Abstract
Background
Low-dose methoxyflurane and nitrous oxide (N2O; 50:50 with oxygen) are both self-administered, self-titrated, rapid-acting, nonnarcotic, and noninvasive inhalational agents with similar onset times of pain relief. The aim of this review was to compare the clinical efficacy, safety, and tolerability of these analgesics in emergency care.
Materials and methods
A systematic literature search and review according to Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were performed using Embase, Medline, the Cochrane Library, several clinical trial registers, and emergency-medicine conference material.
Results
Although both compounds have been used for many years in emergency care, the search found only a few controlled studies and no head-to-head trials performed in this setting. Two double-blind, randomized studies comparing their respective study medication (low-dose methoxyflurane or N2O) to placebo were identified that could be compared in an indirect approach by using placebo as a bridging comparator. Both agents provided rapid pain relief to trauma patients, with no significant differences between them; both treatments were generally well tolerated.
Conclusion
Both low-dose methoxyflurane and N2O are suitable options for the pain treatment of trauma patients in the emergency setting. Due to the ease of administration and portability, inhaled low-dose methoxyflurane, however, may not only offer advantages in emergency situations in remote or difficult-to-reach locations and mass-casualty situations but also be of significant value in urban and rural environments.
Keywords: methoxyflurane, nitrous oxide, trauma, pain treatment, emergency
Introduction
Acute pain is common in emergency care, and is experienced by up to 90% of trauma patients. It is often undertreated in both prehospital and emergency department (ED) settings.1,2 Inadequate pain management is associated with physiological and psychological stress, which can impact therapy and rehabilitation, resulting in diminished quality of life of the patient.3 Main analgesics in emergency settings include paracetamol, nonsteroidal anti-inflammatory drugs, ketamine, nitrous oxide (N2O), and opiates.4 Recently, the inhalational analgesic low-dose methoxyflurane, which has been used extensively in emergency settings in Australia and New Zealand for over 30 years, was approved in some European countries (Belgium, France, Ireland, and the UK) for emergency relief of moderate–severe pain in conscious adult patients with trauma and associated pain. Low-dose methoxyflurane is self-administered in analgesic doses at a maximum of two 3 mL vials in a single administration via a handheld inhaler; a green, whistle-shaped, single-use device.5 It provides rapid, short-term pain relief (within six to ten inhalations),5 and has been shown effective and safe in emergency care6–9 and for minor surgical, radiological, and dental procedures.10,11
Both low-dose methoxyflurane and N2O (50:50 with oxygen; “N2O” hereafter) are self-administered, self-titrated, rapid-acting, nonnarcotic, noninvasive inhalational agents with similar onset times of pain relief.7,12 Our systematic review of the literature found only a few controlled studies and no head-to-head trials of these two analgesics performed in the emergency setting. To compare clinical efficacy, safety, and tolerability in emergency care, we thus carried out an indirect treatment comparison.
Materials and methods
This systematic review was carried out and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.13
Literature search
Searches were undertaken from database inception to December 22, 2015 using the Embase, Medline and Medline In-Process databases, and Cochrane Central Register of Controlled Trials. In addition, conference material of the 3 years prior to the search date from the European Society for Emergency Medicine and the International Conference on Emergency Medicine was screened, and the clinical trial register of the US National Institutes of Health (www.clinical-trials.gov), World Health Organization International Clinical Trials Registry Platform, and Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) were searched for relevant unpublished trial data. Primary search facets were study design (facets according to Scottish Intercollegiate Guidelines Network),14 emergency and trauma-associated pain, and intervention, using both medical subject heading and free text terms (for detailed search strategies, see Tables S1 and S2).
Study selection
Studies were required to fulfill the following criteria to be included in the review: 1) a population of adult patients (≥18 years), 2) trauma-induced pain in the prehospital or hospital setting, 3) randomized controlled trial or observational study design, 4) intervention low-dose methoxyflurane and/or N2O, and 5) English language publication. The screening process was performed independently by two reviewers; any disagreement was resolved by a third reviewer. Abstracts of all records identified in the search were checked for eligibility, and full-text copies of potentially relevant studies were rescreened.
Assessment of methodological quality
Risk-of-bias assessments were performed using the Cochrane Collaboration’s tool (shown in Table S3).15 Study grade for adequacy of concealment of random allocation and Jadad score for study quality and reporting16 were also determined. To rule out the impact of clinical heterogeneity, studies were fully assessed for baseline comparability before they were included in the analysis.
Data extraction
Data extraction was carried out independently by two reviewers; a third reviewer resolved any disagreements. Information regarding study characteristics, type of pain scale employed, definition of pain relief, and patient data, including demographics, type and location of trauma, pain intensity at different time points, lack of pain relief, and occurrence of adverse events (AEs), was collected. Some of the pain-intensity and pain-relief data were extracted from illustrations in the relevant publications using graph digitization. Median pain scores from one study were obtained by post hoc calculations.8
Outcome parameters
The primary efficacy outcome was change in pain intensity under pain medication from baseline at 5, 10, and 15 minutes after start of inhalation, as measured by the 11-point numeric rating scale (NRS; from 0 = no pain to 10 = worst imaginable pain) or the 100 mm visual analog scale (VAS; from 0 = no pain to 100 = worst pain). As these pain scales are interchangeable for acute-pain measurements in the ED17,18 but not interconvertible, the ensuing risk of variability was reduced by calculating standardized median differences with 95% CIs between treatments for change in pain intensity from baseline instead of weighted mean differences. As a second outcome, the proportion of patients experiencing no pain relief under study medication was calculated. AE documentation was used for tolerability assessment.
Statistical analysis
Statistical analyses were performed using Stata for Windows (version 11.0; StataCorp, College Station, TX, USA). We did not identify any head-to-head clinical trial between low-dose methoxyflurane and N2O in our systematic review. Adjusted indirect comparison has been increasingly used in systematic reviews to evaluate relative effects of interventions, due to a lack of head-to-head randomized trials. As the evidence network included only two studies connected through a bridging comparator (placebo), adjusted indirect treatment comparison using the method by Bucher et al19 was conducted. Indirect comparison methods are used to measure the effect of treatment A compared with treatment B based on the results of trials of A and of B versus the same control (placebo or active treatment). These methods provide extrapolations based on the hypothesis that the effects that A and B would have (compared with a control in a head-to-head trial) are the same as those observed in trials used in the indirect comparison.19,20 For the adjusted indirect comparison of intervention A versus intervention B to be valid, trials that compare intervention A versus placebo should be on average similar to trials that compare intervention B versus placebo in terms of moderators of relative treatment effect (baseline characteristics). The significance for treatment differences in this indirect comparison was at the two-sided 5% level.
