.05). Pre-treatment with the PLC inhibitor U73122 largely abolished the positive effects of melatonin on PLC/IP3/Ca2+ pathway activation, downstream BDNF levels, and neuronal apoptosis (p<0.05).
Conclusions
Promotion of BDNF expression and anti-apoptotic effects in neonatal hemolytic hyperbilirubinemia by melatonin largely operates via a PLC-mediated mechanism.
MeSH Keywords: Brain-Derived Neurotrophic Factor; Hyperbilirubinemia, Neonatal; Melatonin; Receptor, trkB
Background
Neonatal hyperbilirubinemia, which is due to a physiological imbalance between the synthesis and elimination of bilirubin, occurs in most newborn infants [1,2]. In certain cases, elevated circulating bilirubin levels can result in acute bilirubin encephalopathy (ABE), chronic bilirubin encephalopathy (CBE, kernicterus), and permanent neurological injury to various subcortical brain structures [2–4]. Although phototherapy and exchange transfusion can be used at an early stage to manage bilirubin toxicity [5], effective therapeutic interventions for rescuing neurological damage from bilirubin-induced encephalopathy are still lacking.
To address this challenge, melatonin (N-acetyl-5-methoxy-tryptamine), a serotonin-derived indolamine synthesized by the pineal gland, has been shown to be an effective neuroprotective agent across various models of perinatal brain injury due to its potent antioxidant properties and ability to cross the blood-brain barrier (BBB) [6]. Notably, a group of 12 perinatal neuroscience research groups from the U.S., U.K., Holland, Sweden, and New Zealand have recommended melatonin as the most promising novel neuroprotective agent for neonatal encephalopathy [7]. In order to illustrate melatonin’s neuroprotective effects in neonatal hyperbilirubinemia, a recent study by Pazar et al. applied melatonin therapy in a phenylhydrazine hydrochloride (PHZ)-induced rodent model of hyperbilirubinemia [8]. They found that melatonin was able to partially rescue PHZ-induced neonatal hyperbilirubinemia-driven neuronal apoptosis and neuroprotective brain-derived neurotrophic factor (BDNF) downregulation [8]. Although Pazar et al. suggested that NF-κB downregulation was responsible for melatonin’s positive effects [8], they failed to elucidate the molecular mechanism(s) by which melatonin promotes BDNF expression and anti-apoptotic effects in neonatal hemolytic hyperbilirubinemia.
That being said, previous research has revealed that brain BDNF expression is heavily driven by phospholipase (PLC)/inositol-1, 4, 5-trisphosphate (IP3)/Ca2+ pathway activation [9]. Moreover, the melatonin receptor MT1 has also been linked to PLC/IP3/Ca2+ pathway activation [10]. On the basis of these previous findings, we hypothesized that melatonin promotes BDNF expression and anti-apoptotic effects in neonatal hemolytic hyperbilirubinemia through a PLC-mediated mechanism.
Material and Methods
Ethics statement
All experimental procedures were approved by the Ethics Committee of Yongchuan Hospital (Chongqing Medical University, Chongqing, China). All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animal subjects
Neonatal Sprague-Dawley rat pups (15–35 g; n=120), along with their mothers, were housed together at room temperature (21±2°C) under a 12-h light-dark cycle. The rat mothers received standard chow and water ad libitum, while the neonates fed off their mother’s milk. As BBB permeability to bilirubin is highest on the tenth day of life [11], experiments were performed in neonates age 10–11 days.
Construction of neonatal hemolytic hyperbilirubinemia model
The neonatal hemolytic hyperbilirubinemia model was constructed as previously described by Pazar et al., with minor modifications [8]. Prior to model construction, the PLC inhibitor U73122 (Biomol Research Laboratories, Plymouth Meeting, PA, USA) was diluted in PBS with 0.05% dimethylsulfoxide (DMSO). Four experimental groups – a control group (n=30), a PHZ group (n=30), a PHZ + melatonin group (n=30), and a PHZ + melatonin+U73122 group (n=30) – were constructed as detailed in Table 1. Briefly, based on the procedure of Sfaello et al. and U73122 dosing recommendations [12], the PHZ + melatonin+U73122 group was intracerebrally (i.c.) injected with U73122 (1 μg). After 30 min, the PHZ + melatonin group and the PHZ + melatonin+U73122 group were injected intraperitoneally (i.p.) with melatonin (10 mg/kg), while the PHZ group was injected i.p. with saline (10 ml/kg). After 30 min, phenylhydrazine hydrochloride (PHZ; Sigma, St. Louis, MO, USA) was prepared in a saline solution and then injected i.p. (75 mg/kg) into the PHZ + melatonin, PHZ + melatonin+U73122, and PHZ groups. This procedure was repeated on the second day. On the third day, U73122 (1 μg), melatonin (10 mg/kg), and/or saline (10 ml/kg) were injected according to their respective groups. The control group received saline (10 ml/kg) for all i.p. injections.
Table 1.
