Abstract
Early adolescents have difficulties performing asthma self-management behaviors, placing them at-risk for poor asthma control and reduced quality of life. This paper describes the development and plans for testing an interactive mobile health (mHealth) tool for early adolescents, ages 12–15 years, and their caregivers to help improve asthma management. Applying Interactive Mobile health to Asthma Care in Teens (AIM2ACT) is informed by the Pediatric Self-management model, which posits that helpful caregiver support is facilitated by elucidating disease management behaviors and allocating treatment responsibility in the family system, and subsequently engaging in collaborative caregiver-adolescent asthma management. The AIM2ACT intervention was developed through iterative feedback from an advisory board composed of adolescent-caregiver dyads. A pilot randomized controlled trial of AIM2ACT will be conducted with 50 early adolescents with poorly controlled asthma and a caregiver. Adolescent-caregiver dyads will be randomized to receive the AIM2ACT smartphone application (AIM2ACT app) or a self-guided asthma control condition for a 4-month period. Feasibility and acceptability data will be collected throughout the trial. Efficacy outcomes, including family asthma management, lung function, adolescent asthma control, asthma-related quality of life, and self-efficacy for asthma management, will be collected at baseline, post-treatment, and 4-month follow-up. Results from the current study will inform the utility of mHealth to foster the development of asthma self-management skills among early adolescents.
Keywords: asthma, mobile phone, mobile health, adolescent, self-management
Introduction
Asthma affects approximately 8.3% of children in the United States and is one of the most common chronic pediatric conditions.1 Adolescents with persistent asthma experience impaired health and quality of life due to complications that are largely preventable via self-management behaviors.2 Unfortunately, many adolescents fail to perform the asthma self-management behaviors that help to control asthma symptoms and enhance quality of life.3 The treatment regimen for persistent asthma is complex, making self-management arduous for adolescents.4 Exceedingly low adherence to daily preventative medications,5–7 difficulty in monitoring and responding to symptoms, and challenges with trigger avoidance are widespread.8,9
Responsibilities for managing asthma transition from caregivers to youth during early adolescence.5,10,11 Caregivers often expect adolescents to assume increased levels of responsibility for their asthma care; however, early adolescents are unlikely to possess the requisite skills needed to manage asthma without caregiver assistance.6 Discrepancies in the allocation of asthma management responsibilities between parties can also result in a situation where management tasks are poorly executed or left unattended by both the caregiver and adolescent.5,11,12 Therefore, the presence of helpful caregiver support can determine whether adolescents with asthma are successful in developing and mastering self-management behaviors. Family conflict is related to poor asthma outcomes including poor adherence and frequent emergency department visits.13–15 Alternatively, helpful caregiver involvement is related to improved asthma control, quality of life, adherence, and lung function.15–17 The centrality of helpful caregiver support in developing asthma self-management skills18 is illustrated best in the Pediatric Self-management Model.19 This model posits that youth self-management behaviors are influenced by the presence of helpful caregiver monitoring and involvement in disease management and the relationship quality between the caregiver and adolescent. Based on this model, helpful caregiver support is facilitated by: 1) elucidating disease management behaviors and the allocation of treatment responsibility in the family system and 2) subsequently engaging in collaborative caregiver-adolescent asthma management as early adolescents develop and master self-management behaviors.
Mobile Health (mHealth) technologies can improve disease outcomes20 and may be an especially powerful tool to deliver effective behavioral health interventions to facilitate helpful family support as early adolescents with asthma develop and master self-management behaviors. Smartphones are owned and habitually carried by approximately 50% of 12–17 year-olds and 75% of adults ages 30–49.21,22 mHealth technologies can obtain near real time data that can be used to inform tailored mHealth interventions that are dynamic, user-centric, and continuously adapted.23 mHealth technology also presents a unique opportunity to provide a safe and structured environment to foster communication between adolescents and caregivers while avoiding emotional escalation and conflict that can occur when dyads try to navigate asthma management on their own.
There is a clear need for a mHealth intervention that targets helpful family support as early adolescents with persistent asthma transition to taking more responsibility for their asthma care. This study aims to develop the AIM2ACT intervention and test the AIM2ACT smartphone application (AIM2ACT app), a novel mHealth tool for youth with poorly controlled asthma and their caregivers. We outline the development of intervention components that will be used in a pilot randomized controlled trial (RCT) that will evaluate feasibility, acceptability, and efficacy of the AIM2ACT intervention.
Design and Methods
Objectives
Consistent with Obesity-Related Behavioral Intervention Trials (ORBIT) consortium guidelines,24 the current study includes two phases. Phase I focuses on collaborating with a family advisory board to develop the AIM2ACT intervention, a mHealth tool informed by the Pediatric Self-Management Model19 designed to increase helpful family support as early adolescents develop and master self-management behaviors. Phase II testing involves conducting a 4-month RCT of AIM2ACT in 50 early adolescents with persistent asthma and their caregivers to evaluate feasibility, acceptability, and efficacy. Families will be randomized to receive the AIM2ACT app or to a self-guided asthma control condition.
AIM2ACT Design and Development
AIM2ACT intervention materials were developed over a 15-month period. Development was primarily focused on building the AIM2ACT app, which uses existing technology provided by MEI Research through the patented PiLR Health system.25 PiLR is a cloud-based platform that collects, stores, processes, and reports on objective behavioral data from mobile devices.26 Additional features were developed for the purpose of this study, including presentation of skills-training videos and interactive behavioral contracting between adolescents and caregivers. The AIM2ACT app was tailored to our targeted population by gathering feedback from advisory board meetings composed of families of youth with persistent asthma.
