Highlights
-
•
Moraxella osloensis is a gram-negative coccobacillus, which part of normal flora in human skin and mucus.
-
•
Moraxella osloensis rarely causes infections in human, especially in immunocompromised.
-
•
Proper microbiologic investigation is important to diagnose Moraxella osloensis.
-
•
Accurate diagnosis and proper treatment are crucial for the resolution of infections caused by Moraxella osloensis.
Keywords: Moraxella osloensis, Osteomyelitis, Case report
Abstract
Introduction
Moraxella osloensis is a gram-negative coccobacillus, that is saprophytic on skin and mucosa, and rarely causing human infections. Reported cases of human infections usually occur in immunocompromised patients.
Presentation of case
We report the second case of M. osloensis-caused-osteomyelitis in literature, occurring in a young healthy man. The organism was identified by sequencing analysis of the 16S ribosomal RNA gene. Our patient was treated successfully with surgical debridement and intravenous third-generation cephalosporins.
Discussion
M. osloensis has been rarely reported to cause local or invasive infections. Our case report is the second case in literature and it is different from the previously reported case in that our patient has no chronic medical problems, no history of trauma, with unique presentation and features on the MRI and intraoperative finding.
Conclusion
Proper diagnosis is essential for appropriate treatment of osteomyelitis. RNA gene sequence analysis is the primary method of M. osloensis diagnosis. M. osloensis is usually susceptible to simple antibiotics.
1. Introduction
Moraxella consists of gram negative aerobic, non-motile, oxidase-positive and asaccharolytic coccobacilli [1], [2]. Moraxella osloensis species is one of seven species of Moraxella genus; with M. atlantae, M. canis, M. catarrhalis, M. lacunata, M. lincolnii and M. nonliquefaciens [1]. Moraxella osloensis is part of normal flora in the skin, mucus membranes and respiratory tract of humans. Infection with this organism is rare, and few cases in literature were reported. Of the cases reported to be caused by infection with Moraxella osloensis include endocarditis, septic arthritis, bacteremia, and meningitis [3], [4], [5].
There is only one reported case in literature for osteomyelitis caused by Moraxella osloensis in 1981 in paraplegic psychological bedridden male patient with multiple comorbidities and multiple decubitus ulcers [6]. We report a case of femur osteomyelitis caused by Moraxella osloensis in a young healthy adult with no other focus of infection, who presented to our hospital which is a public tertiary orthopedic center.
This work has been reported in line with the SCARE criteria [7].
2. Case report
A 30-year-old male laborer with no significant past medical history presented to our hospital October 2016 with chronic sinus discharge from his left thigh. He mentioned that his problem started two months ago when he started complaining of pain in his left thigh, associated with swelling, redness and hotness, and noticed a small boil with intermittent serous discharge at his thigh, which was diagnosed as a subcutaneous abscess by a general practitioner, that was treated in a primary health care center with local drainage and multiple short courses of antibiotics (Cloxacillin 500 mg TID for 14 days, then Ciprofloxacin 500 mg BID for another 14 days) with no benefit. The drainage was not sent for culture by the treating physician. During this period, the patient’s pain used to improve temporarily after each drainage, however, after few days, the pain and local symptoms recur. Therefore, he kept visiting the emergency department with tender indurated skin at the posterior aspect of the thigh, where an Ultrasound was performed showing a 10 cm homogenous anechoic collection, with surrounding subcutaneous edema. Again, a needle aspiration was attempted, but no pus came out. And for two months the patient kept suffering until he was referred to orthopedics service in our institution.
At presentation, the patient was afebrile with no constitutional symptoms and with chronic sinus discharge at the posterior aspect of his left thigh (Fig. 1). Initial lab investigations were normal with normal inflammatory markers; WBC = 9.3*10^3/ul, ESR = 21 mm/hr, CRP < 5 mg/dl. Left femur radiographs and MRI showed features of chronic osteomyelitis with sinus tract communicating with skin at the posterior thigh type II-A according to Cierny-Mader classification of osteomyelitis (superficial osteomyelitis in a normal host) (Fig. 2, Fig. 3, Fig. 4). Swab from the discharge was taken for gram stain and culture, and the patient was started on cefazolin empirically. Quantiferon test for TB was negative, and serology tests for Malaria, Brucella, Salmonella, Aspergillus, and CMV were negative.