Results
Search results and study selection
Literature searches yielded 808 references, which included 120 duplicates (Figure 1). Twenty publications were deemed eligible; however, further assessment revealed that the eleven included observational studies did not provide data suitable for a quantitative efficacy analysis. These studies were thus excluded, leaving four randomized controlled trials7,12,21,22 for analysis.
Figure 1.
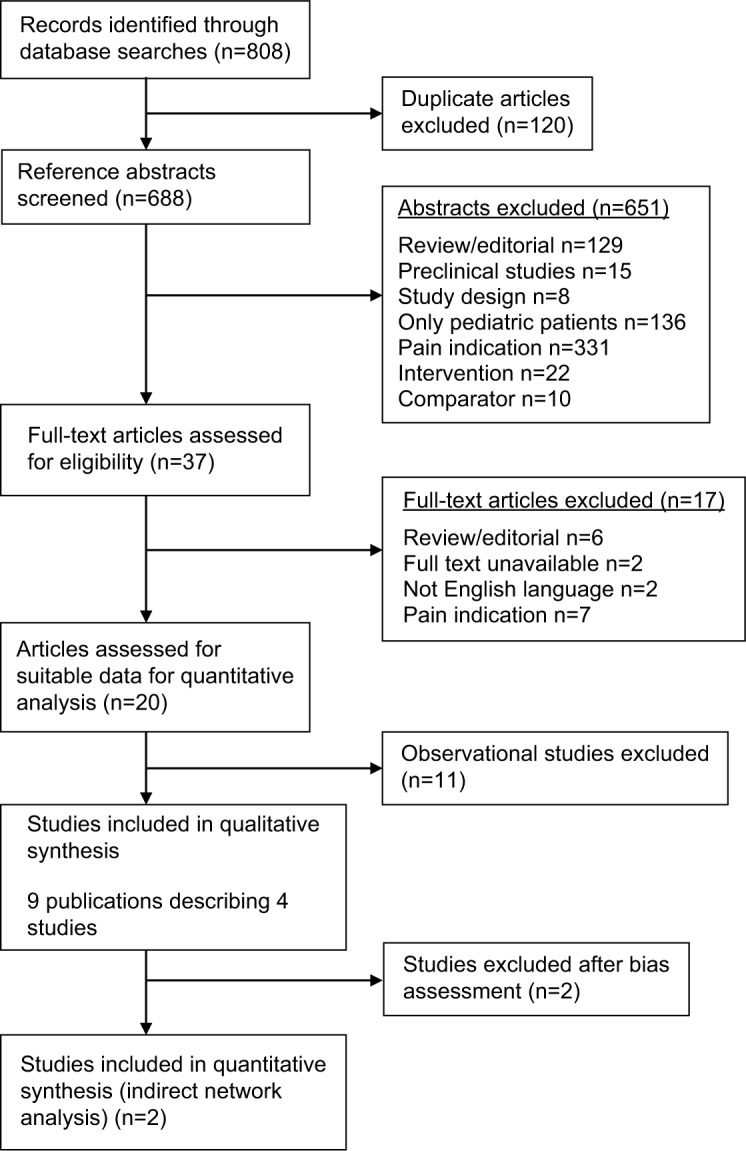
Flowchart of study selection.
Qualitative assessment
Two of the studies7,12 were well conducted with a low risk of bias, according to the Cochrane critical appraisal list (Table S3). Both received an allocation-concealment grade of A and a maximum Jadad score of 5. One study21 reported that physicians and data collectors were not blinded, and received a Jadad score of 3. The fourth study22 was described in a conference abstract, and did not report allocation concealment, baseline characteristics, withdrawals, or statistical analysis details. It compared methoxyflurane with tramadol, and lacked a bridging comparator between the two compounds of interest: methoxyflurane and N2O. It was decided to exclude the latter two studies from the quantitative analysis. The two double-blind, randomized studies included both compared their respective study medication (low-dose methoxyflurane or N2O) to placebo;7,12 an adjusted indirect comparison was thus used to compare the two treatments.
Study characteristics
Table 1 lists the main study characteristics. In the first study, patients presenting at six EDs in the UK with at least moderate pain due to minor trauma (physical wound or injury, such as fractures, lacerations, burns, dislocations, contusions, or injury due to foreign bodies) received either low-dose methoxyflurane or placebo for pain relief.7 For ethical reasons, rescue medication could be requested at any time during and after treatment. The study population included adolescent and adult patients. Data of the adult subgroup were published separately,8 and are used in this network analysis. The second study setting included ambulances from two firefighter emergency services in France.12 Patients with moderate traumatic pain received blinded treatment of either N2O or medical air (placebo) for the first 15 minutes in the ambulance; thereafter, all placebo patients also received N2O. Table 2 provides baseline demographic and clinical characteristics of the patients. The proportion of patients with back trauma was considerably higher in the N2O study. Baseline pain intensity was comparable between the studies.
Table 1.
Characteristics of included studies
| Coffey et al7 | Ducassé et al12 | |
|---|---|---|
| Study setting | Multicenter, emergency department | Multicenter, ambulance |
| Treatment | Methoxyflurane 3 mL inhaler | Premixed 50% N2Oa inhalation 9 L/min |
| Comparator | Placebo 5 mL inhaler | Medical air inhalation 9 L/min |
| Inclusion criteria | ≥12 years of age | ≥18 years of age |
| Pain score ≥4–≤7 (NRS) at time of admission, due to minor trauma | Pain score 4–6 (NRS), due to trauma | |
| Pain assessment | Before first inhalation (baseline), at 5, 10, 15, 20, and 30 minutes, then every 30 minutes until rescue-medication use or discharge | At baseline, every 5 minutes for 30 minutes, then every 15 minutes until arrival at ED |
| Follow-up duration | 14±2 days | No follow-up |
| Type of pain scale | VAS 100 | NRS |
| Definition of pain relief | Reduction in pain intensity by 30% of baseline score (clinically significant) | Pain score ≤3 (NRS) 15 minutes after first inhalation |
Note:
With oxygen.