AIM2ACT Advisory Board Meetings
Methods
Advisory board participants included youth with asthma between the ages of 12–15, and their caregiver. Participants completed a demographic questionnaire and the Asthma Control Test27 (see Efficacy Outcomes section). Three advisory board meetings were conducted with nine adolescent-caregiver dyads. These meetings were facilitated by the primary investigator, study coordinator, and a graduate student. Advisory board meetings were used to elicit constructive feedback and gauge participant interest, relevance, and potential feasibility and acceptability of the AIM2ACT app (e.g., language used). Specific goals for each advisory board meeting were as follows. The first advisory board meeting focused on introducing the overarching goals of AIM2ACT, presenting wireframes of core AIM2ACT app functionality (e.g., goal setting, behavioral contracting), and presenting initial version of skills-training videos. During the second advisory board, we solicited feedback on surveys embedded within AIM2ACT, using a beta version of the smartphone application, and presented refined versions of skills-training videos. Families completed final beta testing of the AIM2ACT app during the third and final advisory board meeting and were encouraged to think-aloud to provide feedback. Intervention materials for the self-guided asthma control condition were also presented during final advisory board meetings to obtain feedback on language and presentation of materials, and to ensure the control condition accurately mirrored the AIM2ACT app without the mHealth components. Families were compensated for their participation in advisory board meetings.
Results
Nine families participated in the advisory board meetings; six families participated in two or more meetings. Family demographic characteristics are presented in Table 1. During the first advisory board meetings, families perceived the goals of the project as important and felt the framework for using the application was coherent and easy to follow. A number of participants also provided constructive feedback on the initial versions of AIM2ACT skills training videos. Suggestions included using a more engaging multimedia platform for generating videos, replacing text-based information with visual content wherever possible, and reducing the length of the videos to sustain attention. Refined skills-training videos were created, using animated visuals and voiceover, and presented during second advisory board meetings. Families provided feedback on a beta version of the AIM2ACT application during the second advisory board meetings, citing issues with wording and the visuals used in the app. Following final beta testing during the third and final advisory board meetings, families generated additional examples to include in the different components of the behavioral contracts on the AIM2ACT app (e.g., goals and rewards). Families suggested the AIM2ACT app initiate a second assessment of needs, half way through the project period, to show families their overall progress in the intervention. Participant feedback from advisory boards was integrated into final components of the AIM2ACT app.
Table 1.
Advisory Board Demographic Variables
| Variables | n (%) or mean ± SD |
|---|---|
| Youth Age | 13.33 (1.32) |
| Youth Gender | |
| Male | 3 (33.3%) |
| Female | 6 (66.7%) |
| Child | |
| Race/Ethnicity | |
| Caucasian/White | 2 (22.2%) |
| African American | 5 (55.6%) |
| Puerto Rican | 1 (11.1%) |
| Other Latino | 1 (11.1%) |
| Parent Relationship | |
| Biological Mother | 6 (66.7%) |
| Biological Father | 1 (11.1%) |
| Step, Adoptive, or | 1 (11.1%) |
| Foster Mother Grandmother | 1 (11.1%) |
| Parent | |
| Race/Ethnicity | |
| Caucasian/White | 2 (22.2%) |
| African American | 4 (44.4%) |
| Other Latino | 1 (11.1%) |
| Mixed or Multi-Racial | 2 (22.2%) |
| ACT Score | 20.44 (2.30) |
Completed by caregiver
Completed by adolescent
Information obtained from pharmacy records
Pilot Randomized Controlled Trial of AIM2ACT
Participants
Phase II of the study will include a RCT of 50 adolescents between the ages of 12 and 15 years that have a persistent asthma diagnosis, poorly controlled asthma, and live in the same residence as their participating caregiver for the study. Families will be excluded from the study if the adolescent has a developmental disability, the adolescent or caregiver cannot read or speak English, or if the adolescent is currently enrolled in an asthma management intervention.
Recruitment
Families will be recruited from pediatric clinics, through local advertisements, and brochures distributed to clinics and schools. Using an established, IRB-approved mechanism, families attending clinic visits will be asked to complete a “Consent-to-Contact” form that enables study staff to call and screen for eligibility. Staff will provide information about the project, determine initial eligibility, and schedule an in-person baseline visit at their home or at our research lab. Youth with persistent asthma and a current controller medication prescription will be included due to pervasiveness of symptoms.28 Participants meeting inclusion criteria will complete the in-person baseline assessment visit to obtain caregiver consent and child assent.
Treatment Allocation
We anticipate that we will need to screen approximately 60 dyads to obtain 50 for treatment allocation via minimization. Minimization is a recommended treatment allocation method for pilot studies to achieve balance across treatment conditions on variables deemed important by the investigator. 29–31 Minim, a computer minimization program, will be used to allocate families to 1 of 2 groups with stratification factors of race/ethnicity (African American; Non-Latino White; Other) and child sex (male/female).32 Half of the dyads will be allocated to the AIM2ACT app, and half to a self-guided asthma control condition. Dyads will be notified of their assignment at the end of the baseline assessment period.
