Abstract
Purpose
Using a clustered randomized controlled trial (RCT) design, we evaluated whether support to keep Kenyan orphaned adolescents in school reduces the risk of HIV infection.
Methods
Participants included 835 orphaned boys and girls in Grades 7 and 8 (mean age at baseline=15 years) in western Kenya. Primary schools (N=26) were randomized to study condition. Intervention participants received school uniforms, payment of tuition when they transitioned into high school, and nurse visits to monitor school absenteeism and provide assistance to stay in school. Annual surveys were conducted from 2011 through 2014, and HIV and herpes simplex virus 2 (HSV-2) biomarker data were collected at baseline and endline. Data were analyzed using survey logistic regression or generalized estimating equations controlling for age, gender, and socioeconomic status.
Results
Intervention and control groups were equivalent at baseline and did not differ on new HIV or HSV-2 incidence at endline. The school support intervention increased school retention but had few HIV-related effects, except increased circumcision among male participants, and reduced likelihood of transactional sex.
Conclusions
Despite a strong study design, we found no relative reduction in HIV or HSV-2 infection after three years of intervention implementation. New incidence of HIV was lower than expected in this region among youth whose average age at endline was 18 years (range=14-23). Although support for secondary school promises many benefits for vulnerable youth, our study adds to the growing body of research showing weak evidence for its effectiveness as HIV prevention.
Keywords: School Support, HIV, Orphans, Kenya
Worldwide, there are 17.8 million orphans who have lost one or both parents to AIDS. About 11.6 million of these orphans are living in Sub-Saharan Africa (SSA), including 2.6 million in Kenya alone [1]. Compared to their non-orphan counterparts, orphans are at increased risk of psychological distress, exploitation, early marriage and pregnancy, sexual trafficking, poverty, and school dropout, which leaves adolescent orphans particularly vulnerable to HIV infection [2,3].
Over the past few decades, HIV/AIDS prevention efforts, including those targeting vulnerable adolescent populations such as orphans, have shifted towards considering whether structural social and economic factors may help drive the HIV epidemic [4,5]. Social protection or social safety net programs in the form of conditional cash transfer (CCT) to improve educational and health outcomes have garnered tremendous attention [6]. CCT programs generally provide small amounts of cash, uniforms, and/or school tuition to the poorest households, conditional on certain behaviors, with the most common one being child school enrollment. The conceptual framework for these programs posits that cash transfers and/or direct school support will increase the likelihood of adolescents staying enrolled in school, and that this, in turn, will lower social risk factors and behaviors leading to HIV and other sexually transmitted infections [7]. Such incentives are also assumed to alleviate economic burdens to households, and beneficiaries of the transfers are expected to be less likely to depend on older sex partners or transactional sex for school fees [8].
Several studies evaluating this structural approach to HIV prevention have been conducted in recent years [1,7]. We limit our review here to the four rigorous randomized controlled trials (RCTs) testing the relationship between cash transfers and/or direct school support and HIV risk among adolescents in sub-Saharan Africa. These studies included three major domains of study outcomes: 1) educational outcomes; 2) proxy indicators of risk for HIV such as early sexual debut, marriage, and transactional sex; and 3) biomarker data of HIV and Herpes Simplex Virus-2 (HSV-2) infections. The first domain is important, since it would be hard to support the conceptual theory that school provides a protective environment for orphans against HIV risk in the absence of effects on school retention. A Kenya study provided two school uniforms to students in upper primary school and found reduced school dropout among Kenyan boys and girls after both a three and seven year follow-up [9,10]. A Zimbabwe orphaned girl study provided a comprehensive school support program of school tuition, fees and uniforms from Grade 6 through high school, and found reduced dropout and increases in the highest grade achieved among the intervention compared to the control group [11-13]. In South Africa, a large CCT study of girls in Grades 8-11 found no impact on school enrollment [14], while a Malawi CCT study that also included secondary school fees found improved school enrollment for girls in the treatment group compared to controls [8,15].
In terms of HIV risk indicators, school support trials have shown variable results. The Kenya uniform subsidy intervention found a lower likelihood of marriage and pregnancy for treatment females after both three and seven-year follow-up [9,10]. The Zimbabwe orphan girls' school support study found reduced likelihood of marriage and an increase in SES with the intervention [11,12]. The South African CCT study found an increased likelihood of 12-month sexual abstinence and reduced likelihood of intimate partner violence and unprotected sex [14]. The Malawi girls study found program effects on frequent sexual intercourse and having an older sexual partner [15].
Likewise, the effect of CCT interventions on HIV and HSV-2 biomarker outcomes have been mixed. The Kenya uniform subsidy intervention found no effects on girls' or boys' biomarker outcomes after seven years, although uniforms combined with an HIV education program reduced HSV-2 prevalence among girls only [9,10]. The Zimbabwean orphan girls' school support study found no treatment effects on HIV or HSV-2 biomarkers after 5 years, neither did the South African CCT girls study after three years follow-up [12,14]. Only the Malawi CCT study reported a reduction in HIV infection, although not HSV-2 infection, after 18 months follow-up [15]. For all studies, HIV prevalence among the control group was low: less than 1% in Kenya, 4% in South Africa, 4.1% in Zimbabwe, and 3% in Malawi.
Differences in impacts on sexual risk behaviors and biomarkers may be due to differences in study setting, target population, type or size of intervention, or study design. Although conducted in different countries and with different ethnic groups, participants in all studies were similar in age. Regarding important design issues, three studies (in Kenya, Zimbabwe, and Malawi) all conducted biological testing at endline but not baseline [10,12,15], thus failing to establish HIV-infection by condition prior to the intervention. This design factor may be particularly problematic for orphan youth, since they are at greater risk for being HIV-infected through maternal to child transmission compared to non-orphans [16], but it begs caution across all three studies, given the relatively low HIV and HSV-2 prevalence found among participants.