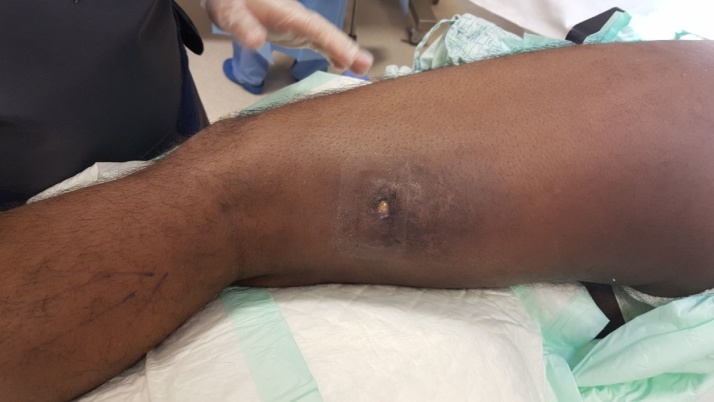
Fig. 1.
A clinical photograph illustrating the sinus on the postero-lateral aspect of the patient’s thigh.
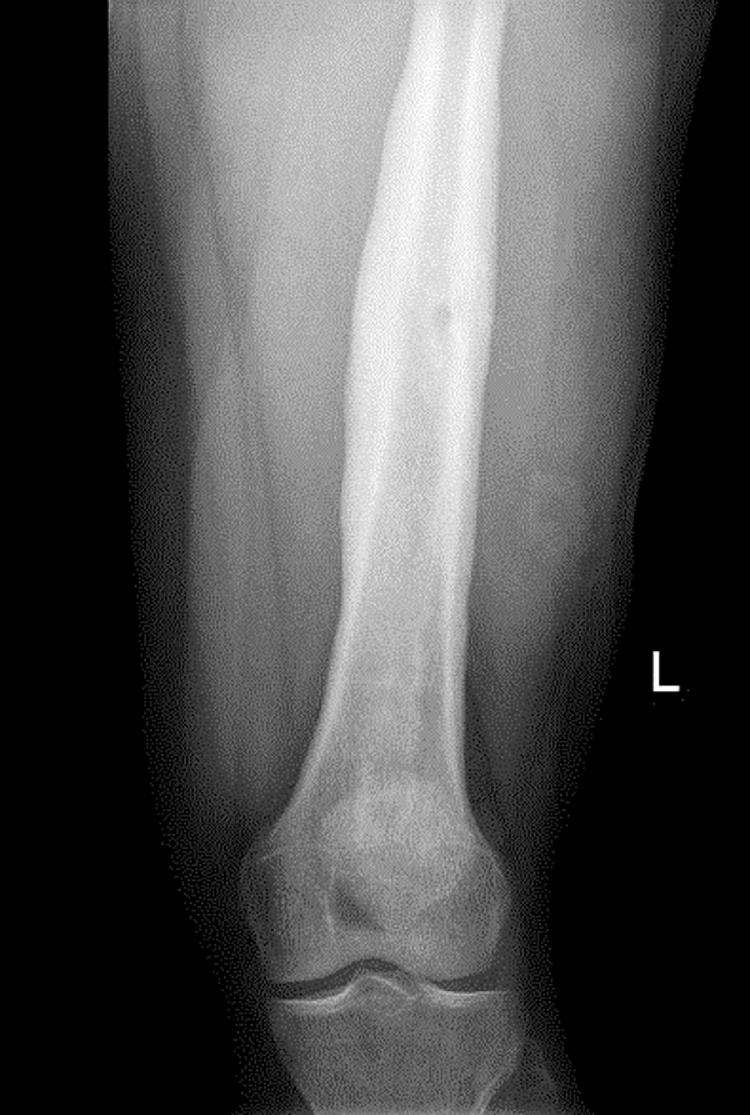
Fig. 2.
Antero-posterior X-ray view of the left femur, showing mid shaft periosteal thickening & small area of lucency in cortex may suggest osteomyelitis.
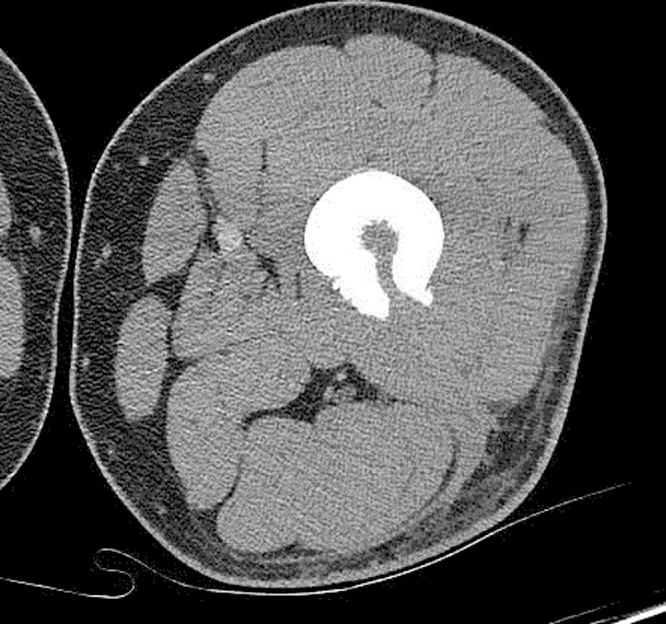
Fig. 3.