AIM2ACT Smartphone Application
Assessing asthma management and allocation of treatment responsibility
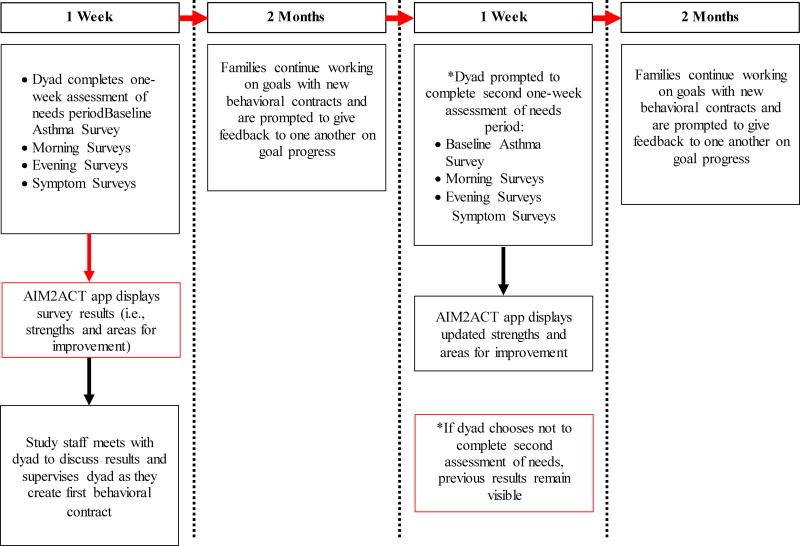
Participants in this condition will use the AIM2ACT app for a 4-month period, summarized as a flow chart in Figure 1. The application starts with a one-week real-time assessment of needs period to elucidate a family’s asthma management behaviors and allocation of treatment responsibility. Assessments will be delivered to both adolescents and caregivers via semi-random prompts twice daily from the AIM2ACT app. Application prompts will occur once in the morning and once in the evening to capture asthma management tasks that occur during these time periods (e.g., medication use). Adolescents will be asked to complete an assessment after they experience asthma symptoms while caregivers will be asked to complete an assessment after their child has had asthma symptoms.
Figure 1.
AIM2ACT Application flow chart
Real-time assessments will measure asthma management behaviors that are: 1) Clearly defined; 2) Likely to occur on a daily or frequent basis; and 3) Have clear implications to inform the supportive behavioral management component of the AIM2ACT intervention. Assessed asthma management behaviors include starting treatment when symptoms occur, taking daily controller medications, taking preventative medication before trigger exposure, noticing signs and symptoms of an asthma episode, and having medications present when away from home. Assessments capture the frequency of engagement, adolescent and caregiver involvement, and barriers to completing these asthma management behaviors. This information is collected via validated asthma management questionnaires and questions developed by the research team for assessing asthma management behaviors.5,33–37
Data from both adolescents and caregivers will be coded and analyzed by an algorithm designed to determine a family’s asthma management strengths and areas for improvement. This will be accomplished by calculating rates of endorsed asthma management behaviors and dyadic agreement on the allocation of treatment responsibility. Strengths and areas for improvement in five areas of asthma management: 1) Acting on asthma; 2) Being prepared for asthma; 3) Preventing asthma; 4) Talking about asthma; 5) Managing asthma as a team, will be presented to families through the AIM2ACT app at the end of the one-week assessment of needs period.
Collaborative asthma management
The AIM2ACT app will facilitate collaborative asthma management by automatically guiding dyads through a structured process that includes the evidence-based supportive behavioral management strategies of proximal goal setting, contingency management, and problem solving communication. The overarching goal of the AIM2ACT application is to improve adolescent self-efficacy for asthma self-management. To accomplish this goal, the application includes components that are informed by the four different domains of the Pediatric Self-Management Model19: 1) Individual; 2) Family; 3) Community; and 4) Health care system. The model suggests self-management behaviors are linked to non-modifiable and modifiable variables that fall within each of the four domains. See Table 2 for a description of AIM2ACT app components that target modifiable influences within each domain of this model. The application will also allow dyads flexibility in all collaborative asthma management components to accommodate potential differences in asthma management goals across families from different racial and ethnic backgrounds.
Table 2.
AIM2ACT App Components from the Pediatric Self-Management Model
| Domain | Modifiable Influences/Processes |
Description of AIM2ACT App Component |
|---|---|---|
| Family | Parental monitoring and supervision | •Goal setting: Caregivers will be encouraged to talk to their adolescent about the goal the adolescent chooses and to give their adolescent an opportunity to choose a goal they feel motivated to accomplish |
| Parental involvement | •Allocation of treatment responsibility: The app will help to allocate treatment responsibility by allowing dyads to include both adolescent and caregiver responsibilities when creating a plan | |
| Family functioning | •Skills-training: Videos will be included to guide families through problem solving communication and outline strategies that can be implemented to mitigate conflicts that may arise when dyads create a plan and give feedback on goal progress | |
| Behavioral Management | •Behavioral contracting: Adolescents are encouraged to choose rewards that will motivate them reach their goals; Caregivers approve this reward in the contract as a means to reinforce the behavior | |
| Individual/Family | Disease and treatment knowledge | •Skills-training: Five educational videos will be presented to dyads to explain the importance of the five areas of asthma management assessed in the app. These videos will discuss the importance of medication usage, self-monitoring, teamwork, and communication in asthma self-management. |
| Determining health care needs | •Assessment of needs: The app will use daily surveys to collect data on asthma care and show dyads their strengths and areas for improvement | |
| Community | Peer support | •Goal setting: Adolescents will be given the opportunity to choose goals that involve talking to their peers about their asthma so they can support adolescents if they need treatment or in the event of an emergency |
| Health Care System | Patient-provider communication | •Study letters: A letter will be mailed to the family’s primary care provider detailing the adolescent’s study outcomes and strengths and weakness in areas of asthma care. Providers will be encouraged to talk to families about this letter. |
The application will guide dyads to engage in proximal goal setting by requesting that the adolescent select an asthma management goal from the list of strengths and areas for improvement automatically populated based on the one-week real-time assessment of needs period. Dyads will also be able to write in an unlisted asthma management goal to accommodate family preferences. The AIM2ACT app will then ask dyads to construct their initial behavioral contract, which is referred to as the adolescent’s “plan.” Study staff will be present the first time dyads complete a contract to further establish rapport, encourage engagement, and provide assistance, if necessary. The AIM2ACT app will automatically guide dyads through successive behavioral contracts over the remainder of the 4-month treatment period. The study staff will not be present when a family completes subsequent contracts.