Construction of the neonatal hemolytic hyperbilirubinemia model.
| Day 1 | Day 2 | Day 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time point | 8: 00 AM | 8: 30 AM | 9: 00 AM | 8: 00 AM | 8: 30 AM | 9: 00 AM | 8: 00 AM | 8: 30 AM | 2: 00 PM |
| Control group | Saline* | Saline | Saline | Saline | Saline | Saline | Saline | Saline | Sacrifice |
| PHZ group | Saline | Saline | PHZ# | Saline | Saline | PHZ# | Saline | Saline | Sacrifice |
| PHZ + melatonin group | Saline | Melatonin‡ | PHZ# | Saline | Melatonin@ | PHZ# | Saline | Melatonin@ | Sacrifice |
| PHZ + melatonin + U73122 group | U73122§ | Melatonin‡ | PHZ# | U73122§ | Melatonin@ | PHZ# | U73122§ | Melatonin@ | Sacrifice |
10 ml/kg saline intraperitoneal (i.p.) injection;
75 mg/kg PHZ intraperitoneal (i.p.) injection;
10 mg/kg melatonin intraperitoneal (i.p.) injection;
1 μg U73122 intracerebral (i.c.) injection.
Six hours after the final injection, all neonates were sacrificed by rapid, non-anesthetized decapitation using sharp surgical scissors, and trunk blood was immediately collected, as previously described [13,14]. Trunk blood samples were immediately centrifuged at 1500 g for 10 min (4°C) and then stored at −80°C until later analysis. The hippocampus and cortex were dissected out, immediately placed in liquid nitrogen, and then stored at −80°C until later analysis, as previously described [15,16].
Assessment of serum hemoglobin, hematocrit, and bilirubin levels
Trunk blood was assayed for hemoglobin and hematocrit (serum markers of hemolysis) and total and direct bilirubin (serum markers of hyperbilirubinemia) [17,18]. Hemoglobin and hematocrit were assayed with an automated blood cell counter (Beckman-Coulter LH 750 Hematology Analyzer, Fullerton, CA, USA). Total and direct bilirubin were assayed via a diazo reagent-based method. Assays were performed in triplicate.
Validation of bilirubin-induced neurotoxicity by serum S100B and tau assays
Serum S100B and tau protein levels were assayed as previously described by Pazar et al. [8]. Briefly, enzyme-linked immunosorbent assay (ELISA) kits were used to assess serum S100B and tau protein levels according to the manufacturer’s instructions (BioSource, Camarillo, CA, USA). Assays were performed in triplicate.
Serum and brain BDNF assays
Serum and brain tissue brain-derived neurotrophic factor (BDNF) levels were assayed as previously described by Pazar et al. and Pandya et al., respectively [8,19]. Briefly, an ELISA kit was used to assess serum BDNF levels according to the manufacturer’s instructions (BioSource, Camarillo, CA, USA). The BDNF Emax ImmunoAssay System (Promega, Madison, WI, USA) was used to assay hippocampal and cortical brain tissue BDNF levels. Assays were performed in triplicate.
Measurement of PLC activity and IP3 content
PLC activity and IP3 content in hippocampal brain tissue were assayed as previously described by Domijan et al. with minor modifications [20]. Briefly, hippocampal brain samples were first homogenized in ice-cold 10% trichloroacetic acid (10 volumes). The homogenate was then centrifuged at 1000 g for 10 min. The resulting supernatant was then assayed for PLC activity and IP3 content using the EnzChek Direct PLC kit (Invitrogen Molecular Probes, Eugene, OR, USA) and an IP3 ELISA kit (Cusabio, Wuhan, China), respectively, according to the manufacturer’s instructions.
Western blotting
Western blotting was performed as previously described by Liu et al. [21]. Briefly, hippocampal brain samples were homogenized in ice-cold RIPA lysis buffer (Beyotime) with protease and phosphatase inhibitors (Beyotime). The homogenate was centrifuged at 15000 g for 30 min. The protein samples (80 μg each) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The protein samples were incubated with the following primary rabbit antibodies overnight (at 4°C): anti-calcium/calmodulin-dependent protein kinase type IV (CaMKIV) (diluted 1: 500, Cell Signaling, Danvers, MA, USA), anti-phopho-CaMKIVThr196 (diluted 1: 100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-cAMP response element binding protein (CREB) (diluted 1: 1000, Millipore), anti-phospho-CREBSer133 (diluted 1: 1000, Sigma), and anti-β-actin (diluted 1: 1000; Cell Signaling). After washing 3 times with PBST, the blots were treated for 1 h with matching horseradish peroxidase-conjugated secondary antibodies (1: 10 000, from matching suppliers). After washing, protein bands were detected with an enhanced chemiluminescent reagent (Pierce ECL, Rockford, IL, USA).