Abbreviations: ED, emergency department; NRS, numeric rating scale (0 = no pain to 10 = worst imaginable pain); VAS, visual analog scale (0 = no pain to 100 = worst pain)
Table 2.
Demographic and clinical characteristics of study populations at baseline
| Methoxyflurane (n=102) | Placebo (n=101) | N2O (n=30) | Placebo (n=30) | |
|---|---|---|---|---|
| Age (years) | 35 (18–74) | 30 (18–84) | 37 (26–66) | 29 (23–50) |
| Male sex | 53 (52%) | 51 (50.5%) | 20 (67%) | 19 (63%) |
| Trauma site | ||||
| Upper limb | 21 (20.6%) | 37 (36.6%) | 11 (37%) | 8 (27%) |
| Lower limb | 60 (58.8%) | 57 (56.4%) | 12 (40%) | 15 (50%) |
| Back | 5 (4.9%) | 2 (2%) | 6 (20%) | 7 (23%) |
| Chest | 8 (7.8%) | 0 | 1 (3%) | 0 |
| Other | 8 (7.8%) | 5 (5%) | 0 | 0 |
| Pain intensity | 68 (25–100)a | 70 (10–100)a | 6 (5–6)b | 6 (5–6)b |
Notes:
Visual analog scale (0 = no pain to 100 = worst pain);
numeric rating scale (0 = no pain to 10 = worst imaginable pain). Data are median (range) or number of patients (%).
Efficacy outcomes
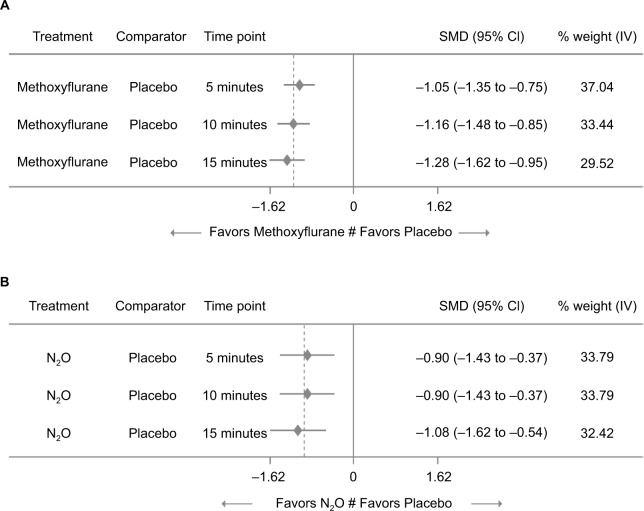
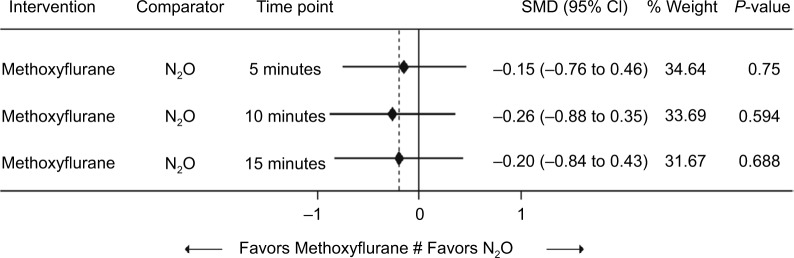
Figure 2 shows the comparison between low-dose methoxyflurane and placebo and between N2O and placebo for change in pain intensity from baseline, which favored both active treatments at all three time points. The indirect treatment comparison between low-dose methoxyflurane and N2O revealed no significant differences between the two agents at 5, 10, or 15 minutes after start of inhalation, although data were in favor of low-dose methoxyflurane (Figure 3).
Figure 2.
Treatment differences for change in pain intensity from baseline between low-dose methoxyflurane and placebo (A) and N2O and placebo (B).
Abbreviations: IV, inverse variance; SMD, standardized median difference.
Figure 3.
Comparison between low-dose methoxyflurane and N2O regarding change in pain intensity from baseline (indirect network analysis).
Abbreviation: SMD, standardized median difference.
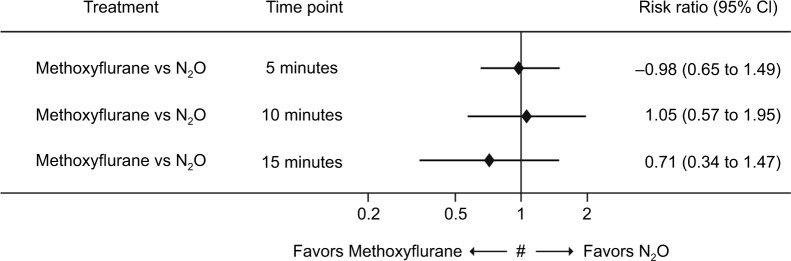
Figure 4 shows the proportion of patients not experiencing pain relief under low-dose methoxyflurane or N2O treatment at 5, 10, and 15 minutes after treatment. Numbers were not significantly different between the two treatments. At 15 minutes, the proportion of patients experiencing pain relief was greater under low-dose methoxyflurane; however, statistical significance could not be achieved.
Figure 4.
Comparison between low-dose methoxyflurane and N2O regarding proportion of patients not experiencing pain relief.
Safety
Data pertaining to tolerability and safety outcomes were not reported in a similar time frame in the two studies, and thus could not be compared. The N2O study evaluated safety at baseline, every 5 minutes for 30 minutes, and then every 15 minutes until arrival at the ED.12 AEs or withdrawal due to an AE were not reported for patients under N2O or placebo during the first 15 minutes of blinded treatment. When all patients received N2O treatment in the following 15 minutes, four patients (6.7%) experienced nausea, which was fully reversible 5 minutes after the end of inhalation.
The low-dose methoxyflurane study documented all AEs from enrollment up to 16 days after ED discharge.8 Treatment-related AEs were reported for 42.2% of low-dose methoxyflurane and 14.9% of placebo patients. Most were mild and transient in nature. Notable treatment differences to placebo were observed for the most frequently reported AEs under low-dose methoxyflurane (dizziness 36.3% vs 10.9% for placebo, headache 19.6% vs 12.9%) and for somnolence (4.9% vs 1%). None of the patients under low-dose methoxyflurane withdrew due to an AE. There were no observable effects on cardiovascular or respiratory parameters and no renal or hepatic concerns.