Two months into the 4-month project period, dyads will be asked to complete another one-week assessment of needs period using the same assessments and protocol outlined for the first week of the project period. Updated strengths and areas for improvement will be presented to dyads to show families how their overall asthma management has changed over the past two months.
The AIM2ACT app will prompt dyads to complete successive behavioral contracts for asthma management goals until dyads agree that the adolescent has mastered the behavior. Dyads will be asked to define the length of the behavioral contract (maximum of 7 days, minimum of 3 days) and the allocation of treatment responsibility in accomplishing the asthma management goal (e.g., caregiver responsible for checking-in with adolescent once daily). The length of the behavioral contract is referred to as the “goal period.” Dyads will also be asked to indicate what reward the adolescent will receive if the asthma management goal is met. In order to facilitate a collaborative asthma management process, the application will ask that both adolescents and caregivers independently agree upon the asthma management goal, behavioral contract length, and allocation of treatment responsibility. The application will then suggest that adolescents and caregivers track progress towards asthma management goals by logging when steps towards goal progress are completed.
Finally, at the end of each goal period, the AIM2ACT app will ask dyads to engage in problem solving communication. Dyads will first be asked to report whether the asthma management goal was accomplished. The application will suggest that family members provide feedback to one another to facilitate communication about the behavioral contracting process; dyads will be required to send positive and constructive feedback to one another about their behavior during the behavioral contract. Feedback can be selected from a prepopulated list in the AIM2ACT app or be written in directly. Dyads will be asked to discuss their feedback prior to beginning a new behavior contract. If an unsuccessful goal attempt is reported, the application will suggest families use collaborative problem solving skills learned via the AIM2ACT app (see below) to arrive at a new asthma management goal.
Skills-training
Adolescents and their caregivers need skills training to understand how to work together to develop a behavioral contract and how to achieve the goals articulated in the contract. At each step of the initial assessment and collaborative asthma management process described above, the AIM2ACT app will prompt both adolescents and caregivers to view animated skills-training videos explaining how to (a) use the application, (b) complete the realtime assessment process, and (c) collaborate on asthma management. The AIM2ACT app contains separate skills-training videos for adolescents and caregivers. Skills-training videos for the adolescent are developmentally appropriate and will present the application as a novel way to positively demonstrate autonomy in asthma management. To caregivers, the AIM2ACT app will be presented as a tool that can aid in transitioning asthma management responsibility to early adolescents. Videos will teach the supportive behavioral management skills to successfully engage in collaborative asthma management (e.g., problem solving communication). The application will also provide the dyads rationale and practical examples of goal setting, behavioral contracting, and problem solving communication. Videos will always be available to users from the AIM2ACT application. The adolescent introduction video and a caregiver skills-training video that will be used in the AIM2ACT app are available in the electronic version of this manuscript (please see supplementary material).
If a family fails to reach acceptable levels of engagement (see intervention feasibility and acceptability section) then study staff will complete a one-time call to families to assess for technological difficulties and encourage participation. Any assistance or technological difficulties will be documented and used to improve the AIM2ACT app upon completion of the pilot RCT.
Self-guided asthma control
A self-guided asthma control condition will be used that accounts for staff attention and isolates the effect of a mHealth intervention approach. After randomization, participants in this condition will report on asthma management behaviors and the allocation of treatment responsibility using a paper and pencil versions of questions asked in the AIM2ACT application for a one-week period. Participants will then meet with study staff to receive a paper copy of feedback related to family asthma management strengths and areas for improvement and will be given general information on supportive behavioral management techniques they can use to target their identified areas for improvement. Participants will be asked to use these strategies to target improved asthma management over the remainder of the 4-month treatment period. It is anticipated that this one-time meeting will have minimal impact on asthma management or behavior change. We anticipate this condition will optimize participant recruitment and retention, and will be perceived as beneficial.
Incorporation of Healthcare Providers
To incorporate the family’s healthcare team, the adolescent’s primary care provider will receive a letter detailing: 1) Study objectives; 2) The family’s asthma management strengths and areas for improvement; and 3) Information describing their patient’s outcomes over the course of the project. Families will receive a copy of this letter at the end of the project period.
Study Outcomes
Outcomes include change in the family’s asthma management (primary outcome), prescription refill history, lung function, adolescent asthma control, asthma-related quality of life, and self-efficacy for asthma management from baseline to post-treatment and 4-month follow-up. Feasibility, acceptability, and utilization of AIM2ACT will be measured throughout the RCT. Study personnel involved in assessments will remain blind to treatment assignment throughout the trial.
Efficacy Outcomes
Outcome measures will be administered at baseline, post-intervention, and at 4-month follow-up. See Table 3 for efficacy outcomes that will be collected.
Family asthma management will be assessed with the Family Asthma Management System Scale (FAMSS), a validated family interview that assesses core aspects of asthma management including medication adherence, symptom assessment and response, and integration of asthma into the family system. Interrater reliability will be assessed using standardized procedures. 38
Medication adherence will be calculated using the adolescent’s Prescription Refill History from their pharmacy records. Families will provide consent to obtain this information from their pharmacy.39
Pulmonary Function, including FEV1 and FEV1/FVC will be assessed via portable spirometry measurements. Youth will be trained and instructed to use the AM1+, a portable spirometer, for a two-week assessment period at each outcome time point. The AM1+ is an accurate and validated hand-held computerized spirometer that stores up to 1200 assessments before data is downloaded. Similar devices have been successfully utilized in numerous research protocols.38–40 Children will use the AM1+ immediately following assessment visits.
Asthma Control will be assessed via the Asthma Control Test administered to the youth participants.27
Child Asthma-related Quality of Life will be assessed via the adolescent-report Pediatric Asthma Quality of Life Questionnaire.41
Asthma management self-efficacy for asthma self-management behaviors will be assessed via the adolescent-report Asthma Management Efficacy questionnaire.42
Family communication will be assessed via the Decision Making Involvement Scale (DMIS) parent and child version.43
Table 3.