TUNEL assay for apoptosis
Neuronal apoptosis levels were assayed as previously described by Pazar et al. with minor modifications [8]. Briefly, hippocampal and cortical brain tissues were immediately fixed in 4% paraformaldehyde, embedded in paraffin, and sliced into 5-μm sections. TUNEL staining was performed with the ApopTag Plus Peroxidase In Situ Apoptosis Kit (Millipore, Billerica, MA, USA). TUNEL-positive cells were counted in the hippocampus and cortex samples using a light microscope (Olympus BX51, Olympus Optical Company, Shanghai, China) at 400× magnification. The apoptotic index (%) was derived from 10 randomly-selected high-power fields (HPFs) for each sample.
Statistical analysis
Statistical analysis was performed using SPSS version 15 (SPSS, Chicago, IL, USA) and MS Excel (Microsoft, Redmond, Washington, USA). All data are reported as means ± standard errors of the mean (SEMs). Student’s t test was used for pairwise comparisons, while one-way analysis of variance (ANOVA) with Bonferroni correction was used for multiple comparisons. A p-value of 0.05 was set as the significance threshold.
Results
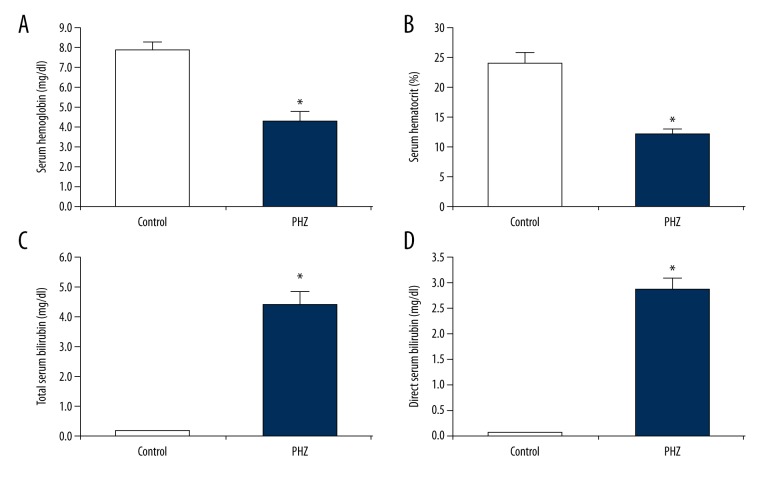
The aim of this study was to determine if melatonin promotes BDNF expression in neonatal hemolytic hyperbilirubinemia through a PLC-based mechanism. First, we established the presence of PHZ-induced neonatal hemolytic hyperbilirubinemia in our rat model through assaying serum hemoglobin, hematocrit, and bilirubin levels following PHZ administration. We found that PHZ significantly decreased serum hemoglobin (46% decrease, p<0.05, Figure 1A) and hematocrit (49% decrease, p<0.05, Figure 1B). We also found that PHZ drastically increased total and direct serum bilirubin (25× and 56× fold-changes, respectively, p<0.05; Figure 1C, 1D).
Figure 1.
Validation of PHZ-induced neonatal hemolytic hyperbilirubinemia. Following PHZ administration, significant decreases in (A) serum hemoglobin and (B) serum hematocrit validated PHZ-induced hemolysis. Moreover, following PHZ administration, significant increases in (C) total serum bilirubin and (D) direct serum bilirubin validated PHZ-induced hyperbilirubinemia. All experiments were performed in triplicate. Data are reported as means ± standard errors of the mean (SEMs). * p<0.05 vs. control group.
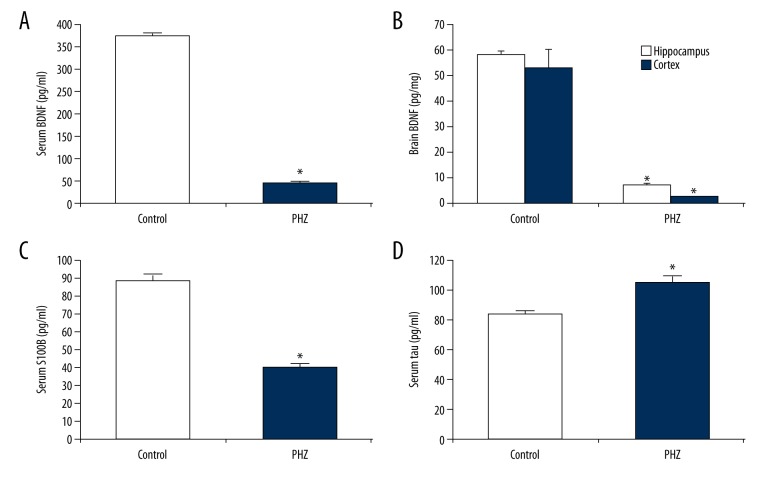
Next, we established the presence of neonatal bilirubin-induced neurotoxicity through assaying several well-established serum markers of neonatal bilirubin-induced neurotoxicity: serum BDNF, brain BDNF, serum S100B, and tau protein [22]. We found that PHZ-induced neonatal hemolytic hyperbilirubinemia significantly decreased serum BDNF (88% decrease, p<0.05, Figure 2A), brain BDNF (hippocampus, 88% decrease; cortex, 95% decrease, p<0.05, Figure 2B), and serum S100B levels (54% decrease, p<0.05, Figure 2C). We also found that PHZ-induced neonatal hemolytic hyperbilirubinemia significantly increased serum tau protein levels (25% increase, p<0.05; Figure 2D).