Discussion
Low-dose methoxyflurane and N2O both provided rapid analgesia for trauma patients in emergency care, with no significant differences between the two agents. The proportion of patients experiencing pain relief at 15 minutes after start of inhalation was greater under low-dose methoxyflurane, but statistical significance was not achieved.
Although both compounds have been used for many years in emergency care, the number of controlled studies was surprisingly small. Head-to-head trials between methoxyflurane and N2O in the emergency setting have not been performed; comparative data were only available from an older observational study in volunteers23 and two studies in dentistry.11,24 The synthesis of the data identified in our search thus required an indirect approach to treatment comparison. The robustness of such an approach is limited, but indirect analyses offer a first assessment in the absence of head-to-head comparisons. It should be noted that a blinded head-to-head trial of these two agents would not be feasible, due to their properties and delivery methods. Low-dose methoxyflurane is an almost colorless, volatile liquid with a characteristic fruity odor that is vaporized in a handheld inhaler. N2O is a homogeneous gas (50:50 mixture with oxygen) compressed into a cylinder that is connected to a demand valve with attached facemask or mouthpiece for delivery to the patient.25
It should also be noted that different pain scales were used in the two studies: the 100 mm VAS7 and the NRS,12 which are not interconvertible. The NRS is a whole-number scale with no intermediate pain scores, whereas the VAS considers point differences as well. However, correlation analyses of acute-pain data obtained in EDs found strong correlations between the NRS and VAS, and showed that they were interchangeable for acute-pain measurement in adult patients.17,18 In the present analysis, standardized median differences were computed to limit the risk of variability arising from the use of different assessment scales.
Besides analgesic effectiveness, safety profile and ease of handling (in particular in prehospital settings) will influence the decision on which analgesic to use in emergency care. A comparison of safety data of the two studies included in the indirect treatment analysis was not possible, due to the different time periods analyzed. In the following paragraphs, general safety information for the two compounds is provided.
Low-dose methoxyflurane is contraindicated in patients hypersensitive to the agent or to any fluorinated anesthetics, patients with malignant hyperthermia, patients showing signs of liver damage after previous methoxyflurane use or halogenated hydrocarbon anesthesia, and those with clinically significant renal impairment, an altered level of consciousness, clinically evident cardiovascular instability, or with clinically evident respiratory depression.5 The most common adverse reactions are related to the central nervous system (eg, dizziness, headache, somnolence) and are mostly transient. Dosages should not exceed 6 mL for a single administration, because methoxyflurane can cause significant nephrotoxicity at high doses.5 Renal damage probably occurs due to the production of fluoride ions when methoxyflurane is metabolized in the liver and kidneys,26,27 and has been reported for anesthetic methoxyflurane doses.28 However, when used at analgesic doses, the maximum 6 mL of methoxyflurane produce a minimum alveolar concentration (MAC) of 0.59 MAC hours,29 which is well below the exposure level of >2 MAC hours associated with renal damage.26 A review by Dayan concluded that methoxyflurane in low-dose analgesic concentrations has a safety margin of at least 2.7- to eightfold based on MAC hours or serum fluoride concentrations.29 Clinical laboratory evaluations did not indicate any nephrotoxicity in a recent trial in the emergency setting,7,8 and a large data-linkage study in the prehospital setting did not observe any increased risk for renal disease in the 17,629 patients administered low-dose methoxyflurane compared to 118,141 patients not receiving methoxyflurane during ambulance transport.30 Cardiovascular and respiratory parameters were not affected by analgesic methoxyflurane doses in emergency care either,8,31 and a retrospective study of case reports of ambulance patients did not show any negative effects of low-dose analgesic concentrations on pulse rate, systolic blood pressure, or respiratory rate.32 Although the latter study did not find any particular pattern to the patients’ systolic blood pressure after low-dose methoxyflurane administration across different age-groups, caution should be exercised in the elderly, due to possible reduction in blood pressure.5
N2O is contraindicated in any condition where gas is entrapped within the body and where expansion might be dangerous. Clinical examples are pneumothorax, air embolism, pneumocephalus, decompression sickness, following a recent dive, following air encephalography, severe bullous emphysema, use during myringoplasty, gross abdominal distension, and in patients who have recently received intraocular gas injections.25 There is a risk of additive effects when coadministered with centrally acting depressant medications.25 N2O can increase intracranial pressure in patients with head injury,33 which should preclude its use in traumatic brain injury. The side-effect profile of N2O includes dizziness, euphoria, disorientation, sedation, generalized tingling, nausea, and vomiting, which are generally minor and rapidly reversible.25 A systematic review of the safety of N2O in the prehospital setting found that none of the investigated AEs (nausea, vomiting, dizziness, drowsiness, headache, hypotension, and oxygen desaturation) was significantly associated with N2O treatment.34
Low-dose methoxyflurane and N2O are both self-administered, self-titrated, nonnarcotic, noninvasive inhalational agents with similar onset times of pain relief. Both provide rapid and well tolerated analgesia for trauma patients in emergency care, but there are differences regarding ease of administration, handling, and portability. Low-dose methoxyflurane is easily administered via a handheld inhaler, whereas self-administration of N2O can be more difficult. Many patients (especially those in pain) do not have the inspiratory capability to trigger the demand valve for successful inhalation of N2O. Elderly patients in particular find this difficult, and if edentulous might also encounter problems obtaining a competent fit of the alternative face mask. The small methoxyflurane inhaler is also easier to handle than the heavier and bulkier N2O cylinder. Easy portability is especially desired for emergency care in remote or difficult-to-reach locations and mass-casualty situations, but is also important for urban/rural paramedics who carry heavy backpacks and in addition often also monitoring equipment. The weighty N2O cylinder is thus an additional challenge that should not be underestimated. Furthermore, special precautions for storage are required for N2O cylinders. N2O is nonflammable, but strongly supports combustion due to the high oxygen concentration within the mixture, and should not be stored near stocks of combustible material.25 The cylinders also require temperatures above 10°C for at least 24 hours before use to avoid separation of the gas mixture, which could result in hypoxic concentrations being delivered as the cylinder empties.
Conclusion
Low-dose methoxyflurane and N2O both provide rapid and well-tolerated analgesia via a noninvasive administration route, and are suitable options for the pain treatment of trauma patients in emergency care. Due to the ease of administration and portability, inhaled low-dose methoxyflurane may not only offer advantages in emergency situations in remote or difficult-to-reach locations and in mass-casualty situations but also be of significant value in urban and rural environments.