The current RCT was developed following a pilot RCT of 100 orphan boys and girls in western Kenya which found that a 1-year intervention of school fee, uniform, and community visitor support delayed sexual debut, reduced school dropout and attitudes supporting early sex, and increased pro-social bonding and gender equity attitudes [17]. These program impacts, however, disappeared at the 2-year follow-up [18], leading to design and intervention adjustments (i.e., randomization of schools rather than households and selecting students by grade level rather than by age; lengthening the duration of the follow-up to three years to follow students from upper primary into high school; using a no-treatment control group design with biomarkers at baseline and endline; and using nurses with bachelor's degrees to monitor students rather than lay community visitors) [19]. The purpose of this paper is to report program effects on HIV risk from the subsequent RCT. We hypothesized that intervention orphans would be more likely to stay in school, have lower self-reported sexual risk behaviors, and have lower likelihood of HIV and HSV-2 infection compared to controls.
Methods
Study Design and Setting
The RCT was conducted in Siaya County, Nyanza Province in Kenya. Nyanza has the highest prevalence of both HIV and orphanhood in Kenya [20]. The study was longitudinal with annual repeated measures collected over 4 years. We selected 26 primary schools with at least 20 orphans in Grades 7 and 8 per school in 2011 and that were at least 10km apart to avoid control group perceptions of relative depravation. Orphans were defined as individuals who had lost one or both parents to death from any cause. We invited all orphans in grades 7 or 8 in the 26 schools to participate in the study (n=923); of these, 837 students completed both the student survey and biomarker testing at the baseline. Stratified randomization procedures were conducted assigning 13 schools to be intervention (E) schools (n=411 students) and 13 to be control (C) schools (n=426 students).
Intervention
The study tested a structural intervention, as opposed to a health education program, providing participating students in intervention schools with a school uniform in Grades 7 and 9, and payment of secondary school fees. In addition, nurse research staff members visited schools in order to monitor intervention study participants' school attendance and to assist them with resolving absenteeism problems. Support continued for 36 months (2012-2014) or until the student dropped out of school.
Human subjects protection
All study participation was voluntary. We obtained written informed permission from either a surviving parent or custodial guardian and written assent from all participants. The institutional review boards of the Pacific Institute for Research and Evaluation (US) and Moi University (Kenya) reviewed and approved all study procedures. Participating schools in the control condition were provided cash incentives of $240 annually to use for their school development projects. Participants received small incentives ($3) for participating in the survey and biomarker testing.
Biomarkers of HIV and HSV-2 infections
We conducted serologic testing to detect antibodies against HIV and HSV-2 as biomarkers of infection at the 2011 baseline and the 2014 endline [21,22]. Venous blood samples were collected at baseline; whole blood was used immediately for HIV antibody testing, and serum was prepared for HSV-2 serology. At endline, blood was obtained by finger stick; whole blood was used for HIV antibody testing, and dried blood spots (DBS) were prepared for HSV-2 serology. Rapid HIV testing was conducted at both time points, and results were immediately disclosed; pre- and post-test counselling followed established health system protocols, with guardians present for disclosure to minor adolescents. For HSV-2 serology, we tested serum obtained at baseline using HSV-2 ELISA (Kalon Biological, Guildford, UK) [21]. To test DBS obtained endline, a 6mm disc was punched from a spot into 150 μL phosphate-buffered saline and eluted at 4°C overnight. DBS eluates were tested using the HerpeSelect HSV-2 ELISA (Focus Diagnostics, Cypress, CA), which has been widely used for HSV-2 serology from DBS specimens [21-24]. For both Kalon and Focus tests, we applied a study-designed algorithm using a higher cut off (1.5) than that specified by the manufacturers' (1.0) to maximize test specificity and reduce the likelihood of false positive results. HSV-2 results were disclosed (with guardians for minors) along with counseling to participants testing positive for HSV-2, and an additional follow-up contact was made to assess and monitor participant well-being [22]. New HIV and HSV-2 infections, defined as positive tests from participants who were negative at baseline, were used as outcomes.
Survey Measures
We conducted the annual survey using audio computer-assisted self-interview (ACASI) on personal digital assistant (PDA) devices. The questionnaire was originally developed from several validated instruments and has been used in previous studies in SSA [11,12,17]. The following self-reported measures were used: School dropout, indicating whether or not the participant was out of school at endline; Highest grade achieved at endline; School absence, measured by the frequency of school absence during the last term; Sexual debut, a dichotomous response to the question “Have you ever had sexual intercourse?”; Ever married, a dichotomous variable based on currently or having ever married or cohabited with a sexual partner; and Ever pregnant, among girls by whether they were pregnant or had ever given birth or had a miscarriage, abortion, or stillbirth. Sexual risk behavior measures, restricted to those who reported sexual debut, were: Transactional sex (“Did you receive favors, gifts, or money in return for sex? Did you give favors, gifts, or money in return for sex?” Yes or No), Age of sexual debut, Forced sex (“The first or only time you had sexual intercourse, did you want it to happen?” Yes or No), Lifetime number of sexual partners, Condom use in the last year (“how often did you or your partner use condoms?” Yes or No), and New circumcision (a dichotomous measure who were circumcised at endline, but uncircumcised at baseline and asked of boys only). Quality of life (QALY) was based on the EQ-5D assessment instrument measuring functional problems (range=1-3) on five items: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [25]. Future Schooling Expectations was measured by questions about perceived chances of completing secondary school and beyond (1=no chance to 5=almost certain).