Axial view of CT scan showing features suggestive of osteomyelitis with tiny involucrum seen in the marrow at the level of the cortical defect.
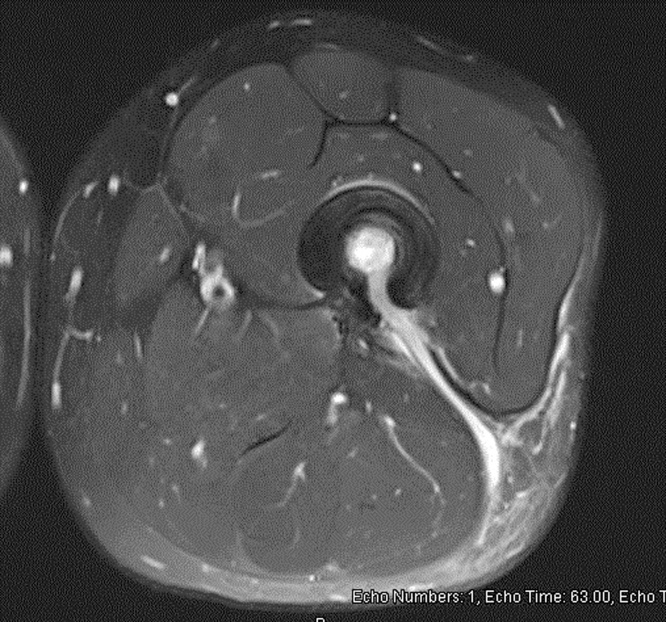
Fig. 4.
MRI T1 with intravenous contrast, showing femur osteomyelitis and sinus tract communicating with the posterior aspect of the femur.
Several days later, the Gram-stained smear of colonies revealed gram-negative coccobacilli. The isolate was oxidase positive and did not oxidize glucose. Following 16S ribosomal RNA sequencing identified the organism as Moraxella oselensis. And as in-vitro susceptibility testing for this organism is not routinely done in our hospital, the patient continued Cefazolin 1 g TID for another ten days. Then antibiotic was changed to intravenous Ampicillin-Sulbactam every 6 h, and posted for bone debridement and biopsy as recommended by ID team as sinus discharge did not stop.
2.1. Intra-operative findings
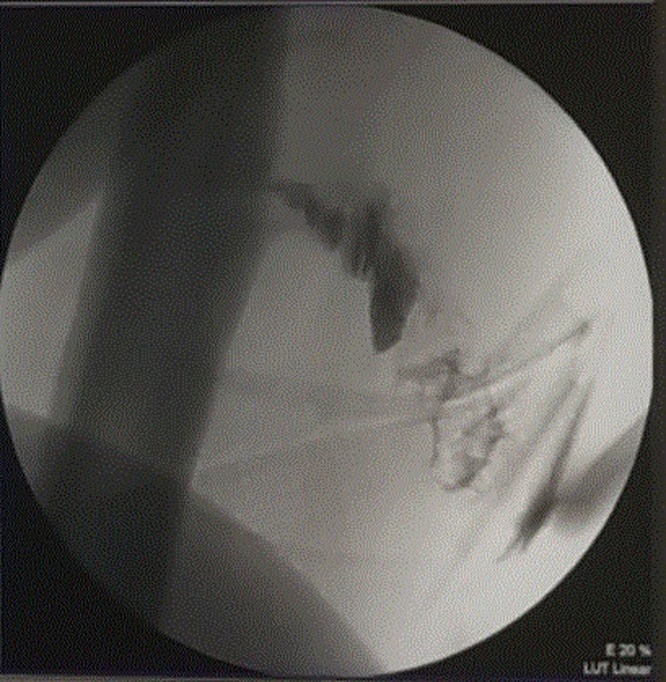
Sinogram showed two sinus tracts, one is superior and another one is inferior, with no contrast connected to the intramedullary cavity of the femur (Fig. 5). A 3 cm skin incision, and excision of the upper sinus tract and curettage for the lower tract were done. Afterwards, drilling for the two bony sinuses in the femur was performed, no intramedullary pus was appreciated. Bone biopsy was sent for culture and histopathology. The tissues were washed with copious amount of normal saline, and Vacuum dressing was applied.