Supplementary material
Table S1.
Search strategy for Embase and Medline databases
| Query | Facet | Hits | |
|---|---|---|---|
| 1 | “clinical trial”/exp OR “randomization”/de OR “controlled study”/de OR “comparative study”/de OR “single blind procedure”/de OR “double blind procedure”/de OR “crossover procedure”/de OR “placebo”/de OR “clinical trial” OR “clinical trials” OR “controlled clinical trial” OR “controlled clinical trials” OR “randomised controlled trial” OR “randomized controlled trial” OR “randomised controlled trials” OR “randomized controlled trials” OR “randomisation” OR “randomization” OR rct OR “random allocation” OR “randomly allocated” OR “allocated randomly” OR placebo* OR “prospective study”/de OR allocated NEAR/2 random OR random* NEAR/1 assign* OR random* OR (single OR double OR triple OR treble) NEAR/1 (blind* OR mask*) NOT (“case study”/de OR “case report” OR “abstract report”/de OR “letter”/de) | Study design | 6,893,787 |
| 2 | nrct OR “n rct” OR n?rct OR non NEAR/2 random* | 20,235 | |
| 3 | “controlled clinical trial”/exp OR “intervention study”/exp | 532,584 | |
| 4 | “major clinical study”/exp | 2,425,461 | |
| 5 | “cohort analysis”/exp OR “longitudinal study”/exp OR “retrospective study”/exp OR “follow up”/exp OR “clinical article”/exp | 2,855,582 | |
| 6 | cohort*:ab,ti OR ((“follow up” OR followup) NEXT/1 (study OR studies)):ab,ti | 569,950 | |
| 7 | #2 OR #3 OR #4 OR #5 OR #6 | 5,221,241 | |
| 8 | “letter”/de OR “abstract report”/de OR “case report” OR “case study”/de | 2,902,272 | |
| 9 | #7 NOT #9 | 4,969,978 | |
| 10 | “pain”/exp | Emergency and trauma-associated pain | 947,851 |
| 11 | pain* OR agony OR agoniz* OR nocicept* OR sur* NEAR/3 pain OR proced* NEAR/3 pain | 665,889 | |
| 12 | #10 OR #11 | 1153389 | |
| 13 | wound* OR injur* OR traum* OR casualt* OR fracture* OR laceration* OR burn* OR dislocation* OR contusion* | 2,058,086 | |
| 14 | “injury”/exp | 1,717,928 | |
| 15 | “emergency”/exp | 44,436 | |
| 16 | pre NEAR/3 hospital* OR pre?hospital OR prehospital | 20,049 | |
| 17 | emergenc* | 525,115 | |
| 18 | OR/13-17 | 2,795,186 | |
| 19 | #12 AND #18 | 285,657 | |
| 20 | ambulance/de OR paramed* | 37,507 | |
| 21 | pain NEAR/3 “treatment” OR pain NEAR/3 “management” | 84,426 | |
| 22 | #20 AND #21 | 320 | |
| 23 | #18 OR #22 | 285,795 | |
| 24 | (“methoxyflurane”/exp OR penthrox) OR penthrox:ab,ti | Intervention | 4,158 |
| 25 | paracetamol”/syn OR “paracetamol”:ab,ti | 154,020 | |
| 26 | nitrous oxide”/syn OR “nitrous oxide”:ab,ti OR nitrous NEXT/2 oxide OR n2o | 37,700 | |
| 27 | OR/24-26 | 193,693 | |
| 28 | (#1 OR #9) AND #23 AND #25 | Combined | 4,921 |
| 29 | (#1 OR #9) AND #23 AND (#24 OR #26) | Combined N2O and Penthrox | 628 |
| Total for screening | 628 |
Table S2.
Search strategy for Cochrane database
| Query | Facet | Hits | |
|---|---|---|---|
| 1 | MeSH descriptor: [Pain] explode all trees | Emergency and trauma-associated pain | 33,832 |
| 2 | pain* or agony or agoniz* or nocicept* or sur* near/3 pain or proced* near/3 pain | 93,700 | |
| 3 | #1 or #2 | 99,555 | |
| 4 | MeSH descriptor: [Wounds and Injuries] explode all trees | 16,427 | |
| 5 | wound* or injur* or traum* or casualt* or fracture* or laceration* or burn* or dislocation* or contusion* | 73,688 | |
| 6 | MeSH descriptor: [Emergencies] explode all trees | 662 | |
| 7 | pre near/3 hospital* or pre?hospital or prehospital | 1,277 | |
| 8 | emergenc* | 19,137 | |
| 9 | #4 or #5 or #6 or #7 or #8 | 90,705 | |
| 10 | #3 and #9 | 18,620 | |
| 11 | MeSH descriptor: [Ambulances] this term only | 107 | |
| 12 | paramed* | 780 | |
| 13 | #11 or #12 | 861 | |
| 14 | pain near/3 “treatment” or pain near/3 “management” | 11,915 | |
| 15 | #13 and #14 | 31 | |
| 16 | #10 or #15 | 18,629 | |
| 17 | methoxyflurane or “da 759” or da759 or inhalan or “methoxy flurane” or methoxyfluorane or methoxyfluran or metofan or metofane or “nsc 110432” or nsc110432 or penthrane or pentrane or penthrox or penthrox:ab,ti | Intervention | 71 |
| 18 | paracetamol or “paracetamol”:ab,ti | 4,414 | |
| 19 | nitrous oxide or “nitrous oxide”:ab,ti or nitrous next/2 oxide or n2o | 5,013 | |
| 20 | #17 or #18 or #19 | 9,312 | |
| 21 | #16 and #20 | Combined | 1,139 |
| 22 | #16 and #20 in Trials | 744 | |
| 23 | Trials pertaining to Penthrox and nitrous oxide for screening | 180 |
Table S3.