Statistical Analyses
We conducted significance tests on the main demographic and outcome variables using t-tests for continuous variables and Rao-Scott chi-square tests for categorical variables to compare study condition group equivalence at baseline as well as to compare respondents and non-respondents at endline. To evaluate intervention effects using endline outcomes, we used regression for continuous outcomes and logistic regression for binary outcomes using survey procedures in SAS. We used generalized estimating equations (GEE) to test differential change from baseline to follow-ups over time using longitudinal data. The study is longitudinal with repeated measurements of the same orphans, nested within schools; thus we accounted for clustering in primary schools, and analyzed correlated outcomes with reasonable statistical efficiency [26]. The models assessed condition (intervention vs. control), time (as a continuous variable), and the condition by time interaction, controlling for participant age, participant biological sex, and a count index of household SES (13 items). We initially tested dummy variables for both single versus double orphans and maternal versus paternal orphans and found no statistically significant effect from either classification on any of our outcomes. Therefore, we dropped these variables in our final analyses for the paper. Analyses were conducted using SAS software.
Results
The total study enrollment at baseline was 837 (Figure 1). About half were females and the mean age was 15 years old (range=11-20 years). One study participant turned out to be ineligible for the study and one participant withdrew from the study; thus, 835 was the effective study sample. Four additional participants died. Overall retention was 97-98% at Waves 2-3. At endline, 90% of participants were retained, and differences by condition were not significant.
Figure 1. Study Design Flowchart.
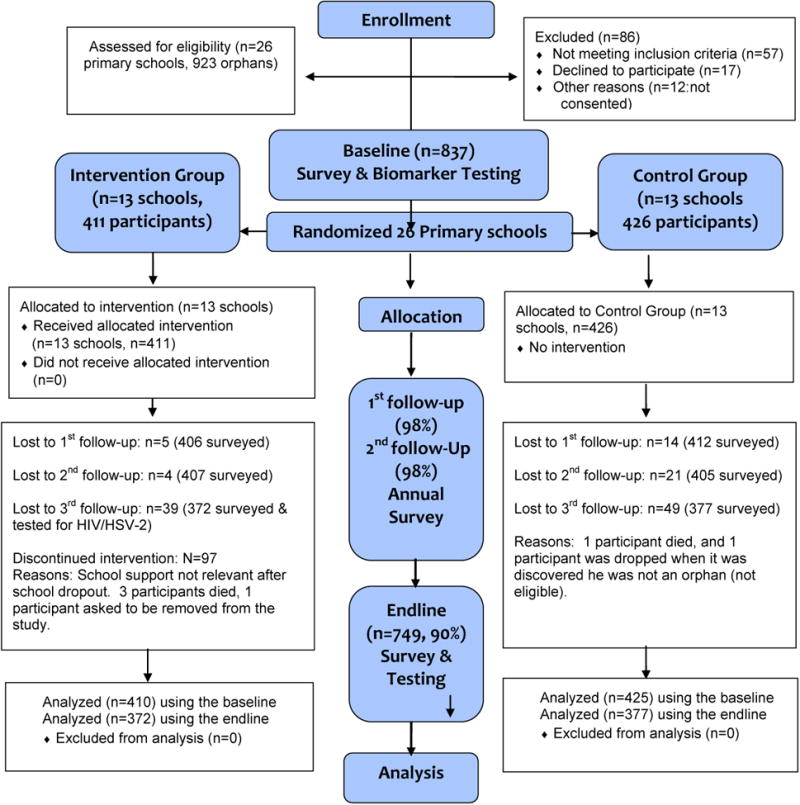
Baseline Equivalence/Attrition (Table 1)
Table 1. Baseline Equivalence between Intervention and Control Groups (All study participants at 2011 & Completers at 2014).
| All Study Participants at 2011 (n=835) | Study Participants who completed Endline at 2014 (n=749) | |||||
|---|---|---|---|---|---|---|
| GROUP | Intervention Group N = 410 | Control Group N= 425 | Intervention Group N = 372 | Control Group N= 377 | ||
| BASELINE VARIABLES | Mean (SD) Number (%) | Mean (SD) Number (%) | Rao-Scott chi-square (p-value) | Mean (SD) Number (%) | Mean (SD) Number (%) | Rao-Scott chi-square (p-value) |
| Demographic Variables | ||||||
| Age in 2011 | 14.8 (1.5) | 14.8 (1.6) | 0.62 (0.53) | 14.7 (1.5) | 14.8 (1.6) | 0.85 (0.39) |
| Gender | ||||||
| Male | 213 (52.0%) | 219 (51.5%) | 0.02 (0.88) | 200(53.8%) | 202 (53.6%) | 0.003 (0.96) |
| Female | 197 (48.0%) | 206 (48.5%) | 172(46.2%) | 175(46.4%) | ||
| Orphan Status | 4.24 (0.24) | 1.22(0.31) | ||||
| Paternal | 199 (48.5%) | 228 (53.7%) | 185(49.7%) | 207(54.9%) | ||
| Maternal | 54 (13.2%) | 47 (11.1%) | 48(12.9%) | 43(11.4%0 | ||
| Double Orphan | 146 (35.6%) | 145 (34.1%) | 129(34.7%) | 123(32.6%) | ||
| Unspecified | 11 (2.7%) | 5 (1.2%) | 10(2.7%) | 4(1.1%) | ||
| SES count index (alpha=.61, 13 items) | 3.82 (1.91) | 3.85 (1.92) | 0.23 (0.82) | 3.81(0.10) | 3.82 (0.10) | 0.09(0.93) |
| Education variables | ||||||
| School Uniform (yes) | 255 (62.5%) | 272 (64.2%) | 0.24 (0.62) | 233(63.0%) | 241 (64.1%) | 0.10(0.75) |
| Future Expectations (1=almost no chance ∼ 5=almost certain) | ||||||
| Graduate from high school | 4.13(1.14) | 3.97(1.22) | -1.92(0.05) | 4.16(0.06) | 3.97(0.06) | -2.16(0.03)* |
| Graduate from College/University | 4.02(1.26) | 3.87(1.32) | -1.72(0.09) | 4.05(0.07) | 3.92(0.07) | -1.48(0.14) |