AIM2ACT Outcome Measures
| Outcome | Measure | Description |
|---|---|---|
| Demographics | Self-reporta | Child age/sex, caregiver marital status, educational level, family income, asthma medications, and child ED visits/hospitalizations |
| Pulmonary Function | AM 1+ Portable spirometerb | FEV1 and FEV1/FVC |
| Child Asthma-related Quality of Life | Pediatric Asthma Quality of Life Questionnaireb | 13-item validated measure of adolescent’s asthma-related quality of life |
| Asthma Control | Asthma Control Testb | Five-item validated scale used to measure asthma control |
| Family Asthma Management | Family Asthma Management System Scalea,b | A validated, semi-structured interview assessing core aspects of asthma management |
| Family Communication | Decision Making Involvement Scale Caregiver- and Adolescent-Reportsa,b | 38-item validated measure assessing caregiver and adolescent involvement and interaction during asthma care decisions |
| Asthma Management Self-Efficacy | Asthma Management Efficacy Parent- and Patient-Questionnairesa,b | 13-item and 14-item validated measures assessing parent and patient asthma management self-efficacy, respectively |
| Asthma Medication Adherence | Pharmacy prescription refill historyc | The number of prescriptions refilled is divided by the number of prescriptions that should occur within a certain period of time |
Completed by caregiver
Completed by adolescent
Information obtained from pharmacy records
Intervention Feasibility, Acceptability and Utilization
Feasibility will be determined by examining enrollment and attrition rates and usage statistics of participants using the AIM2ACT app. Should families opt not to participate or discontinue use, we will attempt to solicit reasons for doing so. Acceptability will be determined by rating scales to assess participants’ satisfaction with application components (e.g., collaborative asthma management components, skills-training videos) and the overall tool. Semi-structured exit interviews with participants (adolescent and caregiver separately) will be conducted at the post treatment time point to obtain detailed feedback about their experience. Specific topics include: perceived usefulness of AIM2ACT components; how effective the AIM2ACT app was in changing asthma self-management behaviors; ways to improve the usability and content of AIM2ACT; suggestions on tailoring AIM2ACT to racial and ethnic minority groups (when applicable). Interviews will be audio recorded and transcribed for review to complement quantitative findings. Utilization will be quantified by calculating the number of times AIM2ACT features (e.g., skills-training videos) are accessed by adolescents or caregivers each week during the intervention.
Power Analysis
Statistical power for the test of primary aims will be adequate if effect sizes are medium or larger (Cohen’s d ≥ .5) However, the primary purpose of the analysis is to evaluate feasibility, acceptability, and utilization of AIM2ACT, and to obtain preliminary estimates of effect sizes. Relevant variables (e.g., season at time of study enrollment) will be examined for potential inclusion as covariates in analyses.
Statistical Analyses
Analyses pertaining to efficacy outcomes at post-treatment and 4-month follow-up will be examined using generalized linear mixed models (GLMM) and standard model building procedures with restricted maximum likelihood estimation, which is preferable to maximum likelihood in this case due to the small sample size. Primary outcomes analyses will follow the intent to treat principle. GLMM allows for all data to contribute to the analysis. Sensitivity analyses will test the assumption that missing data occurred at random. If not, other less powerful but robust methods for handling of missing data will be implemented. An effect size of ≥ .3 at post-treatment will be the criterion for demonstrating preliminary efficacy of the AIM2ACT intervention and proceeding to a fully powered RCT, including a more thorough assessment of mediators and moderators of the treatment effect. This effect size for the planned analyses was selected based on previous pediatric asthma intervention literature.12,44
Rates of successful participant recruitment and attrition will be calculated. Descriptive statistics will be used to examine adolescent and caregiver satisfaction ratings for AIM2ACT components and the overall tool. Standard cutoffs (i.e., mean scores ≥70% of the maximum score) will be used to determine whether AIM2ACT can be deemed as feasible and acceptable. Based on our previous work with this population, attrition of ≤ 15% at 4-month follow-up in the RCT will constitute success. Utilization of the AIM2ACT app will depend on the goals identified by each dyad, but a global minimum level of acceptable engagement applicable to all possible goals will be defined as 5 interactions with the application (e.g., goal setting, behavioral contracting, progress monitoring) per week by both parent and adolescent.
Data Safety Monitoring
An independent Data Safety Monitoring Officer (DSMO) currently oversees participant safety, evaluates performance, monitors data quality, and provides advice regarding the status and continuation of AIM2ACT and its components. For all adverse events, an Adverse Event Record Form is completed that includes a description of the event, a classification of seriousness, an evaluation of potential relationship to the intervention, and an assessment of need for change in the informed consent or study activities. Any adverse events are reviewed. All serious, unexpected events are reported to the IRB within five business days. Fatal or life-threatening adverse events are reported to the funding institute within seven calendar days, and other serious adverse events are reported to the funding institute within 15 calendar days.
Design Considerations and Potential Problems
We anticipate facing several challenges during the course of this study. We acknowledge that 12–15 years of age is a somewhat narrow age which may present as a barrier to recruitment. In anticipation, we plan to use a variety of recruitment strategies including: recruiting in several pediatric medical clinics (i.e., pulmonary, primary care, intensive care unit), sending targeted mailings, using local advertisements, and distributing brochures to local schools. It is possible that families will not meet acceptable levels of engagement with the AIM2ACT app, thereby limiting data quality. To encourage engagement, study staff will complete a one-time call to families who have ≤ 5 interactions with the AIM2ACT app per week to assess for technological difficulties and encourage participation. If the first 5 families fail to reach acceptable levels of intervention engagement, then participant feedback will be reviewed to determine what improvements can be made to the application. Our previous work suggests that a majority of families will be from lower socioeconomic backgrounds. Therefore, we will offer to complete study visits in the participant’s home to reduce burden on the family and will providemobile phones with data plans to adolescents and caregivers who do not own a phone. Finally, it is possible that families will be disappointed upon being randomized to the non-mobile condition. We have created a self-control condition that assesses important domains of asthma management and includes personalized feedback and resources in an attempt to facilitate active participant engagement.