Figure 2.
Validation of PHZ-induced neonatal bilirubin-induced neurotoxicity. Several markers of neonatal bilirubin-induced neurotoxicity – serum BDNF, brain (hippocampus/cortex) BDNF, serum S100B, and serum tau protein – were assayed. Following PHZ administration, significant decreases in (A) serum BDNF, (B) brain BDNF, and (C) serum S100B, as well as significant increases in (D) serum tau protein, validated neonatal bilirubin-induced neurotoxicity. All experiments were performed in triplicate. Data are reported as means ± standard errors of the mean (SEMs). * p<0.05 vs. control group.
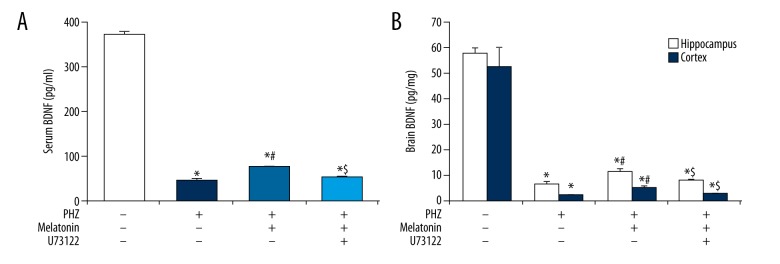
Having validated the rat model of PHZ-induced neonatal hemolytic hyperbilirubinemia, we next assessed the effect of melatonin and PLC upon serum and brain BDNF levels. Melatonin therapy was capable of partially rescuing PHZ-induced serum BDNF downregulation (p<0.05, Figure 3A) and brain BDNF downregulation (p<0.05, Figure 3B). Pre-treatment with the PLC inhibitor U73122 largely abolished the effects of melatonin on serum and brain BDNF expression (p<0.05, Figure 3A, 3B). These findings indicate that the positive effect of melatonin on brain BDNF expression under neonatal hemolytic hyperbilirubinemic conditions is largely driven through upregulated PLC activity.
Figure 3.
Melatonin promotes serum and brain BDNF expression in a PLC-mediated manner. PHZ-induced neonatal hemolytic hyperbilirubinemia significantly decreased (A) serum BDNF levels and (B) brain (hippocampus/cortex) BDNF levels. Melatonin therapy partially rescued (A) serum BDNF levels and (B) brain BDNF levels. Pre-treatment with the PLC inhibitor U73122 abolished the effects of melatonin on (A) serum BDNF levels and (B) brain BDNF levels. All experiments were performed in triplicate. Data are reported as means ± standard errors of the mean (SEMs). * p<0.05 versus control group; # p<0.05 vs. PHZ group, $ p<0.05 vs. PHZ + melatonin group.
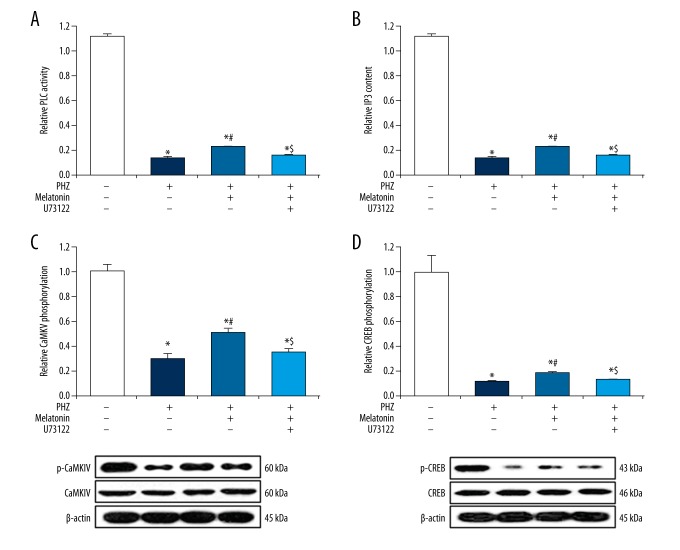
Brain BDNF expression is heavily driven by PLC/IP3/Ca2+ pathway activation [9]. Having demonstrated that PHZ-induced neonatal hemolytic hyperbilirubinemia significantly downregulates brain BDNF expression, we next analyzed hippocampal tissue samples for PLC/IP3/Ca2+ pathway proteins. We found that PHZ-induced neonatal hemolytic hyperbilirubinemia drastically decreased hippocampal PLC activity (91% decrease, p<0.05, Figure 4A), IP3 content (90% decrease, p<0.05, Figure 4B), CaMKIV phosphorylation (70% decrease, p<0.05, Figure 4C), and CREB phosphorylation (89% decrease, p<0.05, Figure 4D). Melatonin therapy was capable of partially rescuing these PHZ-induced effects (p<0.05, Figure 4A–D). Pre-treatment with the PLC inhibitor U73122 largely abolished the positive effects of melatonin on PLC activity, IP3 content, CaMKIV phosphorylation, and CREB phosphorylation (p<0.05, Figure 4A–4D). These findings validate that the positive effect of melatonin on hippocampal brain BDNF expression under neonatal hemolytic hyperbilirubinemic conditions is largely driven through upregulated PLC activity.