| Sexual Risk Behaviors | ||||||
| Sexual Debut (Yes) | 90 (22.0%) | 94 (22.2%) | 0.004 (0.95) | 80(21.5%) | 76(20.3%) | 0.09(0.76) |
| Married/Living together (Yes) | 37 (9.0%) | 46 (10.9%) | 0.90 (0.34) | 36(9.7%) | 36(9.6%) | 0.002(0.96) |
| Pregnancy (Yes, female only) | 1 (0.2%) | 5 (1.2%) | 2.84(0.09) | 1(0.27) | 3(0.80) | 0.90(0.35) |
| QALY | 0.71 (0.18) | 0.69 (0.19) | -1.20(0.23) | 0.71(0.01) | 0.69(0.01) | -1.39(0.16) |
| Circumcision (Yes, male only) | 72 (34.3%) | 45 (20.8%) | 2.10 (0.15) | 70(35.5%) | 40(20.16%) | 2.86(90.10) |
| Biomarker Data | ||||||
| HIV Infection (Positive) | 5 (1.2%) | 5 (1.2%) | 0.004(0.95) | 3(0.81) | 2(0.27) | 0.19(0.67) |
| HSV-2 Infection (Positive vs. Non-positive using the study cutoff 1.5) | 17 (4.2%) | 10 (2.4%) | 2.98(0.08) | 17(4.6%) | 8(2.12%) | 4.76(0.04)* |
p≤.05
p≤.01
There were no significant differences (p<.05) by condition at baseline in the full sample (Table 1). Overall, endline responders were more likely to be male, younger, and virgins at baseline compared to non-responders. Also, intervention group endline responders had higher future expectations of graduating from high school and were more likely to be HSV-2 positive at baseline compared to control responders.
Results of Regression/Logistic Regressions Analysis at Endline (Table 2)
Table 2. Regression/Logistic Regression Analyses at Endline.
| Outcomes | Frequency | Group (ref=Control) |
Age | Gender (ref=male) |
SES |
|---|---|---|---|---|---|
| Freq (%)/Rao-Scott λ2 Mean(Sd)/T test score |
AOR (CI) | AOR (CI) | AOR (CI) | AOR (CI) | |
| Education Outcomes | |||||
| School Dropout (Yes) | 107 (14.3%) E =28 (7.53%) C = 79 (21.0%) λ2=195.89 (p=<.0001)** |
0.30 (0.18-0.52) p < 0.0001** |
1.50 (1.29-1.75) p< 0.0001** |
1.98 (1.19 – 3.28) p=0.008** |
0.94 (0.81 - 1.07) p = 0.34 |
| Highest Grade Achieveda | 9.62 (1.16) E=9.83 (1.09) C=9.41 (1.19) T test=-5.04 (p=<.0001)** |
0.43 p=.0001** |
0.006 p=0.85 |
-0.23 p=0.05 |
0.03 p=0.13 |
| Biomarker Data | |||||
| New HIV Infection | 5 (0.67%) E=2 (0.54) C=3 (0.80) λ2=0.23, p=0.63 |
0.72 (0.15-3.42) p=0.68 |
1.34 (1.04-1.74) p=0.03* |
2.24 (0.37-13.63) p=0.38 |
0.75 (0.48-1.16) p=0.19 |
| New HSV-2 Infection (study cutoff) | 118 (16.30%) E=56 (15.82%) C=62 (16.76%) λ2=0.08, p=0.96 |
0.98(0.54-1.78) p=0.94 |
0.96 (0.82-1.14) p=0.65 |
1.15 (0.76-1.73) p=0.51 |
1.01 (0.95-1.11) p=0.47 |
| Sexual Risk Behavior | |||||
| Marriage (Yes) | 35 (5.17%) E=14 (4.17%) C = 21 (6.16%) λ2=0.62 (p=0.43) |
0.68(0.24-1.97) p=0.48 |
1.54 (1.24-1.91) p<.0001** |
3.13 (1.50-6.56) p=0.0002** |
0.99 (0.75-1.30) p=0.92 |
| Pregnancy (Yes) (Female only) | 49 (14.29%) E = 20(11.70%) C = 29 (16.86%) λ2=2.05 p=0.16 |
0.65(0.35-1.19) p=0.16 |
1.39(1.13-1.71) p=0.002** |
NA | 0.98 (0.84-1.14) p=0.78 |
| Sexual Debut (Yes) | 145 (24.49%) E = 66 (22.68%) C=79 (26.25%) λ2=0.78, p=0.38 |
0.83 (0.55-1.27) p=0.39 |
.34 (1.21-1.48) p=<.0001** |
1.12 (0.77-1.62) P=0.55 |
0.98 (0.95-1.12) p=0.45 |
| Circumcision (Yes) Male only | 182 (62.33) E=90 (69.2%) C=92 (57.1%) λ2=4.49 p=0.03** |
1.66 (1.02-2.70) p=0.04* |
0.98 (0.84-1.15) p=0.85 |
NA | 0.95(0.83-1.09) p=0.48 |
| Among sexually active participants | |||||
| Transactional Sex (Yes) | 61 (23.37%) E=21 (16.7%) C=40 (29.6%) λ2=7.75 (p=0.01)** |
0.49 (0.25-0.96) p=0.03* |
1.13 (0.89-1.41) p=0.50 |
4.69 (2.53-8.67) p=<.0001** |
1.15 (0.97-11.36) p=0.11 |
| Forced Sex (Yes) | 87(33.46%) E=43 (34.1%) C=44 (32.8%) λ2=0.05 (p=0.83) |
0.01(0.62=1.65) p=0.95 |
0.19 (0.85-1.2) p=0.90 |
0.40(0.20-0.78) p=0.01** |
1.04 (0.90-1.20) p=0.59 |
| Age of the First Sexa (9 younger -16 or older) | 14.26 (2.25) E=14.15 (2.37) C=14.36 (2.15) T=0.73 (p=0.46) |
-0.14 p=0.61 |
0.30 p=0.001** |
1.03 p= 0.0002** |
-0.07 p=0.35 |