Discussion
The AIM2ACT intervention uses a mHealth tool designed to help early adolescents diagnosed with persistent asthma develop and master asthma self-management skills. The goals of this study are: 1) to develop AIM2ACT with the help of qualitative feedback from an advisory board comprised of users from the target AIM2ACT audience and 2) to conduct a pilot RCT of AIM2ACT to examine feasibility, acceptability, and efficacy of AIM2ACT in improving relevant asthma outcomes. A primary goal of this study is to generate preliminary estimates of effect sizes to inform future work.
AIM2ACT builds upon a burgeoning body of research that has sought to leverage technology to improve asthma outcomes in youth.45,46 The majority of published technology-based interventions in pediatric asthma use computer- or web-based asthma programs to deliver asthma education or monitor symptoms.47–49 Findings from these studies suggest that technology-based interventions can be viable, feasible, and acceptable intervention modalities among youth diagnosed with asthma. However, examination of the follow-up effects of some of these interventions show difficulties in sustaining intervention gains49 or engagement,48 leading to calls for additional refinement in future studies.46
Recent advances in mobile technologies now allow for the collection of dynamic health-related data which can be leveraged to deliver tailored user-centric interventions that are continuously refined over time. Within pediatric asthma, a small number of pilot mHealth interventions have been developed. Example mHealth interventions include: 1) delivering tailored disease management text messages following brief motivational interviewing and problem solving skills training,50 2) using a smartphone application to provide contingent reinforcement when youth complete asthma management behaviors,51 and 3) a text messaging system that uses natural language dialogue system to provide asthma self-management assistance.52 Collectively, these pilot mHealth interventions appear feasible, acceptable, and show promise in improving several key asthma outcomes including symptoms, quality of life, and adherence to controller medication.
To our knowledge, AIM2ACT is the first mHealth intervention designed to target early adolescence and explicitly focus on the parent-youth partnership surrounding asthma management. As previously noted, early adolescence is an important developmental period in which asthma management responsibilities transition from parents to youth. This transition is fraught with difficulties, including miscommunication and increased family conflict,12 which can place youth at-risk for poor asthma outcomes that are well documented in adolescents.53 Results from the current study will add to the extant mHealth literature by determining whether a mobile-based tool can facilitate the transition of asthma management responsibilities from parent to adolescents by using a supportive, behavioral skills-based approach.
We will determine whether proceeding to a larger RCT is warranted based on observed levels of intervention engagement, effect size of the intervention on health outcomes, and attrition rates at the conclusion of the current study. We will also: 1) gather advisory board feedback given during AIM2ACT development; 2) collect feedback on participation barriers, usefulness of AIM2ACT content, and ways to further tailor and improve AIM2ACT (e.g., further tailoring of videos); and 3) assess the quality and utility of our outcome measures. If justified, AIM2ACT will be refined for a larger RCT to further examine the efficacy of AIM2ACT, electronically monitor medication adherence, and assess mediators and moderators of treatment outcomes (e.g., race/ethnicity).
Supplementary Material
Acknowledgments
Funding
This project is supported by funding from the National Institutes of Health (1R21HD083830-01A1, PI: Fedele). The RCT described in this paper is registered as NCT02302040.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akinbami LJ, Simon AE, Rossen LM. Changing trends in asthma prevalence among children. Pediatrics. 2016;137(1) doi: 10.1542/peds.2015-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guevara JP, Wolf FM, Grum CM, Clark NM. Effects of educational interventions for self management of asthma in children and adolescents: systematic review and meta-analysis. BMJ. 2003;326(7402):1308–1309. doi: 10.1136/bmj.326.7402.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruzzese JM, Bonner S, Vincent EJ, et al. Asthma education: the adolescent experience. Patient Educ Couns. 2004;55(3):396–406. doi: 10.1016/j.pec.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Drotar D, Bonner MS. Influences on adherence to pediatric asthma treatment: a review of correlates and predictors. J Dev Behav Pediatr. 2009;30(6):574–582. doi: 10.1097/DBP.0b013e3181c3c3bb. [DOI] [PubMed] [Google Scholar]
- 5.McQuaid EL, Penza-Clyve SM, Nassau JH, et al. The asthma responsibility questionnaire: Patterns of family responsibility for asthma management. Child Heal Care. 2001;30(3):183–199. [Google Scholar]