Figure 4.
Melatonin promotes PLC/IP3/Ca2+ pathway activation. PHZ-induced neonatal hemolytic hyperbilirubinemia significantly decreased (A) PLC activity, (B) IP3 content, (C) CaMKIV phosphorylation, and (D) CREB phosphorylation. Melatonin therapy partially rescued (A) PLC activity, (B) IP3 content, (C) CaMKIV phosphorylation, and (D) CREB phosphorylation. Pre-treatment with the PLC inhibitor U73122 abolished effects of melatonin on (A) PLC activity, (B) IP3 content, (C) CaMKIV phosphorylation, and (D) CREB phosphorylation. All experiments were performed in triplicate. Data are reported as means ± standard errors of the mean (SEMs). * p<0.05 vs. control group; # p<0.05 vs. PHZ group; $ p<0.05 vs. PHZ + melatonin group.
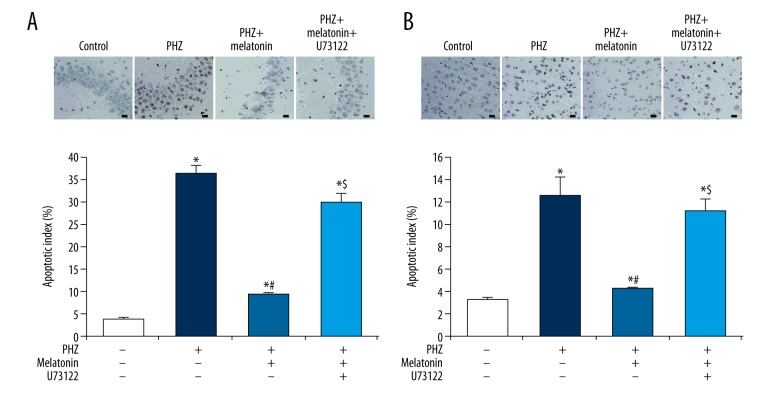
As melatonin has been shown to partially rescue the promotion of neuronal apoptosis by PHZ-induced neonatal hyperbilirubinemia, we next assessed the effects of melatonin and PLC on neuronal apoptosis levels. Consistent with previous findings, we found that PHZ-induced neonatal hemolytic hyperbilirubinemia significantly increased hippocampal apoptosis levels (8.9× fold-change, p<0.05, Figure 5A) and cortical apoptosis levels (2.8× fold-change decrease, p<0.05, Figure 5B). Melatonin therapy partially rescued PHZ-induced hippocampal apoptosis (p<0.05, Figure 5A) and cortical apoptosis (p<0.05, Figure 5B). Pre-treatment with the PLC inhibitor U73122 largely abolished the positive effects of melatonin on hippocampal and cortical apoptosis (p<0.05, Figure 5A, 5B). These findings indicate that the positive effect of melatonin on neuronal apoptosis under neonatal hemolytic hyperbilirubinemic conditions is largely driven by upregulated PLC activity.
Figure 5.
Melatonin reduces neuronal apoptosis in a PLC-mediated manner. (Top panels) Representative TUNEL staining images from the (A) hippocampus and (B) cortex. Black scale bar=10 μm. (Bottom panels) PHZ-induced neonatal hemolytic hyperbilirubinemia significantly increased (A) hippocampal apoptosis levels and (B) cortical apoptosis levels. Melatonin therapy partially rescued (A) hippocampal apoptosis levels and (B) cortical apoptosis levels. Pre-treatment with the PLC inhibitor U73122 abolished effects of melatonin on (A) hippocampal apoptosis levels and (B) cortical apoptosis levels. All experiments were performed in triplicate. Data are reported as means ± standard errors of the mean (SEMs). * p<0.05 vs. control group; # p<0.05 vs. PHZ group, $ p<0.05 vs. PHZ + melatonin group.
Discussion
In this study, we evaluated whether promotion of BDNF expression and anti-apoptotic effects by melatonin in neonatal hemolytic hyperbilirubinemia operates via a PLC-mediated mechanism. Through use of the PLC inhibitor U73122 in a rodent model of neonatal hemolytic hyperbilirubinemia, we determined that the positive effects of melatonin on brain BDNF expression and neuronal apoptosis under neonatal hemolytic hyperbilirubinemic conditions are largely driven by upregulated PLC activity. These findings provide valuable insights for further investigation into the molecular mechanism(s) underlying neonatal hemolytic hyperbilirubinemia, as well as the development of targeted therapeutics for this debilitating condition.