Fig. 5.
Intra-operative sonogram, showing contrast communicating with the posterior aspect of femur.
The surgical pathology specimen culture report was ready after 7 days post-operatively, which showed no growth; this might be explained as the patient had already been on antibiotics for around 16 days (Cefazolin for 10 days, and Ampicillin-Sulbactam for 6 days). Manual sensitivity for Moraxella oseolensis from the previous specimen was tested and showed it was very sensitive to Amoxicillin, Ceftriaxone, and Trimethoprim-Sulphamethoxazole.
Post-operatively, the patient was started on intravenous Ceftriaxone and Vacuum dressing was changed every 3 days, with only minimal serosanguinous collection. After seven days, the wound became dry, the Vacuum dressing was removed, sterile dressing was applied, and the patient was discharged home on daily intravenous 2 g Ceftriaxone to be given in a special clinic for outpatient intravenous drugs administration, to complete a total duration of six weeks of treatment.
The patient sinus discharge stopped, and complete wound healing was observed and documented on his visit to the outpatient clinic after 21 days post-operatively. He was followed up clinically for more than 3 months, the inflammatory markers maintained their normal range values during the post-operative period (WBC = 8.8*10^3/ul, ESR = 6 mm/hr, CRP <5 mg/dl), and the patient stayed asymptomatic during this period.
3. Discussion
The species M. osloensis was proposed in 1967 to distinguish a subset of organisms that had formerly been classified as Moraxella nonliquefaciens. This organism is a gram negative aerobic, oxidase-positive coccobacillus that has been isolated from environmental sources in the hospital, including anesthetic agents [8], and sink traps; suggesting that this bacterium is capable of spreading to patients from the environment. The clinical significance of infections due to this organism are less well studied. It has been rarely reported to cause local or invasive infections, including meningitis, endocarditis, bacteremia, pneumonia, peritonitis, and catheter associated infection [3], [4], [5], [9], [10], [11]. Fewer than 40 cases of infection due to this genus have been described [12]. The vast majority of these cases occurred in immunocompromised patients, including cases with solid tumors or hematological malignancies or transplant recipients. In the musculoskeletal system, only 2 cases of septic arthritis caused by this organism; both were of the knee joint, the first was in 1969 in a 2-year-old girl and was associated with vaginal discharge, and the other of a 45 years old healthy man in 1986 [13], [14]. The only previously reported case report of osteomyelitis caused by M.osloensis occurred in a patient with paraplegia and multiple comorbidities with decubitus ulcers [6]. Our case report is considered the second case in literature and it is different from the previously reported case in that our patient has no chronic medical problems with no history of trauma, with unique presentation and features on the MRI and intraoperative finding.
Identification of an unusual bacterial isolate is a very challenging in a clinical microbiology laboratory. Only after RNA gene sequence analysis, we could diagnose the isolates as M. osloensis [15]. Due to the low number of cases, there are no guidelines of treatment, however, M. osloensis is usually susceptible to penicillin G, cephalosporins, and aminoglycosides [16], [17]. Our patient was treated successfully with surgical debridement and intravenous third-generation cephalosporins alone.
4. Conclusion
As human infection with M.osloensis is still not fully understood. Proper diagnosis and antimicrobial treatment are prudent. This is not a new clinically emerging pathogen, but rather emerging owing to new techniques. Even if this bacterium is sensitive to antibiotics, but it remains a pathogen that can cause lethal diseases such as endocarditis, that seem to occur in immunocompromised patients. We hope that the unique presentation of our case helps to improve the understanding of this organism and its clinical significance and treatment.
Informed consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest
None of the authors have any financial or personal relationship with other people, or organizations, that could inappropriately influence our work; therefore, there is no conflict of interest for all authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This study received ethical approval from the institutional review board (IRB) in the Medical Research Center in Hamad Medical Corporation.
Author contribution
Nidal J. Alkhatib: collecting the data, manuscript writing.
Manaf H. Younis: collecting the data, manuscript writing.
Ahmad S. Alobaidi: manuscript writing and reviewing.
Nebal M. Shaath: principal investigator, manuscript writing and reviewing.
Guarantor
Manaf H. Younis.
Nidal J. Alkhatib.
Ahmad S. Alobaidi.
Nebal M. Shaath.