Cochrane Collaboration checklist for bias assessment
| Cochrane criteria | Coffey et al1 | Ducassé et al2 | Kariman et al3 | Konyakev et al4 |
|---|---|---|---|---|
| Was randomization carried out appropriately? | Low risk; randomization sequence developed by an independent statistician | Low risk; computer-generated randomization list | Low risk; random-number table | Low risk; random-number table |
| Was the concealment of treatment allocation adequate? | Low risk; treatment assembly and allocation by an unblinded team member | Low risk; use of sealed opaque envelopes | Low risk; use of sealed envelopes | Unclear; details not reported |
| Were the groups similar at the outset of the study in terms of prognostic factors? | Low risk; baseline characteristics were well balanced between the groups | Low risk; baseline characteristics were well balanced between the groups | Low risk; baseline characteristics were well balanced between the groups | Unclear; baseline characteristics were not reported |
| Were the care providers, participants, and outcome assessors blind to treatment allocation? If any of these people were not blinded, what might have been the likely impact on the risk of bias? | Low risk; the research nurse, the treating physician, and the patient all remained blind to the treatment administered | Unclear; this was a double-blind trial, but the details pertaining to blinding status were not reported | High risk; the treating physicians and individuals collecting the data were not blinded | Unclear; details pertaining to blinding were not available |
| Were there any unexpected imbalances in dropouts between groups? If so, were they explained or adjusted for? | Low risk; data pertaining to withdrawals were adequately reported | Low risk; no patients withdrew from the study | Low risk; no patients withdrew from the study | Unclear; data pertaining to withdrawals were not reported |
| Is there any evidence to suggest that the authors measured more outcomes than they reported? | Low risk; all outcomes mentioned in NCT01420159 were reported by the publication | Low risk; all outcomes mentioned in NCT01356745 were reported by the publication | Unclear; it was unclear whether more outcomes were measured than reported | Unclear; it was unclear whether more outcomes were measured than reported |
| Did the analysis include ITT analysis? If so, was this appropriate, and were appropriate methods used to account for missing data? | Low risk; modified ITT population was involved in data analysis | Low risk; analysis included ITT population | Low risk; analysis included ITT population | Unclear; details pertaining to analysis type were unclear |
| Additional criteria | ||||
| Jadad score | 5 | 5 | 3 | 2 |
| Allocation-concealment grade | A | A | A | B |
Abbreviation: ITT, intent to treat.
References
- 1.Coffey F, Wright J, Hartshorn S, et al. STOP!: a randomised, double-blind, placebo-controlled study of the efficacy and safety of methoxyflurane for the treatment of acute pain. Emerg Med J. 2014;31:613–618. doi: 10.1136/emermed-2013-202909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ducassé JL, Siksik G, Durand-Béchu M, et al. Nitrous oxide for early analgesia in the emergency setting: a randomized, double-blind multicenter prehospital trial. Acad Emerg Med. 2013;20:178–184. doi: 10.1111/acem.12072. [DOI] [PubMed] [Google Scholar]
- 3.Kariman H, Majidi A, Amini A, Dolatabadi AA, Derakhshanfar H. Nitrous oxide/oxygen compared with fentanyl in reducing pain among adults with isolated extremity trauma: a randomized trial. Emerg Med Australas. 2011;23:761–768. doi: 10.1111/j.1742-6723.2011.01447.x. [DOI] [PubMed] [Google Scholar]
- 4.Konyakev A, Baimagambetov S, Sainov M, Bekmagambetova N, Ahmetova A. The using of methoxyflurane as analgesic in patients with ankle injury. Intensive Care Med. 2013;39:S439. [Google Scholar]
Acknowledgments
Writing and editorial assistance was provided by Elke Grosselindemann and Birgit Brett and paid for by Mundipharma International Ltd. Parexel International performed the systematic review and indirect treatment comparison, which was funded by Mundipharma International Ltd.
Footnotes
Disclosure
KMP has been funded for work undertaken on behalf of Orion Medical in seeking the MHRA license and also for work undertaken for Mundipharma and Galen. MKS is an employee of Parexel, and SD and AE are employees of Mundipharma. IS was an employee of Parexel at the time the work was carried out, and is currently working for Eli Lilly Services India Pvt Ltd. The authors report no other conflicts of interest in this work.
References
- 1.Berben SA, Meijs TH, van Dongen RT, et al. Pain prevalence and pain relief in trauma patients in the accident & emergency department. Injury. 2008;39:578–585. doi: 10.1016/j.injury.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Berben SA, Schoonhoven L, Meijs TH, van Vugt AB, van Grunsven PM. Prevalence and relief of pain in trauma patients in emergency medical services. Clin J Pain. 2011;27:587–592. doi: 10.1097/AJP.0b013e3182169036. [DOI] [PubMed] [Google Scholar]
- 3.Keene DD, Rea WE, Aldington D. Acute pain management in trauma. Trauma. 2011;13:167–179. [Google Scholar]
- 4.Thomas SH. Management of pain in the emergency department. ISRN Emerg Med. 2013;2013:583132. [Google Scholar]
- 5.Electronic Medicines Compendium. Penthrox 3 mL inhalation vapour, liquid [summary of product characteristics] [Accessed September 30, 2016]. Available from: https://www.medicines.org.uk/emc/medicine/31391.
- 6.Grindlay J, Babl F. Review article: efficacy and safety of methoxyflurane analgesia in the emergency department and prehospital setting. Emerg Med Australas. 2009;21:4–11. doi: 10.1111/j.1742-6723.2009.01153.x. [DOI] [PubMed] [Google Scholar]
- 7.Coffey F, Wright J, Hartshorn S, et al. STOP!: a randomised, double-blind, placebo-controlled study of the efficacy and safety of methoxyflurane for the treatment of acute pain. Emerg Med J. 2014;31:613–618. doi: 10.1136/emermed-2013-202909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffey F, Dissmann P, Mirza K, Lomax M. Methoxyflurane analgesia in adult patients in the emergency department: a subgroup analysis of a randomized, double-blind, placebo-controlled study (STOP!) Adv Ther. 2016;33:2012–2031. doi: 10.1007/s12325-016-0405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim KJH, Koh ZX, Zafirah NA, et al. Clinical evaluation of Penthrox (methoxyflurane) and tramadol for the Singapore emergency ambulance service. 2016. [Accessed October 24, 2017]. Available from: http://www.emsasia2016.org/upload/summary/O-041.pdf.