Severe neonatal hyperbilirubinemia, commonly defined as total serum bilirubin levels exceeding 17 mg/dl [23,24], is most commonly produced by ABO blood type heterospecificity or glucose-6-phosphate dehydrogenase (G6PD) deficiency [25,26]. Notably, these commonplace conditions that produce severe neonatal hyperbilirubinemia also produce hemolysis, which has also been shown to contribute to bilirubin-induced neurotoxicity [26]. On this basis, the PHZ-induced rodent model used here is a particularly valid animal model for neonatal hyperbilirubinemia, as PHZ induces acute hemolysis through promoting erythrocytic oxidative stress and hemoglobin precipitation (i.e., Heinz body formation) [27]. In fact, the PHZ-induced rodent model used here displays stronger validity with respect to bilirubin-induced encephalopathy as compared to other animal models of neonatal hyperbilirubinemia, such as glucuronyl transferase-deficient models or bilirubin-albumin displacement models [8]. Here, we confirmed successful construction of the PHZ-induced model of severe neonatal hyperbilirubinemia by verifying significant decreases in serum hemoglobin and hematocrit (indicating acute hemolysis) along with significant increases in total and direct bilirubin (with total bilirubin exceeding 3 mg/dl indicating hyperbilirubinemia in rodents) [28]. Moreover, we also confirmed bilirubin-induced neurotoxicity through verifying significant decreases in serum BDNF, brain BDNF, and serum S100B, as well as significant increases in tau protein, which are all well-established serum markers of neonatal bilirubin-induced neurotoxicity [22]. Interestingly, although serum S100B (a glial marker protein) is usually elevated in human neonatal bilirubin-induced neurotoxicity [22], we found that serum S100B was significantly decreased in our rat model, a paradoxical phenomenon previously observed in neurodegenerative disease states such as Alzheimer’s disease and amyotrophic lateral sclerosis [29].
PHZ-induced neonatal hyperbilirubinemia has been shown to significantly reduce serum BDNF levels, an effect partially reversed through melatonin therapy [8]. Mature BDNF (derived from its precursor pro-BDNF) is a broadly-expressed neurotrophin that displays anti-apoptotic, anti-inflammatory, anti-neurotoxic, and neuroregenerative properties [30]. As mature BDNF is critical to neuroprotection in the neonatal brain, newborns are more susceptible to brain injury on account of decreased brain BDNF levels [30]. On this basis, the ability of melatonin to increase mature BDNF levels in neonatal hyperbilirubinemia is of particular clinical interest [8]. Here, through application of the PLC inhibitor U73122, we showed that the positive effect of melatonin on brain BDNF expression under neonatal hemolytic hyperbilirubinemic conditions is largely driven through upregulated PLC activity. These findings are consistent with previous in vitro research showing that the G protein-coupled melatonin receptors MT1 and MT2/5-HT2C heteromer both couple to the PLC/IP3/Ca2+ pathway [10,31] and that the MT1/MT2 antagonist luzindole significantly suppresses protective effects of melatonin in neural stem cells [32]. Our findings also agree with reports of melatonin’s in vivo reversal of sleep deprivation-induced brain BDNF downregulation and calcium-calmodulin-dependent kinase II (CaMKII) downregulation in the rat cerebral cortex and hippocampus [33], as well as the efficacy of the MT2 agonist agomelatine in increasing serum BDNF levels in patients with severe clinical depression [31,34]. These combined findings suggest that targeting the MT1/MT2-PLC axis with melatonin (or synthetic melatonergic drugs) may be a promising neuroprotective strategy for neonatal hemolytic hyperbilirubinemia and other brain disorders involving BDNF downregulation.
Severe neonatal hyperbilirubinemia (if left untreated) results in kernicterus, a permanent neurologic disorder characterized by dystonia, choreoathetosis, hearing loss, and oculomotor pareses produced by neuropathological damage to the basal ganglia, hippocampus, cerebellum, and brainstem [5]. On a cellular level, unbound or ‘free’ bilirubin crosses the BBB, where it activates glial cells, impairs myelination, and promotes neuronal apoptosis [24]. Accordingly, PHZ-induced neonatal hyperbilirubinemia significantly increases neuronal apoptosis levels [8]. On this basis, the anti-apoptotic effect of melatonin in neonatal hyperbilirubinemia is of particular clinical interest. Here, through application of the PLC inhibitor U73122, we showed that the positive effect of melatonin on neuronal apoptosis under neonatal hemolytic hyperbilirubinemic conditions is largely driven through upregulated PLC activity. Notably, recent research has revealed astrocytic IP3/Ca2+-mediated reductions in neuronal apoptosis, reactive astrogliosis, and tissue loss following cerebral ischemia [35,36]. Therefore, further research should investigate the role of astrocytes (and other glial cells) in the PLC-mediated effects of melatonin on brain BDNF expression and neuronal apoptosis.