| Number of Sexual Partnersa (1∼ 6 or more) | 1.84(1.10) E=1.59 (0.91) C=2.02 (0.99) T=1.69 (p=0.09) |
-0.45 p=0.07 |
0.05 p=0.57 |
-0.60 p=0.01** |
0.03 p=0.62 |
| Condom use at the last year (Yes) | 67(90.54%) E=29 (90.63%) C=38 (90.48%) λ2=0.001 (p=0.98) |
0.012 (-0.12-0.14) p=0.86 |
0.04(0.001-0.09) p=0.04* |
-0.07(-0.20-0.06) p=0.29 |
0.03 (-0.01-0.06) p=0.14 |
Regression analysis was used for continuous outcome.
p≤.05
p≤.01
New HIV and HSV-2 Infections
Survey logistic regression analyses on new HIV and HSV-2 infections showed no significant impact by intervention, gender, or SES. Older age was significantly associated with new HIV infection only.
Education/Sexual Risky Behaviors at Endline
Compared to controls, intervention youth were less likely to drop out of school and achieved a higher average grade level in school (p≤.001). The odds of circumcision for male participants were about two times higher among intervention participants vs. control (p=0.04). Among sexually active participants, intervention participants were less likely than control participants to report transactional sex (p=0.03). Gender was significantly associated with transactional sex, with more females than males reporting that they had both received (32 girls vs. 9 boys) and given (14 girls vs. 12 boys) gifts for sex.
Longitudinal Study Outcomes (Table 3)
Table 3. Results of GEE Analysis using longitudinal data.
| Experimental Group | Control Group | Study Condition *Time | Condition | Time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T 1 | T 2 | T3 | T 4 | T 1 | T 2 | T 3 | T 4 | Estimate (p value) |
Estimate p-value |
Estimate p-value |
|
| School absence (1=never∼ 5=more than 3 days a month) | 2.16 | 2.00 | 1.96 | 2.16 | 2.18 | 2.41 | 2.61 | 3.11 |
-0.30 p<.0001** |
-0.05 p=0.53 |
0.30 p<.0001 |
| Food Security (# of meals per day, 1∼5) | 2.20 | 2.19 | 2.33 | 2.12 | 2.18 | 2.19 | 2.27 | 2.04 | 0.02 p=0.24 |
0.00 p=0.99 |
-0.03 p=0.03** |
| Future expectation: Chances completing secondary school (1=no chance, 5=almost certain) | 4.13 | 4.01 | 3.78 | 3.87 | 3.97 | 3.70 | 3.44 | 3.51 | 0.06 p=0.08 |
0.19 P=0.01** |
-0.17 p<.0001 |
| Future expectation: Graduating from College/Univ. (1=no chance, 5=almost certain) | 4.02 | 3.96 | 3.75 | 3.77 | 3.87 | 3.63 | 3.37 | 3.35 |
0.09 p=0.01** |
0.19 p=0.02* |
-0.19 p<0.000 1** |
| QALY Index (1∼3) | 0.71 | 0.71 | 0.75 | 0.71 | 0.69 | 0.71 | 0.75 | 0.67 | 1.08 p=-.30 |
0.01 P=0.91 |
0.14 p=0.71 |
| Mobile (1=No problem, 2=some problem in walking about, 3=confined to bed) |
1.33 | 1.34 | 1.28 | 1.39 | 1.35 | 1.32 | 1.28 | 1.46 | 0.40 p=0.52 |
0.05 p=0.83 |
4.09 p=0.04* |
| Self-care (1=No problem,2=some problems with self-care, 3=unable to wash or dress myself) |
1.38 | 1.36 | 1.28 | 1.39 | 1.40 | 1.38 | 1.28 | 1.49 | 0.28 p=0.60 |
0.00 p=0.93 |
0.20 p=0.65 |
| Usual Activity (1=No problem, 2=some problems performing usual activities, 3=unable to perform my usual activities) |
1.40 | 1.41 | 1.32 | 1.41 | 1.38 | 1.38 | 1.33 | 1.52 |
7.63 p=0.006** |
4.29 p=0.04* |
1.31 p= 0.25 |
| Pain/Discomfort (1=No problem, 2= moderate pain or discomfort, 3=have extreme pain or discomfort) |
1.64 | 1.68 | 1.31 | 1.71 | 1.76 | 1.62 | 1.51 | 1.76 | 2.05 p=0.15 |
2.04 p=0.15 |
0.00 p=0.96 |
| Anxiety/Depression (1=am not anxious or depressed, 2=moderately anxious or depressed, 3=extremely anxious or depressed) |
1.90 | 1.87 | 1.53 | 1.74 | 1.88 | 1.89 | 1.68 | 1.99 |
8.12 p=0.004** |
1.80 p=0.18 |
8.66 p=0.003 |
p≤.05
p≤.01
Intervention participants were absent from school less frequently and believed they had better chances of completing college/university (p=0.01). Among health-related quality of life items, the intervention group was less likely to report problems with depression/anxiety and performing usual activities compared to the control group.