- 10.Gaskell AL, Jephcott CG, Smithells JR, Sleigh JW. Self-administered methoxyflurane for procedural analgesia: experience in a tertiary Australasian centre. Anaesthesia. 2016;71:417–423. doi: 10.1111/anae.13377. [DOI] [PubMed] [Google Scholar]
- 11.Abdullah WA, Sheta SA, Nooh NS. Inhaled methoxyflurane (Penthrox) sedation for third molar extraction: a comparison to nitrous oxide sedation. Aust Dent J. 2011;56:296–301. doi: 10.1111/j.1834-7819.2011.01350.x. [DOI] [PubMed] [Google Scholar]
- 12.Ducassé JL, Siksik G, Durand-Béchu M, et al. Nitrous oxide for early analgesia in the emergency setting: a randomized, double-blind multicenter prehospital trial. Acad Emerg Med. 2013;20:178–184. doi: 10.1111/acem.12072. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Shamseer L, Clarke M, Ghersi D. Preferred Reporting Items for Systematic Reviews and Meta-analyses protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scottish Intercollegiate Guidelines Network Search filters. [Accessed October 24, 2017]. Available from: http://www.sign.ac.uk/search-filters.html.
- 15.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390–392. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 18.Bahreini M, Jalili M, Moradi-Lakeh M. A comparison of three self-report pain scales in adults with acute pain. J Emerg Med. 2015;48:10–18. doi: 10.1016/j.jemermed.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 19.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 20.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14:417–428. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Kariman H, Majidi A, Amini A, Dolatabadi AA, Derakhshanfar H. Nitrous oxide/oxygen compared with fentanyl in reducing pain among adults with isolated extremity trauma: a randomized trial. Emerg Med Australas. 2011;23:761–768. doi: 10.1111/j.1742-6723.2011.01447.x. [DOI] [PubMed] [Google Scholar]
- 22.Konyakev A, Baimagambetov S, Sainov M, Bekmagambetova N, Ahmetova A. The using of methoxyflurane as analgesic in patients with ankle injury. Intensive Care Med. 2013;39:S439. [Google Scholar]
- 23.Tomlin PJ, Jones BC, Edwards R, Robin PE. Subjective and objective sensory responses to inhalation of nitrous oxide and methoxyflurane. Br J Anaesth. 1973;45:719–725. doi: 10.1093/bja/45.7.719. [DOI] [PubMed] [Google Scholar]
- 24.Edmunds DH, Rosen M. Inhalation sedation for conservative dentistry: a comparison between nitrous oxide and methoxyflurane. Br Dent J. 1975;139:398–402. doi: 10.1038/sj.bdj.4803643. [DOI] [PubMed] [Google Scholar]
- 25.Medicines and Healthcare Products Regulatory Agency Entonox [summary of product characteristics] 2016. [Accessed November 9, 2016]. Available from: http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1464324146707.pdf.
- 26.Cousins MJ, Mazze RI. Methoxyflurane nephrotoxicity: a study of dose response in man. JAMA. 1973;225:1611–1616. doi: 10.1001/jama.225.13.1611. [DOI] [PubMed] [Google Scholar]
- 27.Mazze RI, Cousins MJ. Biotransformation of methoxyflurane. Int Anesthesiol Clin. 1974;12:93–105. doi: 10.1097/00004311-197412020-00011. [DOI] [PubMed] [Google Scholar]
- 28.Crandell WB, Pappas SG, Macdonald A. Nephrotoxicity associated with methoxyflurane anesthesia. Anesthesiology. 1966;27:591–607. doi: 10.1097/00000542-196609000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Dayan AD. Analgesic use of inhaled methoxyflurane: evaluation of its potential nephrotoxicity. Hum Exp Toxicol. 2016;35:91–100. doi: 10.1177/0960327115578743. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs IG. Health effects of patients given methoxyflurane in the pre-hospital setting: a data linkage study. Open Emerg Med J. 2010;3:7–13. [Google Scholar]
- 31.Johnston S, Wilkes GJ, Thompson JA, Ziman M, Brightwell R. Inhaled methoxyflurane and intranasal fentanyl for prehospital management of visceral pain in an Australian ambulance service. Emerg Med J. 2011;28:57–63. doi: 10.1136/emj.2009.078717. [DOI] [PubMed] [Google Scholar]
- 32.Oxer HF. Effects of Penthrox (methoxyflurane) as an analgesic on cardiovascular and respiratory functions in the pre-hospital setting. J Mil Veterans Health. 2016;24:14–20. [Google Scholar]
- 33.Moss E, McDowall DG. I.C.P. increases with 50% nitrous oxide in oxygen in severe head injuries during controlled ventilation. Br J Anaesth. 1979;51:757–761. doi: 10.1093/bja/51.8.757. [DOI] [PubMed] [Google Scholar]
- 34.Faddy SC, Garlick SR. A systematic review of the safety of analgesia with 50% nitrous oxide: can lay responders use analgesic gases in the prehospital setting? Emerg Med J. 2005;22:901–906. doi: 10.1136/emj.2004.020891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Search strategy for Embase and Medline databases
| Query | Facet | Hits | |
|---|---|---|---|
| 1 | “clinical trial”/exp OR “randomization”/de OR “controlled study”/de OR “comparative study”/de OR “single blind procedure”/de OR “double blind procedure”/de OR “crossover procedure”/de OR “placebo”/de OR “clinical trial” OR “clinical trials” OR “controlled clinical trial” OR “controlled clinical trials” OR “randomised controlled trial” OR “randomized controlled trial” OR “randomised controlled trials” OR “randomized controlled trials” OR “randomisation” OR “randomization” OR rct OR “random allocation” OR “randomly allocated” OR “allocated randomly” OR placebo* OR “prospective study”/de OR allocated NEAR/2 random OR random* NEAR/1 assign* OR random* OR (single OR double OR triple OR treble) NEAR/1 (blind* OR mask*) NOT (“case study”/de OR “case report” OR “abstract report”/de OR “letter”/de) | Study design | 6,893,787 |
| 2 | nrct OR “n rct” OR n?rct OR non NEAR/2 random* | 20,235 | |
| 3 | “controlled clinical trial”/exp OR “intervention study”/exp | 532,584 | |
| 4 | “major clinical study”/exp | 2,425,461 | |
| 5 | “cohort analysis”/exp OR “longitudinal study”/exp OR “retrospective study”/exp OR “follow up”/exp OR “clinical article”/exp | 2,855,582 | |
| 6 | cohort*:ab,ti OR ((“follow up” OR followup) NEXT/1 (study OR studies)):ab,ti | 569,950 | |
| 7 | #2 OR #3 OR #4 OR #5 OR #6 | 5,221,241 | |
| 8 | “letter”/de OR “abstract report”/de OR “case report” OR “case study”/de | 2,902,272 | |
| 9 | #7 NOT #9 | 4,969,978 | |
| 10 | “pain”/exp | Emergency and trauma-associated pain | 947,851 |
| 11 | pain* OR agony OR agoniz* OR nocicept* OR sur* NEAR/3 pain OR proced* NEAR/3 pain | 665,889 | |
| 12 | #10 OR #11 | 1153389 | |
| 13 | wound* OR injur* OR traum* OR casualt* OR fracture* OR laceration* OR burn* OR dislocation* OR contusion* | 2,058,086 | |
| 14 | “injury”/exp | 1,717,928 | |
| 15 | “emergency”/exp | 44,436 | |
| 16 | pre NEAR/3 hospital* OR pre?hospital OR prehospital | 20,049 | |
| 17 | emergenc* | 525,115 | |
| 18 | OR/13-17 | 2,795,186 | |
| 19 | #12 AND #18 | 285,657 | |
| 20 | ambulance/de OR paramed* | 37,507 | |
| 21 | pain NEAR/3 “treatment” OR pain NEAR/3 “management” | 84,426 | |
| 22 | #20 AND #21 | 320 | |
| 23 | #18 OR #22 | 285,795 | |
| 24 | (“methoxyflurane”/exp OR penthrox) OR penthrox:ab,ti | Intervention | 4,158 |
| 25 | paracetamol”/syn OR “paracetamol”:ab,ti | 154,020 | |
| 26 | nitrous oxide”/syn OR “nitrous oxide”:ab,ti OR nitrous NEXT/2 oxide OR n2o | 37,700 | |
| 27 | OR/24-26 | 193,693 | |
| 28 | (#1 OR #9) AND #23 AND #25 | Combined | 4,921 |
| 29 | (#1 OR #9) AND #23 AND (#24 OR #26) | Combined N2O and Penthrox | 628 |
| Total for screening | 628 |
Table S2.