There are several limitations to this study. First, although the rodent model of PZH-induced neonatal hyperbilirubinemia used here aims to mimic the acute hemolysis, hyperbilirubinemia, and neurotoxicity observed in human neonates, there may be physiological differences between the 2 species that affect translation of the current findings to humans. Second, the scope of this study was to examine a single mechanism by which melatonin positively affects BDNF expression under neonatal hemolytic hyperbilirubinemic conditions. Therefore, longer-term preclinical and clinical studies that measure a larger set of experimental parameters are still needed to examine the potential efficacy of melatonin in treating neonatal bilirubin-induced neurotoxicity. Third, although PLC activity appears to be largely responsible for the positive effect of melatonin on BDNF expression, other pathways may also contribute to the effect of melatonin on BDNF expression.
Conclusions
In conclusion, promotion of BDNF expression and anti-apoptotic effects by melatonin in neonatal hemolytic hyperbilirubinemia largely operates via a PLC-mediated mechanism. These findings provide valuable insights for further investigation into the molecular mechanism(s) underlying neonatal hemolytic hyperbilirubinemia, as well as the development of targeted therapies for this debilitating condition. Longer-term preclinical and clinical studies that measure a larger set of experimental parameters are still needed to examine the potential efficacy of melatonin in treating neonatal bilirubin-induced neurotoxicity.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Yu Z-B, Han S-P, Chen C. Bilirubin nomograms for identification of neonatal hyperbilirubinemia in healthy term and late-preterm infants: A systematic review and meta-analysis. World J Pediatr. 2014;10(3):211–18. doi: 10.1007/s12519-014-0495-8. [DOI] [PubMed] [Google Scholar]
- 2.Petersen JP, Henriksen TB, Hollegaard MV, et al. Extreme neonatal hyperbilirubinemia and a specific genotype: A population-based case-control study. Pediatrics. 2014;134(3):510–15. doi: 10.1542/peds.2014-0035. [DOI] [PubMed] [Google Scholar]
- 3.Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134(3):504–9. doi: 10.1542/peds.2014-0987. [DOI] [PubMed] [Google Scholar]
- 4.Koziol LF, Budding DE, Chidekel D. Hyperbilirubinemia: Subcortical mechanisms of cognitive and behavioral dysfunction. Pediatr Neurol. 2013;48(1):3–13. doi: 10.1016/j.pediatrneurol.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Ingelfinger JR, Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage – mechanisms and management approaches. N Engl J Med. 2013;369(21):2021–30. doi: 10.1056/NEJMra1308124. [DOI] [PubMed] [Google Scholar]
- 6.Gitto E, Marseglia L, Manti S, et al. Protective role of melatonin in neonatal diseases. Oxid Med Cell Longev. 2013;2013:980374. doi: 10.1155/2013/980374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson NJ, Tan S, Groenendaal F, et al. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J Pediatr. 2012;160(4):544–552.e4. doi: 10.1016/j.jpeds.2011.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pazar A, Kolgazi M, Memisoglu A, et al. The neuroprotective and anti-apoptotic effects of melatonin on hemolytic hyperbilirubinemia-induced oxidative brain damage. J Pineal Res. 2016;60(1):74–83. doi: 10.1111/jpi.12292. [DOI] [PubMed] [Google Scholar]
- 9.Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: Mechanisms and functions. Physiology. 2005;20(1):70–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- 10.Tosini G, Owino S, Guillaume JL, Jockers R. Understanding melatonin receptor pharmacology: Latest insights from mouse models, and their relevance to human disease. Bioessays. 2014;36(8):778–87. doi: 10.1002/bies.201400017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roger C, Koziel V, Vert P, Nehlig A. Autoradiographic mapping of local cerebral permeability to bilirubin in immature rats: Effects of hyperbilirubinemia. Pediatr Res. 1996;39(1):64–71. doi: 10.1203/00006450-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Sfaello I, Baud O, Arzimanoglou A, Gressens P. Topiramate prevents excitotoxic damage in the newborn rodent brain. Neurobiol Dis. 2005;20(3):837–48. doi: 10.1016/j.nbd.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Dalgakiran F, Witcomb LA, McCarthy AJ, et al. Non-invasive model of neuropathogenic Escherichia coli infection in the neonatal rat. J Vis Exp. 2014;2018;(92):e5. doi: 10.3791/52018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Victoria NC, Karom MC, Eichenbaum H, Murphy AZ. Neonatal injury rapidly alters markers of pain and stress in rat pups. Dev Neurobiol. 2014;74(1):42–51. doi: 10.1002/dneu.22129. [DOI] [PubMed] [Google Scholar]