- 6.McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. J Pediatr Psychol. 2003;28(5):323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- 7.Naimi DR, Freedman TG, Ginsburg KR, Bogen D, Rand CS, Apter AJ. Adolescents and asthma: why bother with our meds? J Allergy Clin Immunol. 2009;123(6):1335–1341. doi: 10.1016/j.jaci.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butz AM, Tsoukleris M, Donithan M, et al. Patterns of inhaled antiinflammatory medication use in young underserved children with asthma. Pediatrics. 2006;118(6):2504–2513. doi: 10.1542/peds.2006-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forero R, Bauman A, Young L, Larkin P. Asthma prevalence and management in Australian adolescents: results from three community surveys. J Adolesc Heal. 1992;13(8):707–712. doi: 10.1016/1054-139x(92)90068-m. http://www.ncbi.nlm.nih.gov/pubmed/1290773. [DOI] [PubMed] [Google Scholar]
- 10.Bruzzese JM, Stepney C, Fiorino EK, et al. Asthma self-management is sub-optimal in urban Hispanic and African American/black early adolescents with uncontrolled persistent asthma. J Asthma. 2012;49(1):90–97. doi: 10.3109/02770903.2011.637595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walders N, Drotar D, Kercsmar C. The allocation of family responsibility for asthma management tasks in African-American adolescents. J Asthma. 2000;37(1):89–99. doi: 10.3109/02770900009055432. http://www.ncbi.nlm.nih.gov/pubmed/10724302. [DOI] [PubMed] [Google Scholar]
- 12.Duncan CL, Hogan MB, Tien KJ, et al. Efficacy of a parent-youth teamwork intervention to promote adherence in pediatric asthma. J Pediatr Psychol. 2013;38(6):617–628. doi: 10.1093/jpepsy/jss123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiese B, Everhart RS. Medical adherence and childhood chronic illness: family daily management skills and emotional climate as emerging contributors. Curr Opin Pediatr. 2006;18(5):551–557. doi: 10.1097/01.mop.0000245357.68207.9b. [DOI] [PubMed] [Google Scholar]
- 14.B B, FS W, SL O, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy, Asthma Immunol. 2000;85(5):416–421. doi: 10.1016/s1081-1206(10)62557-4. http://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,uid&db=cin20&AN=107005298&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- 15.Rhee H, Ph D, P PN, et al. Family Support and Asthma Outcomes in Adolescents?: Barriers to Adherence as a Mediator. J Adolesc Heal. 2010;47(5):472–478. doi: 10.1016/j.jadohealth.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartlett SJ, Lukk P, Butz A, Lampros-Klein F, Rand CS. Enhancing Medication Adherence Among Inner-City Children with Asthma: Results from Pilot Studies. J Asthma. 2002;39(1):47–54. doi: 10.1081/JAS-120000806. [DOI] [PubMed] [Google Scholar]
- 17.Duncan CL, Hogan MB, Tien KJ, et al. Efficacy of a parent-youth teamwork intervention to promote adherence in pediatric asthma. J Pediatr Psychol. 2013;38(6):617–617. doi: 10.1093/jpepsy/jss123. Christina.Duncan@mail.wvu.edu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaugars AS, Klinnert MD, Bender BG. Family influences on pediatric asthma. J Pediatr Psychol. 2004;29(7):475–491. doi: 10.1093/jpepsy/jsh051. [DOI] [PubMed] [Google Scholar]
- 19.Modi AC, Pai AL, Hommel KA, et al. Pediatric self-management: a framework for research, practice, and policy. Pediatrics. 2012;129(2):e473–85. doi: 10.1542/peds.2011-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fedele DA, Cushing CC, Fritz A, Amaro CM, Ortega A. Mobile health interventions for improving health outcomes in youth: A meta-analysis. JAMA Pediatr. 2017 doi: 10.1001/jamapediatrics.2017.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pew Research Center. [Accessed March 14, 2017];Teens, Technology and Friendships. http://www.pewinternet.org/2015/08/06/teens-technology-and-friendships/ Published 2015.
- 22.Pew Research Center. [Accessed March 14, 2017];Demographics of Mobile Device Ownership and Adoption in the United States. http://www.pewinternet.org/fact-sheet/mobile/ Published 2017.
- 23.Heron KE, Smyth JM. Ecological momentary interventions: incorporating mobile technology into psychosocial and health behaviour treatments. Br J Heal Psychol. 2010;15(Pt 1):1–39. doi: 10.1348/135910709x466063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czajkowski SM, Powell LH, Adler N, et al. From ideas to efficacy: The ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. 2015;34(10):971–982. doi: 10.1037/hea0000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNabb WL, Wilson-Pessano SR, Jacobs AM. Critical self-management competencies for children with asthma. J Pediatr Psychol. 1986;11(1):103–117. doi: 10.1093/jpepsy/11.1.103. http://www.ncbi.nlm.nih.gov/pubmed/3958864. [DOI] [PubMed] [Google Scholar]
- 26.Moon J, Sieling J, Bond D, Thomas G. The Obesity Society Annual Scientific Meeting. Atlanta, GA: 2013. Coordinating sensor and subjective data in mHealth studies. [Google Scholar]
- 27.Liu AH, Zeiger R, Sorkness C, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119(4):817–825. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 28.National Heart Lung and Blood Institute. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 29.Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials. Control Clin Trials. 2002;23(6):662–674. doi: 10.1016/S0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 30.Altman DG, Bland JM. Treatment allocation by minimisation. BMJ. 2005;330(7495) doi: 10.1136/bmj.330.7495.843. http://www.bmj.com/content/330/7495/843.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saghaei M. An overview of randomization and minimization programs for randomized clinical trials. J Med Signals Sens. 2011;1(1):55–61. http://www.ncbi.nlm.nih.gov/pubmed/22606659. [PMC free article] [PubMed] [Google Scholar]
- 32.Evans S, Royston P, Day S. [Accessed March 19, 2017];Minim: allocation by minimisation in clinical trials. https://www-users.york.ac.uk/~mb55/guide/minim.htm Published 2004.