Discussion
The purpose of this study was to conduct a rigorous impact evaluation of a school support intervention on HIV prevention among orphan boys and girls in western Kenya. The three year school support program did not result in lower HIV or HSV-2 infection among Kenyan orphans. HIV prevalence in the total sample was lower than expected, with only five new incident cases after three years. Since we conducted HIV testing at baseline, new HIV cases are known to have been acquired sexually rather than through mother-to-child transmission. Although the study lacked power to detect HIV incidence by condition, we found 116 new cases of HSV-2 after three years, using a more stringent cutoff than the manufacturer. The lack of intervention effect on HSV-2 incidence as a marker of sexual risk adds to questions about the likelihood that school support reduces HIV risk among orphans.
Positive and significant program impacts on educational outcomes is requisite to supporting the underlying theory of this intervention, and the study indeed found reduced school dropout and reduced school absenteeism among intervention participants compared to the control group. However, despite the intervention's direct impacts on schooling, there were few effects on HIV-related risk factors as hypothesized -- only transactional sex and circumcision. School fee support reduced the odds for engaging in transactional sex among intervention participants compared to controls. Notably, we found that girls were just as likely as boys to report that they gave gifts for sex as boys. Recent studies in Tanzania, Malawi, and Kenya have suggested that while some young women engage in transactional sex due to survival needs, gift giving is also normative in dating relationships [27-29]. Our findings of both boys and girls giving and receiving gifts may suggest that, for some, gifts and favors were given in the context of intimate relationships rather than transactional sex. Other studies, however, suggest that transactional sex, while increasingly seen as normal and acceptable [27], may lessen a young woman's ability to negotiate safe sex practices [29]. Thus, our finding of a significant reduction in transactional sex with school support likely indicates an important beneficial outcome.
In addition, we found a significant impact on circumcision rates among male participants, with intervention participants being about two times more likely to be newly circumcised by the end of the study than controls. Male circumcision has been widely recommended and implemented as an HIV prevention tool in African countries including Kenya [30]. Kenya's Ministry of Health promoted the implementation of voluntary medical male circumcision, prioritizing high HIV prevalence and non-circumcising ethnic groups such as the Luos in the Nyanza area [31]. Since intervention group participants were more likely to stay in school, they would have been more likely to be included in public health campaigns targeting schools.
Despite using a rigorous experimental design, we found no impacts on biomarkers of HIV/HSV-2 infections. All other RCTs of school support also have found no significant impact on HIV infection except for one study conducted in Malawi [15]. That study, however, also noted low HIV prevalence and could not determine whether infection was already present prior to intervention because biomarkers were not collected at baseline. Further, while each of the previous school support/CCT studies found an impact on a few sexual risk factors, these factors were inconsistent across studies, suggesting no clear underlying mechanism of association between schooling and sexual risk behaviors.
It is possible that the beneficial impact of school support takes longer to materialize, as students grow older. Given the escalation in risk behaviors and sexually transmitted infections in later adolescence and young adulthood, it may be important to examine the longer term impacts of school support interventions, but a clear rationale for investing in this line of research is lacking. It is also possible that combining school support with other interventions might be useful, as suggested by Duflo and colleagues who found a reduction in HSV-2 infection with both uniform provision and an enhanced HIV-prevention program [10]. However, the evidence for maintaining the effectiveness of school-based prevention programs over time is bleak [32,33]. On the other hand, while there is scant support for linking schooling and HIV prevention, other important benefits are much more likely to accrue for vulnerable youth who stay in school, including improved employment opportunities, better health, greater child survival, and citizens who are more able to participate in democratic forms of government [34-36].
There are several limitations to this study. Our sample is limited to Luo youth in the Nyanza area, which has limited generalizability. The average age of participants was just under 15 years at baseline, while the median age of sexual debut in Siaya County is 16.6 years [37]. However, the average age at endline was 18 years and since almost 19% of the sample was HSV-2 positive, a majority of youth were likely to have been sexually active. Although all students resided in Siaya District at baseline, many had scattered throughout the country by endline, suggesting geographic variability in contexts of HIV and HSV-2 prevalence. We did not measure participant exposure to school-based prevention programs, but since a standard HIV prevention curriculum has been in place in all Kenya schools since 2005 [32,38], students from both conditions should have had an equivalent opportunity for exposure to such programs. Although ACASI can be expected to help reduce bias [39], self-reported survey measurement of sexual risk was found to be inconsistent with biomarker measurement at baseline [22,40].
In summary, our school support intervention removed financial barriers to educational access by providing school fees and uniforms to orphan adolescents. The value of the school support given in this study was more generous compared to that of CCT studies, and implementation fidelity of fees and uniforms was high [19]. However, despite helping orphaned adolescents stay in school, intervention effects on HIV risk outcomes were very limited after three years of program implementation, and no differences on biomarkers were detected. Our study adds to the growing body of research showing weak evidence for keeping youth in school as HIV prevention.
Acknowledgments
This study was funded by the National Institute of Mental Health, National Institutes of Health (R01MH09225, Hyunsan Cho, P.I.). We thank Janet Itindi, Benson Milimo, Carolyn Atieno, David Okumu, Everlyn,Aloo, James Oguta, Mark Wanyama, Joan Amani, and Bonita Iritani, Shane Hartman for their important contributions to the project.