- 15.Mela V, Díaz F, Borcel E, et al. Long term hippocampal and cortical changes induced by maternal deprivation and neonatal leptin treatment in male and female rats. PLoS One. 2015;10(9):e0137283. doi: 10.1371/journal.pone.0137283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson O, Jiang L, Ersson L, et al. Environmental neurotoxin interaction with proteins: Dose-dependent increase of free and protein-associated BMAA (b-N-methylamino-L-alanine) in neonatal rat brain. Sci Rep. 2015;5:15570. doi: 10.1038/srep15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colella MP, de Paula EV, Machado-Neto JA, et al. Elevated hypercoagulability markers in hemoglobin SC disease. Haematologica. 2015;100(4):466–71. doi: 10.3324/haematol.2014.114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med. 2001;344(8):581–90. doi: 10.1056/NEJM200102223440807. [DOI] [PubMed] [Google Scholar]
- 19.Pandya CD, Hoda MN, Kutiyanawalla A, et al. Differential effects upon brain and serum BDNF levels in rats as response to continuous and intermittent administration strategies of two second generation antipsychotics. Schizophr Res. 2013;151(1):287–88. doi: 10.1016/j.schres.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Domijan A-M, Kovac S, Abramov AY. Lipid peroxidation is essential for phospholipase C activity and the inositol-trisphosphate-related Ca2+ signal. J Cell Sci. 2014;127(1):21–26. doi: 10.1242/jcs.138370. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Guo H, Sayed MDS, et al. cAMP/PKA/CREB/GLT1 signaling involved in the antidepressant-like effects of phosphodiesterase 4D inhibitor (GEBR-7b) in rats. Neuropsychiatr Dis Treat. 2016;12:219–27. doi: 10.2147/NDT.S90960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okumus N, Turkyilmaz C, Onal EE, et al. Tau and S100B proteins as biochemical markers of bilirubin-induced neurotoxicity in term neonates. Pediatr Neurol. 2008;39(4):245–52. doi: 10.1016/j.pediatrneurol.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Chou H-C, Chien C-T, Tsao P-N, et al. Prediction of severe neonatal hyperbilirubinemia using cord blood hydrogen peroxide: A prospective study. PloS One. 2014;9(1):e86797. doi: 10.1371/journal.pone.0086797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brites D, Fernandes A. Bilirubin-induced neural impairment: A special focus on myelination, age-related windows of susceptibility and associated co-morbidities. Semin Fetal Neonatal Med. 2015;20(1):14–19. doi: 10.1016/j.siny.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Sgro M, Kandasamy S, Shah V, et al. Severe neonatal hyperbilirubinemia decreased after the 2007 Canadian Guidelines. J Pediatr. 2016;171:43–47. doi: 10.1016/j.jpeds.2015.12.067. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan M, Bromiker R, Hammerman C. Hyperbilirubinemia, hemolysis, and increased bilirubin neurotoxicity. Semin Perinatol. 2014;38(7):429–37. doi: 10.1053/j.semperi.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Singh R, Pandey K, Meena AK, Jain A. Molecular mechanism of phenylhydrazine induced haematotoxicity: A review. American Journal of Phytomedicine and Clinical Therapeutics. 2014;2(3):390–94. [Google Scholar]
- 28.Ortega FD, Azpeitia GG, Pidre MC, Rosales JC. Apertura reversible de la barrera hematoencefálica inducida por hipercapnia en hiperbilirrubinemia experimental. An Esp Pediatr. 1997;46:374–77. [in Spanish] [PubMed] [Google Scholar]
- 29.Steiner J, Bogerts B, Schroeter ML, Bernstein H-G. S100B protein in neurodegenerative disorders. Clin Chem Lab Med. 2011;49(3):409–24. doi: 10.1515/CCLM.2011.083. [DOI] [PubMed] [Google Scholar]
- 30.Chen A, Xiong L-J, Tong Y, Mao M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury (Review) Biomed Rep. 2013;1(2):167–76. doi: 10.3892/br.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamal M, Gbahou F, Guillaume J-L, et al. Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J Biol Chem. 2015;290(18):11537–46. doi: 10.1074/jbc.M114.559542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Li X, Chan MT, et al. Melatonin antagonizes interleukin-18-mediated inhibition on neural stem cell proliferation and differentiation. J Cell Mol Med. 2017;21(9):2163–71. doi: 10.1111/jcmm.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Zhang H-Q, Liang X-Y, et al. Melatonin ameliorates cognitive impairment induced by sleep deprivation in rats: role of oxidative stress, BDNF and CaMKII. Behav Brain Res. 2013;256:72–81. doi: 10.1016/j.bbr.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 34.Gupta K, Gupta R, Bhatia M, et al. Effect of agomelatine and fluoxetine on HAM-D score, serum brain-derived neurotrophic factor, and tumor necrosis factor-α level in patients with major depressive disorder with severe depression. J Clin Pharmacol. 2017;57(12):1519–26. doi: 10.1002/jcph.963. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Xie Y, Zhang N, et al. Disruption of IP 3 R2-mediated Ca2+ signaling pathway in astrocytes ameliorates neuronal death and brain damage while reducing behavioral deficits after focal ischemic stroke. Cell Calcium. 2015;58(6):565–76. doi: 10.1016/j.ceca.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakers C, Petzold GC. Astrocytic calcium release mediates peri-infarct depolarizations in a rodent stroke model. J Clin Invest. 2017;127(2):511–15. doi: 10.1172/JCI89354. [DOI] [PMC free article] [PubMed] [Google Scholar]