References
- 1.Schreckenberger P.C., Daneshvar M.I., Weyant R.S., Hollis D.G. Acinetobacter, Achromobacter, Chryseobacterium, Moraxella, and other nonfermentative gram-negative rods. In: Murray P.R., Baron E.J., Jorgensen J.H., Pfaller M.A., editors. Manual of Clinical Microbiology. American Society for Microbiology; Washington: 2003. pp. 754–757. [Google Scholar]
- 2.Weyant R.S., Moss C.W., Weaver R.E., Hollis D.G., Jordan J.G., Cook E.C., Daneshvar M.I. Williams & Wilkins; Baltimore: 1995. Identification of Unusual Pathogenic Gram-Negative Aerobic and Facultatively Anaerobic Bacteria; pp. 390–405. [Google Scholar]
- 3.Buchman A.L., Pickett M.J., Mann L., Ament M.E. Central venous catheter infection caused by Moraxella osloensis in a patient receiving home parenteral nutrition. Diagn. Microbiol. Infect. Dis. 1993;17:163–166. doi: 10.1016/0732-8893(93)90028-6. [DOI] [PubMed] [Google Scholar]
- 4.Vuori-Holopainen E., Salo E., Saxen H., Vaara M., Tarkka E., Peltola H. Clinical “pneumococcal pneumonia” due to Moraxella osloensis: case report and a review. Scand. J. Infect. Dis. 2001;33:625–627. doi: 10.1080/00365540110026737. [DOI] [PubMed] [Google Scholar]
- 5.Shah S.S., Ruth A., Coffin S.E. Infection due to Moraxella osloensis: case report and review of the literature. Clin. Infect. Dis. 2000;30:179–181. doi: 10.1086/313595. [DOI] [PubMed] [Google Scholar]
- 6.Sugarman B., Clarridge J. Osteomyelitis caused by Moraxella osloensis. J. Clin. Microbiol. 1982;15:1148–1149. doi: 10.1128/jcm.15.6.1148-1149.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., the SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Bennett S.N., McNeil M.M., Bland L.A. Postoperative infections traced to contamination of an intravenous anesthetic, propofol. N. Engl. J. Med. 1995;333:147–154. doi: 10.1056/NEJM199507203330303. [DOI] [PubMed] [Google Scholar]
- 9.Berger U., Kreissel M. Menigitis due to Moraxella osloensis. Infection. 1974;2:166–168. doi: 10.1007/BF01642239. [DOI] [PubMed] [Google Scholar]
- 10.Fritsche D., Karte H., del Solar E. Moraxella osloensis as pathogen in septicemia. Infection. 1976;4:53–54. doi: 10.1007/BF01638351. [DOI] [PubMed] [Google Scholar]
- 11.Han X.Y., Tarrand J.J. Moraxella osloensis blood and catheter infections during anticancer chemotherapy: clinical and microbiologic studies of 10 cases. Am. J. Clin. Pathol. 2004;121:581–587. doi: 10.1309/QBB3-AVCM-GWA3-K1XK. [DOI] [PubMed] [Google Scholar]
- 12.Dien Bard J., Lewinski M., Summanen P.H., Deville J.G. Sepsis with prolonged hypotension due to Moraxella osloensis in a non-immunocompromised child. J. Med. Microbiol. 2011;60:138–141. doi: 10.1099/jmm.0.016378-0. [DOI] [PubMed] [Google Scholar]
- 13.Feigin R.D., San Joaquin V., Middelkamp J.N. Septic arthiritis due to Moraxella osleoensis. J. Pediatr. 1969;75(JulY (1)):116–117. doi: 10.1016/s0022-3476(69)80109-5. [DOI] [PubMed] [Google Scholar]
- 14.Schonholtz G.J., Scott W.O. Moraxella septic arthritis of the knee joint: a case report. Arthroscopy. 1986;2(2):96–97. doi: 10.1016/s0749-8063(86)80021-4. [DOI] [PubMed] [Google Scholar]
- 15.Clarridge J.E., 3rd Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004;17:840–862. doi: 10.1128/CMR.17.4.840-862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham D.R., Band J.D., Thornsberry C., Hollis D.G., Weaver R.E. Infections caused by Moraxella, Moraxella urethralis, Moraxella-like groups M-5 and M-6, and Kingella kingae in the United States, 1953–1980. Rev. Infect. Dis. 1990;12:423–431. doi: 10.1093/clinids/12.3.423. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal S.L., Freundlich L.F., Gilardi G.L., Clodomar F.Y. In vitro antibiotic sensitivity of Moraxella species. Chemotherapy. 1978;24:360–363. doi: 10.1159/000237808. [DOI] [PubMed] [Google Scholar]