Abbreviations
- ACASI
audio computer-assisted self-interview
- CCT/UCT
conditional/unconditional cash transfers
- DBS
dried blood spots
- GEE
generalized estimating equations
- HSV-2
Herpes Simplex Virus-2
- OVC
orphans and vulnerable children
- PDA
personal digital assistant
- QALY
Quality of life
- RCT
randomized controlled trials
- SES
socioeconomic status
- SSA
Sub-Saharan Africa
Footnotes
Disclosure of potential conflicts: None
Clinical trials registry site and number: ClinicalTrials.gov NCT01501864
Implications and Contribution: Three years of experimentally testing support to stay in school as an intervention showed few effects on HIV-related outcomes and no impact on HIV or HSV-2 biomarkers. Given similar findings from most other rigorously implemented trials, the evidence for school support as HIV prevention appears to be weak.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller E, Samson S. HIV-Sensitive Social Protection: State of the Evidence 2012 in sub-Saharan Africa. Cape Town, South Africa: UNICEF and the Economic Policy Research Institute; [Google Scholar]
- 2.Kidman R, Anglewicz P. Are adolescent orphans more likely to be HIV-positive? A pooled data analyses across 19 countries in sub-Saharan Africa. J Epidemiol Community Health. 2016;70(8):791–7. doi: 10.1136/jech-2015-206744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luseno W, Zhang L, Rusakaniko S, Cho H, Hallfors D. HIV infection and related risk behaviors: does school support level the playing field between orphans and nonorphans in Zimbabwe? AIDS Care. 2015;27(9):1191–5. doi: 10.1080/09540121.2015.1036726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pettifor A, MacPhail C, Nguyen N, Rosenberg M. Can money prevent the spread of HIV? A review of cash payments for HIV prevention. AIDS Behav. 2012;16(7):1729–38. doi: 10.1007/s10461-012-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta GG, Parkhurst JO, Ogden JA, Aggleton P, Mahal A. Structural approaches to HIV prevention. Lancet. 2008;372:764–775. doi: 10.1016/S0140-6736(08)60887-9. [DOI] [PubMed] [Google Scholar]
- 6.World Bank. The State of Social Safety Nets 2014. Washington D.C.: World Bank; 2014. Available at: http://www.worldbank.org/en/topic/safetynets/publication/the-state-of-social-safety-nets-2014. [Google Scholar]
- 7.United Nations Development Programme (UNDP) Cash Transfers and HIV Prevention. New York: UNDP; 2014. Discussion Paper. [Google Scholar]
- 8.Baird S, McIntosh C, Ozler B. Schooling, income, and HIV risk: experimental evidence from a cash transfer program; XVIII International AIDS Conference; Vienna, Austria. July 18-23, 2010.; [Accessed December 15, 2010]. Available at http://pag.aids2010.org/session.aspx?s=70#2< http://pag.aids2010.org/session.aspx?s=70>. [Google Scholar]
- 9.Duflo E, Dupas P, Kremer M, Sinei S. Education and HIV/AIDS Prevention: Evidence from a randomized evaluation in Western Kenya. World Bank Policy Research Working Paper 4024, Background Paper to the 2007 World Development Report; 2006. [Google Scholar]
- 10.Duflo E, Dupas P, Kremer M. Education, HIV, and Early Fertility: Experimental Evidence from Kenya. Am Econ Rev. 2015;105(9):2757–97. doi: 10.1257/aer.20121607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallfors DD, Cho H, Rusakaniko S, Iritani BJ, Mapfumo J, Halpern CT. Supporting Adolescent Orphan Girls to Stay in School as HIV Risk Prevention: Evidence from a Randomized Controlled Trial in Zimbabwe. American Journal of Public Health. 2011;101(6):1082–1088. doi: 10.2105/AJPH.2010.300042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallfors DD, Cho H, Rusakaniko S, Mapfumo J, Iritani B, Zhang L, et al. The Impact of School Subsidies on HIV-Related Outcomes Among Adolescent Female Orphans. J Adolesc Health. 2015;56(1):79–84. doi: 10.1016/j.jadohealth.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iritani BJ, Cho H, Rusakaniko S, Mapfumo J, Hartman S, Hallfors DD. Educational Outcomes for Orphan Girls in Rural Zimbabwe: Effects of a School Support Intervention. Health Care Women Int. 2016;37(3):301–22. doi: 10.1080/07399332.2015.1017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pettifor A, MacPhail C, Hughes JP, et al. The effect of a conditional cash transfer on HIV incidence in young women in rural South Africa (HPTN 068): a phase 3, randomised controlled trial. Lancet Global Health. 2016;4(12):e978–e988. doi: 10.1016/S2214-109X(16)30253-4. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baird SJ, Garfein RS, McIntosh CT, Ozler B. Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. Lancet. 2012;379(9823):1320–9. doi: 10.1016/S0140-6736(11)61709-1. [DOI] [PubMed] [Google Scholar]
- 16.Ferrand RA, Corbett EL, Wood R, Hargrove J, Ndhlovu CE, Cowan FM, et al. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS. 2009;23(15):2039–46. doi: 10.1097/QAD.0b013e32833016ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho H, Hallfors DD, Mbai II, Itindi J, Milimo BW, Halpern CT, et al. Keeping adolescent orphans in school to prevent human immunodeficiency virus infection: evidence from a randomized controlled trial in Kenya. J Adolesc Health. 2011;48(5):523–6. doi: 10.1016/j.jadohealth.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallfors DD, Cho H, Mbai I, Milimo B, Itindi J. Process and outcome evaluation of a community intervention for orphan adolescents in western Kenya. J Community Health. 2012;37(5):1101–9. doi: 10.1007/s10900-012-9548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallfors D, Cho H, Hartman S, Mbai I, Atieno C. Process Evaluation of a Clinical trial to Test School Support as HIV Prevention Among Orphans in Western Kenya. doi: 10.1007/s11121-017-0827-8. Unpublished manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National AIDS Control Council of Kenya. Kenya AIDS Response Progress Report, 2014 Progress Towards Zero. Nairobi: National AIDS Control Council of Kenya; 2014. [Google Scholar]