- 33.Varni JW, Burwinkle TM, Rapoff MA, Kamps JL, Olson N. The PedsQL in pediatric asthma: reliability and validity of the Pediatric Quality of Life Inventory generic core scales and asthma module. J Behav Med. 2004;27(3):297–318. doi: 10.1023/b:jobm.0000028500.53608.2c. http://www.ncbi.nlm.nih.gov/pubmed/15259457. [DOI] [PubMed] [Google Scholar]
- 34.Logan D, Zelikovsky N, Labay L, Spergel J. The Illness Management Survey: identifying adolescents’ perceptions of barriers to adherence. J Pediatr Psychol. 2003;28(6):383–392. doi: 10.1093/jpepsy/jsg028. http://www.ncbi.nlm.nih.gov/pubmed/12904450. [DOI] [PubMed] [Google Scholar]
- 35.McQuaid EL, Walders N, Kopel SJ, Fritz GK, Klinnert MD. Pediatric asthma management in the family context: the family asthma management system scale. J Pediatr Psychol. 2005;30(6):492–502. doi: 10.1093/jpepsy/jsi074. [DOI] [PubMed] [Google Scholar]
- 36.Brodzinsky DM, Elias MJ, Steiger C, Simon J, Gill M, Hitt JC. Coping scale for children and youth: Scale development and validation. J Appl Dev Psychol. 1992;13(2):195–214. doi: 10.1016/0193-3973(92)90029-H. [DOI] [Google Scholar]
- 37.Yeatts KB, Stucky B, Thissen D, et al. Construction of the Pediatric Asthma Impact Scale (PAIS) for the Patient-Reported Outcomes Measurement Information System (PROMIS) J Asthma. 2010;47(3):295–302. doi: 10.3109/02770900903426997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fritz GK, Adams SK, McQuaid EL, et al. Symptom perception in pediatric asthma: resistive loading and in vivo assessment compared. Chest. 2007;132(3):884–889. doi: 10.1378/chest.06-2140. [DOI] [PubMed] [Google Scholar]
- 39.McQuaid EL, Koinis Mitchell D, Walders N, et al. Pediatric asthma morbidity: the importance of symptom perception and family response to symptoms. J Pediatr Psychol. 2007;32(2):167–177. doi: 10.1093/jpepsy/jsj112. [DOI] [PubMed] [Google Scholar]
- 40.Feldman JM, McQuaid EL, Klein RB, et al. Symptom perception and functional morbidity across a 1-year follow-up in pediatric asthma. Pediatr Pulmonol. 2007;42(4):339–347. doi: 10.1002/ppul.20584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Qual Life Res. 1996;5(1):27–34. doi: 10.1007/BF00435966. [DOI] [PubMed] [Google Scholar]
- 42.Bursch B, Schwankovsky L, Gilbert J, Zeiger R. Construction and validation of four childhood asthma self-management scales: parent barriers, child and parent self-efficacy, and parent belief in treatment efficacy. J Asthma. 1999;36(1):115–128. doi: 10.3109/02770909909065155. http://www.ncbi.nlm.nih.gov/pubmed/10077141. [DOI] [PubMed] [Google Scholar]
- 43.Miller VA, Harris D. Measuring children’s decision-making involvement regarding chronic illness management. J Pediatr Psychol. 2012;37(3):292–306. doi: 10.1093/jpepsy/jsr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naar-King S, Ellis D, King PS, et al. Multisystemic Therapy for high-risk African American adolescents with asthma: A randomized clinical trial. J Consult Clin Psychol. 2014;82(3):536–545. doi: 10.1037/a0036092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosnaim GS, Powell LH, Rathkopf M. A Review of Published Studies Using Interactive Internet Tools or Mobile Devices to Improve Asthma Knowledge or Health Outcomes. Pediatr Allergy Immunol Pulmonol. 2012;25(2):55–63. doi: 10.1089/ped.2011.0112. [DOI] [Google Scholar]
- 46.Nickels A, Dimov V. Innovations in Technology: Social Media and Mobile Technology in the Care of Adolescents with Asthma. Curr Allergy Asthma Rep. 2012;12(6):607–612. doi: 10.1007/s11882-012-0299-7. [DOI] [PubMed] [Google Scholar]
- 47.van der Meer V, van Stel HF, Detmar SB, Otten W, Sterk PJ, Sont JK. Internet-Based Self-Management Offers an Opportunity To Achieve Better Asthma Control in Adolescents. Chest. 2007;132(1):112–119. doi: 10.1378/chest.06-2787. [DOI] [PubMed] [Google Scholar]
- 48.Homer C, Susskind O, Alpert HR, et al. An evaluation of an innovative multimedia educational software program for asthma management: report of a randomized, controlled trial. [Accessed March 20, 2017];Pediatrics. 2000 106(1 Pt 2):210–215. http://www.ncbi.nlm.nih.gov/pubmed/10888694. [PubMed] [Google Scholar]
- 49.Guendelman S, Meade K, Benson M, Chen YQ, Samuels S. Improving asthma outcomes and self-management behaviors of inner-city children: a randomized trial of the Health Buddy interactive device and an asthma diary. [Accessed March 20, 2017];Arch Pediatr Adolesc Med. 2002 156(2):114–120. doi: 10.1001/archpedi.156.2.114. http://www.ncbi.nlm.nih.gov/pubmed/11814370. [DOI] [PubMed] [Google Scholar]
- 50.Seid M, D’Amico EJ, Varni JW, et al. The in vivo adherence intervention for at risk adolescents with asthma: report of a randomized pilot trial. J Pediatr Psychol. 2012;37(4):390–403. doi: 10.1093/jpepsy/jsr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mosnaim G, Li H, Martin M, et al. A tailored mobile health intervention to improve adherence and asthma control in minority adolescents. J allergy Clin Immunol Pract. 2015;3(2):288–290. doi: 10.1016/j.jaip.2014.10.011. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rhee H, Allen J, Mammen J, Swift M. Mobile phone-based asthma self-management aid for adolescents (mASMAA): a feasibility study. Patient Prefer Adherence. 2014;8:63–72. doi: 10.2147/PPA.S53504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhee H, Belyea MJ, Brasch J. Family support and asthma outcomes in adolescents: barriers to adherence as a mediator. J Adolesc Heal. 2010;47(5):472–478. doi: 10.1016/j.jadohealth.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.