Search strategy for Cochrane database
| Query | Facet | Hits | |
|---|---|---|---|
| 1 | MeSH descriptor: [Pain] explode all trees | Emergency and trauma-associated pain | 33,832 |
| 2 | pain* or agony or agoniz* or nocicept* or sur* near/3 pain or proced* near/3 pain | 93,700 | |
| 3 | #1 or #2 | 99,555 | |
| 4 | MeSH descriptor: [Wounds and Injuries] explode all trees | 16,427 | |
| 5 | wound* or injur* or traum* or casualt* or fracture* or laceration* or burn* or dislocation* or contusion* | 73,688 | |
| 6 | MeSH descriptor: [Emergencies] explode all trees | 662 | |
| 7 | pre near/3 hospital* or pre?hospital or prehospital | 1,277 | |
| 8 | emergenc* | 19,137 | |
| 9 | #4 or #5 or #6 or #7 or #8 | 90,705 | |
| 10 | #3 and #9 | 18,620 | |
| 11 | MeSH descriptor: [Ambulances] this term only | 107 | |
| 12 | paramed* | 780 | |
| 13 | #11 or #12 | 861 | |
| 14 | pain near/3 “treatment” or pain near/3 “management” | 11,915 | |
| 15 | #13 and #14 | 31 | |
| 16 | #10 or #15 | 18,629 | |
| 17 | methoxyflurane or “da 759” or da759 or inhalan or “methoxy flurane” or methoxyfluorane or methoxyfluran or metofan or metofane or “nsc 110432” or nsc110432 or penthrane or pentrane or penthrox or penthrox:ab,ti | Intervention | 71 |
| 18 | paracetamol or “paracetamol”:ab,ti | 4,414 | |
| 19 | nitrous oxide or “nitrous oxide”:ab,ti or nitrous next/2 oxide or n2o | 5,013 | |
| 20 | #17 or #18 or #19 | 9,312 | |
| 21 | #16 and #20 | Combined | 1,139 |
| 22 | #16 and #20 in Trials | 744 | |
| 23 | Trials pertaining to Penthrox and nitrous oxide for screening | 180 |
Table S3.
Cochrane Collaboration checklist for bias assessment
| Cochrane criteria | Coffey et al1 | Ducassé et al2 | Kariman et al3 | Konyakev et al4 |
|---|---|---|---|---|
| Was randomization carried out appropriately? | Low risk; randomization sequence developed by an independent statistician | Low risk; computer-generated randomization list | Low risk; random-number table | Low risk; random-number table |
| Was the concealment of treatment allocation adequate? | Low risk; treatment assembly and allocation by an unblinded team member | Low risk; use of sealed opaque envelopes | Low risk; use of sealed envelopes | Unclear; details not reported |
| Were the groups similar at the outset of the study in terms of prognostic factors? | Low risk; baseline characteristics were well balanced between the groups | Low risk; baseline characteristics were well balanced between the groups | Low risk; baseline characteristics were well balanced between the groups | Unclear; baseline characteristics were not reported |
| Were the care providers, participants, and outcome assessors blind to treatment allocation? If any of these people were not blinded, what might have been the likely impact on the risk of bias? | Low risk; the research nurse, the treating physician, and the patient all remained blind to the treatment administered | Unclear; this was a double-blind trial, but the details pertaining to blinding status were not reported | High risk; the treating physicians and individuals collecting the data were not blinded | Unclear; details pertaining to blinding were not available |
| Were there any unexpected imbalances in dropouts between groups? If so, were they explained or adjusted for? | Low risk; data pertaining to withdrawals were adequately reported | Low risk; no patients withdrew from the study | Low risk; no patients withdrew from the study | Unclear; data pertaining to withdrawals were not reported |
| Is there any evidence to suggest that the authors measured more outcomes than they reported? | Low risk; all outcomes mentioned in NCT01420159 were reported by the publication | Low risk; all outcomes mentioned in NCT01356745 were reported by the publication | Unclear; it was unclear whether more outcomes were measured than reported | Unclear; it was unclear whether more outcomes were measured than reported |
| Did the analysis include ITT analysis? If so, was this appropriate, and were appropriate methods used to account for missing data? | Low risk; modified ITT population was involved in data analysis | Low risk; analysis included ITT population | Low risk; analysis included ITT population | Unclear; details pertaining to analysis type were unclear |
| Additional criteria | ||||
| Jadad score | 5 | 5 | 3 | 2 |
| Allocation-concealment grade | A | A | A | B |
Abbreviation: ITT, intent to treat.