- 21.Luseno WK, Hallfors DD, Cho H, et al. Use of HIV and HSV-2 biomarkers in subsaharan adolescent prevention research: a comparison of two approaches. J Prim Prev. 2014;35:181–191. doi: 10.1007/s10935-014-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallfors DD, Cho H, Mbai II, Millimo BW, Atieno C, Okumu D, et al. Disclosure of HSV-2 serological test results in the context of an adolescent HIV prevention trial in Kenya. Sex Transm Infect. 2015;91(6):395–400. doi: 10.1136/sextrans-2015-052025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdool Karim Q, Kharsany AB, Leask K, et al. Prevalence of HIV, HSV-2 and pregnancy among high school students in rural KwaZulu-Natal, South Africa: a biobehavioural cross-sectional survey. Sex Transm Infect. 2014;90:620–626. doi: 10.1136/sextrans-2014-051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charpentier C, Koyalta D, Ndinaromtan M, et al. Distribution of HIV-1 and HSV-2 epidemics in Chad revealing HSV-2 hot-spot in regions of high-risk HIV spread. J Infect Dev Ctries. 2011;5:64–67. doi: 10.3855/jidc.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jelsma J, Hansen K, De Weerdt W, De Cock P, Kind P. How do Zimbabweans value health states? Popul Health Metr. 2003;1(1):11. doi: 10.1186/1478-7954-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang K, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 27.Wamoyi J, Fenwick A, Urassa M, Zaba B, Stones W. “Women's bodies are shops”: beliefs about transactional sex and implications for understanding gender power and HIV prevention in Tanzania. Arch Sexual Beh. 2011;40(1):5–15. doi: 10.1007/s10508-010-9646-8. [DOI] [PubMed] [Google Scholar]
- 28.Poulin M, Dovell K, Watkins SC. Men with money and the “vulnerable women” client category in an AIDS epidemic. World Development. 2016;85:16–30. [Google Scholar]
- 29.Luke N. Economic Status, Informal Exchange, and Sexual Risk in Kisumu, Kenya. Economic Development and Cultural Change. 2008;56(2):375–396. doi: 10.1086/522896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization, UNAIDS. New Data on Male Circumcision and HIV Prevention: Policy and Programme Implications. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 31.Rosenberg J, Cole C, May M, Weintraub R. Cases in Global Health delivery: Voluntary Medical Male Circumcision in Nyanza Province, Kenya. Lancet Commission on Global Surgery. 2015 Available at: http://www.globalsurgery.info/wpcontent/uploads/2015/08/Kenya-Circumcision-Teaching-Case.pdf.
- 32.Matthews EJ, Puffer ES, Meade CS, Broverman SA. Implementation of a School-Based HIV Prevention Curriculum Following National Dissemination in Nyanza Province, Kenya. East African Medical Journal. 2014;91(5):152–160. [PubMed] [Google Scholar]
- 33.Mason-Jones AJ, Sinclair D, Mathews C, Kagee A, Hillman A, Lombard C. School-based interventions for preventing HIV, sexually transmitted infections, and pregnancy in adolescents. The Cochrane database of systematic reviews. 2016;11 doi: 10.1002/14651858.CD006417.pub3. CD006417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gakidou E, Cowling K, Lozano R, Murray CJ. Increased educational attainment and its effect on child mortality in 175 countries between 1970 and 2009: a systematic analysis. Lancet. 2010;376(9745):959–974. doi: 10.1016/S0140-6736(10)61257-3. [DOI] [PubMed] [Google Scholar]
- 35.Psacharopoulos G, Patrinos HA. Returns to investment in education: a further update. Education Economics. 2004;12(2):111–134. [Google Scholar]
- 36.Wyndow P, Li J, Mattes E. Female empowerment as a core driver of democratic development: A dynamic panel model from 1980 to 2005. World Development. 2013;52(1):34–54. [Google Scholar]
- 37.Kenya National Bureau of Statistics. Kenya Demographic Health Survey 2014. Nairobi: Kenya National Bureau of Statistics; 2014. [Google Scholar]
- 38.Maticka-Tyndale E, Mungwete R, Jayeoba O. Replicating impact of a primary school HIV prevention programme: primary school action for better health, Kenya. Health Education Research. 2014;29(4):611–623. doi: 10.1093/her/cyt088. [DOI] [PubMed] [Google Scholar]
- 39.Langhaug LF, Sherr L, Cowan FM. How to improve the validity of sexual behaviour reporting: systematic review of questionnaire delivery modes in developing countries. Trop Med Int Health. 2010;15(3):362–81. doi: 10.1111/j.1365-3156.2009.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho H, Luseno W, Halpern C, Zhang L, Mbai I, Milimo B, et al. Discordance of HIV and HSV-2 biomarkers and self-reported sexual behaviour among orphan adolescents in Western Kenya. Sex Transm Infect. 2015;91(4):260–5. doi: 10.1136/sextrans-2014-051720. [DOI] [PMC free article] [PubMed] [Google Scholar]


